Liver Organoids as an In Vitro Model to Study Primary Liver Cancer
Abstract
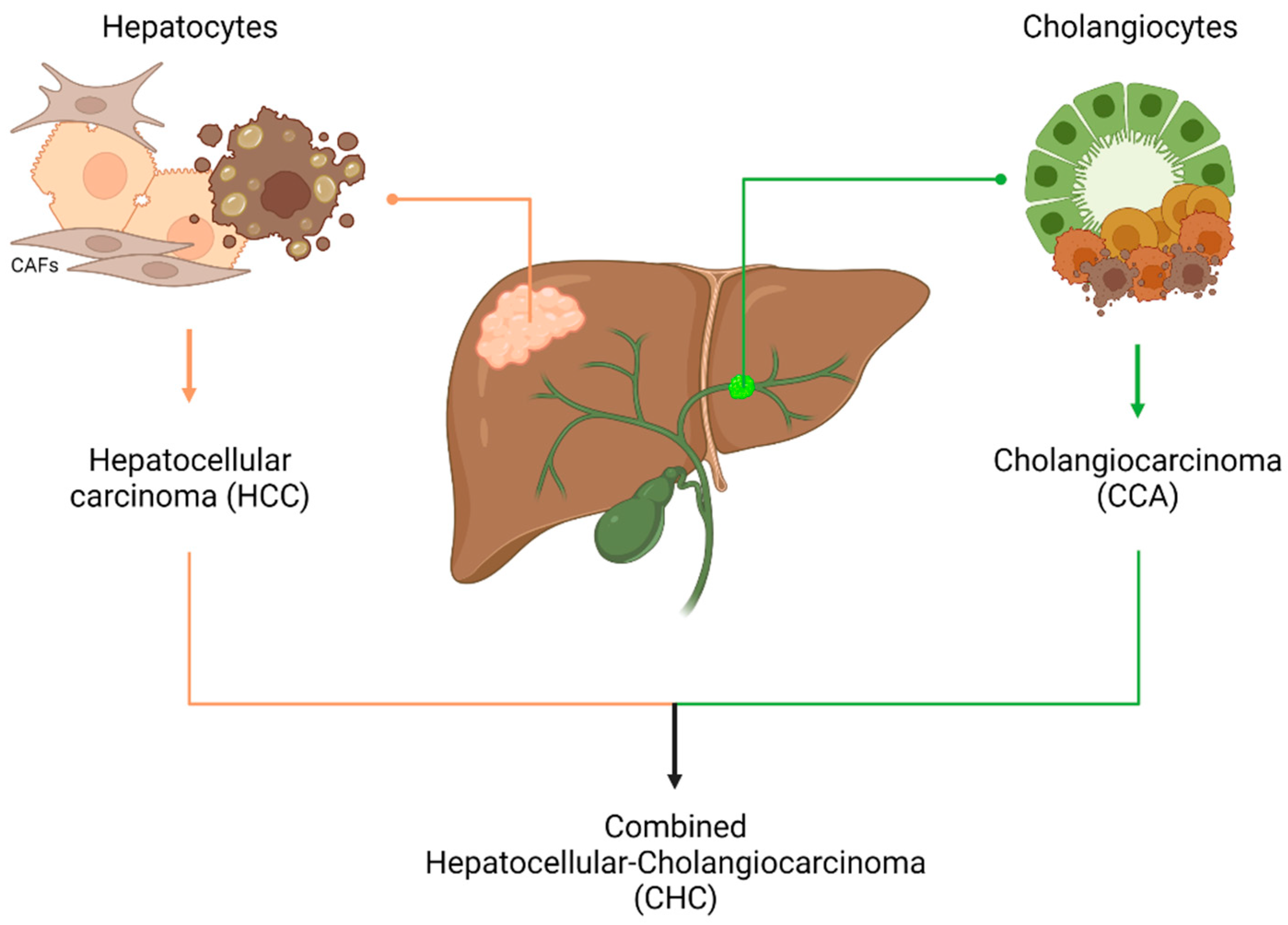
1. Introduction
2. Traditional In Vitro Model to Study Liver Cancer
3. Three-Dimensional Cell Culture (3D)
3.1. Spheroids
3.2. Scaffold-Based 3D Systems
3.3. 3D-Bioprinting and Organs-on-a-Chip
3.4. Organoids
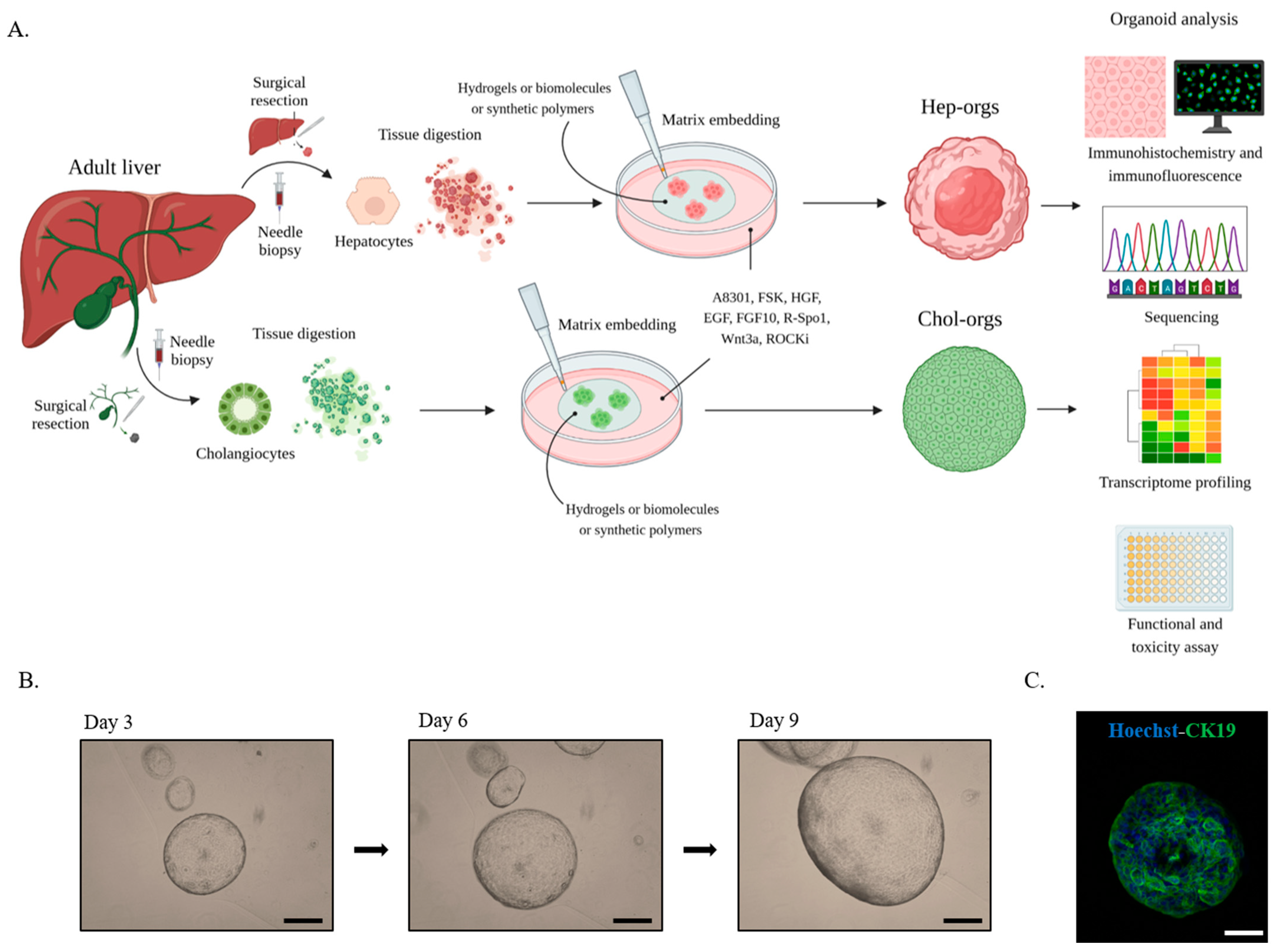
4. Liver Organoids
5. Liver organoids Characterization
6. Liver Organoids Potential Applications
7. Conclusions and Future Directions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, L.; Hui, L. Progress in human liver organoids. J. Mol. Cell Biol. 2020, 12, 607–617. [Google Scholar] [CrossRef] [PubMed]
- Sia, D.; Villanueva, A.; Friedman, S.L.; Llovet, J.M. Liver Cancer Cell of Origin, Molecular Class, and Effects on Patient Prognosis. Gastroenterology 2017, 152, 745–761. [Google Scholar] [CrossRef] [PubMed]
- Chung, B.K.; Karlsen, T.H.; Folseraas, T. Cholangiocytes in the pathogenesis of primary sclerosing cholangitis and development of cholangiocarcinoma. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 1390–1400. [Google Scholar] [CrossRef] [PubMed]
- Zajkowska, M.; Mroczko, B. Chemokines in Primary Liver Cancer. Int. J. Mol. Sci. 2022, 23, 8846. [Google Scholar] [CrossRef] [PubMed]
- Gigante, E.; Paradis, V.; Ronot, M.; Cauchy, F.; Soubrane, O.; Ganne-Carrié, N.; Nault, J.-C. New insights into the pathophysiology and clinical care of rare primary liver cancers. JHEP Rep. 2021, 3, 100174. [Google Scholar] [CrossRef] [PubMed]
- Paradis, V.; Zucman-Rossi, J. Pathogenesis of primary liver carcinomas. J. Hepatol. 2023, 78, 448–449. [Google Scholar] [CrossRef]
- Wang, G.; Wang, Q.; Liang, N.; Xue, H.; Yang, T.; Chen, X.; Qiu, Z.; Zeng, C.; Sun, T.; Yuan, W.; et al. Oncogenic driver genes and tumor microenvironment determine the type of liver cancer. Cell Death Dis. 2020, 11, 313. [Google Scholar] [CrossRef]
- Gentile, D.; Donadon, M.; Lleo, A.; Aghemo, A.; Roncalli, M.; di Tommaso, L.; Torzilli, G. Surgical Treatment of Hepatocholangiocarcinoma: A Systematic Review. Liver Cancer 2020, 9, 15–27. [Google Scholar] [CrossRef]
- Cutolo, C.; Dell’Aversana, F.; Fusco, R.; Grazzini, G.; Chiti, G.; Simonetti, I.; Bruno, F.; Palumbo, P.; Pierpaoli, L.; Valeri, T.; et al. Combined Hepatocellular-Cholangiocarcinoma: What the Multidisciplinary Team Should Know. Diagnostics 2022, 12, 890. [Google Scholar] [CrossRef]
- Li, X.; Ramadori, P.; Pfister, D.; Seehawer, M.; Zender, L.; Heikenwalder, M. The immunological and metabolic landscape in primary and metastatic liver cancer. Nat. Rev. Cancer 2021, 21, 541–557. [Google Scholar] [CrossRef]
- Elvevi, A.; Laffusa, A.; Scaravaglio, M.; Rossi, R.E.; Longarini, R.; Stagno, A.M.; Cristoferi, L.; Ciaccio, A.; Cortinovis, D.L.; Invernizzi, P.; et al. Clinical treatment of cholangiocarcinoma: An updated comprehensive review. Ann. Hepatol. 2022, 27, 100737. [Google Scholar] [CrossRef] [PubMed]
- Satriano, L.; Lewinska, M.; Rodrigues, P.M.; Banales, J.M.; Andersen, J.B. Metabolic rearrangements in primary liver cancers: Cause and consequences. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 748–766. [Google Scholar] [CrossRef] [PubMed]
- Maier, C.F.; Zhu, L.; Nanduri, L.K.; Kühn, D.; Kochall, S.; Thepkaysone, M.-L.; William, D.; Grützmann, K.; Klink, B.; Betge, J.; et al. Patient-derived organoids of cholangiocarcinoma. Int. J. Mol. Sci. 2021, 22, 8675. [Google Scholar] [CrossRef] [PubMed]
- Blidisel, A.; Marcovici, I.; Coricovac, D.; Hut, F.; Dehelean, C.; Cretu, O. Experimental models of hepatocellular carcinoma—A preclinical perspective. Cancers 2021, 13, 3651. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Hernandez-Gea, V. Hepatocellular carcinoma: Reasons for phase III failure and novel perspectives on trial design. Clin. Cancer Res. 2014, 20, 2072–2079. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Ricci, S.; Mazzaferro, V.; Hilgard, P.; Gane, E.; Blanc, J.-F.; De Oliveira, A.C.; Santoro, A.; Raoul, J.-L.; Forner, A.; et al. Sorafenib in Advanced Hepatocellular Carcinoma. N. Engl. J. Med. 2008, 359, 378–390. [Google Scholar] [CrossRef] [PubMed]
- Malik, I.A.; Rajput, M.; Werner, R.; Fey, D.; Salehzadeh, N.; von Arnim, C.A.F.; Wilting, J. Differential in vitro effects of targeted therapeutics in primary human liver cancer: Importance for combined liver cancer. BMC Cancer 2022, 22, 1193. [Google Scholar] [CrossRef] [PubMed]
- Carotenuto, M.; Sacco, A.; Forgione, L.; Normanno, N. Genomic alterations in cholangiocarcinoma: Clinical significance and relevance to therapy. Explor. Target. Anti-Tumor Ther. 2022, 3, 200–223. [Google Scholar] [CrossRef]
- Nuciforo, S.; Heim, M.H. Organoids to model liver disease. JHEP Rep. 2021, 3, 100198. [Google Scholar] [CrossRef]
- Vicent, S.; Lieshout, R.; Saborowski, A.; Verstegen, M.M.A.; Raggi, C.; Recalcati, S.; Invernizzi, P.; van der Laan, L.J.W.; Alvaro, D.; Calvisi, D.F.; et al. Experimental models to unravel the molecular pathogenesis, cell of origin and stem cell properties of cholangiocarcinoma. Liver Int. 2019, 39 (Suppl. S1), 79–97. [Google Scholar] [CrossRef]
- Tharehalli, U.; Svinarenko, M.; Lechel, A. Remodelling and Improvements in Organoid Technology to Study Liver Carcinogenesis in a Dish. Stem Cells Int. 2019, 2019, 3831213. [Google Scholar] [CrossRef]
- Brooks, A.; Liang, X.; Zhang, Y.; Zhao, C.-X.; Roberts, M.S.; Wang, H.; Zhang, L.; Crawford, D.H. Liver organoid as a 3D in vitro model for drug validation and toxicity assessment. Pharmacol. Res. 2021, 169, 105608. [Google Scholar] [CrossRef]
- Nuciforo, S.; Fofana, I.; Matter, M.S.; Blumer, T.; Calabrese, D.; Boldanova, T.; Piscuoglio, S.; Wieland, S.; Ringnalda, F.; Schwank, G.; et al. Organoid Models of Human Liver Cancers Derived from Tumor Needle Biopsies. Cell Rep. 2018, 24, 1363–1376. [Google Scholar] [CrossRef]
- Jensen, C.; Teng, Y. Is It Time to Start Transitioning From 2D to 3D Cell Culture? Front. Mol. Biosci. 2020, 7, 33. [Google Scholar] [CrossRef]
- Prior, N.; Inacio, P.; Huch, M. Liver organoids: From basic research to therapeutic applications. Gut 2019, 68, 2228–2237. [Google Scholar] [CrossRef] [PubMed]
- Bouwmeester, M.C.; Bernal, P.N.; Oosterhoff, L.A.; van Wolferen, M.E.; Lehmann, V.; Vermaas, M.; Buchholz, M.; Peiffer, Q.C.; Malda, J.; van der Laan, L.J.W.; et al. Bioprinting of Human Liver-Derived Epithelial Organoids for Toxicity Studies. Macromol. Biosci. 2021, 21, 2100327. [Google Scholar] [CrossRef] [PubMed]
- Gunti, S.; Hoke, A.T.K.; Vu, K.; London, N.R., Jr. Organoid and Spheroid Tumor Models: Techniques and Applications. Cancers 2021, 13, 874. [Google Scholar] [CrossRef] [PubMed]
- Massa, A.; Varamo, C.; Vita, F.; Tavolari, S.; Peraldo-Neia, C.; Brandi, G.; Rizzo, A.; Cavalloni, G.; Aglietta, M. Evolution of the Experimental Models of Cholangiocarcinoma. Cancers 2020, 12, 2308. [Google Scholar] [CrossRef]
- Ingelman-Sundberg, M.; Lauschke, V.M. 3D human liver spheroids for translational pharmacology and toxicology. Basic Clin. Pharmacol. Toxicol. 2022, 130 (Suppl. S1), 5–15. [Google Scholar] [CrossRef] [PubMed]
- Fiorini, E.; Veghini, L.; Corbo, V. Modeling Cell Communication in Cancer With Organoids: Making the Complex Simple. Front. Cell Dev. Biol. 2020, 8, 166. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, J.; Yin, W.; Sun, D.; Man, Z.; Jiang, S.; Ran, X.; Su, Y.; Wang, Y.; Dong, J. Generation of multicellular tumor spheroids with micro-well array for anticancer drug combination screening based on a valuable biomarker of hepatocellular carcinoma. Front. Bioeng. Biotechnol. 2022, 10, 1087656. [Google Scholar] [CrossRef] [PubMed]
- Raggi, C.; Gammella, E.; Correnti, M.; Buratti, P.; Forti, E.; Andersen, J.B.; Alpini, G.; Glaser, S.; Alvaro, D.; Invernizzi, P.; et al. Dysregulation of Iron Metabolism in Cholangiocarcinoma Stem-like Cells. Sci. Rep. 2017, 7, 17667. [Google Scholar] [CrossRef]
- Kozlowski, M.T.; Crook, C.J.; Ku, H.T. Towards organoid culture without Matrigel. Commun. Biol. 2021, 4, 1387. [Google Scholar] [CrossRef] [PubMed]
- Langhans, S.A. Three-Dimensional in Vitro Cell Culture Models in Drug Discovery and Drug Repositioning. Front. Pharmacol. 2018, 9, 6. [Google Scholar] [CrossRef]
- Benton, G.; Arnaoutova, I.; George, J.; Kleinman, H.K.; Koblinski, J. Matrigel: From discovery and ECM mimicry to assays and models for cancer research. Adv. Drug Deliv. Rev. 2014, 79–80, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Turtoi, M.; Anghelache, M.; Bucatariu, S.-M.; Deleanu, M.; Voicu, G.; Safciuc, F.; Manduteanu, I.; Fundueanu, G.; Simionescu, M.; Calin, M. A novel platform for drug testing: Biomimetic three-dimensional hyaluronic acid-based scaffold seeded with human hepatocarcinoma cells. Int. J. Biol. Macromol. 2021, 185, 604–619. [Google Scholar] [CrossRef] [PubMed]
- Buhome, O.; Wongwattanakul, M.; Daduang, J.; Limpaiboon, T. 3D Silk Fibroin-Gelatin/Hyaluronic Acid/Heparan Sulfate Scaffold Enhances Expression of Stemness and EMT Markers in Cholangiocarcinoma. Vivo 2022, 36, 1155–1167. [Google Scholar] [CrossRef]
- Datta, P.; Dey, M.; Ataie, Z.; Unutmaz, D.; Ozbolat, I.T. 3D bioprinting for reconstituting the cancer microenvironment. Npj Precis. Oncol. 2020, 4, 18. [Google Scholar] [CrossRef]
- Augustine, R.; Kalva, S.N.; Ahmad, R.; Zahid, A.A.; Hasan, S.; Nayeem, A.; McClements, L.; Hasan, A. 3D Bioprinted cancer models: Revolutionizing personalized cancer therapy. Transl. Oncol. 2021, 14, 101015. [Google Scholar] [CrossRef]
- Bae, J.; Han, S.; Park, S. Recent Advances in 3D Bioprinted Tumor Microenvironment. BioChip J. 2020, 14, 137–147. [Google Scholar] [CrossRef]
- Sun, L.; Yang, H.; Wang, Y.; Zhang, X.; Jin, B.; Xie, F.; Jin, Y.; Pang, Y.; Zhao, H.; Lu, X.; et al. Application of a 3D Bioprinted Hepatocellular Carcinoma Cell Model in Antitumor Drug Research. Front. Oncol. 2020, 10, 878. [Google Scholar] [CrossRef]
- Xie, F.; Sun, L.; Pang, Y.; Xu, G.; Jin, B.; Xu, H.; Lu, X.; Xu, Y.; Du, S.; Wang, Y.; et al. Three-dimensional bio-printing of primary human hepatocellular carcinoma for personalized medicine. Biomaterials 2021, 265, 120416. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Jin, B.; Sun, H.; Wang, Y.; Zhao, H.; Sang, X.; Yang, H.; Mao, Y. Exploring the function of stromal cells in cholangiocarcinoma by three-dimensional bioprinting immune microenvironment model. Front. Immunol. 2022, 13, 941289. [Google Scholar] [CrossRef] [PubMed]
- Shirure, V.S.; Hughes, C.C.; George, S.C. Engineering Vascularized Organoid-on-a-Chip Models. Annu. Rev. Biomed. Eng. 2021, 23, 141–167. [Google Scholar] [CrossRef] [PubMed]
- Telles-Silva, K.A.; Pacheco, L.; Komatsu, S.; Chianca, F.; Caires-Júnior, L.C.; Araujo, B.H.S.; Goulart, E.; Zatz, M. Applied Hepatic Bioengineering: Modeling the Human Liver Using Organoid and Liver-on-a-Chip Technologies. Front. Bioeng. Biotechnol. 2022, 10, 109. [Google Scholar] [CrossRef]
- Saydmohammed, M.; Jha, A.; Mahajan, V.; Gavlock, D.; Shun, T.Y.; DeBiasio, R.; Lefever, D.; Li, X.; Reese, C.; Kershaw, E.E.; et al. Quantifying the progression of non-alcoholic fatty liver disease in human biomimetic liver microphysiology systems with fluorescent protein biosensors. Exp. Biol. Med. 2021, 246, 2420–2441. [Google Scholar] [CrossRef]
- Kanabekova, P.; Kadyrova, A.; Kulsharova, G. Microfluidic Organ-on-a-Chip Devices for Liver Disease Modeling In Vitro. Micromachines 2022, 13, 428. [Google Scholar] [CrossRef]
- Lancaster, M.A.; Knoblich, J.A. Organogenesis in a dish: Modeling development and disease using organoid technologies. Science 2014, 345, 1247125. [Google Scholar] [CrossRef]
- Clevers, H. Modeling Development and Disease with Organoids. Cell 2016, 165, 1586–1597. [Google Scholar] [CrossRef]
- Tuveson, D.A.; Clevers, H. Cancer modeling meets human organoid technology. Science 2019, 364, 952–955. [Google Scholar] [CrossRef]
- Lo, Y.-H.; Karlsson, K.; Kuo, C.J. Applications of Organoids for Cancer Biology and Precision Medicine. Nat. Cancer 2020, 1, 761–773. [Google Scholar] [CrossRef] [PubMed]
- Bar-Ephraim, Y.E.; Kretzschmar, K.; Clevers, H. Organoids in immunological research. Nat. Rev. Immunol. 2019, 20, 279–293. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, R.; Bae, S.D.W.; Zhou, G.; Read, S.A.; Ahlenstiel, G.; George, J.; Qiao, L. Application of organoids in translational research of human diseases with a particular focus on gastrointestinal cancers. Biochim. Biophys. Acta Rev. Cancer 2020, 1873, 188350. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Vries, R.G.; Snippert, H.J.; Van De Wetering, M.; Barker, N.; Stange, D.E.; Van Es, J.H.; Abo, A.; Kujala, P.; Peters, P.J.; et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal Niche. Nature 2009, 459, 262–265. [Google Scholar] [CrossRef] [PubMed]
- Lancaster, M.A.; Renner, M.; Martin, C.-A.; Wenzel, D.; Bicknell, L.S.; Hurles, M.E.; Homfray, T.; Penninger, J.M.; Jackson, A.P.; Knoblich, J.A. Cerebral organoids model human brain development and microcephaly. Nature 2013, 501, 373–379. [Google Scholar] [CrossRef]
- Gao, D.; Vela, I.; Sboner, A.; Iaquinta, P.J.; Karthaus, W.R.; Gopalan, A.; Dowling, C.; Wanjala, J.N.; Undvall, E.A.; Arora, V.K.; et al. Organoid cultures derived from patients with advanced prostate cancer. Cell 2014, 159, 176–187. [Google Scholar] [CrossRef]
- Boj, S.F.; Hwang, C.-I.; Baker, L.A.; Chio, C.I.; Engle, D.D.; Corbo, V.; Jager, M.; Ponz-Sarvise, M.; Tiriac, H.; Spector, M.S.; et al. Organoid Model of Human and Mouse Pancreatic Ductal Adenocarcinoma. Cell 2016, 160, 324–338. [Google Scholar] [CrossRef]
- Van De Wetering, M.; Francies, H.E.; Francis, J.M.; Bounova, G.; Iorio, F.; Pronk, A.; Van Houdt, W.; Van Gorp, J.; Taylor-Weiner, A.; Kester, L.; et al. Prospective derivation of a living organoid biobank of colorectal cancer patients. Cell 2015, 161, 933–945. [Google Scholar] [CrossRef]
- Sachs, N.; de Ligt, J.; Kopper, O.; Gogola, E.; Bounova, G.; Weeber, F.; Balgobind, A.V.; Wind, K.; Gracanin, A.; Begthel, H.; et al. A Living Biobank of Breast Cancer Organoids Captures Disease Heterogeneity. Cell 2018, 172, 373–386.e10. [Google Scholar] [CrossRef]
- Lee, S.H.; Hu, W.; Matulay, J.T.; Silva, M.V.; Owczarek, T.B.; Kim, K.; Chua, C.W.; Barlow, L.M.J.; Kandoth, C.; Williams, A.B.; et al. Tumor evolution and drug response in patient-derived organoid models of bladder cancer. Cell 2019, 173, 515–528.e17. [Google Scholar] [CrossRef]
- Huch, M.; Dorrell, C.; Boj, S.F.; Van Es, J.H.; Li, V.S.W.; Van De Wetering, M.; Sato, T.; Hamer, K.; Sasaki, N.; Finegold, M.J.; et al. In vitro expansion of single Lgr5+ liver stem cells induced by Wnt-driven regeneration. Nature 2013, 494, 247–250. [Google Scholar] [CrossRef]
- Broutier, L.; Mastrogiovanni, G.; Verstegen, M.M.A.; Francies, H.E.; Gavarró, L.M.; Bradshaw, C.R.; Allen, G.E.; Arnes-Benito, R.; Sidorova, O.; Gaspersz, M.P.; et al. Human Primary Liver CANCER–derived Organoid Cultures for Disease Modeling and Drug Screening. Nat. Med. 2017, 23, 1424–1435. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Gehart, H.; Artegiani, B.; Löpez-Iglesias, C.; Dekkers, F.; Basak, O.; Van Es, J.; Chuva de Sousa Lopes, S.M.; Begthel, H.; Korving, J.; et al. Long-Term Expansion of Functional Mouse and Human Hepatocytes as 3D Organoids. Cell 2018, 175, 1591–1606.e19. [Google Scholar] [CrossRef] [PubMed]
- Hindley, C.J.; Cordero-Espinoza, L.; Huch, M. Organoids from adult liver and pancreas: Stem cell biology and biomedical utility. Dev. Biol. 2016, 420, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Gamboa, C.M.; Wang, Y.; Xu, H.; Kalemba, K.; Wondisford, F.; Sabaawy, H. Optimized 3D Culture of Hepatic Cells for Liver Organoid Metabolic Assays. Cells 2021, 10, 3280. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zeng, A.; Liu, Z.; Wu, C.; Song, L. Liver organoids: From fabrication to application in liver diseases. Front. Physiol. 2022, 13, 1489. [Google Scholar] [CrossRef]
- Dong, R.; Zhang, B.; Zhang, X. Liver organoids: An in vitro 3D model for liver cancer study. Cell Biosci. 2022, 12, 152. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Zhang, B.; He, Y.; Bao, J. Liver Organoids: Formation Strategies and Biomedical Applications. Tissue Eng. Regen. Med. 2021, 18, 573–585. [Google Scholar] [CrossRef]
- Huch, M.; Gehart, H.; van Boxtel, R.; Hamer, K.; Blokzijl, F.; Verstegen, M.M.; Ellis, E.; van Wenum, M.; Fuchs, S.A.; de Ligt, J.; et al. Long-term culture of genome-stable bipotent stem cells from ADULT human liver. Cell 2014, 160, 299–312. [Google Scholar] [CrossRef]
- Peng, W.C.; Logan, C.Y.; Fish, M.; Anbarchian, T.; Aguisanda, F.; Álvarez-Varela, A.; Wu, P.; Jin, Y.; Zhu, J.; Li, B.; et al. Inflammatory Cytokine TNFα Promotes the Expansion of Primary Hepatocytes in 3D Culture. Cell 2018, 175, 1607–1619.e15. [Google Scholar] [CrossRef]
- Broutier, L.; Andersson-Rolf, A.; Hindley, C.J.; Boj, S.F.; Clevers, H.; Koo, B.-K.; Huch, M. Culture and establishment of self-renewing human and mouse adult liver and pancreas 3D organoids and their genetic manipulation. Nat. Protoc. 2016, 11, 1724–1743. [Google Scholar] [CrossRef]
- Pettinato, G.; Lehoux, S.; Ramanathan, R.; Salem, M.M.; He, L.-X.; Muse, O.; Flaumenhaft, R.; Thompson, M.T.; Rouse, E.A.; Cummings, R.D.; et al. Generation of fully functional hepatocyte-like organoids from human induced pluripotent stem cells mixed with Endothelial Cells. Sci. Rep. 2019, 9, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Schneeberger, K.; Spee, B.; Costa, P.; Sachs, N.; Clevers, H.; Malda, J. Converging biofabrication and organoid technologies: The next frontier in hepatic and intestinal tissue engineering? Biofabrication 2017, 9, 013001. [Google Scholar] [CrossRef] [PubMed]
- Caiazza, C.; Parisi, S.; Caiazzo, M. Liver organoids: Updates on disease modeling and biomedical applications. Biology 2021, 10, 835. [Google Scholar] [CrossRef] [PubMed]
- Buske, P.; Przybilla, J.; Loeffler, M.; Sachs, N.; Sato, T.; Clevers, H.; Galle, J. On the biomechanics of stem cell niche formation in the gut—Modelling growing organoids. FEBS J. 2012, 279, 3475–3487. [Google Scholar] [CrossRef]
- Willemse, J.; van Tienderen, G.; van Hengel, E.; Schurink, I.; van der Ven, D.; Kan, Y.; de Ruiter, P.; Rosmark, O.; Westergren-Thorsson G., G.; Schneeberger, K.; et al. Hydrogels derived from decellularized liver tissue support the growth and differentiation of cholangiocyte organoids. Biomaterials 2022, 284, 121473. [Google Scholar] [CrossRef]
- Yoon, S.-M.; Gerasimidou, D.; Kuwahara, R.; Hytiroglou, P.; Yoo, J.E.; Park, Y.N.; Theise, N.D. Epithelial cell adhesion molecule (EpCAM) marks hepatocytes newly derived from stem/progenitor cells in humans. Hepatology 2010, 53, 964–973. [Google Scholar] [CrossRef]
- Wu, H.-J.; Chu, P.-Y. Role of Cancer Stem Cells in Cholangiocarcinoma and Therapeutic Implications. Int. J. Mol. Sci. 2019, 20, 4154. [Google Scholar] [CrossRef]
- Salas, P.J.; Forteza, R.; Mashukova, A. Multiple roles for keratin intermediate filaments in the regulation of epithelial barrier function and apico-basal polarity. Tissue Barriers 2016, 4, e1178368. [Google Scholar] [CrossRef]
- Ryu, H.S.; Lee, K.; Shin, E.; Kim, S.H.; Jing, J.; Jung, H.Y.; Lee, H.; Jang, J.-J. Comparative analysis of immunohistochemical markers for differential diagnosis of hepatocelluar carcinoma and cholangiocarcinoma. Tumori J. 2012, 98, 478–484. [Google Scholar] [CrossRef]
- Liu, L.-Z.; Yang, L.-X.; Zheng, B.; Dong, P.-P.; Liu, X.-Y.; Wang, Z.-C.; Zhou, J.; Fan, J.; Wang, X.-Y.; Gao, Q. CK7/CK19 index: A potential prognostic factor for postoperative intrahepatic cholangiocarcinoma patients. J. Surg. Oncol. 2018, 117, 1531–1539. [Google Scholar] [CrossRef] [PubMed]
- Pallerla, S.R.; Hoan, N.X.; Rachakonda, S.; Meyer, C.G.; Van Tong, H.; Toan, N.L.; Linh, L.T.K.; Giang, D.P.; Kremsner, P.G.; Bang, M.H.; et al. Custom gene expression panel for evaluation of potential molecular markers in hepatocellular carcinoma. BMC Med. Genom. 2022, 15, 235. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhang, Y.; Guo, K.; Wang, N.; Jin, H.; Liu, Y.; Qin, W. Heat shock proteins in hepatocellular carcinoma: Molecular mechanism and therapeutic potential. Int. J. Cancer 2016, 138, 1824–1834. [Google Scholar] [CrossRef] [PubMed]
- Ben Wang, B.; Lan, T.; Xiao, H.; Chen, Z.-H.; Wei, C.; Chen, L.-F.; Guan, J.-F.; Yuan, R.-F.; Yu, X.; Hu, Z.-G.; et al. The expression profiles and prognostic values of HSP70s in hepatocellular carcinoma. Cancer Cell Int. 2021, 21, 286. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Shi, Y.; Kaseb, A.O.; Qi, X.; Zhang, Y.; Chi, J.; Lu, Q.; Gao, H.; Jiang, H.; Wang, H.; et al. Chimeric Antigen Receptor-Glypican-3 T-Cell Therapy for Advanced Hepatocellular Carcinoma: Results of Phase I Trials. Clin. Cancer Res. 2020, 26, 3979–3989. [Google Scholar] [CrossRef]
- Liu, P.; Lu, D.; Al-Ameri, A.; Wei, X.; Ling, S.; Li, J.; Zhu, H.; Xie, H.; Zhu, L.; Zheng, S.; et al. Glutamine synthetase promotes tumor invasion in hepatocellular carcinoma through mediating epithelial–mesenchymal transition. Hepatol. Res. 2020, 50, 246–257. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Kaufman, P.D. Ki-67: More than a proliferation marker. Chromosoma 2018, 127, 175–186. [Google Scholar] [CrossRef]
- Sobecki, M.; Mrouj, K.; Colinge, J.; Gerbe, F.; Jay, P.; Krasinska, L.; Dulic, V.; Fisher, D. Cell-Cycle Regulation Accounts for Variability in Ki-67 Expression Levels. Cancer Res 2017, 77, 2722–2734. [Google Scholar] [CrossRef]
- Miller, I.; Min, M.; Yang, C.; Tian, C.; Gookin, S.; Carter, D.; Spencer, S.L. Ki67 is a Graded Rather than a Binary Marker of Proliferation versus Quiescence. Cell Rep. 2018, 24, 1105–1112.e5. [Google Scholar] [CrossRef]
- Garwain, O.; Sun, X.; Iyer, D.R.; Li, R.; Zhu, L.J.; Kaufman, P.D. The chromatin-binding domain of Ki-67 together with p53 protects human chromosomes from mitotic damage. Proc. Natl. Acad. Sci. USA 2021, 118, e2021998118. [Google Scholar] [CrossRef]
- Artegiani, B.; van Voorthuijsen, L.; Lindeboom, R.G.; Seinstra, D.; Heo, I.; Tapia, P.; López-Iglesias, C.; Postrach, D.; Dayton, T.; Oka, R.; et al. Probing the Tumor Suppressor Function of BAP1 in CRISPR-Engineered Human Liver Organoids. Cell Stem Cell 2019, 24, 927–943.e6. [Google Scholar] [CrossRef] [PubMed]
- Hendriks, D.; Artegiani, B.; Hu, H.; Chuva de Sousa Lopes, S.; Clevers, H. Establishment of human fetal hepatocyte organoids and CRISPR–Cas9-based gene knockin and knockout in organoid cultures from human liver. Nat. Protoc. 2020, 16, 182–217. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.; Muramatsu, T.; Kanai, Y.; Ojima, H.; Sukeda, A.; Hiraoka, N.; Arai, E.; Sugiyama, Y.; Matsuzaki, J.; Uchida, R.; et al. Establishment of Patient-Derived Organoids and Drug Screening for Biliary Tract Carcinoma. Cell Rep. 2019, 27, 1265–1276.e4. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, R.; Bae, S.D.W.; Qiao, L.; George, J. Developing liver organoids from induced pluripotent stem cells (iPSCs): An alternative source of organoid generation for liver cancer research. Cancer Lett. 2021, 508, 13–17. [Google Scholar] [CrossRef]
- Romal, S.; Hossain, T.; Mahmoudi, T. Generation, Maintenance and HBV Infection of Human Liver Organoids. Bio-protocol 2022, 12, e4358. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, V.; Simoneau, C.R.; Erickson, A.L.; Meyers, N.L.; Baron, J.L.; Cooper, S.; McDevitt, T.C.; Ott, M. Modelling T-cell immunity against hepatitis C virus with liver organoids in a microfluidic coculture system. Open Biol. 2022, 12, 210320. [Google Scholar] [CrossRef] [PubMed]
- Park, S.E.; Georgescu, A.; Huh, D. Organoids-on-a-chip. Science 2019, 364, 960–965. [Google Scholar] [CrossRef]
- McCarron, S.; Bathon, B.; Conlon, D.M.; Abbey, D.; Rader, D.J.; Gawronski, K.; Brown, C.D.; Olthoff, K.M.; Shaked, A.; Raabe, T.D. Functional Characterization of Organoids Derived From Irreversibly Damaged Liver of Patients With NASH. Hepatology 2021, 74, 1825–1844. [Google Scholar] [CrossRef]
- Grgurevic, I.; Bozin, T.; Mikus, M.; Kukla, M.; O’Beirne, J. Hepatocellular carcinoma in non-alcoholic fatty liver disease: From epidemiology to diagnostic approach. Cancers 2021, 13, 5844. [Google Scholar] [CrossRef]
- Wongjarupong, N.; Assavapongpaiboon, B.; Susantitaphong, P.; Cheungpasitporn, W.; Treeprasertsuk, S.; Rerknimitr, R.; Chaiteerakij, R. Non-alcoholic fatty liver disease as a risk factor for cholangiocarcinoma: A systematic review and meta-analysis. BMC Gastroenterol. 2017, 17, 149. [Google Scholar] [CrossRef]
- Huby, T.; Gautier, E.L. Immune cell-mediated features of non-alcoholic steatohepatitis. Nat. Rev. Immunol. 2021, 22, 429–443. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, H.; Deng, P.; Tao, T.; Liu, H.; Wu, S.; Chen, W.; Qin, J. Modeling Human Nonalcoholic Fatty Liver Disease (NAFLD) with an Organoids-on-a-Chip System. ACS Biomater. Sci. Eng. 2020, 6, 5734–5743. [Google Scholar] [CrossRef] [PubMed]
- Takebe, T.; Zhang, B.; Radisic, M. Synergistic Engineering: Organoids Meet Organs-on-a-Chip. Cell Stem Cell 2017, 21, 297–300. [Google Scholar] [CrossRef] [PubMed]
- Cheon, D.-J.; Orsulic, S.; Vesely, M.D.; Kershaw, M.H.; Schreiber, R.D.; Smyth, M.J.; Liu, S.; Leach, S.D.; Almendro, V.; Marusyk, A.; et al. Mouse Models of cancer. Annu. Rev. Pathol. Mech. Dis. 2011, 6, 95–119. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Mun, S.J.; Shin, Y.; Lee, S.; Son, M.J. Advances in liver organoids: Model systems for liver disease. Arch. Pharmacal Res. 2022, 45, 390–400. [Google Scholar] [CrossRef]
- LeSavage, B.L.; Suhar, R.A.; Broguiere, N.; Lutolf, M.P.; Heilshorn, S.C. Next-generation cancer organoids. Nat. Mater. 2022, 21, 143–159. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Kalyani, F.S.; Liu, L.; Cheng, T.; Chen, L. Tumor organoids: Synergistic applications, current challenges, and future prospects in cancer therapy. Cancer Commun. 2021, 41, 1331–1353. [Google Scholar] [CrossRef]
- Lv, W.; Zhou, H.; Aazmi, A.; Yu, M.; Xu, X.; Yang, H.; Huang, Y.Y.S.; Ma, L. Constructing biomimetic liver models through biomaterials and vasculature engineering. Regen. Biomater. 2022, 9, rbac079. [Google Scholar] [CrossRef]

| Characteristics | 2D Cell Culture | 3D Cell Culture |
|---|---|---|
| Cell morphology | Flat and elongated morphology | Predisposition to maintaining natural cell shape |
| Type of interaction | Adjacent cells interactions on a monolayer | Cell-cell and cell-extracellular matrix interactions |
| Exposure to culture medium substances | Equal exposure to culture medium’s nutrients and growth factors | Exposure to additional medium factors based on gradient |
| Drug sensitivity | High sensibility, superior to reality | Greater resistance More realistic representation of therapeutic potential |
| Expression levels | Different expression levels compared to in vivo levels | More accurately identification of in vivo gene expression levels |
| Use and analysis | High repeatability and easy data interpretation | Difficulty in reproducing experiments and data interpretation |
| Cost | Low | Expensive |
| Components | Concentrations | Functions |
|---|---|---|
| B-27 | 1:50 | Serum-free supplement, without vitamin A, it increases differentiated cell vitality during long term expansion culture condition. |
| N-2 | 1:100 | Serum-free supplement, it promotes neuronal primary cell cultures’ growth. |
| Nicotinammide | 10 mM | Anti-inflammatory agent, it controls cell metabolism, mitochondria functionality and energy production. |
| N-acetil-L-cisteine | 1.25 mM | Mucolytic agent, with cytoprotective, anti-inflammatory and antioxidant effects, through NF-Kb and HIF-1α regulation and ROS levels modulation. |
| Forskolin | 10 µM | Diterpenes, agonist of cAMP pathway, it has an anti-inflammatory effect and promotes mRNA expression in primary hepatocytes; it supports long-term expansion of organoids. |
| Y-27632 (ROCKi) | 10 µM | Rho-kinase inhibitor, it blocks apoptosis process. |
| A83-01 | 5 µM | TGF-β signalling inhibitor, it blocks the epithelial to mesenchimal transition TGF-β induced; it supports long-term expansion of organoids culture. |
| [Leu15]-Gastrin I | 10 nM | Essential for digestive system, gastrin stimulates the production of gastric acid from paretial cells and prolong the survival time of liver organoids. |
| FGF-10 | 100 ng/mL | Growth factors with mitogen effect, they promote cell proliferation, differentiation, and survival. |
| EGF | 50 ng/mL | |
| HGF | 25 ng/mL | |
| Noggin | 100 ng/mL | Bone morphogenic protein (BMP) inhibitor. |
| R-Spo1 | 10% | Agonist of WNT/β-catenin and WNT/PCP pathways and ligand of LGR5+ receptor, it improves efficiency of organoids expansion. |
| Wnt3a | 30% | Agonist of WNT pathway, it promotes stem cell LGR5+ proliferation, essential for organoids expansion. |
| Scaffold | Materials | Advantages | Disadvantages |
|---|---|---|---|
| Natural | Matrigel, Cultrex Basement Membrane Extract (BME) | Commercially available; widely used in the majority of developed protocols | Indeterminate culture system with no control over mechanical properties and a lot-to-lot variability; may not include all chemical signals required for differentiation; immunogenicity |
| Decellularized tissue | Developed organoids can be large and still retain mechanical qualities and natural chemical signals | Difficult preparation, limited by donors’ resources | |
| Biomacromolecules (collagen, alginate, hyaluronic acid, silk) | Low cost and wide availability | Lack of retained structural information, absence of the required chemical signals, and lot-to-lot variability | |
| Synthetic | PEG, PLA; PVA PLGA, PCL | Improved control over mechanical and chemical features; easily reproducible experiments; variable degradation rate | It requires the functionalization using peptides that are attached to the cell membrane; potential cytotoxic issues |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Siervi, S.; Turato, C. Liver Organoids as an In Vitro Model to Study Primary Liver Cancer. Int. J. Mol. Sci. 2023, 24, 4529. https://doi.org/10.3390/ijms24054529
De Siervi S, Turato C. Liver Organoids as an In Vitro Model to Study Primary Liver Cancer. International Journal of Molecular Sciences. 2023; 24(5):4529. https://doi.org/10.3390/ijms24054529
Chicago/Turabian StyleDe Siervi, Silvia, and Cristian Turato. 2023. "Liver Organoids as an In Vitro Model to Study Primary Liver Cancer" International Journal of Molecular Sciences 24, no. 5: 4529. https://doi.org/10.3390/ijms24054529
APA StyleDe Siervi, S., & Turato, C. (2023). Liver Organoids as an In Vitro Model to Study Primary Liver Cancer. International Journal of Molecular Sciences, 24(5), 4529. https://doi.org/10.3390/ijms24054529






