PPP3R1 Promotes MSCs Senescence by Inducing Plasma Membrane Depolarization and Increasing Ca2+ Influx
Abstract
1. Introduction
2. Results
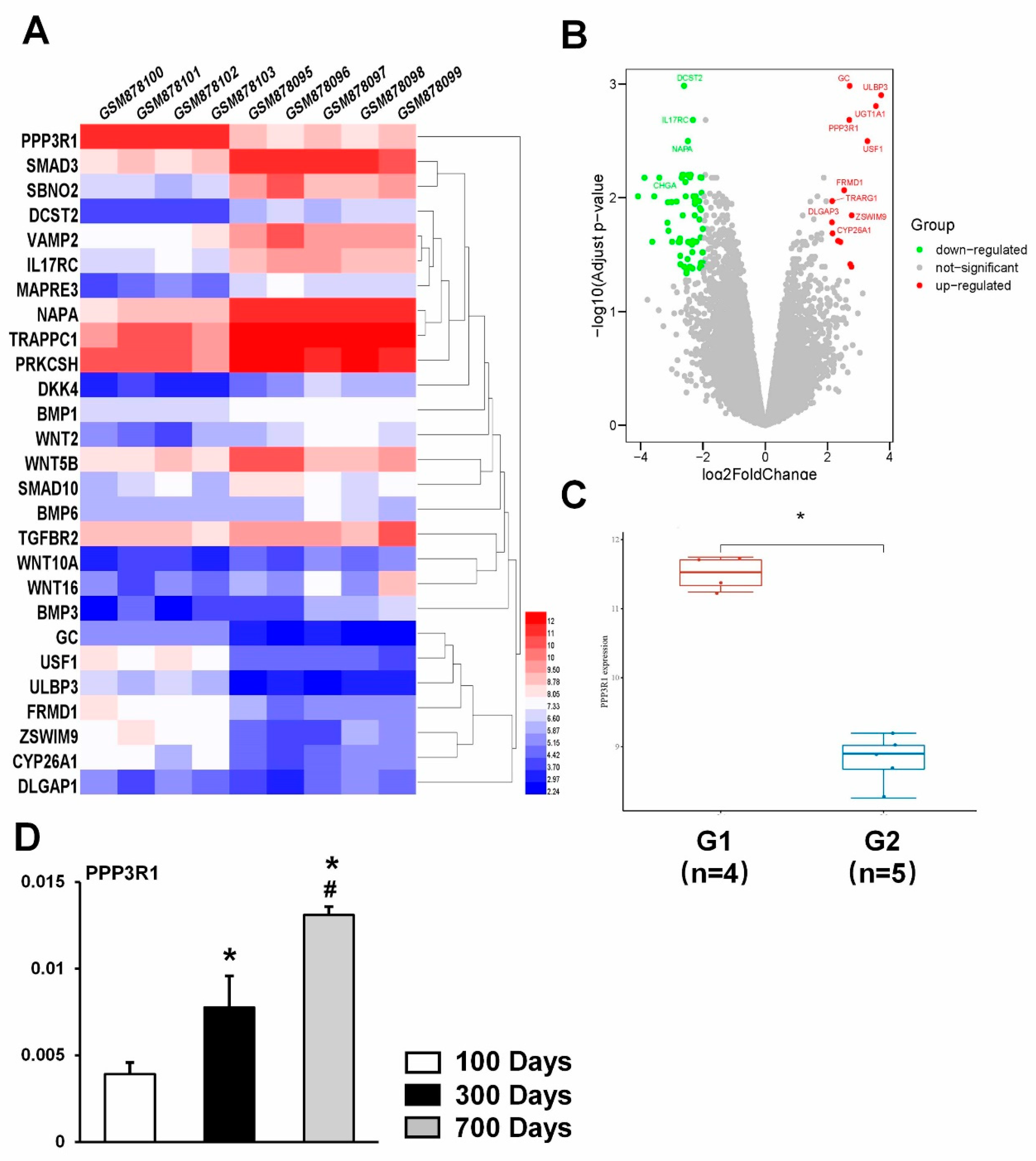
2.1. PPP3R1 Is Activated in Senescent BMSCs
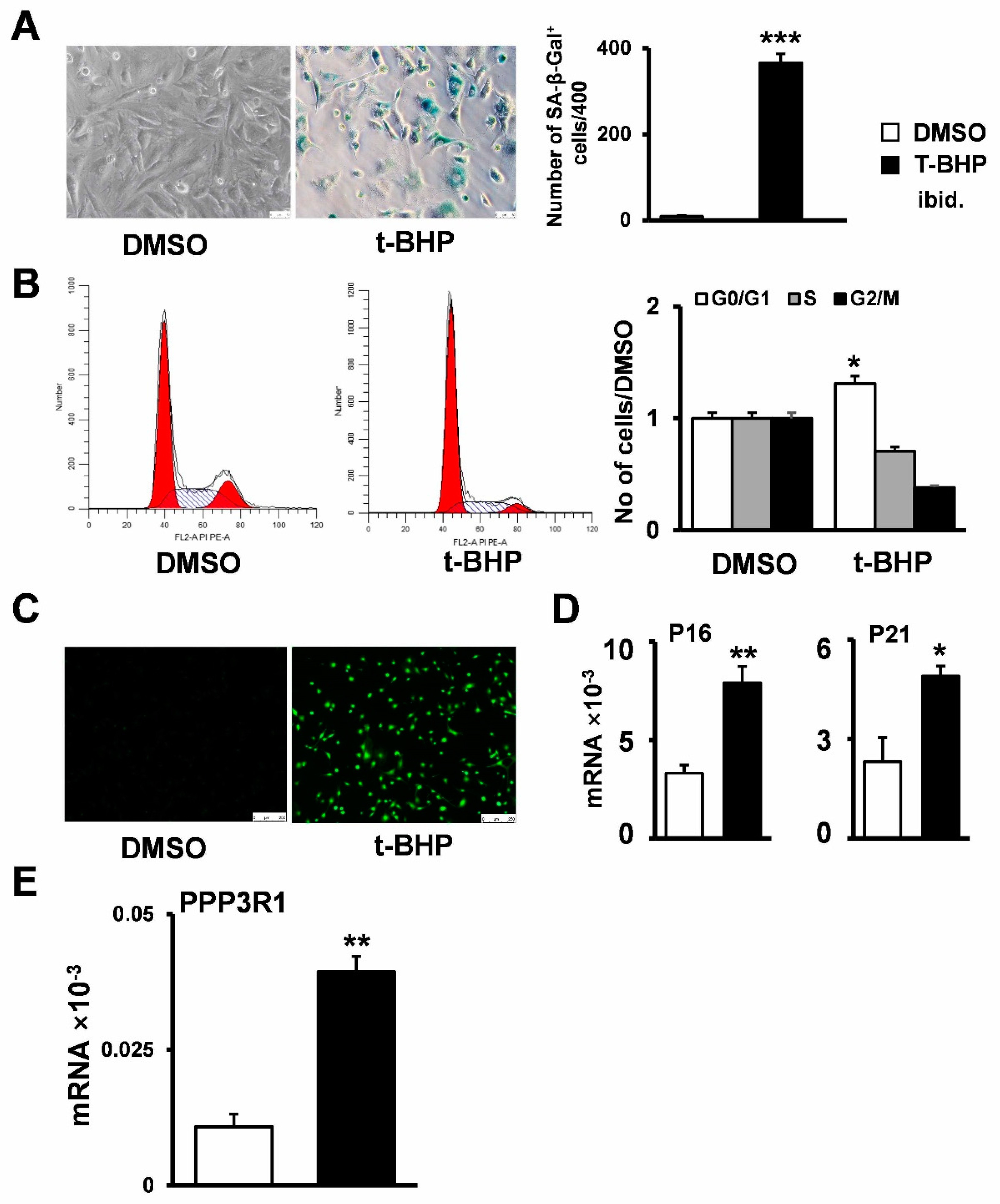
2.2. Establishment of Senescent Stromal Stem Cell Lines
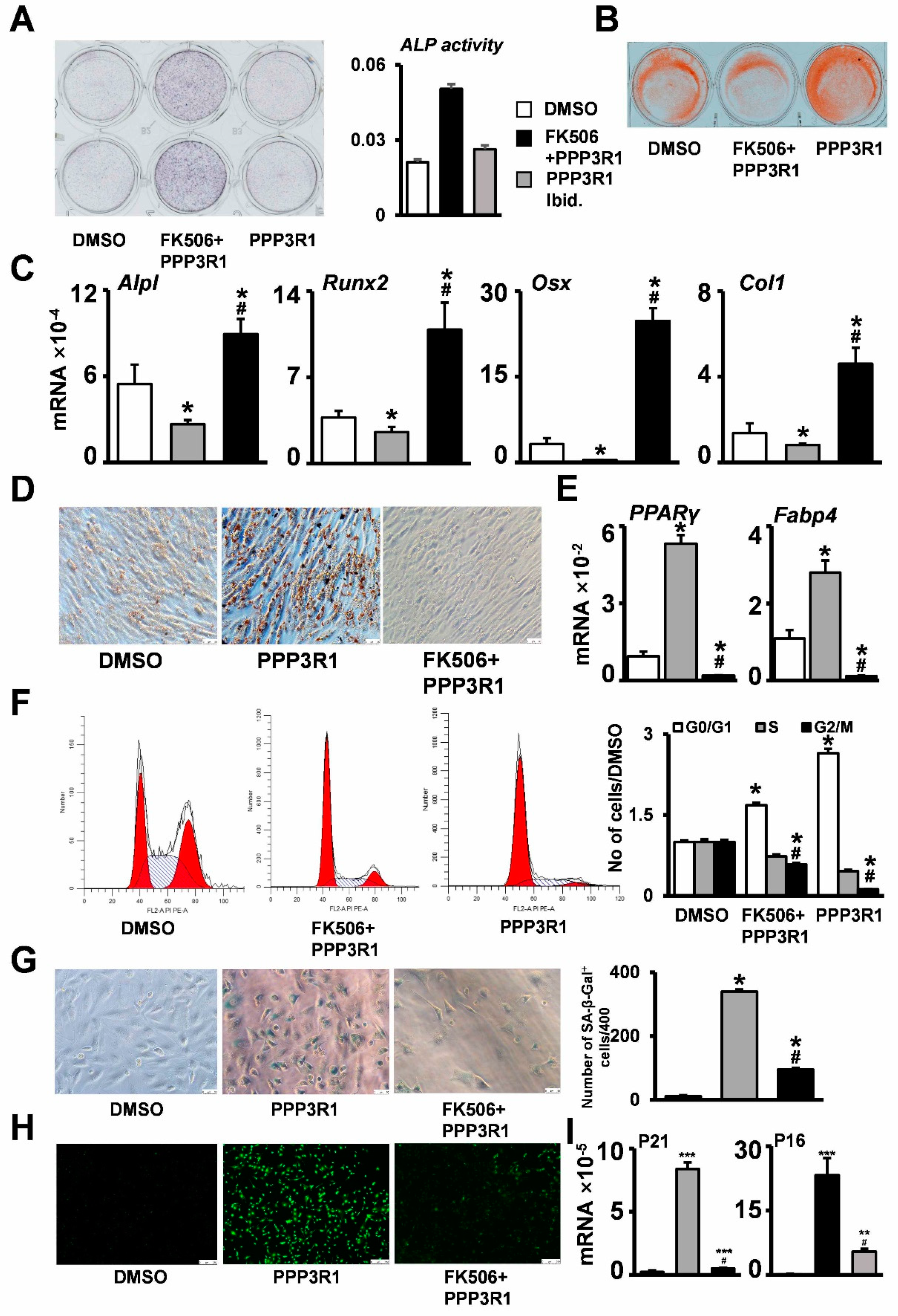
2.3. PPP3R1 Inhibition Enables C3H10T1/2 Escape from Senescence
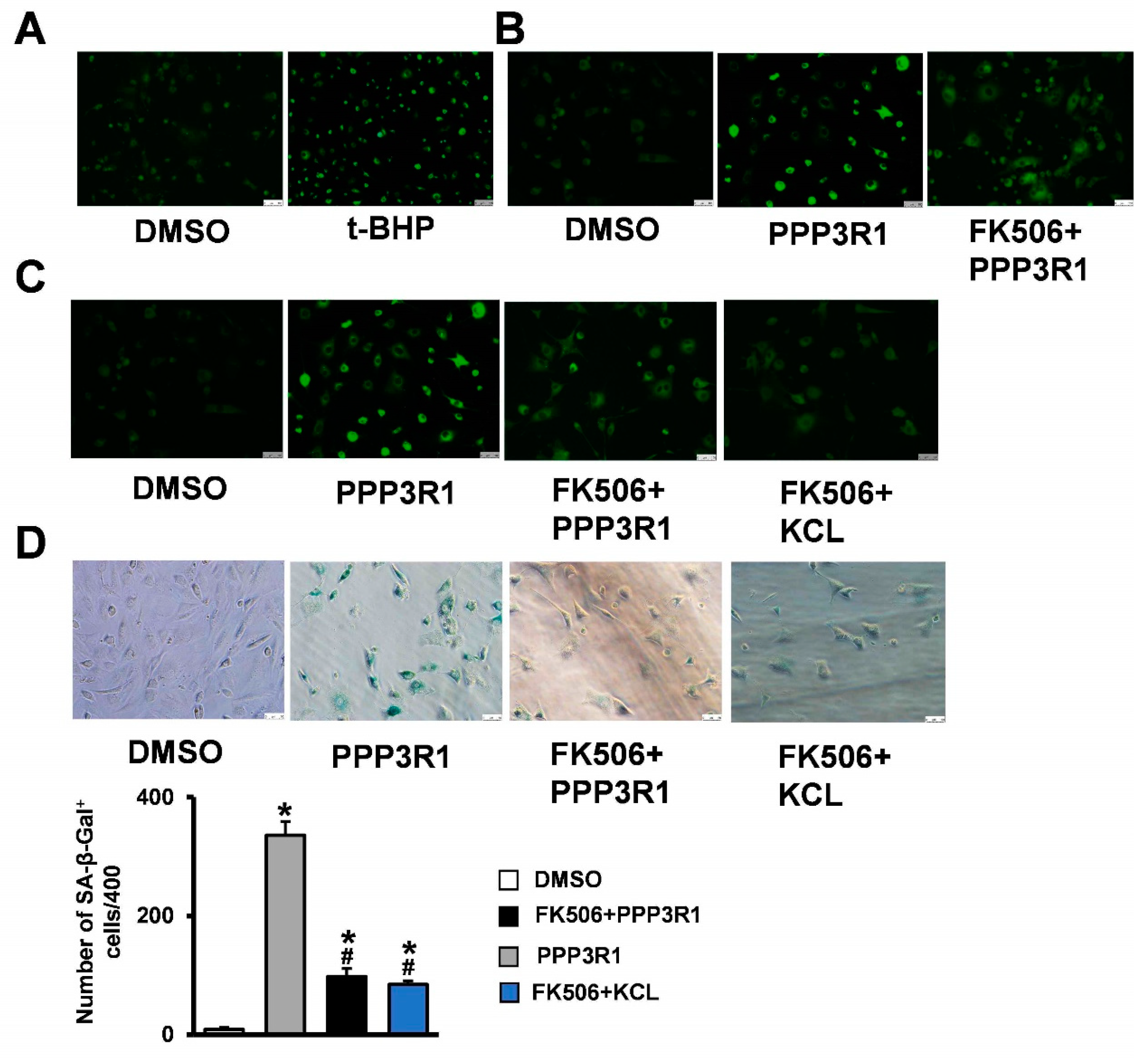
2.4. Plasma Membrane Depolarization Induces C3H10T1/2 Cell Senescence
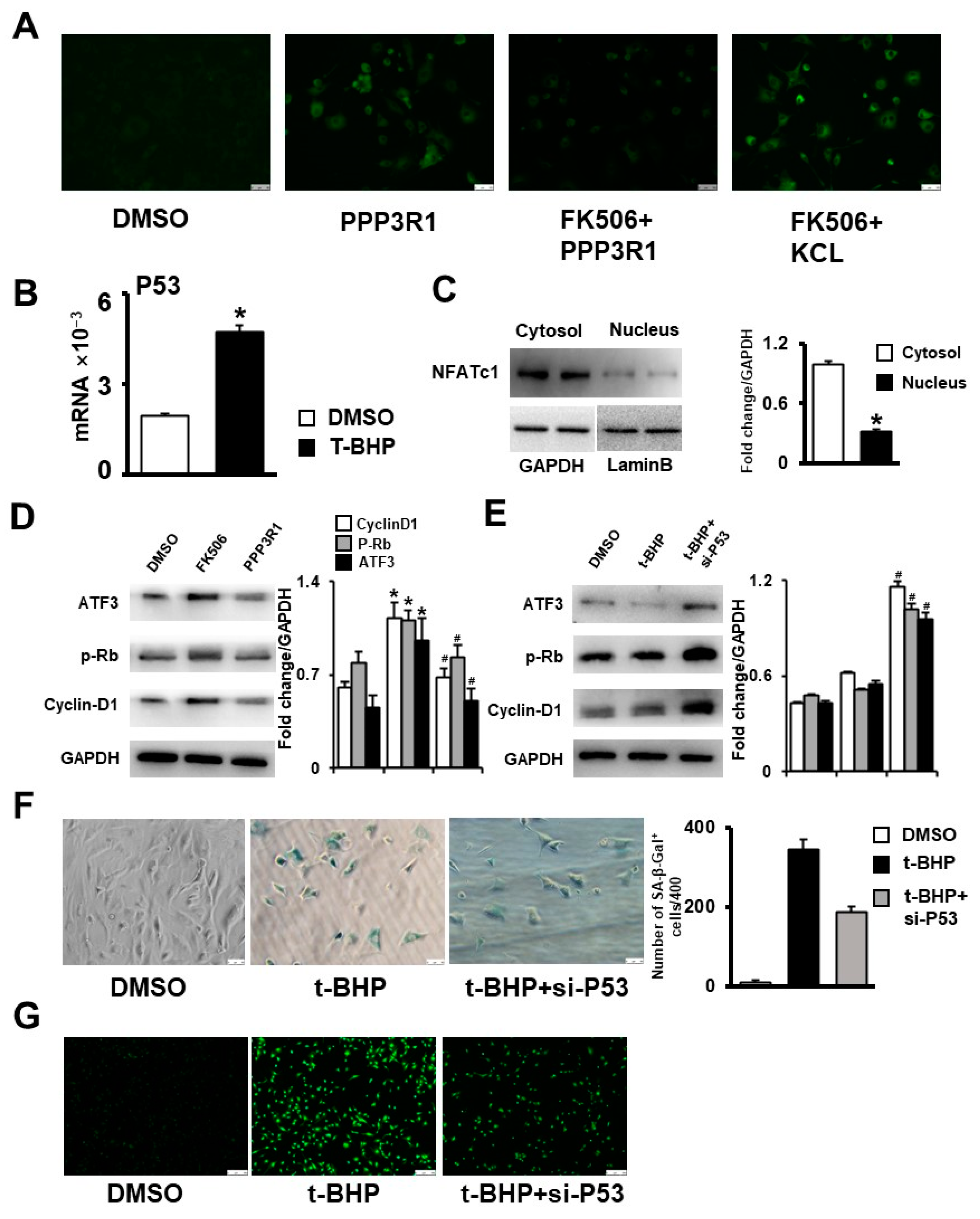
2.5. Plasma Membrane Depolarization Increases Ca2+ Influx and Activates NFAT/ATF3/p53 Signaling, Thereby Inducing MSC Senescence
3. Discussion
4. Materials and Methods
4.1. Microarray Analysis
4.2. Cell Culture and Treatment
4.3. Cell Staining
4.4. Western Blotting
4.5. RNA Extraction and Gene Expression Analysis
4.6. Cell Cycle Assay
4.7. Plasma Membrane Potential Measurement
4.8. Cytosolic Calcium Level Measurements
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Föger-Samwald, U.; Kerschan-Schindl, K.; Butylina, M.; Pietschmann, P. Age Related Osteoporosis: Targeting Cellular Senescence. Int. J. Mol. Sci. 2022, 23, 2701. [Google Scholar] [CrossRef] [PubMed]
- Farr, J.N.; Fraser, D.G.; Wang, H.; Jaehn, K.; Ogrodnik, M.B.; Weivoda, M.M.; Drake, M.T.; Tchkonia, T.; LeBrasseur, N.K.; Kirkland, J.L.; et al. Identification of Senescent Cells in the Bone Microenvironment. J. Bone Miner. Res. 2016, 31, 1920–1929. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, P.; El-Jawhari, J.J.; Giannoudis, P.V.; Burska, A.N.; Ponchel, F.; Jones, E.A. Age-related Changes in Bone Marrow Mesenchymal Stromal Cells: A Potential Impact on Osteoporosis and Osteoarthritis Development. Cell Transplant. 2017, 26, 1520–1529. [Google Scholar] [CrossRef] [PubMed]
- Khong, S.M.L.; Lee, M.; Kosaric, N.; Khong, D.M.; Dong, Y.; Hopfner, U.; Aitzetmüller, M.M.; Duscher, D.; Schäfer, R.; Gurtner, G.C. Single-Cell Transcriptomics of Human Mesenchymal Stem Cells Reveal Age-Related Cellular Subpopulation Depletion and Impaired Regenerative Function. Stem Cells 2019, 37, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Di Micco, R.; Krizhanovsky, V.; Baker, D.; di Fagagna, F.D. Cellular senescence in ageing: From mechanisms to therapeutic opportunities. Nat. Rev. Mol. Cell Biol. 2021, 22, 75–95. [Google Scholar] [CrossRef]
- Ren, X.; Hu, B.; Song, M.; Ding, Z.; Dang, Y.; Liu, Z.; Zhang, W.; Ji, Q.; Ren, R.; Ding, J.; et al. Maintenance of Nucleolar Homeostasis by CBX4 Alleviates Senescence and Osteoarthritis. Cell Rep. 2019, 26, 3643–3656.e7. [Google Scholar] [CrossRef]
- Baker, N.; Boyette, L.B.; Tuan, R.S. Characterization of bone marrow-derived mesenchymal stem cells in aging. Bone 2015, 70, 37–47. [Google Scholar] [CrossRef]
- Infante, A.; Rodríguez, C.I. Osteogenesis and aging: Lessons from mesenchymal stem cells. Stem Cell Res. Ther. 2018, 9, 244. [Google Scholar] [CrossRef]
- Gamradt, S.C.; Abe, N.; Bahamonde, M.E.; Lee, Y.-P.; Nelson, S.D.; Lyons, K.M.; Lieberman, J.R. Tracking Expression of Virally Mediated BMP-2 in Gene Therapy for Bone Repair. Clin. Orthop. Relat. Res. 2006, 450, 238–245. [Google Scholar] [CrossRef]
- Tominaga, K.; Suzuki, H.I. TGF-β Signaling in Cellular Senescence and Aging-Related Pathology. Int. J. Mol. Sci. 2019, 20, 5002. [Google Scholar] [CrossRef]
- Yeh, C.-H.; Chou, Y.-J.; Kao, C.-H.; Tsai, T.-F. Mitochondria and Calcium Homeostasis: Cisd2 as a Big Player in Cardiac Ageing. Int. J. Mol. Sci. 2020, 21, 9238. [Google Scholar] [CrossRef]
- Hamilton, S.; Terentyev, D. Altered Intracellular Calcium Homeostasis and Arrhythmogenesis in the Aged Heart. Int. J. Mol. Sci. 2019, 20, 2386. [Google Scholar] [CrossRef]
- Calvo-Rodriguez, M.; Hernando-Pérez, E.; López-Vázquez, S.; Núñez, J.; Villalobos, C.; Núñez, L. Remodeling of Intracellular Ca2+ Homeostasis in Rat Hippocampal Neurons Aged In Vitro. Int. J. Mol. Sci. 2020, 21, 1549. [Google Scholar] [CrossRef]
- Parys, J.B.; Pereira, C.F.; Villalobos, C. The Eighth ECS Workshop on “Calcium Signaling in Aging and Neurodegenerative Diseases”. Int. J. Mol. Sci. 2019, 20, 6263. [Google Scholar] [CrossRef]
- Zöphel, D.; Hof, C.; Lis, A. Altered Ca2+ Homeostasis in Immune Cells during Aging: Role of Ion Channels. Int. J. Mol. Sci. 2020, 22, 110. [Google Scholar] [CrossRef]
- Winslow, M.M.; Pan, M.; Starbuck, M.; Gallo, E.M.; Deng, L.; Karsenty, G.; Crabtree, G.R. Calcineurin/NFAT Signaling in Osteoblasts Regulates Bone Mass. Dev. Cell 2006, 10, 771–782. [Google Scholar] [CrossRef]
- Laurin, L.-P.; Nachman, P.H.; Foster, B.J. Calcineurin Inhibitors in the Treatment of Primary Focal Segmental Glomerulosclerosis: A Systematic Review and Meta-analysis of the Literature. Can. J. Kidney Health Dis. 2017, 4, 2054358117692559. [Google Scholar] [CrossRef]
- El-Gowelli, H.M.; El-Mas, M.M. Central modulation of cyclosporine-induced hypertension. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2015, 388, 351–361. [Google Scholar] [CrossRef]
- Warnier, M.; Flaman, J.-M.; Chouabe, C.; Wiel, C.; Gras, B.; Griveau, A.; Blanc, E.; Foy, J.-P.; Mathot, P.; Saintigny, P.; et al. The SCN9A channel and plasma membrane depolarization promote cellular senescence through Rb pathway. Aging Cell 2018, 17, e12736. [Google Scholar] [CrossRef]
- Zhou, L.; Chen, X.; Liu, T.; Gong, Y.; Chen, S.; Pan, G.; Cui, W.; Luo, Z.-P.; Pei, M.; Yang, H.; et al. Melatonin reverses H2 O2 -induced premature senescence in mesenchymal stem cells via the SIRT1-dependent pathway. J. Pineal Res. 2015, 59, 190–205. [Google Scholar] [CrossRef]
- Carroll, B.; Nelson, G.; Rabanal-Ruiz, Y.; Kucheryavenko, O.; Dunhill-Turner, N.A.; Chesterman, C.C.; Zahari, Q.; Zhang, T.; Conduit, S.E.; Mitchell, C.A.; et al. Persistent mTORC1 signaling in cell senescence results from defects in amino acid and growth factor sensing. J. Cell Biol. 2017, 216, 1949–1957. [Google Scholar] [CrossRef] [PubMed]
- Courtney, M.; Lambert, J.; Nicholls, D. The interactions between plasma membrane depolarization and glutamate receptor activation in the regulation of cytoplasmic free calcium in cultured cerebellar granule cells. J. Neurosci. 1990, 10, 3873–3879. [Google Scholar] [CrossRef] [PubMed]
- Wiel, C.; Lallet-Daher, H.; Gitenay, D.; Gras, B.; Le Calvé, B.; Augert, A.; Ferrand, M.; Prevarskaya, N.; Simonnet, H.; Vindrieux, D.; et al. Endoplasmic reticulum calcium release through ITPR2 channels leads to mitochondrial calcium accumulation and senescence. Nat. Commun. 2014, 5, 3792. [Google Scholar] [CrossRef] [PubMed]
- Martin, N.; Bernard, D. Calcium signaling and cellular senescence. Cell Calcium 2018, 70, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Hogan, P.G.; Chen, L.; Nardone, J.; Rao, A. Transcriptional regulation by calcium, calcineurin, and NFAT. Genes Dev. 2003, 17, 2205–2232. [Google Scholar] [CrossRef]
- Wu, X.; Nguyen, B.-C.; Dziunycz, P.; Chang, S.; Brooks, Y.; Lefort, K.; Hofbauer, G.F.L.; Dotto, G.P. Opposing roles for calcineurin and ATF3 in squamous skin cancer. Nature 2010, 465, 368–372. [Google Scholar] [CrossRef]
- d’Adda di Fagagna, F. Living on a break: Cellular senescence as a DNA-damage response. Nature Reviews. Cancer 2008, 8, 512–522. [Google Scholar]
- Mair, W.; Morantte, I.; Rodrigues, A.P.C.; Manning, G.; Montminy, M.; Shaw, R.J.; Dillin, A. Lifespan extension induced by AMPK and calcineurin is mediated by CRTC-1 and CREB. Nature 2011, 470, 404–408. [Google Scholar] [CrossRef]
- Sama, D.M.; Norris, C.M. Calcium dysregulation and neuroinflammation: Discrete and integrated mechanisms for age-related synaptic dysfunction. Ageing Res. Rev. 2013, 12, 982–995. [Google Scholar] [CrossRef]
- Reese, L.C.; Zhang, W.; Dineley, K.T.; Kayed, R.; Taglialatela, G. Selective induction of calcineurin activity and signaling by oligomeric amyloid beta. Aging Cell 2008, 7, 824–835. [Google Scholar] [CrossRef]
- Garrigos, M.; Deschamps, S.; Viel, A.; Lund, S.; Champeil, P.; Møller, J.V.; le Maire, M. Detection of Ca2+-binding proteins by electrophoretic migration in the presence of Ca2+ combined with 45Ca2+ overlay of protein blots. Anal. Biochem. 1991, 194, 82–88. [Google Scholar] [CrossRef]
- Rusnak, F.; Mertz, P. Calcineurin: Form and Function. Physiol. Rev. 2000, 80, 1483–1521. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, J.; Yuan, J.; Dang, Y.; Yang, C.; Chen, X.; Xu, J.; Yu, L. Characterization of a human regulatory subunit of protein phosphatase 3 gene (PPP3RL) expressed specifically in testis. Mol. Biol. Rep. 2005, 32, 41–45. [Google Scholar] [CrossRef]
- Newell, E.W.; Schlichter, L.C. Integration of K+ and Cl− currents regulate steady-state and dynamic membrane potentials in cultured rat microglia. J. Physiol. 2005, 567, 869–890. [Google Scholar] [CrossRef]
- Lallet-Daher, H.; Wiel, C.; Gitenay, D.; Navaratnam, N.; Augert, A.; Le Calvé, B.; Verbeke, S.; Carling, D.; Aubert, S.; Vindrieux, D.; et al. Potassium Channel KCNA1 Modulates Oncogene-Induced Senescence and Transformation. Cancer Res. 2013, 73, 5253–5265. [Google Scholar] [CrossRef]
- Catterall, W.A. Voltage-gated calcium channels. Cold Spring Harb. Perspect. Biol. 2011, 3, a003947. [Google Scholar] [CrossRef]
- Chen, A.; Jin, J.; Cheng, S.; Liu, Z.; Yang, C.; Chen, Q.; Liang, W.; Li, K.; Kang, D.; Ouyang, Z.; et al. mTORC1 induces plasma membrane depolarization and promotes preosteoblast senescence by regulating the sodium channel Scn1a. Bone Res. 2022, 10, 25. [Google Scholar] [CrossRef]
- Fei, D.; Zhang, Y.; Wu, J.; Zhang, H.; Liu, A.; He, X.; Wang, J.; Li, B.; Wang, Q.; Jin, Y. Cav1.2 regulates osteogenesis of bone marrow-derived mesenchymal stem cells via canonical Wnt pathway in age-related osteoporosis. Aging Cell 2019, 18, e12967. [Google Scholar] [CrossRef]
- Tu, X.; Joeng, K.S.; Nakayama, K.I.; Nakayama, K.; Rajagopal, J.; Carroll, T.J.; McMahon, A.P.; Long, F. Noncanonical Wnt signaling through G protein-linked PKCdelta activation promotes bone formation. Dev. Cell 2007, 12, 113–127. [Google Scholar] [CrossRef]
| Primer | Forward | Reverse |
|---|---|---|
| GAPDH | GCACAGTCAAGGCCGAGAAT | GCCTTCTCCATGGTGGTGAA |
| PPP3R1 | CCAACACGGTCCCCATTA | TTGGCAAAGCGGACTTTC |
| P16 | CAAGAGCGGGGACATCAAGACATC | CACAAAGACCACCCAGCGGAAC |
| P21 | TCCTGGTGATGTCCGACCTGTTC | ACGAAGTCAAAGTTCCACCGTTCTC |
| P53 | ACCGCCGACCTATCCTTACCATC | GGCACAAACACGAACCTCAAAGC |
| Alp | ACACCAATGTAGCCAAGAATGTCA | GATTCGGGCAGCGGTTACT |
| Runx2 | CCGGTCTCCTTCCAGGAT | GGGAACTGCTGTGGCTTC |
| Osx | CCCTTCTCAAGCACCAATGG | AAGGGTGGGTAGTCA TTTGCATA |
| Col1 | GAGCGGAGAGTACTGGATCG | GCTTCTTTTCCTTGGGGTTC |
| PPARγ | AGCCCTTTACCACAGTTGATTTCTCC | GCAGGTTCTACTTTGATCGCACTTTG |
| FABP4 | GACGACAGGAAGGTGAAGAGCATC | GGAAGTCACGCCTTTCATAACACATTC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Gong, W.; Chen, J.; Zhang, Y.; Ma, Y.; Tu, X. PPP3R1 Promotes MSCs Senescence by Inducing Plasma Membrane Depolarization and Increasing Ca2+ Influx. Int. J. Mol. Sci. 2023, 24, 4421. https://doi.org/10.3390/ijms24054421
Li M, Gong W, Chen J, Zhang Y, Ma Y, Tu X. PPP3R1 Promotes MSCs Senescence by Inducing Plasma Membrane Depolarization and Increasing Ca2+ Influx. International Journal of Molecular Sciences. 2023; 24(5):4421. https://doi.org/10.3390/ijms24054421
Chicago/Turabian StyleLi, Molin, Weimin Gong, Jie Chen, Yining Zhang, Yufei Ma, and Xiaolin Tu. 2023. "PPP3R1 Promotes MSCs Senescence by Inducing Plasma Membrane Depolarization and Increasing Ca2+ Influx" International Journal of Molecular Sciences 24, no. 5: 4421. https://doi.org/10.3390/ijms24054421
APA StyleLi, M., Gong, W., Chen, J., Zhang, Y., Ma, Y., & Tu, X. (2023). PPP3R1 Promotes MSCs Senescence by Inducing Plasma Membrane Depolarization and Increasing Ca2+ Influx. International Journal of Molecular Sciences, 24(5), 4421. https://doi.org/10.3390/ijms24054421





