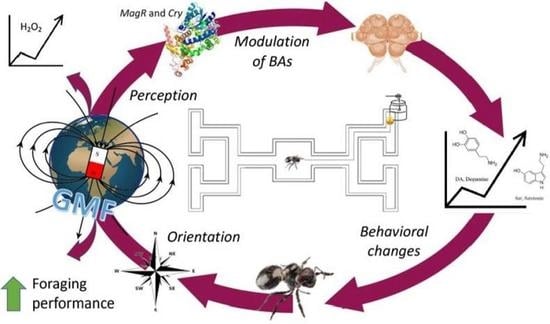
The Geomagnetic Field (GMF) Is Necessary for Black Garden Ant (Lasius niger L.) Foraging and Modulates Orientation Potentially through Aminergic Regulation and MagR Expression
Abstract
1. Introduction
2. Results and Discussion
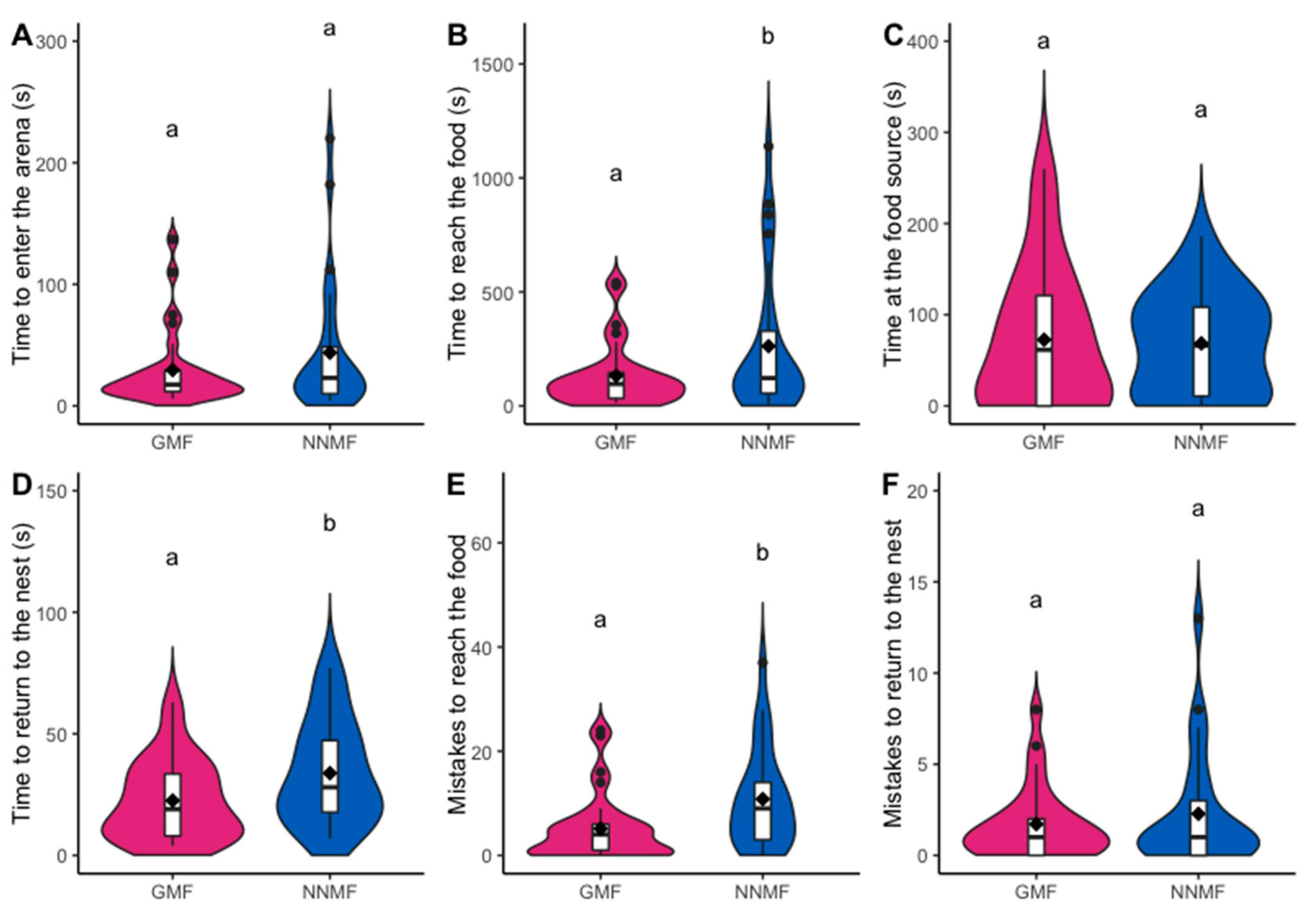
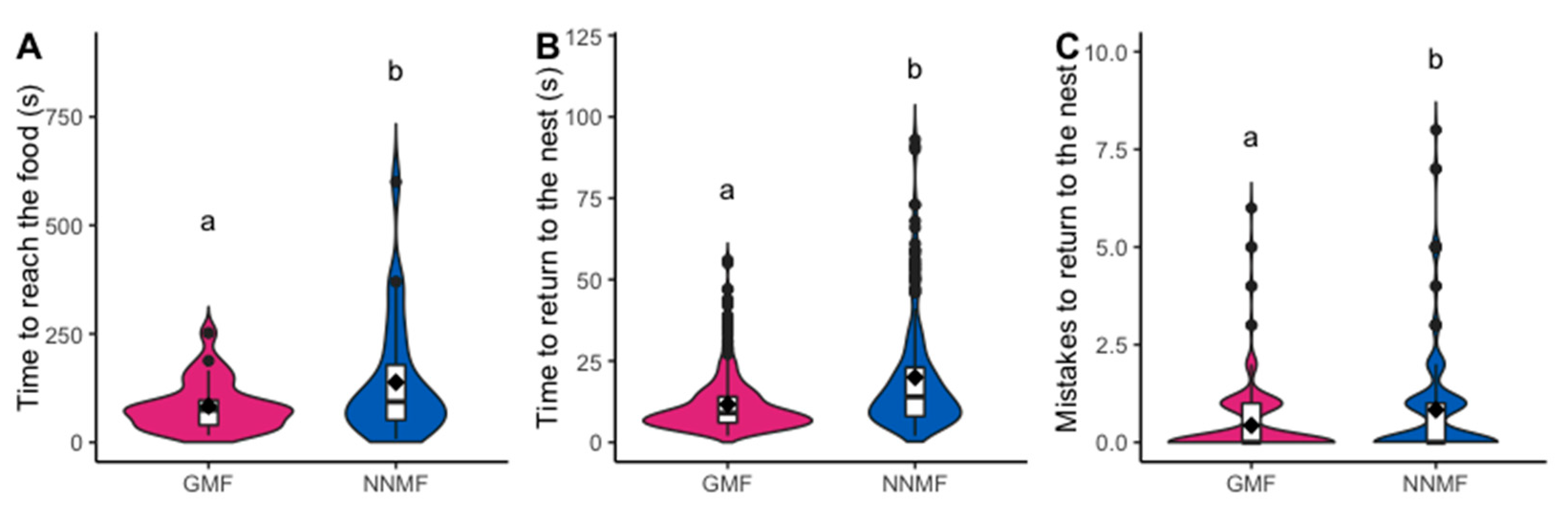
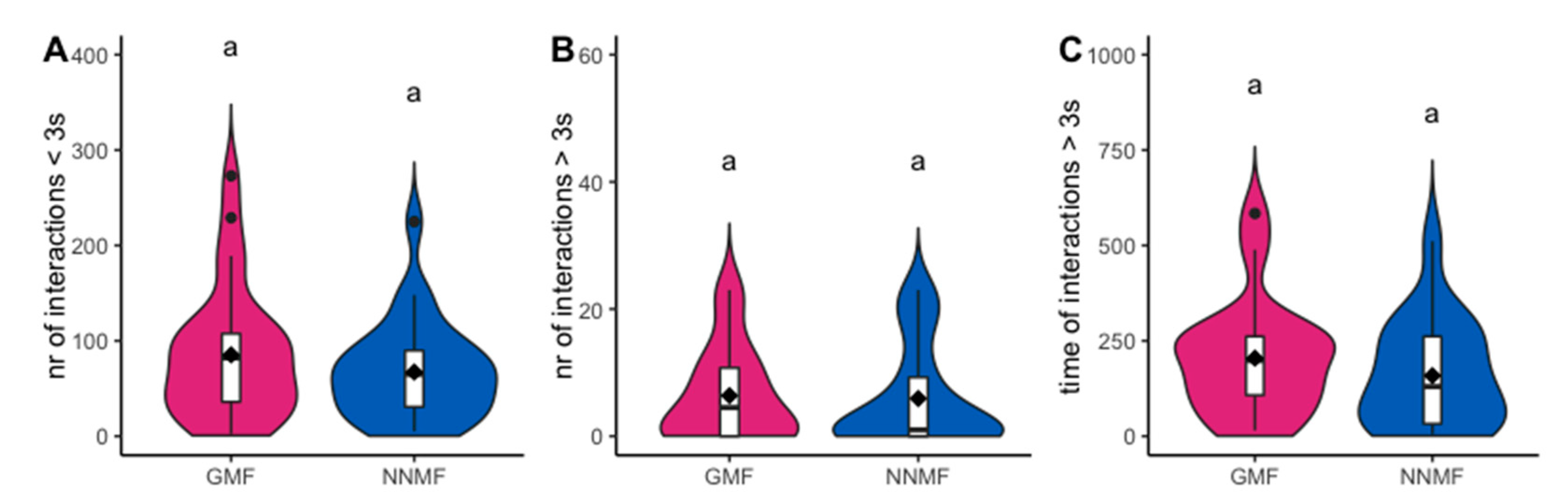
2.1. The GMF Is Necessary for the Orientation Abilities of Lasius niger
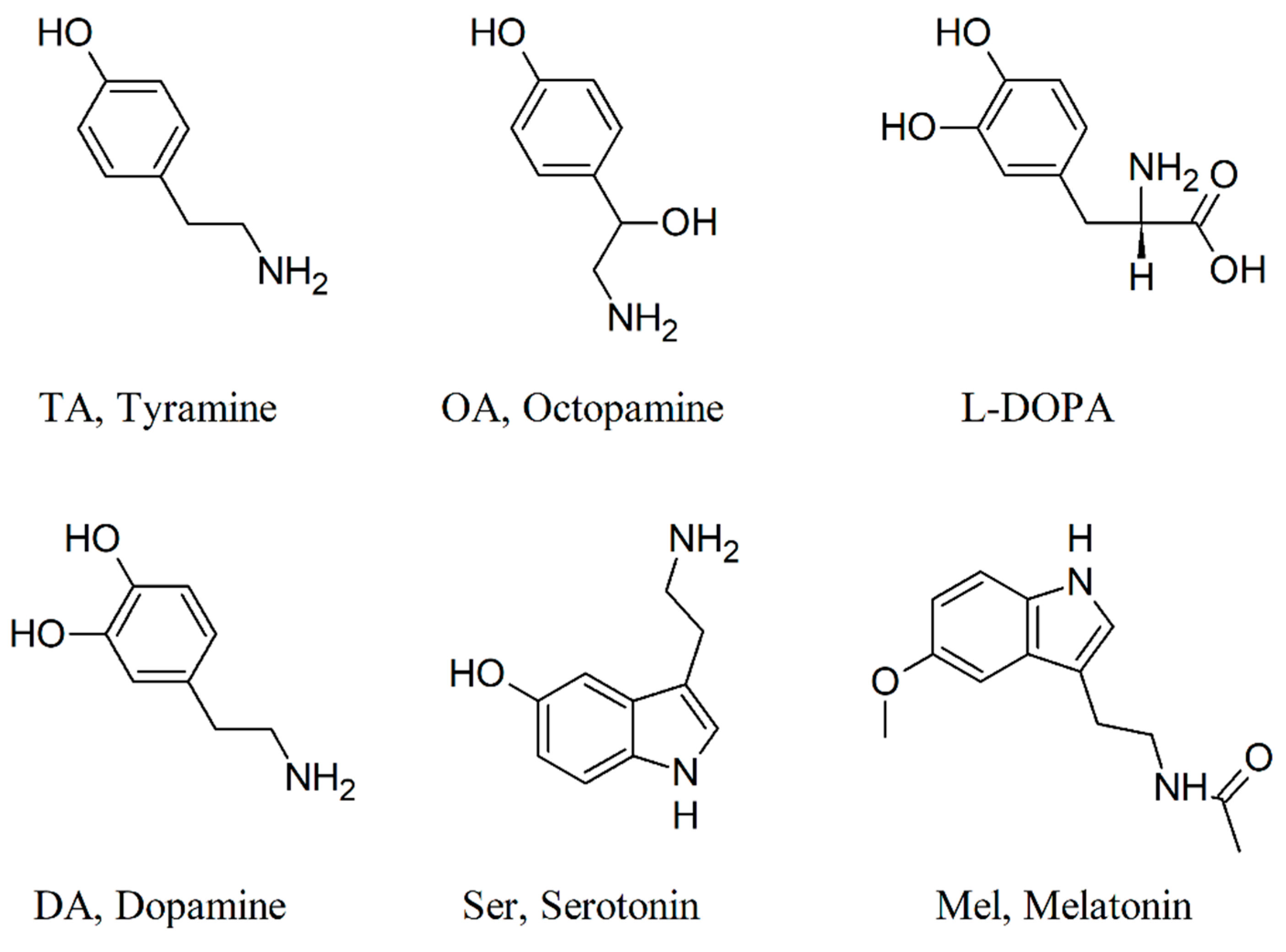
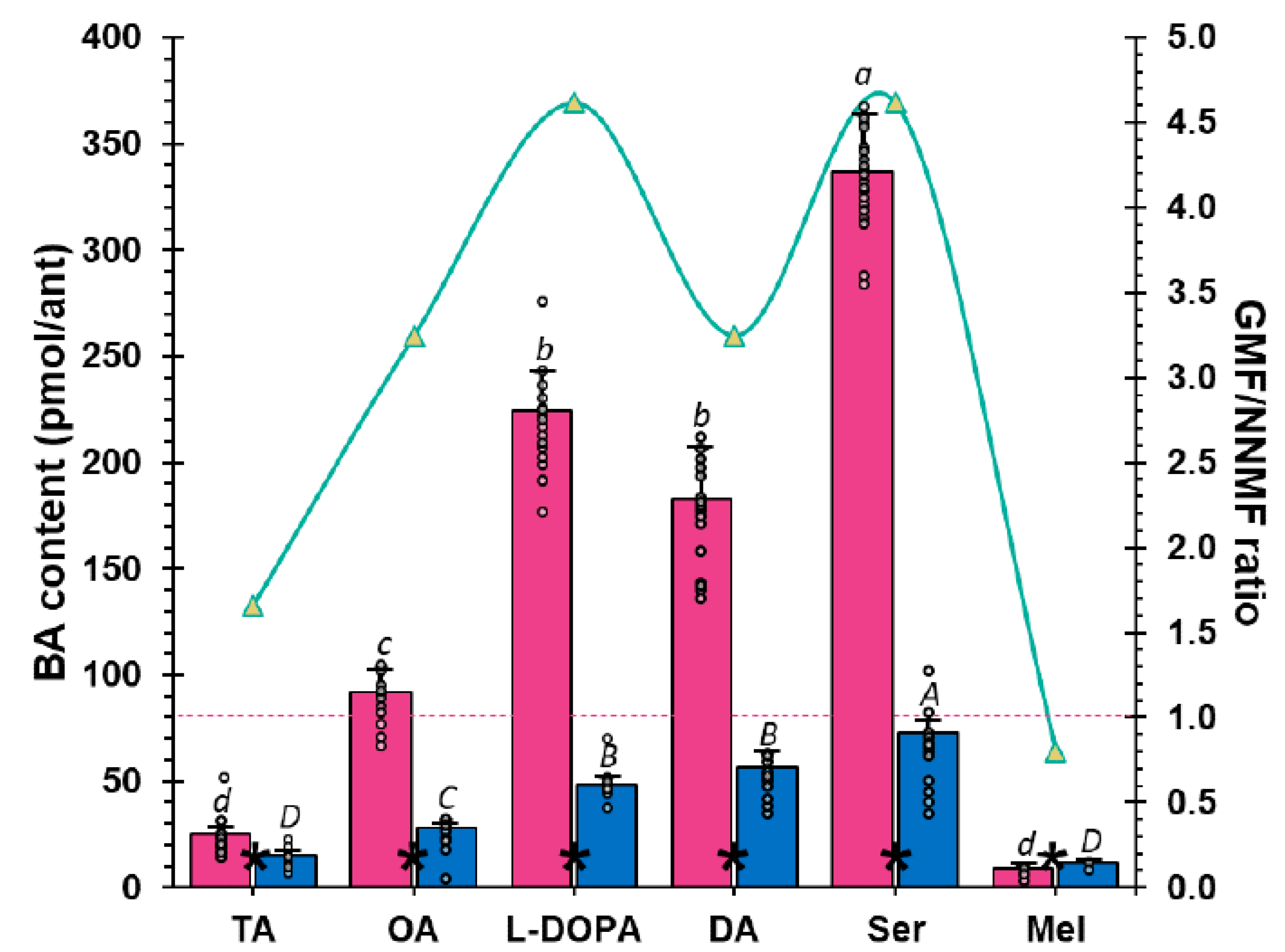
2.2. The GMF Modulates Lasius niger Brain Biogenic Amines
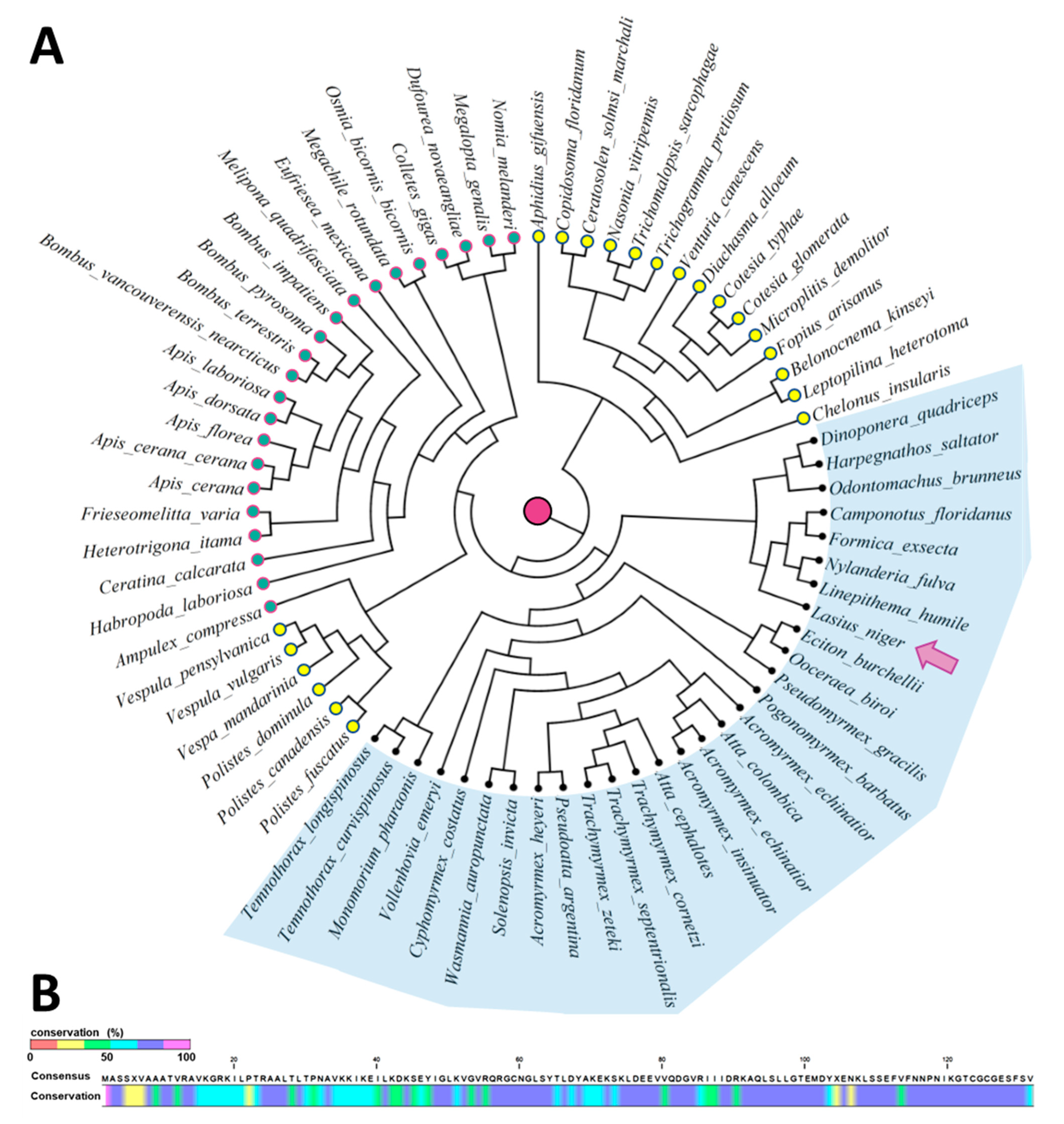
2.3. MagR Is a Highly Conserved Protein in Ants
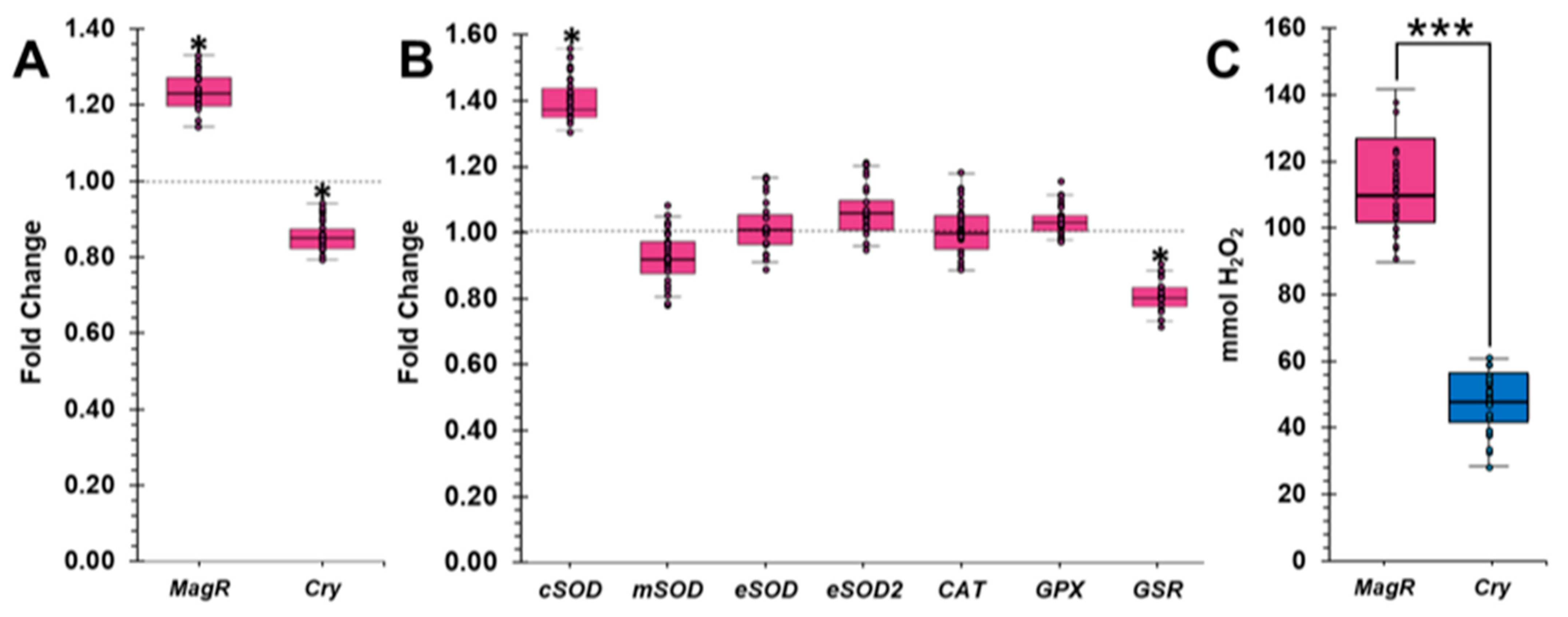
2.4. GMF Influences the Expression of MagR and Cry in Lasius niger
2.5. GMF Modulates the Oxidative Stress of Lasius niger
3. Materials and Methods
3.1. Animal Material and Experimental Conditions
3.2. Locomotor Activity and Orientation Test
3.2.1. Experimental Setup
3.2.2. Training Procedure
3.2.3. Behavioral Tests
3.2.4. Parameter Monitoring
3.3. Extraction of Brain Biogenic Amines
3.4. Protein Phylogenetic Analysis
3.5. Gene Expression Analysis by qRT-PCR
3.6. Hydrogen Peroxide Quantification
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jardine, M. Sunscreen for the young earth. Science 2010, 327, 1206–1207. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Zhang, S.; Chen, P.; Liu, H.; Yin, H.; Li, H. Magnetotactic bacteria, magnetosomes and their application. Microbiol. Res. 2012, 167, 507–519. [Google Scholar] [CrossRef] [PubMed]
- Nagy, P.; Fischl, G. Effect of static magnetic field on growth and sporulation of some plant pathogenic fungi. Bioelectromagn. J. 2004, 25, 316–318. [Google Scholar] [CrossRef] [PubMed]
- Maffei, M.E. Magnetoreception in plants. In Bioelectromagnetism; CRC Press: Boca Raton, FL, USA, 2022; pp. 191–214. ISBN 100318135X. [Google Scholar]
- Wajnberg, E.; Acosta-Avalos, D.; Alves, O.C.; de Oliveira, J.F.; Srygley, R.B.; Esquivel, D.M. Magnetoreception in eusocial insects: An update. J. Roy. Soc. Interf. 2010, 7 (Suppl. S2), S207–S225. [Google Scholar] [CrossRef] [PubMed]
- Traniello, J.F.A.; Robson, S.K. Trail and territorial communication in social insects. In Chemical Ecology of Insects 2; Springer: Berlin/Heidelberg, Germany, 1995; pp. 241–286. [Google Scholar]
- Phillips, J.B. Two magnetoreception pathways in a migratory salamander. Science 1986, 233, 765–767. [Google Scholar] [CrossRef] [PubMed]
- Quinn, T.P. Evidence for celestial and magnetic compass orientation in lake migrating sockeye salmon fry. J. Comp. Physiol. 1980, 137, 243–248. [Google Scholar] [CrossRef]
- Lohmann, K.J. Magnetic orientation by hatchling loggerhead sea turtles (Caretta caretta). J. Exp. Biol. 1991, 155, 37–49. [Google Scholar] [CrossRef]
- Walcott, C.; Green, R.P. Orientation of homing pigeons altered by a change in the direction of an applied magnetic field. Science 1974, 184, 180–182. [Google Scholar] [CrossRef]
- Johnsen, S.; Lohmann, K.J. The physics and neurobiology of magnetoreception. Nat. Rev. Neurosci. 2005, 6, 703–712. [Google Scholar] [CrossRef]
- Zadeh-Haghighi, H.; Simon, C. Magnetic field effects in biology from the perspective of the radical pair mechanism. J. R. Soc. Interface 2022, 19, 20220325. [Google Scholar] [CrossRef]
- Jinping, G.; Haoyu, W.; Matysik, J.; Xiaojie, W. Recent advances in magnetosensing cryptochrome model systems. ACTA Chim. Sin. 2018, 76, 597–604. [Google Scholar]
- Hore, P.J.; Mouritsen, H. The radical-pair mechanism of magnetoreception. Annu. Rev. Biophys. 2016, 45, 299–344. [Google Scholar] [CrossRef]
- Castello, P.; Jimenez, P.; Martino, C.F. The role of pulsed electromagnetic fields on the radical pair mechanism. Bioelectromagnetics 2021, 42, 491–500. [Google Scholar] [CrossRef]
- Pooam, M.; Arthaut, L.-D.; Burdick, D.; Link, J.; Martino, C.F.; Ahmad, M. Magnetic sensitivity mediated by the Arabidopsis blue-light receptor cryptochrome occurs during flavin reoxidation in the dark. Planta 2019, 249, 319–332. [Google Scholar] [CrossRef]
- Körnig, A.; Winklhofer, M.; Baumgartner, J.; Gonzalez, T.P.; Fratzl, P.; Faivre, D. Magnetite crystal orientation in magnetosome chains. Adv. Funct. Mater. 2014, 24, 3926–3932. [Google Scholar] [CrossRef]
- Kempster, R.M.; McCarthy, I.D.; Collin, S.P. Phylogenetic and ecological factors influencing the number and distribution of electroreceptors in elasmobranchs. J. Fish Biol. 2012, 80, 2055–2088. [Google Scholar] [CrossRef]
- Qin, S.; Yin, H.; Yang, C.; Dou, Y.; Liu, Z.; Zhang, P.; Yu, H.; Huang, Y.; Feng, J.; Hao, J. A magnetic protein biocompass. Nat. Mater. 2016, 15, 217–226. [Google Scholar] [CrossRef]
- Pooam, M.; Jourdan, N.; El Esawi, M.; Sherrard, R.M.; Ahmad, M. HEK293 cell response to static magnetic fields via the radical pair mechanism may explain therapeutic effects of pulsed electromagnetic fields. PLoS ONE 2020, 15, e0243038. [Google Scholar] [CrossRef]
- Lohmann, K.J. A candidate magnetoreceptor. Nat. Mater. 2016, 15, 136–138. [Google Scholar] [CrossRef]
- Parmagnani, A.S.; D’Alessandro, S.; Maffei, M.E. Iron-sulfur complex assembly: Potential players of magnetic induction in plants. Plant Sci. 2022, 325, 111483. [Google Scholar] [CrossRef]
- Barbero, F. Cuticular lipids as a cross-talk among ants, plants and butterflies. Int. J. Mol. Sci. 2016, 17, 1966. [Google Scholar] [CrossRef]
- Casacci, L.P.; Bonelli, S.; Balletto, E.; Barbero, F. Multimodal signaling in myrmecophilous butterflies. Front. Ecol. Evol. 2019, 7, 454. [Google Scholar] [CrossRef]
- Banks, A.N.; Srygley, R.B. Orientation by magnetic field in leaf-cutter ants, Atta colombica (Hymenoptera: Formicidae). Ethology 2003, 109, 835–846. [Google Scholar] [CrossRef]
- Wehner, R.; Michel, B.; Antonsen, P. Visual navigation in insects: Coupling of egocentric and geocentric information. J. Exp. Biol. 1996, 199, 129–140. [Google Scholar] [CrossRef]
- Fleischmann, P.N.; Grob, R.; Müller, V.L.; Wehner, R.; Rössler, W. The geomagnetic field is a compass cue in Cataglyphis ant navigation. Curr. Biol. 2018, 28, 1440–1444. [Google Scholar] [CrossRef]
- Czaczkes, T.J.; Franz, S.; Witte, V.; Heinze, J. Perception of collective path use affects path selection in ants. Anim. Behav. 2015, 99, 15–24. [Google Scholar] [CrossRef]
- Czaczkes, T.J.; Grüter, C.; Ratnieks, F.L.W. Negative feedback in ants: Crowding results in less trail pheromone deposition. J. R. Soc. Interface 2013, 10, 20121009. [Google Scholar] [CrossRef]
- Evison, S.E.F.; Petchey, O.L.; Beckerman, A.P.; Ratnieks, F.L.W. Combined use of pheromone trails and visual landmarks by the common garden ant Lasius niger. Behav. Ecol. Sociobiol. 2008, 63, 261–267. [Google Scholar] [CrossRef]
- Popp, S.; Buckham-Bonnett, P.; Evison, S.E.F.; Robinson, E.J.H.; Czaczkes, T.J. No evidence for tactile communication of direction in foraging Lasius ants. Insectes Soc. 2018, 65, 37–46. [Google Scholar] [CrossRef]
- Sakamoto, Y.; Sakiyama, T. Ant Lasius niger joining one-way trails go against the flow. Sci. Rep. 2022, 12, 2361. [Google Scholar] [CrossRef]
- Agliassa, C.; Narayana, R.; Bertea, C.M.; Rodgers, C.T.; Maffei, M.E. Reduction of the geomagnetic field delays Arabidopsis thaliana flowering time through downregulation of flowering-related genes. Bioelectromagnetics 2018, 39, 361–374. [Google Scholar] [CrossRef]
- Libersat, F.; Pflueger, H.J. Monoamines and the orchestration of behavior. Bioscience 2004, 54, 17–25. [Google Scholar] [CrossRef]
- Kamhi, J.F.; Traniello, J.F.A. Biogenic amines and collective organization in a superorganism: Neuromodulation of social behavior in ants. Brain. Behav. Evol. 2013, 82, 220–236. [Google Scholar] [CrossRef]
- Kamhi, J.F.; Arganda, S.; Moreau, C.S.; Traniello, J.F.A. Origins of aminergic regulation of behavior in complex insect social systems. Front. Syst. Neurosci. 2017, 11, 74. [Google Scholar] [CrossRef]
- Downer, R.G.H.; Hiripi, L. Biogenic amines in insects. In Insect Neurochemistry and Neurophysiology 1993; CRC Press: Boca Raton, FL, USA, 2019; pp. 23–38. ISBN 1351073591. [Google Scholar]
- Mannino, G.; Abdi, G.; Maffei, M.E.; Barbero, F. Origanum vulgare terpenoids modulate Myrmica scabrinodis brain biogenic amines and ant behaviour. PLoS ONE 2018, 13, e0209047. [Google Scholar] [CrossRef]
- Farooqi, M.K.; Ali, M.; Amir, M. Melatonin and Serotonin: Their Synthesis and Effects in Insects. Chronobiol. Med. 2022, 4, 24–28. [Google Scholar] [CrossRef]
- Raza, M.F.; Wang, T.; Li, Z.; Nie, H.; Giurfa, M.; Husain, A.; Hlaváč, P.; Kodrik, M.; Ali, M.A.; Rady, A. Biogenic amines mediate learning success in appetitive odor conditioning in honeybees. J. King Saud Univ. 2022, 34, 101928. [Google Scholar] [CrossRef]
- Qi, Y.; Wang, L.; Lu, Y. Biogenic amine signaling systems in the red imported fire ant, Solenopsis invicta—Possible contributors to worker division of labor. Gen. Comp. Endocrinol. 2018, 262, 59–70. [Google Scholar] [CrossRef]
- Mannino, G.; Pernici, C.; Serio, G.; Gentile, C.; Bertea, C.M. Melatonin and phytomelatonin: Chemistry, biosynthesis, metabolism, distribution and bioactivity in plants and animals—An overview. Int. J. Mol. Sci. 2021, 22, 9996. [Google Scholar] [CrossRef]
- Xue, L.; Hu, T.; Guo, Z.; Yang, C.; Wang, Z.; Qin, S.; Yang, P.; Xie, C.; Xu, J.; Li, N. A Novel Biomimetic Magnetosensor Based on Magneto-Optically Involved Conformational Variation of MagR/Cry4 Complex. Adv. Electron. Mater. 2020, 6, 1901168. [Google Scholar] [CrossRef]
- Parmagnani, A.S.; Mannino, G.; Maffei, M.E. Transcriptomics and Metabolomics of Reactive Oxygen Species Modulation in Near-Null Magnetic Field-Induced Arabidopsis thaliana. Biomolecules 2022, 12, 1824. [Google Scholar] [CrossRef]
- Aron, S.; Beckers, R.; Deneubourg, J.-L.; Pasteels, J.M. Memory and chemical communication in the orientation of two mass-recruiting ant species. Insectes Soc. 1993, 40, 369–380. [Google Scholar] [CrossRef]
- Pereira, M.C.; de Carvalho Guimarães, I.; Acosta-Avalos, D.; Antonialli Junior, W.F. Can altered magnetic field affect the foraging behaviour of ants? PLoS ONE 2019, 14, e0225507. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.B.; Vander Meer, R.K. Magnetic orientation in the fire ant, Solenopsis invicta. Naturwissenschaften 1993, 80, 568–570. [Google Scholar] [CrossRef]
- Traniello, J.F.A.; Fujita, M.S.; Bowen, R. V Ant foraging behavior: Ambient temperature influences prey selection. Behav. Ecol. Sociobiol. 1984, 15, 65–68. [Google Scholar] [CrossRef]
- Beckers, R.; Deneubourg, J.-L.; Goss, S. Trail laying behaviour during food recruitment in the ant Lasius niger (L.). Insectes Soc. 1992, 39, 59–72. [Google Scholar] [CrossRef]
- Mc Cabe, S.; Farina, W.M.; Josens, R.B. Antennation of nectar-receivers encodes colony needs and food-source profitability in the ant Camponotus mus. Insectes Soc. 2006, 53, 356–361. [Google Scholar] [CrossRef]
- Lenoir, A. An informational analysis of antennal communication during trophallaxis in the ant Myrmica rubra L. Behav. Process. 1982, 7, 27–35. [Google Scholar] [CrossRef]
- Verlinden, H. Dopamine signalling in locusts and other insects. Insect Biochem. Mol. Biol. 2018, 97, 40–52. [Google Scholar] [CrossRef]
- Schoofs, L.; De Loof, A.; Van Hiel, M.B. Neuropeptides as regulators of behavior in insects. Annu. Rev. Entomol. 2017, 62, 35–52. [Google Scholar] [CrossRef]
- Sasaki, K.; Okada, Y.; Shimoji, H.; Aonuma, H.; Miura, T.; Tsuji, K. Social evolution with decoupling of multiple roles of biogenic amines into different phenotypes in Hymenoptera. Front. Ecol. Evol. 2021. 9, 659160. [CrossRef]
- Muscedere, M.L.; Johnson, N.; Gillis, B.C.; Kamhi, J.F.; Traniello, J.F.A. Serotonin modulates worker responsiveness to trail pheromone in the ant Pheidole dentata. J. Comp. Physiol. A 2012, 198, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Vander Meer, R.K.; Preston, C.A.; Hefetz, A. Queen regulates biogenic amine level and nestmate recognition in workers of the fire ant, Solenopsis invicta. Naturwissenschaften 2008, 95, 1155–1158. [Google Scholar] [CrossRef] [PubMed]
- Xie, C. Searching for unity in diversity of animal magnetoreception: From biology to quantum mechanics and back. Innovation 2022, 3, 100229. [Google Scholar] [CrossRef] [PubMed]
- Bouly, J.P.; Schleicher, E.; Dionisio-Sese, M.; Vandenbussche, F.; Van Der Straeten, D.; Bakrim, N.; Meier, S.; Batschauer, A.; Galland, P.; Bittl, R. Cryptochrome blue light photoreceptors are activated through interconversion of flavin redox states. J. Biol. Chem. 2007, 282, 9383–9391. [Google Scholar] [CrossRef] [PubMed]
- Berwal, M.; Ram, C. Superoxide dismutase: A stable biochemical marker for abiotic stress tolerance in higher plants. In Abiotic and Biotic Stress in Plants; BoD—Books on Demand GmbH: Norderstedt, Germany, 2018; pp. 1–10. [Google Scholar]
- Kaushal, J.; Mehandia, S.; Singh, G.; Raina, A.; Arya, S.K. Catalase enzyme: Application in bioremediation and food industry. Biocatal. Agric. Biotechnol. 2018, 16, 192–199. [Google Scholar] [CrossRef]
- Creissen, G.P.; Edwards, E.A.; Mullineaux, P.M. Glutathione reductase and ascorbate peroxidase. In Causes of Photooxidative Stress and Amelioration of Defense Systems in Plants; CRC Press: Boca Raton, FL, USA, 2019; pp. 343–364. ISBN 1351070452. [Google Scholar]
- Seifert, B. The Ants of Central and North Europe; Iutra Verlags-und Vertriebsgesellschaft: Tauer, Germany, 2018; ISBN 3936412073. [Google Scholar]
- Cammaerts, M.C.; Cammaerts, D. Light thresholds for colour vision in workers of the ant Myrmica sabuleti (Hymenoptera: Formicidae). Belg. J. Zool. 2009, 139, 40–49. [Google Scholar]
- Friard, O.; Gamba, M. BORIS: A free, versatile open-source event-logging software for video/audio coding and live observations. Methods Ecol. Evol. 2016, 7, 1325–1330. [Google Scholar] [CrossRef]
- Magara, G.; Prearo, M.; Vercelli, C.; Barbero, R.; Micera, M.; Botto, A.; Caimi, C.; Caldaroni, B.; Bertea, C.M.; Mannino, G. Modulation of antioxidant defense in farmed rainbow trout (Oncorhynchus mykiss) fed with a diet supplemented by the waste derived from the supercritical fluid extraction of basil (Ocimum basilicum). Antioxidants 2022, 11, 415. [Google Scholar] [CrossRef]
- Campobenedetto, C.; Agliassa, C.; Mannino, G.; Vigliante, I.; Contartese, V.; Secchi, F.; Bertea, C.M. A biostimulant based on seaweed (Ascophyllum nodosum and Laminaria digitata) and yeast extracts mitigates water stress effects on tomato (Solanum lycopersicum L.). Agriculture 2021, 11, 557. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mannino, G.; Casacci, L.P.; Bianco Dolino, G.; Badolato, G.; Maffei, M.E.; Barbero, F. The Geomagnetic Field (GMF) Is Necessary for Black Garden Ant (Lasius niger L.) Foraging and Modulates Orientation Potentially through Aminergic Regulation and MagR Expression. Int. J. Mol. Sci. 2023, 24, 4387. https://doi.org/10.3390/ijms24054387
Mannino G, Casacci LP, Bianco Dolino G, Badolato G, Maffei ME, Barbero F. The Geomagnetic Field (GMF) Is Necessary for Black Garden Ant (Lasius niger L.) Foraging and Modulates Orientation Potentially through Aminergic Regulation and MagR Expression. International Journal of Molecular Sciences. 2023; 24(5):4387. https://doi.org/10.3390/ijms24054387
Chicago/Turabian StyleMannino, Giuseppe, Luca Pietro Casacci, Giorgia Bianco Dolino, Giuseppe Badolato, Massimo Emilio Maffei, and Francesca Barbero. 2023. "The Geomagnetic Field (GMF) Is Necessary for Black Garden Ant (Lasius niger L.) Foraging and Modulates Orientation Potentially through Aminergic Regulation and MagR Expression" International Journal of Molecular Sciences 24, no. 5: 4387. https://doi.org/10.3390/ijms24054387
APA StyleMannino, G., Casacci, L. P., Bianco Dolino, G., Badolato, G., Maffei, M. E., & Barbero, F. (2023). The Geomagnetic Field (GMF) Is Necessary for Black Garden Ant (Lasius niger L.) Foraging and Modulates Orientation Potentially through Aminergic Regulation and MagR Expression. International Journal of Molecular Sciences, 24(5), 4387. https://doi.org/10.3390/ijms24054387