A Nucleus Accumbens Tac1 Neural Circuit Regulates Avoidance Responses to Aversive Stimuli
Abstract
1. Introduction
2. Results
2.1. NAcTac1 Neurons Regulate Avoidance Behavior in Response to Aversive Stimuli
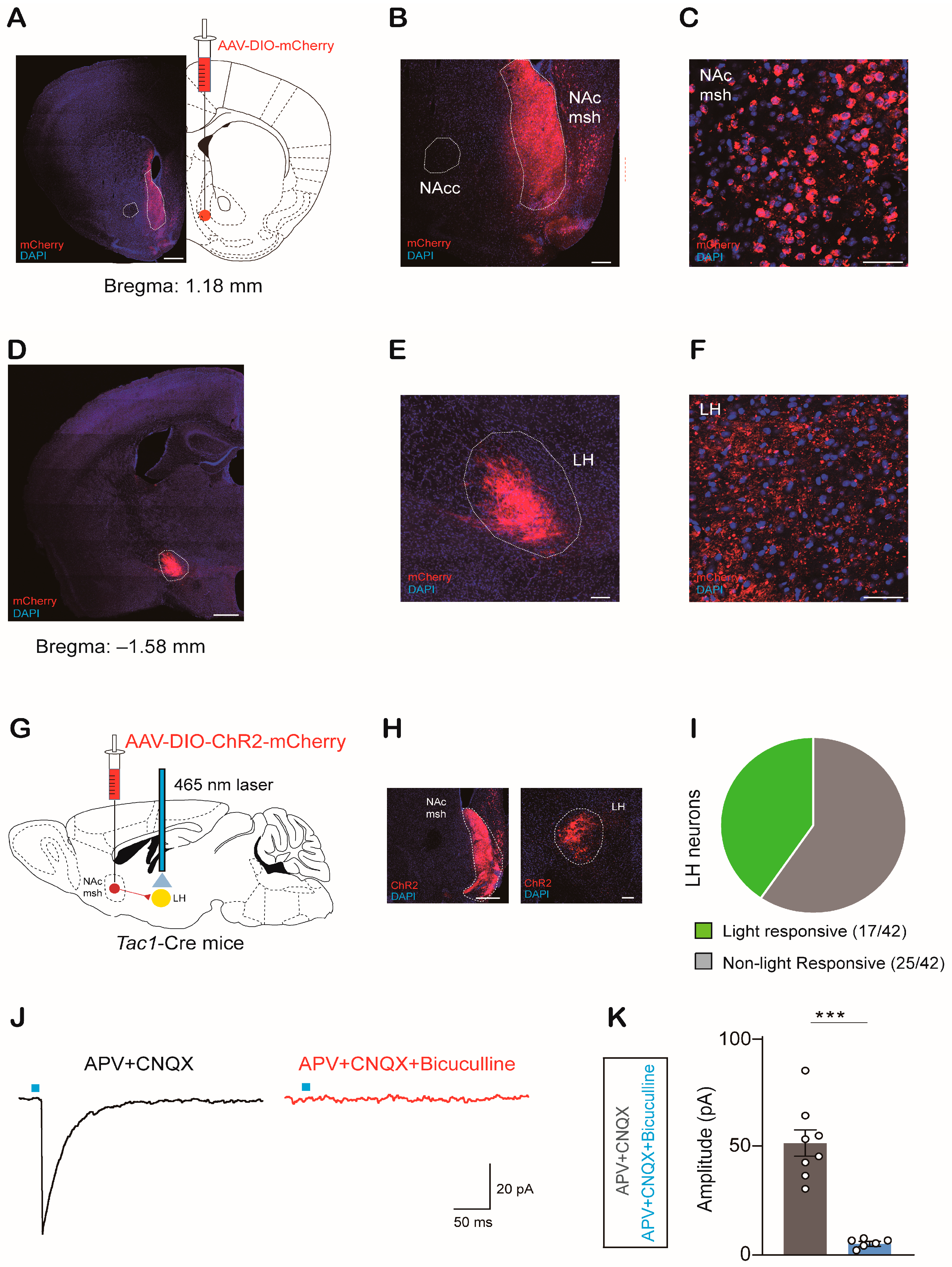
2.2. NAcTac1 Neurons Project to the LH
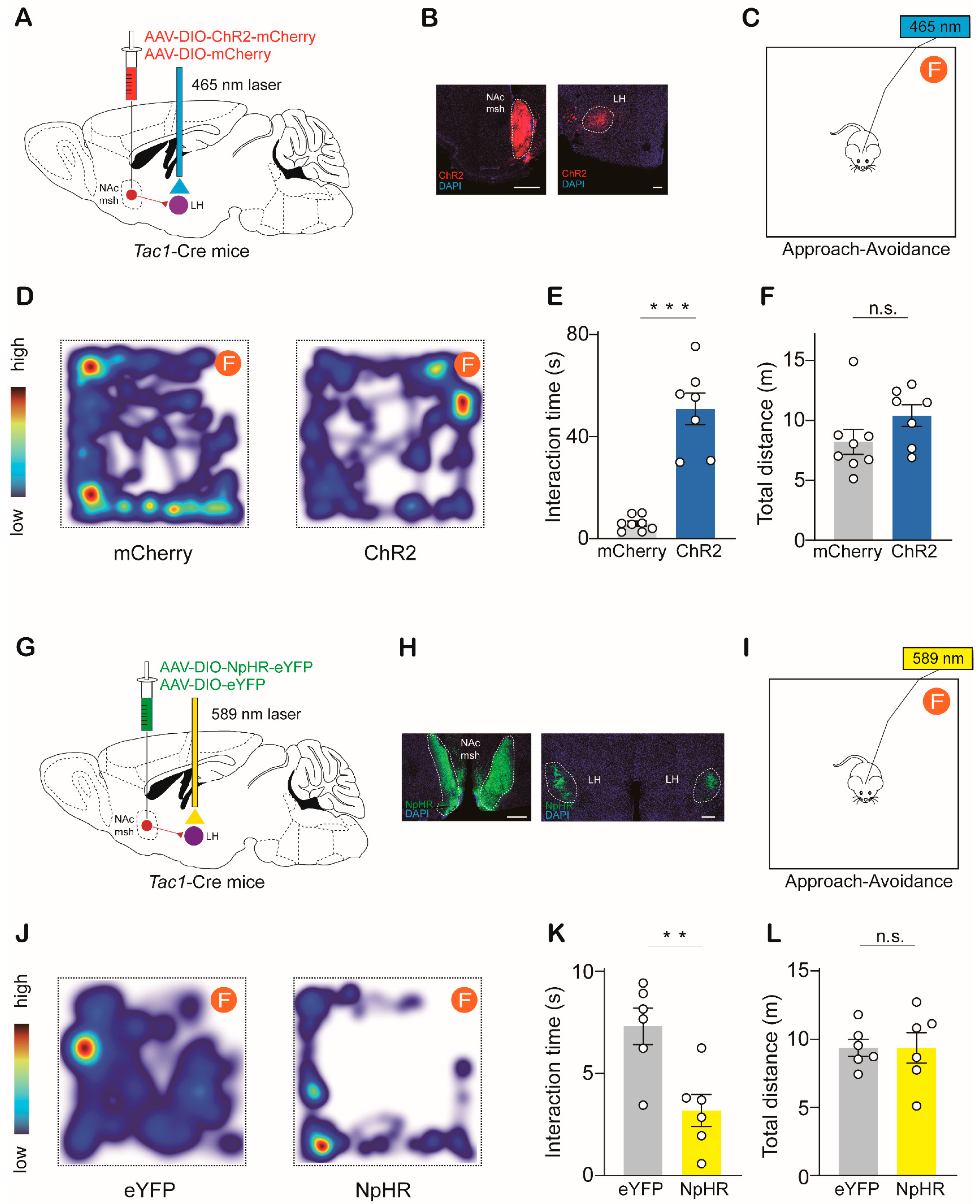
2.3. NAcTac1-to-LH Projection Mediates Avoidance Behaviors in Response to Aversive Stimuli
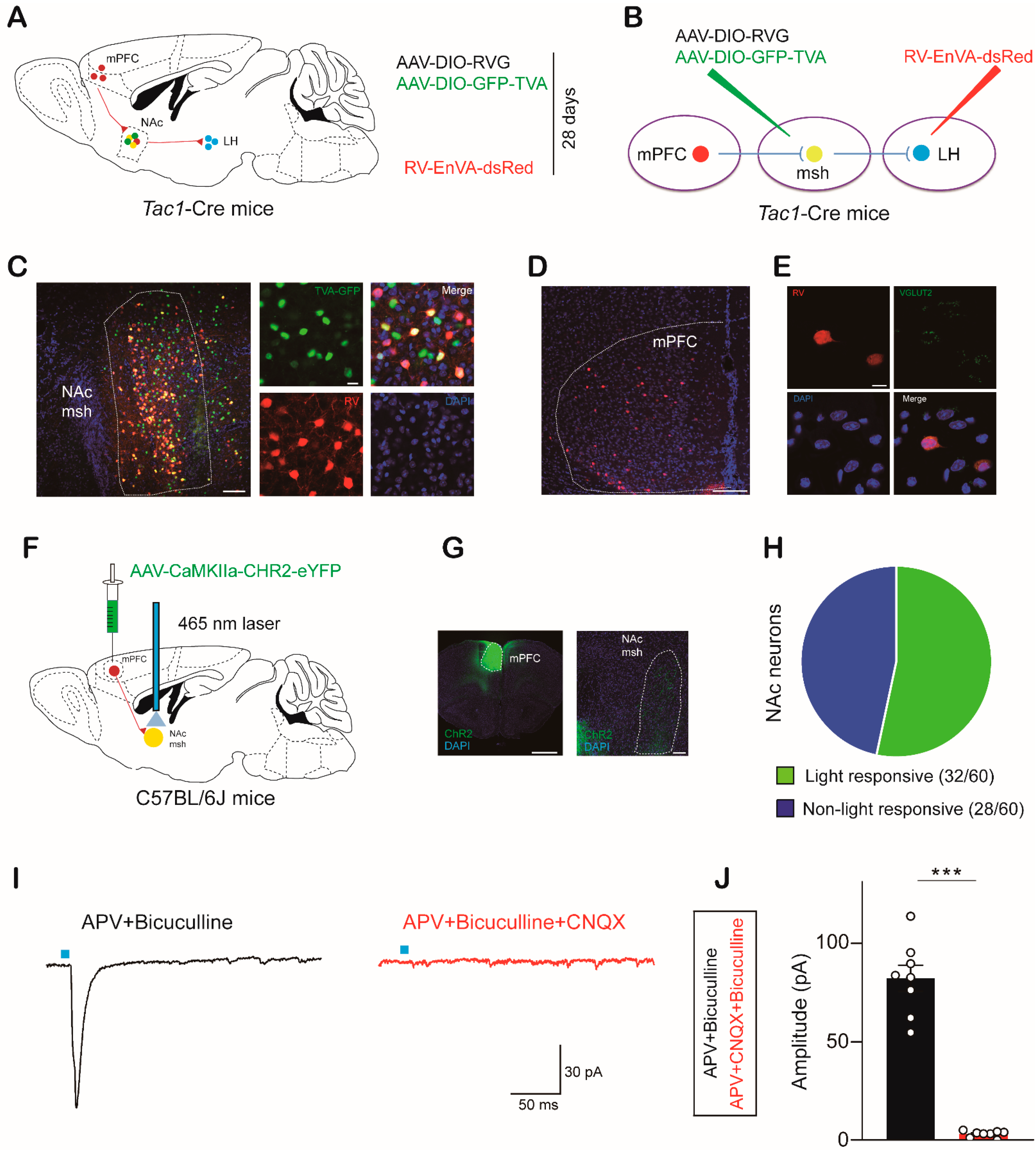
2.4. mPFCGlut Inputs Activate NAc Neurons
2.5. The mPFCGlut-to-NAc Circuit Modulates Avoidance Behaviors in Response to Aversive Stimuli
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Viral Vector Generation
4.3. Viral Tracing
4.4. Stereotaxic Injection
4.5. Implantation of Optical Fibers
4.6. Immunohistochemistry
4.7. Ex Vivo Electrophysiology
4.8. Behavioral Assays
4.9. Approach-Avoidance Test
4.10. RTPA Assay
4.11. Olfaction Test
4.12. Quantification and Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mongeau, R.; Miller, G.A.; Chiang, E.; Anderson, D.J. Neural Correlates of Competing Fear Behaviors Evoked by an Innately Aversive Stimulus. J. Neurosci. 2003, 23, 3855–3868. [Google Scholar] [CrossRef]
- Mogenson, G.J.; Jones, D.L.; Yim, C.Y. From motivation to action: Functional interface between the limbic system and the mo-tor system. Prog. Neurobiol. 1980, 14, 69–97. [Google Scholar] [CrossRef]
- Hu, H. Reward and Aversion. Annu. Rev. Neurosci. 2016, 39, 297–324. [Google Scholar] [CrossRef]
- Tye, K.M. Neural Circuit Motifs in Valence Processing. Neuron 2018, 100, 436–452. [Google Scholar] [CrossRef]
- Wise, R.A.; Rompre, P.P. Brain dopamine and reward. Annu. Rev. Psychol. 1989, 40, 191–225. [Google Scholar] [CrossRef]
- Berridge, K.C.; Kringelbach, M.L. Pleasure Systems in the Brain. Neuron 2015, 86, 646–664. [Google Scholar] [CrossRef]
- Luo, M.; Zhou, J.; Liu, Z. Reward processing by the dorsal raphe nucleus: 5-HT and beyond. Learn. Mem. 2015, 22, 452–460. [Google Scholar] [CrossRef]
- Klawonn, A.M.; Malenka, R.C. Nucleus Accumbens Modulation in Reward and Aversion. Cold Spring Harb. Symp. Quant. Biol. 2018, 83, 119–129. [Google Scholar] [CrossRef]
- Volkow, N.D.; Wise, R.A.; Baler, R. The dopamine motive system: Implications for drug and food addiction. Nat. Rev. Neurosci. 2017, 18, 741–752. [Google Scholar] [CrossRef]
- Carlezon, W.A., Jr.; Thomas, M.J. Biological substrates of reward and aversion: A nucleus accumbens activity hypothesis. Neuropharmacology 2009, 56, 122–132. [Google Scholar] [CrossRef]
- Kenny, P.J. Common cellular and molecular mechanisms in obesity and drug addiction. Nat. Rev. Neurosci. 2011, 12, 638–651. [Google Scholar] [CrossRef]
- Mccutcheon, J.E.; Ebner, S.R.; Loriaux, A.L.; Roitman, M.F. Encoding of Aversion by Dopamine and the Nucleus Accumbens. Front. Neurosci. 2012, 6, 137. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.K.; Sanchez, M.I.; Hoerbelt, P.; Fenno, L.E.; Malenka, R.C.; Deisseroth, K.; Ting, A.Y. A Molecular Calcium Integrator Reveals a Striatal Cell Type Driving Aversion. Cell 2020, 183, 2003–2019.e16. [Google Scholar] [CrossRef]
- He, Z.-X.; Yin, Y.-Y.; Xi, K.; Xing, Z.-K.; Cao, J.-B.; Liu, T.-Y.; Liu, L.; He, X.-X.; Yu, H.-L.; Zhu, X.-J. Nucleus Accumbens Tac1-Expressing Neurons Mediate Stress-Induced Anhedonia-like Behavior in Mice. Cell Rep. 2020, 33, 108343. [Google Scholar] [CrossRef]
- Castro, D.C.; Bruchas, M.R. A Motivational and Neuropeptidergic Hub: Anatomical and Functional Diversity within the Nu-cleus Accumbens Shell. Neuron 2019, 102, 529–552. [Google Scholar] [CrossRef]
- Al-Hasani, R.; McCall, J.G.; Shin, G.; Gomez, A.M.; Schmitz, G.P.; Bernardi, J.M.; Pyo, C.O.; Park, S.I.; Marcinkiewcz, C.M.; Crowley, N.A.; et al. Distinct Subpopulations of Nucleus Accumbens Dy-norphin Neurons Drive Aversion and Reward. Neuron 2015, 87, 1063–1077. [Google Scholar] [CrossRef]
- Blomeley, C.; Garau, C.; Burdakov, D. Accumbal D2 cells orchestrate innate risk-avoidance according to orexin signals. Nat. Neurosci. 2017, 21, 29–32. [Google Scholar] [CrossRef]
- De Jong, J.W.; Afjei, S.A.; Dorocic, I.P.; Peck, J.R.; Liu, C.; Kim, C.K.; Tian, L.; Deisseroth, K.; Lammel, S. A Neural Circuit Mechanism for Encoding Aversive Stimuli in the Mesolimbic Dopamine System. Neuron 2018, 101, 133–151.e7. [Google Scholar] [CrossRef]
- Zhu, Y.; Wienecke, C.F.R.; Nachtrab, G.; Chen, X. A thalamic input to the nucleus accumbens mediates opiate dependence. Nature 2016, 530, 219–222. [Google Scholar] [CrossRef]
- Zhou, K.; Xu, H.; Lu, S.; Jiang, S.; Hou, G.; Deng, X.; He, M.; Zhu, Y. Reward and aversion processing by input-defined parallel nucleus accumbens circuits in mice. Nat. Commun. 2022, 13, 1–12. [Google Scholar] [CrossRef]
- Christoffel, D.J.; Walsh, J.J.; Heifets, B.D.; Hoerbelt, P.; Neuner, S.; Sun, G.; Ravikumar, V.K.; Wu, H.; Halpern, C.H.; Malenka, R.C. Input-specific modulation of murine nucleus accumbens differentially regulates hedonic feeding. Nat. Commun. 2021, 12, 1–12. [Google Scholar] [CrossRef]
- Beas, B.S.; Wright, B.; Skirzewski, M.; Leng, Y.; Hyun, J.H.; Koita, O.; Ringelberg, N.; Kwon, H.-B.; Buonanno, A.; Penzo, M.A. The locus coeruleus drives disinhibition in the midline thalamus via a dopaminergic mechanism. Nat. Neurosci. 2018, 21, 963–973. [Google Scholar] [CrossRef] [PubMed]
- Gerfen, C.R.; Engber, T.M.; Mahan, L.C.; Susel, Z.; Chase, T.N.; Monsma, F.J., Jr.; Sibley, D.R. D1 and D2 dopamine recep-tor-regulated gene expression of striatonigral and striatopallidal neurons. Science 1990, 250, 1429–1432. [Google Scholar] [CrossRef] [PubMed]
- Kreitzer, A.C. Physiology and Pharmacology of Striatal Neurons. Annu. Rev. Neurosci. 2009, 32, 127–147. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.J.; Lobo, M.K.; Spencer, S.; Kalivas, P.W. Cocaine-induced adaptations in D1 and D2 accumbens projection neurons (a dichotomy not necessarily synonymous with direct and indirect pathways). Curr. Opin. Neurobiol. 2013, 23, 546–552. [Google Scholar] [CrossRef] [PubMed]
- Steiner, H.; Gerfen, C.R. Role of dynorphin and enkephalin in the regulation of striatal output pathways and behavior. Exp. Brain Res. 1998, 123, 60–76. [Google Scholar] [CrossRef] [PubMed]
- Surmeier, D.J.; Song, W.J.; Yan, Z. Coordinated expression of dopamine receptors in neostriatal medium spiny neurons. J. Neurosci. 1996, 16, 6579–6591. [Google Scholar] [CrossRef]
- Anderson, K.D.; Reiner, A. Extensive co-occurrence of substance P and dynorphin in striatal projection neurons: An evolu-tionarily conserved feature of basal ganglia organization. J. Comp. Neurol. 1990, 295, 339–369. [Google Scholar] [CrossRef]
- Blomeley, C.P.; Kehoe, L.A.; Bracci, E. Substance P mediates excitatory interactions between striatal projection neurons. J. Neurosci. 2009, 29, 4953–4963. [Google Scholar] [CrossRef]
- Francis, T.C.; Yano, H.; Demarest, T.G.; Shen, H.; Bonci, A. High-Frequency Activation of Nucleus Accumbens D1-MSNs Drives Excitatory Potentiation on D2-MSNs. Neuron 2019, 103, 432–444.e3. [Google Scholar] [CrossRef]
- Tejeda, H.A.; Wu, J.; Kornspun, A.R.; Pignatelli, M.; Kashtelyan, V.; Krashes, M.J.; Lowell, B.B.; Carlezon, W.A.; Bonci, A. Pathway- and Cell-Specific Kappa-Opioid Receptor Modulation of Excitation-Inhibition Balance Differentially Gates D1 and D2 Accumbens Neuron Activity. Neuron 2017, 93, 147–163. [Google Scholar] [CrossRef]
- Pignatelli, M.; Tejeda, H.A.; Barker, D.J.; Bontempi, L.; Wu, J.; Lopez, A.; Ribeiro, S.P.; Lucantonio, F.; Parise, E.M.; Torres-Berrio, A.; et al. Cooperative synaptic and intrinsic plasticity in a disynaptic limbic circuit drive stress-induced anhedonia and passive coping in mice. Mol. Psychiatry 2020, 26, 1860–1879. [Google Scholar] [CrossRef] [PubMed]
- Britt, J.P.; Benaliouad, F.; McDevitt, R.A.; Stuber, G.D.; Wise, R.A.; Bonci, A. Synaptic and behavioral profile of multiple glu-tamatergic inputs to the nucleus accumbens. Neuron 2012, 76, 790–803. [Google Scholar] [CrossRef]
- Russo, S.J.; Nestler, E.J. The brain reward circuitry in mood disorders, Nature reviews. Neuroscience 2013, 14, 609–625. [Google Scholar]
- Monosov, I.E.; Hikosaka, O. Regionally distinct processing of rewards and punishments by the primate ventromedial pre-frontal cortex. J. Neurosci. 2012, 32, 10318–10330. [Google Scholar] [CrossRef]
- Friedman, A.; Homma, D.; Gibb, L.G.; Amemori, K.-I.; Rubin, S.J.; Hood, A.S.; Riad, M.H.; Graybiel, A.M. A Corticostriatal Path Targeting Striosomes Controls Decision-Making under Conflict. Cell 2015, 161, 1320–1333. [Google Scholar] [CrossRef]
- Weele, C.M.V.; Siciliano, C.A.; Matthews, G.A.; Namburi, P.; Izadmehr, E.M.; Espinel, I.C.; Nieh, E.H.; Schut, E.H.S.; Padilla-Coreano, N.; Burgos-Robles, A.; et al. Dopamine enhances signal-to-noise ratio in cortical-brainstem encoding of aversive stimuli. Nature 2018, 563, 397–401. [Google Scholar] [CrossRef]
- Moscarello, J.M.; LeDoux, J.E. Active avoidance learning requires prefrontal suppression of amygdala-mediated defensive reactions. J. Neurosci. 2013, 33, 3815–3823. [Google Scholar] [CrossRef]
- Joel, D.; Tarrasch, R.; Feldon, J.; Weiner, I. Effects of electrolytic lesions of the medial prefrontal cortex or its subfields on 4-arm baited, 8-arm radial maze, two-way active avoidance and conditioned fear tasks in the rat. Brain Res. 1997, 765, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Holson, R.R. Mesial prefrontal cortical lesions and timidity in rats. I. Reactivity to aversive stimuli. Physiol. Behav. 1986, 37, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.A.; Hirokawa, K.E.; Sorensen, S.A.; Gu, H.; Mills, M.; Ng, L.L.; Bohn, P.; Mortrud, M.; Ouellette, B.; Kidney, J.; et al. Anatomical characterization of Cre driver mice for neural circuit mapping and manipulation. Front. Neural Circuits 2014, 8, 76. [Google Scholar] [CrossRef]
- Madisen, L.; Zwingman, T.A.; Sunkin, S.M.; Oh, S.W.; Zariwala, H.A.; Gu, H.; Ng, L.L.; Palmiter, R.D.; Hawrylycz, M.J.; Jones, A.R.; et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat. Neurosci. 2009, 13, 133–140. [Google Scholar] [CrossRef]
- Deyama, S.; Yamamoto, J.; Machida, T.; Tanimoto, S.; Nakagawa, T.; Kaneko, S.; Satoh, M.; Minami, M. Inhibition of glutama-tergic transmission by morphine in the basolateral amygdaloid nucleus reduces pain-induced aversion. Neurosci. Res. 2007, 59, 199–204. [Google Scholar] [CrossRef]
- Lammel, S.; Ion, D.I.; Roeper, J.; Malenka, R.C. Projection-Specific Modulation of Dopamine Neuron Synapses by Aversive and Rewarding Stimuli. Neuron 2011, 70, 855–862. [Google Scholar] [CrossRef]
- Sorg, B.A.; Davidson, D.L.; Hochstatter, T.; Sylvester, P.W. Repeated cocaine decreases the avoidance response to a novel aver-sive stimulus in rats. Psychopharmacology 2002, 163, 9–19. [Google Scholar] [CrossRef]
- Burgos-Robles, A.N.; Kimchi, E.Y.; Izadmehr, E.M.; Porzenheim, M.J.; Ramos-Guasp, W.A.; Nieh, E.H.; Felix-Ortiz, A.C.; Namburi, P.; Leppla, C.A.; Presbrey, K.N.; et al. Amygdala inputs to prefrontal cortex guide behavior amid conflicting cues of reward and punishment. Nat. Neurosci. 2017, 20, 824–835. [Google Scholar] [CrossRef]
- Euston, D.R.; Gruber, A.J.; McNaughton, B.L. The Role of Medial Prefrontal Cortex in Memory and Decision Making. Neuron 2012, 76, 1057–1070. [Google Scholar] [CrossRef]
- Bravo-Rivera, C.; Roman-Ortiz, C.; Brignoni-Perez, E.; Sotres-Bayon, F.; Quirk, G.J. Neural structures mediating expression and extinction of platform-mediated avoidance. J. Neurosci. 2014, 34, 9736–9742. [Google Scholar] [CrossRef]
- Berthoud, H.-R.; Münzberg, H. The lateral hypothalamus as integrator of metabolic and environmental needs: From electrical self-stimulation to opto-genetics. Physiol. Behav. 2011, 104, 29–39. [Google Scholar] [CrossRef]
- Stuber, G.D.; Wise, R.A. Lateral hypothalamic circuits for feeding and reward. Nat. Neurosci. 2016, 19, 198–205. [Google Scholar] [CrossRef]
- Jennings, J.H.; Rizzi, G.; Stamatakis, A.M.; Ung, R.L.; Stuber, G.D. The inhibitory circuit architecture of the lateral hypothala-mus orchestrates feeding. Science 2013, 341, 1517–1521. [Google Scholar] [CrossRef]
- Lazaridis, I.; Tzortzi, O.; Weglage, M.; Märtin, A.; Xuan, Y.; Parent, M.; Johansson, Y.; Fuzik, J.; Fürth, D.; Fenno, L.E.; et al. A hypothalamus-habenula circuit controls aversion. Mol. Psychiatry 2019, 24, 1351–1368. [Google Scholar] [CrossRef]
- Stamatakis, A.M.; Van Swieten, M.; Basiri, M.L.; Blair, G.A.; Kantak, P.; Stuber, G.D. Lateral Hypothalamic Area Glutamatergic Neurons and Their Projections to the Lateral Habenula Regulate Feeding and Reward. J. Neurosci. 2016, 36, 302–311. [Google Scholar] [CrossRef]
- Lecca, S.; Meye, F.J.; Trusel, M.; Tchenio, A.; Harris, J.; Schwarz, M.K.; Burdakov, D.; Georges, F.; Mameli, M. Aversive stimuli drive hypothalamus-to-habenula excitation to promote escape behavior. Elife 2017, 6, e30697. [Google Scholar] [CrossRef]
- Lee, A.T.; Vogt, D.; Rubenstein, J.L.; Sohal, V.S. A class of GABAergic neurons in the prefrontal cortex sends long-range projec-tions to the nucleus accumbens and elicits acute avoidance behavior. J. Neurosci. 2014, 34, 11519–11525. [Google Scholar] [CrossRef]
- Meredith, G.; Agolia, R.; Arts, M.; Groenewegen, H.; Zahm, D. Morphological differences between projection neurons of the core and shell in the nucleus accumbens of the rat. Neuroscience 1992, 50, 149–162. [Google Scholar] [CrossRef]
- Bertran-Gonzalez, J.; Bosch-Bouju, C.; Maroteaux, M.; Matamales, M.; Herve, D.; Valjent, E.; Girault, J.-A. Opposing Patterns of Signaling Activation in Dopamine D1 and D2 Receptor-Expressing Striatal Neurons in Response to Cocaine and Haloperidol. J. Neurosci. 2008, 28, 5671–5685. [Google Scholar] [CrossRef]
- Kupchik, Y.M.; Brown, R.; Heinsbroek, J.; Lobo, M.K.; Schwartz, D.J.; Kalivas, P.W. Coding the direct/indirect pathways by D1 and D2 receptors is not valid for accumbens projections. Nat. Neurosci. 2015, 18, 1230–1232. [Google Scholar] [CrossRef]
- Kupchik, N.M.; Bridges, E.P. Improving Outcomes from In-Hospital Cardiac Arrest. AJN Am. J. Nurs. 2015, 115, 51–54. [Google Scholar] [CrossRef]
- Liu, Z.; Le, Q.; Lv, Y.; Chen, X.; Cui, J.; Zhou, Y.; Cheng, D.; Ma, C.; Su, X.; Xiao, L.; et al. A distinct D1-MSN subpopulation down-regulates dopamine to promote negative emotional state. Cell Res. 2021, 32, 139–156. [Google Scholar] [CrossRef]
- Shen, C.J.; Zheng, D.; Li, K.X.; Yang, J.M.; Pan, H.Q.; Yu, X.D.; Fu, J.Y.; Zhu, Y.; Sun, Q.X.; Tang, M.Y.; et al. Cannabinoid CB(1) receptors in the amygdalar cholecystokinin glutamatergic afferents to nucleus accum-bens modulate depressive-like behavior. Nat. Med. 2019, 25, 337–349. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Z.-X.; Xi, K.; Liu, K.-J.; Yue, M.-H.; Wang, Y.; Yin, Y.-Y.; Liu, L.; He, X.-X.; Yu, H.-L.; Xing, Z.-K.; et al. A Nucleus Accumbens Tac1 Neural Circuit Regulates Avoidance Responses to Aversive Stimuli. Int. J. Mol. Sci. 2023, 24, 4346. https://doi.org/10.3390/ijms24054346
He Z-X, Xi K, Liu K-J, Yue M-H, Wang Y, Yin Y-Y, Liu L, He X-X, Yu H-L, Xing Z-K, et al. A Nucleus Accumbens Tac1 Neural Circuit Regulates Avoidance Responses to Aversive Stimuli. International Journal of Molecular Sciences. 2023; 24(5):4346. https://doi.org/10.3390/ijms24054346
Chicago/Turabian StyleHe, Zi-Xuan, Ke Xi, Kai-Jie Liu, Mei-Hui Yue, Yao Wang, Yue-Yue Yin, Lin Liu, Xiao-Xiao He, Hua-Li Yu, Zhen-Kai Xing, and et al. 2023. "A Nucleus Accumbens Tac1 Neural Circuit Regulates Avoidance Responses to Aversive Stimuli" International Journal of Molecular Sciences 24, no. 5: 4346. https://doi.org/10.3390/ijms24054346
APA StyleHe, Z.-X., Xi, K., Liu, K.-J., Yue, M.-H., Wang, Y., Yin, Y.-Y., Liu, L., He, X.-X., Yu, H.-L., Xing, Z.-K., & Zhu, X.-J. (2023). A Nucleus Accumbens Tac1 Neural Circuit Regulates Avoidance Responses to Aversive Stimuli. International Journal of Molecular Sciences, 24(5), 4346. https://doi.org/10.3390/ijms24054346



