Adjuvant Therapy for Renal Cell Carcinoma: Hype or Hope?
Abstract
1. Introduction
2. Adjuvant Therapy in RCC
2.1. Rationale Use in RCC
2.2. Risk Classification
2.3. Efficacy Outcome
3. Adjuvant Tyrosine Kinase Inhibitors
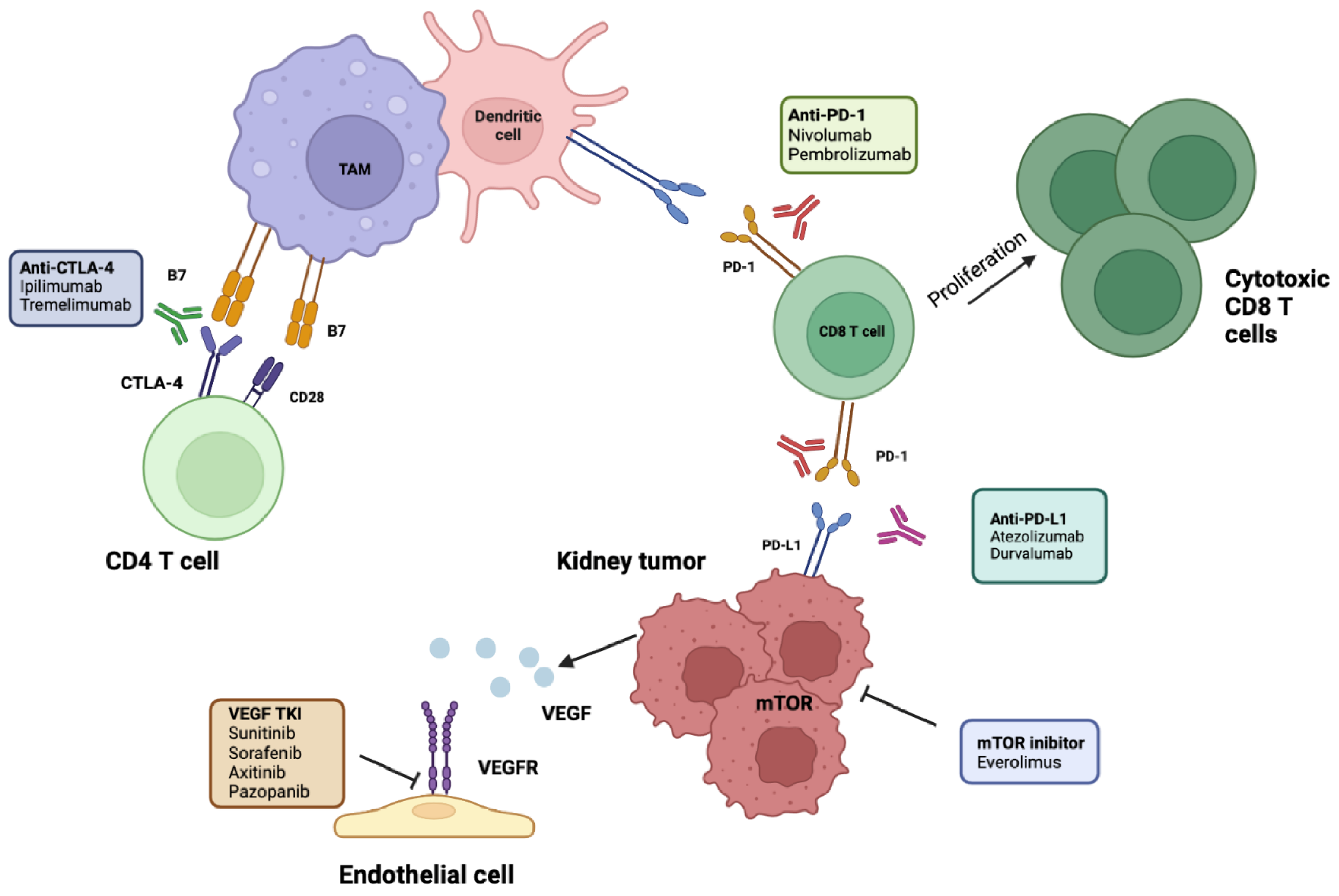
4. Rational Use of Immunotherapy in an Adjuvant Setting
Adjuvant Immune Checkpoint Inhibitors
5. Current Challenges and Future Perspective
Author Contributions
Funding
Conflicts of Interest
References
- Makino, T.; Kadomoto, S.; Izumi, K.; Mizokami, A. Epidemiology and Prevention of Renal Cell Carcinoma. Cancers 2022, 14, 4059. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Padala, S.A.; Barsouk, A.; Thandra, K.C.; Saginala, K.; Mohammed, A.; Vakiti, A.; Rawla, P.; Barsouk, A. Epidemiology of Renal Cell Carcinoma. World J. Oncol. 2020, 11, 79–87. [Google Scholar] [CrossRef]
- Chowdhury, N.; Drake, C.G. Kidney Cancer: An Overview of Current Therapeutic Approaches. Urol. Clin. North Am. 2020, 47, 419–431. [Google Scholar] [CrossRef] [PubMed]
- Eisen, T.; Frangou, E.; Oza, B.; Ritchie, A.W.; Smith, B.; Kaplan, R.; Davis, I.D.; Stockler, M.R.; Albiges, L.; Escudier, B.; et al. Adjuvant Sorafenib for Renal Cell Carcinoma at Intermediate or High Risk of Relapse: Results from the SORCE Randomized Phase III Intergroup Trial. J. Clin. Oncol. 2020, 38, 4064–4075. [Google Scholar] [CrossRef]
- Gross-Goupil, M.; Kwon, T.; Eto, M.; Ye, D.; Miyake, H.; Seo, S.; Byun, S.-S.; Lee, J.; Master, V.; Jin, J.; et al. Axitinib versus placebo as an adjuvant treatment of renal cell carcinoma: Results from the phase III, randomized ATLAS trial. Ann. Oncol. 2018, 29, 2371–2378. [Google Scholar] [CrossRef]
- Haas, N.B.; Manola, J.; Uzzo, R.G.; Flaherty, K.T.; Wood, C.G.; Kane, C.; Jewett, M.; Dutcher, J.P.; Atkins, M.B.; Pins, M.; et al. Adjuvant sunitinib or sorafenib for high-risk, non-metastatic renal-cell carcinoma (ECOG-ACRIN E2805): A double-blind, placebo-controlled, randomised, phase 3 trial. Lancet 2016, 387, 2008–2016. [Google Scholar] [CrossRef]
- Motzer, R.J.; Haas, N.B.; Donskov, F.; Gross-Goupil, M.; Varlamov, S.; Kopyltsov, E.; Lee, J.L.; Melichar, B.; Rini, B.I.; Choueiri, T.K.; et al. Randomized Phase III Trial of Adjuvant Pazopanib Versus Placebo After Nephrectomy in Patients with Localized or Locally Advanced Renal Cell Carcinoma. J. Clin. Oncol. 2017, 35, 3916–3923. [Google Scholar] [CrossRef]
- Ravaud, A.; Motzer, R.J.; Pandha, H.S.; George, D.J.; Pantuck, A.J.; Patel, A.; Chang, Y.-H.; Escudier, B.; Donskov, F.; Magheli, A.; et al. Adjuvant Sunitinib in High-Risk Renal-Cell Carcinoma after Nephrectomy. New Engl. J. Med. 2016, 375, 2246–2254. [Google Scholar] [CrossRef]
- Ryan, C.W.; Tangen, C.; Heath, E.I.; Stein, M.N.; Meng, M.; Alva, A.S.; Pal, S.K.; Puzanov, I.; Clark, J.I.; Choueiri, T.K.; et al. EVEREST: Everolimus for renal cancer ensuing surgical therapy—A phase III study (SWOG S0931, NCT01120249). J. Clin. Oncol. 2022, 40, LBA4500. [Google Scholar] [CrossRef]
- FDA Approves Pembrolizumab for Adjuvant Treatment of Renal Cell Carcinoma. FDA. 2021. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-pembrolizumab-adjuvant-treatment-renal-cell-carcinoma (accessed on 2 November 2022).
- American Cancer Society. Survival Rates for Kidney Cancer. Available online: https://www.cancer.org/cancer/kidney-cancer/detection-diagnosis-staging/survival-rates.html (accessed on 12 November 2022).
- Stewart-Merrill, S.B.; Thompson, R.H.; Boorjian, S.A.; Psutka, S.P.; Lohse, C.M.; Cheville, J.C.; Leibovich, B.C.; Frank, I. Oncologic Surveillance After Surgical Resection for Renal Cell Carcinoma: A Novel Risk-Based Approach. J. Clin. Oncol. 2015, 33, 4151–4157. [Google Scholar] [CrossRef]
- Kim, S.P.; Weight, C.J.; Leibovich, B.C.; Thompson, R.H.; Costello, B.A.; Cheville, J.C.; Lohse, C.M.; Boorjian, S.A. Outcomes and Clinicopathologic Variables Associated With Late Recurrence After Nephrectomy for Localized Renal Cell Carcinoma. Urology 2011, 78, 1101–1106. [Google Scholar] [CrossRef]
- Sciarra, A.; Cattarino, S.; Salciccia, S.; Alfarone, A.; Gentilucci, A.; Parente, U.; Mariotti, G.; Innocenzi, M.; Gentile, V. The emerging role of targeted therapy in renal cell carcinoma (RCC): Is it time for a neoadjuvant or an adjuvant approach? Crit. Rev. Oncol. 2011, 81, 151–162. [Google Scholar] [CrossRef]
- Gul, A.; Rini, B.I. Adjuvant therapy in renal cell carcinoma. Cancer 2019, 125, 2935–2944. [Google Scholar] [CrossRef]
- Martini, A.; Fallara, G.; Pellegrino, F.; Cirulli, G.O.; Larcher, A.; Necchi, A.; Montorsi, F.; Capitanio, U. Neoadjuvant and adjuvant immunotherapy in renal cell carcinoma. World J. Urol. 2021, 39, 1369–1376. [Google Scholar] [CrossRef]
- Breda, A.; Konijeti, R.; Lam, J.S. Patterns of recurrence and surveillance strategies for renal cell carcinoma following surgical resection. Expert Rev. Anticancer. Ther. 2007, 7, 847–862. [Google Scholar] [CrossRef]
- Speed, J.M.; Trinh, Q.-D.; Choueiri, T.K.; Sun, M. Recurrence in Localized Renal Cell Carcinoma: A Systematic Review of Contemporary Data. Curr. Urol. Rep. 2017, 18, 15. [Google Scholar] [CrossRef]
- Fuhrman, S.A.; Lasky, L.C.; Limas, C. Prognostic significance of morphologic parameters in renal cell carcinoma. Am. J. Surg. Pathol. 1982, 6, 655–664. [Google Scholar] [CrossRef]
- Leibovich, B.C.; Blute, M.L.; Cheville, J.C.; Lohse, C.M.; Frank, I.; Kwon, E.D.; Weaver, A.L.; Parker, A.S.; Zincke, H. Prediction of progression after radical nephrectomy for patients with clear cell renal cell carcinoma. Cancer 2003, 97, 1663–1671. [Google Scholar] [CrossRef]
- Frank, I.; Blute, M.L.; Cheville, J.C.; Lohse, C.M.; Weaver, A.L.; Zincke, H. An outcome prediction model for patients with clear cell renal cell carcinoma treated with radical nephrectomy based on tumor stage, size, grade and necrosis: The SSIGN score. J. Urol. 2002, 168, 2395–2400. [Google Scholar] [CrossRef]
- Zisman, A.; Pantuck, A.J.; Dorey, F.; Said, J.W.; Shvarts, O.; Quintana, D.; Gitlitz, B.J.; Dekernion, J.B.; Figlin, R.A.; Belldegrun, A.S. Improved Prognostication of Renal Cell Carcinoma Using an Integrated Staging System. J. Clin. Oncol. 2001, 19, 1649–1657. [Google Scholar] [CrossRef] [PubMed]
- Harshman, L.C.; Xie, W.; Moreira, R.B.; Bossé, D.; Ares, G.J.R.; Sweeney, C.J.; Choueiri, T.K. Evaluation of disease-free survival as an intermediate metric of overall survival in patients with localized renal cell carcinoma: A trial-level meta-analysis. Cancer 2017, 124, 925–933. [Google Scholar] [CrossRef]
- Haas, N.B.; Song, Y.; Rogerio, J.W.; Zhang, S.; Adejoro, O.; Carley, C.; Zhu, J.; Bhattacharya, R.; Signorovitch, J.; Sundaram, M. Disease-free survival as a predictor of overall survival in localized renal cell carcinoma (RCC) following first nephrectomy. J. Clin. Oncol. 2021, 39, 4581. [Google Scholar] [CrossRef]
- MC Tacconi, E.; Tuthill, M.; Protheroe, A. Review of Adjuvant Therapies in Renal Cell Carcinoma: Evidence to Date. OncoTargets Ther. 2020, 13, 12301–12316. [Google Scholar] [CrossRef]
- Sawhney, P.; Suyanto, S.; Michael, A.; Pandha, H. Adjuvant therapy for renal cell carcinoma. J. Cancer Metastasis Treat. 2021, 7, 48. [Google Scholar] [CrossRef]
- Motzer, R.J.; Russo, P.; Haas, N.; Doehn, C.; Donskov, F.; Gross-Goupil, M.; Varlamov, S.; Kopyltsov, E.; Lee, J.L.; Lim, H.Y.; et al. Adjuvant Pazopanib Versus Placebo After Nephrectomy in Patients with Localized or Locally Advanced Renal Cell Carcinoma: Final Overall Survival Analysis of the Phase 3 PROTECT Trial. Eur. Urol. 2021, 79, 334–338. [Google Scholar] [CrossRef]
- Massari, F.; Di Nunno, V.; Mollica, V.; Graham, J.; Gatto, L.; Heng, D. Adjuvant Tyrosine Kinase Inhibitors in Treatment of Renal Cell Carcinoma: A Meta-Analysis of Available Clinical Trials. Clin. Genitourin. Cancer 2019, 17, e339–e344. [Google Scholar] [CrossRef]
- Renal Cell Carcinoma Treatment Recommendations. ESMO, eUpdate 2021. Available online: https://www.esmo.org/guidelines/guidelines-by-topic/genitourinary-cancers/renal-cell-carcinoma/eupdate-renal-cell-carcinoma-treatment-recommendations-4 (accessed on 25 November 2022).
- Bakos, O.; Lawson, C.; Rouleau, S.; Tai, L.-H. Combining surgery and immunotherapy: Turning an immunosuppressive effect into a therapeutic opportunity. J. Immunother. Cancer 2018, 6, 86. [Google Scholar] [CrossRef]
- Marconi, L.; Sun, M.; Beisland, C.; Klatte, T.; Ljungberg, B.; Stewart, G.D.; Dabestani, S.; Choueiri, T.K.; Bex, A. Prevalence, Disease-free, and Overall Survival of Contemporary Patients with Renal Cell Carcinoma Eligible for Adjuvant Checkpoint Inhibitor Trials. Clin. Genitourin. Cancer 2021, 19, e92–e99. [Google Scholar] [CrossRef]
- Rotte, A.; Jin, J.Y.; Lemaire, V. Mechanistic overview of immune checkpoints to support the rational design of their combinations in cancer immunotherapy. Ann. Oncol. 2018, 29, 71–83. [Google Scholar] [CrossRef]
- Powles, T.; Tomczak, P.; Park, S.H.; Venugopal, B.; Ferguson, T.; Symeonides, S.N.; Hajek, J.; Gurney, H.; Chang, Y.-H.; Lee, J.L.; et al. Pembrolizumab versus placebo as post-nephrectomy adjuvant therapy for clear cell renal cell carcinoma (KEYNOTE-564): 30-month follow-up analysis of a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2022, 23, 1133–1144. [Google Scholar] [CrossRef]
- Renner, A.; Rojas, C.; Walton-Diaz, A.; Burotto, M. Adjuvant therapy for renal cell carcinoma, finally a new standard? Front. Oncol. 2022, 12, 926661. [Google Scholar] [CrossRef]
- Pal, S.K.; Uzzo, R.; Karam, J.A.; Master, V.A.; Donskov, F.; Suarez, C.; Albiges, L.; Rini, B.; Tomita, Y.; Kann, A.G.; et al. Adjuvant atezolizumab versus placebo for patients with renal cell carcinoma at increased risk of recurrence following resection (IMmotion010): A multicentre, randomised, double-blind, phase 3 trial. Lancet 2022, 400, 1103–1116. [Google Scholar] [CrossRef]
- Bedke, J.; Albiges, L.; Capitanio, U.; Giles, R.H.; Hora, M.; Ljungberg, B.; Marconi, L.; Klatte, T.; Volpe, A.; Abu-Ghanem, Y.; et al. The 2022 Updated European Association of Urology Guidelines on the Use of Adjuvant Immune Checkpoint Inhibitor Therapy for Renal Cell Carcinoma. Eur. Urol. 2023, 83, 10–14. [Google Scholar] [CrossRef]
- Motzer, R.; Russo, P.; Gruenwald, V.; Tomita, Y.; Zurawski, B.; Parikh, O.; Buti, S.; Barthelemy, P.; Goh, J.; Ye, D.; et al. Adjuvant nivolumab plus ipilimumab versus placebo for localised renal cell carcinoma after nephrectomy (CheckMate 914): A double-blind, randomised, phase 3 trial. Ann. Oncol. 2022, 33 (Suppl. 7), S808–S869. [Google Scholar] [CrossRef]
- Allaf, M.; Kim, S.; Harshman, L.; McDermott, D.; Master, V.; Signoretti, S.; Cole, S.; Moon, H.; Adra, N.; Singer, E.; et al. PROSPER: Phase III RandOmized Study Comparing PERioperative nivolumab versus observation in patients with renal cell carcinoma (RCC) undergoing nephrectomy (ECOG-ACRIN EA8143). J. Clin. Oncol. 2021, 39, 15. [Google Scholar] [CrossRef]
- Renal Adjuvant MultiPle Arm Randomised Trial (RAMPART). Available online: https://clinicaltrials.gov/ct2/show/NCT03288532 (accessed on 2 November 2022).
- A Study of Belzutifan (MK-6482) Plus Pembrolizumab (MK-3475) versus Placebo Plus Pembrolizumab in Participants with Clear Cell Renal Cell Carcinoma Post Nephrectomy (MK-6482-022). Available online: https://clinicaltrials.gov/ct2/show/record/NCT05239728 (accessed on 2 November 2022).
- Choueiri, T.K.; Bedke, J.; Karam, J.A.; McKay, R.R.; Motzer, R.J.; Pal, S.K.; Suárez, C.; Uzzo, R.; Liu, H.; Burgents, J.E.; et al. LITESPARK-022: A phase 3 study of pembrolizumab + belzutifan as adjuvant treatment of clear cell renal cell carcinoma (ccRCC). J. Clin. Oncol. 2022, 40, TPS4602. [Google Scholar] [CrossRef]
- Berg, S.A.; McGregor, B.A. The Continuing Question of Adjuvant Therapy in Clear Cell Renal Cell Carcinoma. Cancers 2022, 14, 6018. [Google Scholar] [CrossRef]
- Blick, C.; Ritchie, A.W.S.; Eisen, T.; Stewart, G. Improving outcomes in high-risk, nonmetastatic renal cancer: New data and ongoing trials. Nat. Rev. Urol. 2017, 14, 753–759. [Google Scholar] [CrossRef]
- Bex, A.; Uzzo, R.; Karam, J.; Master, V.; Donskov, F.; Suárez, C.; Albiges, L.; Rini, B.; Tomita, Y.; Kann, A.; et al. LBA66 IMmotion010: Efficacy and Safety from the Phase III Study of Atezolizumab vs Placebo as Adjuvant Therapy in Patients with RCC at Increased Risk of Recurrence After Resection. Ann. Oncol. 2022, 33, S1431–S1432. [Google Scholar] [CrossRef]
- Weber, E.M.; Titman, A.C. Quantifying the association between progression-free survival and overall survival in oncology trials using Kendall’s τ. Stat. Med. 2018, 38, 703–719. [Google Scholar] [CrossRef] [PubMed]
- Klatte, T.; Seligson, D.B.; LaRochelle, J.; Shuch, B.; Said, J.W.; Riggs, S.B.; Zomorodian, N.; Kabbinavar, F.F.; Pantuck, A.J.; Belldegrun, A.S. Molecular Signatures of Localized Clear Cell Renal Cell Carcinoma to Predict Disease-Free Survival after Nephrectomy. Cancer Epidemiol. Biomark. Prev. 2009, 18, 894–900. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.H.; Gillett, M.D.; Cheville, J.C.; Lohse, C.M.; Dong, H.; Webster, W.S.; Krejci, K.G.; Lobo, J.R.; Sengupta, S.; Chen, L.; et al. Costimulatory B7-H1 in renal cell carcinoma patients: Indicator of tumor aggressiveness and potential therapeutic target. Proc. Natl. Acad. Sci. USA 2004, 101, 17174–17179. [Google Scholar] [CrossRef] [PubMed]
| Clinical Trial [Ref.] | No. of Patients | Population | Treatment Arms (n) | Duration of Treatment | Primary Endpoint | Results | Overall Response | Grade 3 or Worse Adverse Events |
|---|---|---|---|---|---|---|---|---|
| ASSURE [7] | 1069 | ≥T1b completely resected, M0 | Sunitinib 50 mg (n = 358) Vs. Sorafenib 400 mg(n = 355) Vs. Placebo (n = 356) | 1 year | DFS | HR, 1.02 (97.5% CI 0.85–1.23; p = 0.8038) HR, 0.97 (97.5% CI, 0.80–1.17; p = 0.7184) | 5-y-OS: HR, 1.06; 97.5% CI, 0.78–1.45; p = 0.66; HR, 0.80; 97.5% CI, 0.58–1.11; p = 0.12 | Sunitinib: 63% Sorafenib: 72% Placebo: 25% |
| S-TRAC [9] | 615 | T3-T4, N0-Nx, M0 or any T, N+, M0 | Sunitinib 50 mg (n = 309) Vs. placebo (n = 306) | 1 year | DFS | HR, 0.76; 95% CI, 0.59 to 0.98; p = 0.03 | mOS HR 0.92, 95% CI, 0.66–1.28; p = 0.6) | Sunitinib: 65% Placebo: 23.3% |
| PROTECT [8] | 1538 | pT2 high grade, pT3-4, N0/+, M0 | Pazopanib 600 mg (n = 769) Vs. Placebo (n = 769) | 1 year | DFS | HR, 0.86; 95% CI, 0.70 to 1.06. p = 0.165 | mOS HR, 1.0, 95% CI, 0.80–1.26; p > 0.9 | Pazopanib: 59% Placebo: 19% |
| ATLAS [6] | 724 | >pT2 and/or N+ M0 | Axitinib 10 mg (n = 363) Vs. Placebo (n = 361) | 1 to 3 years | DFS | HR = 0.870; 95% CI: 0.660 to 1.147; p = 0.3211 | NA | Axitinib: 49% Placebo:12% |
| SORCE [5] | 1711 | Intermediate- or high-risk disease (Leibovich score 3 to 11) | Sorafenib for 1-year followed by 2-year placebo, (n = 642) Vs. 3-year sorafenib (n = 639) Vs. Placebo (n = 430) | 3 years | DFS | HR, 1.01; 95% CI, 0.82 to 1.23. P = 0.946) | HR, 0.92; 95% CI, 0.71 to 1.20; p = 0.541 HR, 1.06; 95% CI, 0.82 to 1.38; p = 0.638 | Sorafenib plus placebo: 59% Sorefenib: 64% Placebo: 29% |
| EVEREST [10] | 2018 | pT1-pT3a N0, pT3-pT4, N0/+, M0 | Everolimus 10 mg (n = 775) Vs. Placebo (n = 770) | 5 years | RFS | HR 0.85, 95% CI, 0.72–1.00; P1-sided = 0.0246 | HR 0.90, 95% CI, 0.71–1.13: P1-sided = 0.178 | Everolimus: 46% Placebo: 11% |
| Clinical Trial [Ref.] | No. of Patients | Tumor Features | Treatment Arms | Duration of Treatment | DFS | RFS | OS | Grade 3 or Worse AEs |
|---|---|---|---|---|---|---|---|---|
| KEYNOTE-564 [34] | 994 | Intermediate–high-risk M0-M1 NED Clear-cell RCC/sarcomatoid | Pembrolizumab Placebo | 1 year | HR 0.63 (95% CI 0.50–0.80 p < 0.0001) | 75.2% (95% CI 70.8–79.1) 65·5% (60.9–69.7) | HR 0.52 (95% CI 0.31–0.86, p = 0.0048) | 32% 18% |
| IMmotion010 [36] | 778 | Intermediate–high-risk M0-M1 NED Clear-cell RCC/sarcomatoid | Atezolizumab Placebo | 1 year | HR 0.93 (95% CI 0.75–1.15, p = 0.50) | NA | HR 0.97 (95% CI 0.67–1.42) | 28% 24% |
| Checkmate-914 [38] | 816 | Intermediate-high risk M0 Clear-cell RCC/sarcomatoid | Nivolumab + Ipilimumab Placebo | At least 24 weeks | HR 0.92 (95% CI 0.71–1.20) | NA | NA | 28.5% 2% |
| PROSPER [39] | 819 | Intermediate-high risk, M0 or M1 NED RCC of any histology | Nivolumab neoadjuvant- adjuvant Placebo | 40 weeks (One dose prior to surgery followed by 9 doses) | NA | HR: 0.97 (95% CI: 0.74–1.28; P1-sided = 0.43) | HR: 1.48; (95% CI: 0.89–2.48; P1-sided = 0.93). | 20% 6% |
| LITESPARK 002 [41] | 1600 | Clear-cell RCC pT2, grade 4 or sarcomatoid, N0, M0 or pT3, any grade, N0, M0, high (pT4, any grade, N0, M0 or pT, any stage/grade, N+, M0) or M1 NED | Belzutifan + pembrolizumab Placebo + pembrolizumab | 1 year | DFS | NA | NA | NA |
| RAMPART [40] | 1750 | Clear-cell and non-clear-cell histological RCC subtypes with high or intermediate risk of relapse (Leibovich score 3–11). | Durvaumab + tremelimumab Placebo | 1 year | DFS and OS | NA | NA | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cosso, F.; Roviello, G.; Nesi, G.; Shabani, S.; Spatafora, P.; Villari, D.; Catalano, M. Adjuvant Therapy for Renal Cell Carcinoma: Hype or Hope? Int. J. Mol. Sci. 2023, 24, 4243. https://doi.org/10.3390/ijms24044243
Cosso F, Roviello G, Nesi G, Shabani S, Spatafora P, Villari D, Catalano M. Adjuvant Therapy for Renal Cell Carcinoma: Hype or Hope? International Journal of Molecular Sciences. 2023; 24(4):4243. https://doi.org/10.3390/ijms24044243
Chicago/Turabian StyleCosso, Federica, Giandomenico Roviello, Gabriella Nesi, Sonia Shabani, Pietro Spatafora, Donata Villari, and Martina Catalano. 2023. "Adjuvant Therapy for Renal Cell Carcinoma: Hype or Hope?" International Journal of Molecular Sciences 24, no. 4: 4243. https://doi.org/10.3390/ijms24044243
APA StyleCosso, F., Roviello, G., Nesi, G., Shabani, S., Spatafora, P., Villari, D., & Catalano, M. (2023). Adjuvant Therapy for Renal Cell Carcinoma: Hype or Hope? International Journal of Molecular Sciences, 24(4), 4243. https://doi.org/10.3390/ijms24044243







