In Silico Analysis of Ferroptosis-Related Genes and Its Implication in Drug Prediction against Fluorosis
Abstract
1. Introduction
2. Result
2.1. Identification of Differential Genes
2.2. Overlap of DEGs and Ferroptosis-Related Genes and Venn Analysis
2.3. Gene Ontology (GO) and KEGG Pathway Analysis of Overlapping Genes
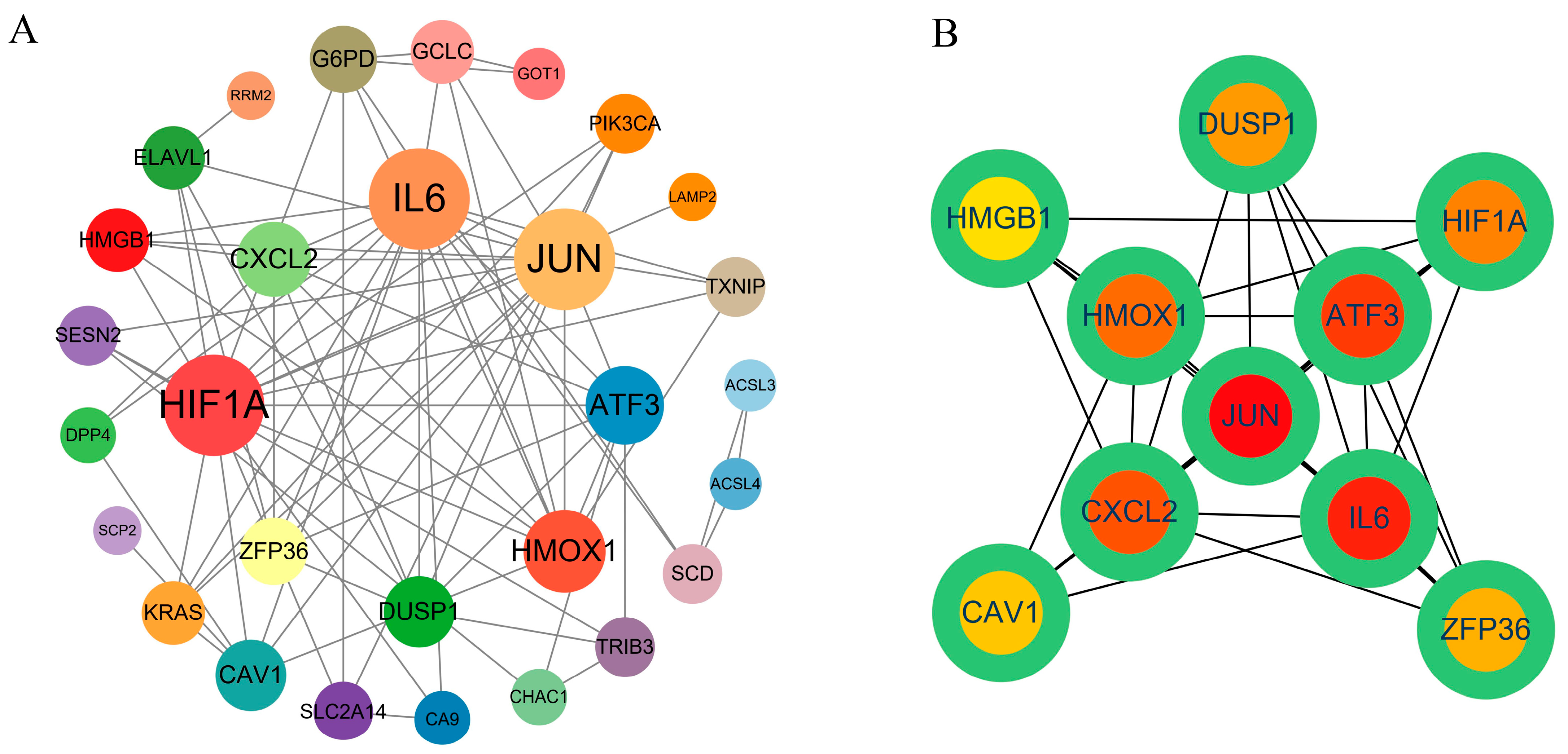
2.4. PPI Network Construction and Hub Gene Screening
2.5. Screening of Potential Pharmacological Targets
2.6. Validation of Potential Therapeutics against CTD Database
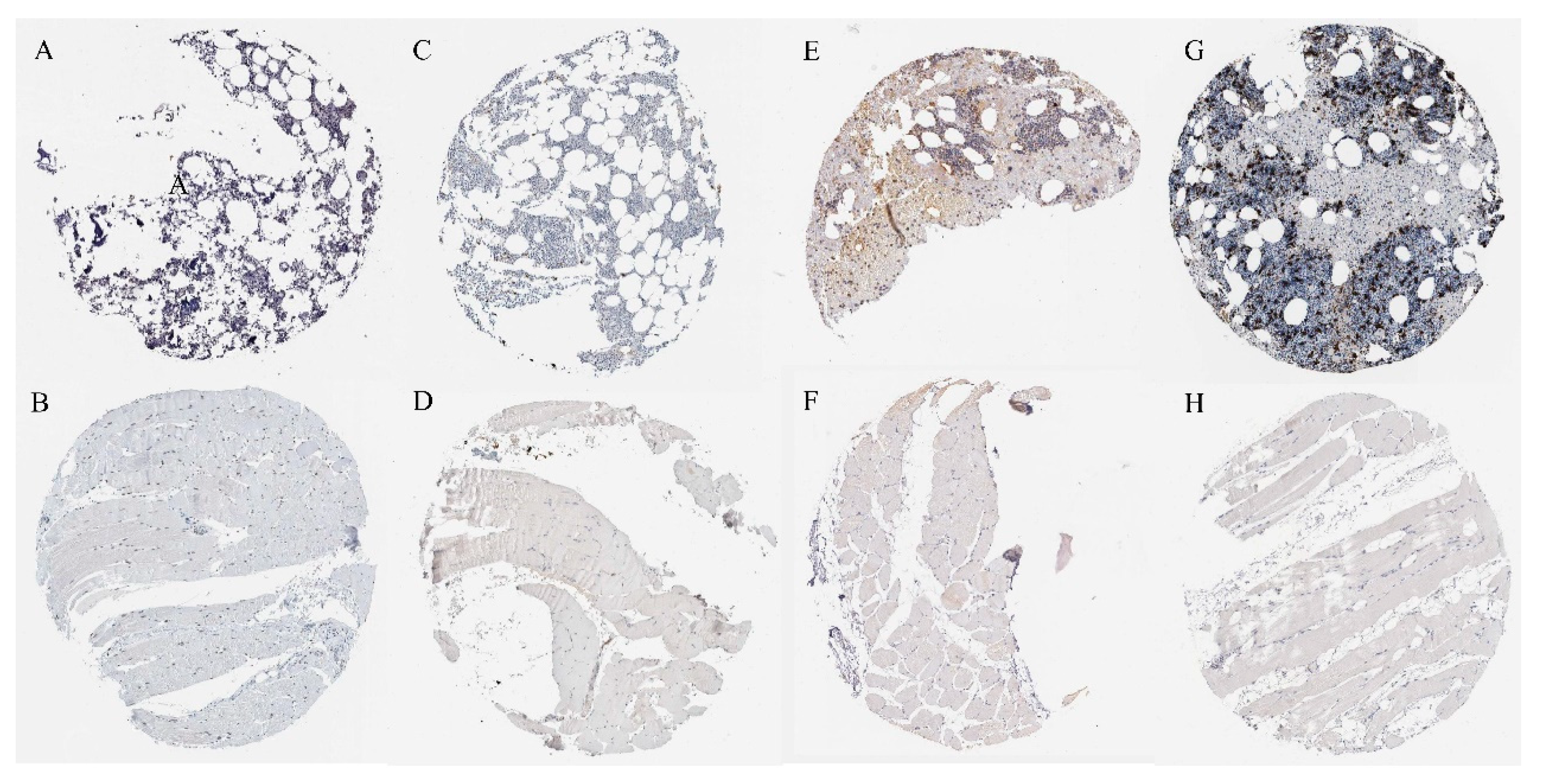
2.7. Verification of Protein Expression of Overlapping Genes
2.8. Molecular Docking Reveals Potential Targets for Small-Molecule Drugs and Proteins
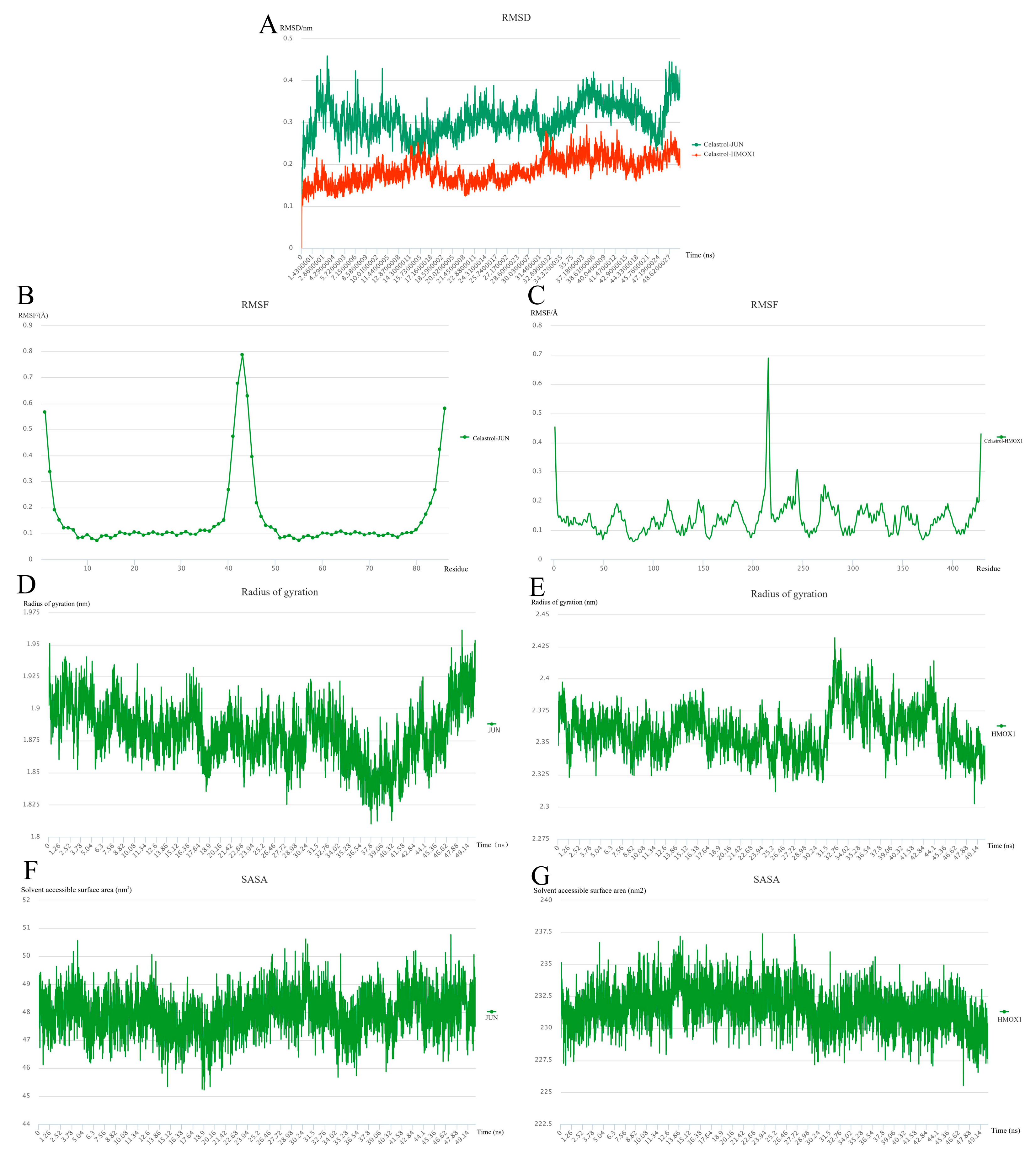
2.9. Molecular Dynamics Simulation
3. Discussion
4. Materials and Methods
4.1. Source of Microarray Data
4.2. Analysis of Differentially Expressed Genes in Samples
4.3. Identification of DEGs Related to Ferroptosis and Venn
4.4. Gene Ontology (GO) Enrichment Analysis of Ferroptosis-Related DEGs Genes and Kyoto Gene Encyclopedia and Genome Path Analysis
4.5. Protein–Protein Interaction (PPI) Network Analysis and Hub Gene Screening
4.6. Screening of Potential Pharmacological Targets
4.7. Validation of Potential Therapeutics against CTD Database
4.8. Immunohistochemical Verification of Overlapping Proteins
4.9. Molecular Docking
4.10. Molecular Dynamics Simulation and Post-Dynamic Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gbadebo, A.M. Groundwater fluoride and dental fluorosis in southwestern Nigeria. Environ. Geochem. Health 2012, 34, 597–604. [Google Scholar] [CrossRef]
- Buzalaf, M.A.R.; Whitford, G.M. Fluoride metabolism. Monogr. Oral. Sci. 2011, 22, 20–36. [Google Scholar]
- Del Bello, L. Fluorosis: An ongoing challenge for India. Lancet Planet. Health 2020, 4, e94–e95. [Google Scholar] [CrossRef]
- Malde, M.K.; Scheidegger, R.; Julshamn, K.; Bader, H.P. Substance flow analysis: A case study of fluoride exposure through food and beverages in young children living in Ethiopia. Environ. Health Perspect. 2011, 119, 579–584. [Google Scholar] [CrossRef]
- Suzuki, M.; Bandoski, C.; Bartlett, J.D. Fluoride induces oxidative damage and SIRT1/autophagy through ROS-mediated JNK signaling. Free Radic. Biol. Med. 2015, 89, 369–378. [Google Scholar] [CrossRef]
- Kebede, A.; Retta, N.; Abuye, C.; Whiting, S.J.; Kassaw, M.; Zeru, T.; Tessema, M.; Kjellevold, M. Dietary Fluoride Intake and Associated Skeletal and Dental Fluorosis in School Age Children in Rural Ethiopian Rift Valley. Int. J. Environ. Res. Public Health 2016, 13, 756. [Google Scholar] [CrossRef]
- Choi, A.L.; Sun, G.; Zhang, Y.; Grandjean, P. Developmental fluoride neurotoxicity: A systematic review and meta-analysis. Environ. Health Perspect. 2012, 120, 1362–1368. [Google Scholar] [CrossRef]
- Sun, Z.; Niu, R.; Su, K.; Wang, B.; Wang, J.; Zhang, J.; Wang, J. Effects of sodium fluoride on hyperactivation and Ca2+ signaling pathway in sperm from mice: An In Vivo study. Arch. Toxicol. 2010, 84, 353–361. [Google Scholar] [CrossRef]
- Zhou, G.; Hu, Y.; Wang, A.; Guo, M.; Du, Y.; Gong, Y.; Ding, L.; Feng, Z.; Hou, X.; Xu, K.; et al. Fluoride Stimulates Anxiety- and Depression-like Behaviors Associated with SIK2-CRTC1 Signaling Dysfunction. J. Agric. Food Chem. 2021, 69, 13618–13627. [Google Scholar] [CrossRef]
- Zhou, G.Y.; Ren, L.J.; Hou, J.X.; Cui, L.X.; Ding, Z.; Cheng, X.M.; Zhu, J.Y.; Cui, R.R.; Ba, Y. Endemic fluorosis in Henan province, China: ERalpha gene polymorphisms and reproductive hormones among women. Asia Pac. J. Clin. Nutr. 2016, 25, 911–919. [Google Scholar]
- Susheela, A.K.; Toteja, G.S. Prevention & control of fluorosis & linked disorders: Developments in the 21(st) Century—Reaching out to patients in the community & hospital settings for recovery. Indian J. Med. Res. 2018, 148, 539–547. [Google Scholar]
- Wang, F.; Li, Y.; Tang, D.; Zhao, J.; Yang, X.; Liu, Y.; Peng, F.; Shu, L.; Wang, J.; He, Z.; et al. Effects of water improvement and defluoridation on fluorosis-endemic areas in China: A meta-analysis. Environ. Pollut. 2021, 270, 116227. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, P.; Zhai, B.; Zhang, M.; Xiang, Y.; Fang, J.; Xu, S.; Gao, Y.; Chen, X.; Sui, X.; et al. The emerging role of ferroptosis in inflammation. Biomed. Pharmacother. 2020, 127, 110108. [Google Scholar] [CrossRef]
- Yang, W.S.; SriRamaratnam, R.; Welsch, M.E.; Shimada, K.; Skouta, R.; Viswanathan, V.S.; Cheah, J.H.; Clemons, P.A.; Shamji, A.F.; Clish, C.B.; et al. Regulation of ferroptotic cancer cell death by GPX4. Cell 2014, 156, 317–331. [Google Scholar] [CrossRef]
- Hassannia, B.; Vandenabeele, P.; Vanden Berghe, T. Targeting Ferroptosis to Iron Out Cancer. Cancer Cell 2019, 35, 830–849. [Google Scholar] [CrossRef]
- Zhao, M.; Li, M.; Zheng, Y.; Hu, Z.; Liang, J.; Bi, G.; Bian, Y.; Sui, Q.; Zhan, C.; Lin, M.; et al. Identification and analysis of a prognostic ferroptosis and iron-metabolism signature for esophageal squamous cell carcinoma. J. Cancer 2022, 13, 1611–1622. [Google Scholar] [CrossRef]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef]
- Friedmann Angeli, J.P.; Schneider, M.; Proneth, B.; Tyurina, Y.Y.; Tyurin, V.A.; Hammond, V.J.; Herbach, N.; Aichler, M.; Walch, A.; Eggenhofer, E.; et al. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat. Cell Biol. 2014, 16, 1180–1191. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, J.; Jiang, L.; Lu, C.; Huang, Z.; Liu, B. Prospects for the Role of Ferroptosis in Fluorosis. Front. Physiol. 2021, 12, 773055. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, X.; Chen, J.; Cao, J.; Feng, C.; Luo, Y.; Lin, Y. Self-recovery study of fluoride-induced ferroptosis in the liver of zebrafish (Danio rerio). Aquat. Toxicol. 2022, 251, 106275. [Google Scholar] [CrossRef]
- Liu, M.; Fan, Y.; Li, D.; Han, B.; Meng, Y.; Chen, F.; Liu, T.; Song, Z.; Han, Y.; Huang, L.; et al. Ferroptosis inducer erastin sensitizes NSCLC cells to celastrol through activation of the ROS-mitochondrial fission-mitophagy axis. Mol. Oncol. 2021, 15, 2084–2105. [Google Scholar] [CrossRef]
- Zhang, X.; Xing, X.; Liu, H.; Feng, J.; Tian, M.; Chang, S.; Liu, P.; Zhang, H. Ionizing radiation induces ferroptosis in granulocyte-macrophage hematopoietic progenitor cells of murine bone marrow. Int. J. Radiat. Biol. 2020, 96, 584–595. [Google Scholar] [CrossRef]
- Bae, Y.S.; Yoon, S.H.; Kim, Y.S.; Oh, S.P.; Song, W.S.; Cha, J.H.; Kim, M.H. Suppression of exaggerated NMDAR activity by memantine treatment ameliorates neurological and behavioral deficits in aminopeptidase P1-deficient mice. Exp. Mol. Med. 2022, 54, 1109–1124. [Google Scholar] [CrossRef]
- Poke, G.; Sadleir, L.G.; Merla, G.; de Valles-Ibanez, G.; Skinner, J.R. GNB5-Related Neurodevelopmental Disorder. In GeneReviews((R)); Adam, M.P., Everman, D.B., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Gripp, K.W., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993. [Google Scholar]
- Soderholm, J.F.; Bird, S.L.; Kalab, P.; Sampathkumar, Y.; Hasegawa, K.; Uehara-Bingen, M.; Weis, K.; Heald, R. Importazole, a small molecule inhibitor of the transport receptor importin-beta. ACS Chem. Biol. 2011, 6, 700–708. [Google Scholar] [CrossRef]
- Fan, S.; Wu, K.; Zhao, M.; Yuan, J.; Ma, S.; Zhu, E.; Chen, Y.; Ding, H.; Yi, L.; Chen, J. LDHB inhibition induces mitophagy and facilitates the progression of CSFV infection. Autophagy 2021, 17, 2305–2324. [Google Scholar] [CrossRef]
- Zhang, T.; He, X.; Caldwell, L.; Goru, S.K.; Ulloa Severino, L.; Tolosa, M.F.; Misra, P.S.; McEvoy, C.M.; Christova, T.; Liu, Y.; et al. NUAK1 promotes organ fibrosis via YAP and TGF-beta/SMAD signaling. Sci. Transl. Med. 2022, 14, eaaz4028. [Google Scholar] [CrossRef]
- Han, L.; Bai, L.; Qu, C.; Dai, E.; Liu, J.; Kang, R.; Zhou, D.; Tang, D.; Zhao, Y. PPARG-mediated ferroptosis in dendritic cells limits antitumor immunity. Biochem. Biophys. Res. Commun. 2021, 576, 33–39. [Google Scholar] [CrossRef]
- Li, C.; Zhang, Y.; Liu, J.; Kang, R.; Klionsky, D.J.; Tang, D. Mitochondrial DNA stress triggers autophagy-dependent ferroptotic death. Autophagy 2021, 17, 948–960. [Google Scholar] [CrossRef]
- Carter, C.J. eIF2B and oligodendrocyte survival: Where nature and nurture meet in bipolar disorder and schizophrenia? Schizophr. Bull. 2007, 33, 1343–1353. [Google Scholar] [CrossRef]
- Rakib, A.; Nain, Z.; Sami, S.A.; Mahmud, S.; Islam, A.; Ahmed, S.; Siddiqui, A.B.F.; Babu, S.; Hossain, P.; Shahriar, A.; et al. A molecular modelling approach for identifying antiviral selenium-containing heterocyclic compounds that inhibit the main protease of SARS-CoV-2: An In Silico investigation. Brief. Bioinform. 2021, 22, 1476–1498. [Google Scholar] [CrossRef]
- Zhu, C.; Bai, G.; Liu, X.; Li, Y. Screening high-fluoride and high-arsenic drinking waters and surveying endemic fluorosis and arsenism in Shaanxi province in western China. Water Res. 2006, 40, 3015–3022. [Google Scholar] [CrossRef]
- Wang, L.F.; Huang, J.Z. Outline of control practice of endemic fluorosis in China. Soc. Sci. Med. 1995, 41, 1191–1195. [Google Scholar]
- Wang, Z.; Yang, X.; Yang, S.; Ren, G.; Ferreri, M.; Su, Y.; Chen, L.; Han, B. Sodium fluoride suppress proliferation and induce apoptosis through decreased insulin-like growth factor-I expression and oxidative stress in primary cultured mouse osteoblasts. Arch. Toxicol. 2011, 85, 1407–1417. [Google Scholar] [CrossRef]
- Lu, Y.; Luo, Q.; Cui, H.; Deng, H.; Kuang, P.; Liu, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; et al. Sodium fluoride causes oxidative stress and apoptosis in the mouse liver. Aging (Albany NY) 2017, 9, 1623–1639. [Google Scholar] [CrossRef]
- Bonola-Gallardo, I.; Irigoyen-Camacho, M.E.; Vera-Robles, L.; Campero, A.; Gomez-Quiroz, L. Enzymatic Activity of Glutathione S-Transferase and Dental Fluorosis Among Children Receiving Two Different Levels of Naturally Fluoridated Water. Biol. Trace Elem. Res. 2017, 176, 40–47. [Google Scholar] [CrossRef]
- Guo, H.; Jiang, Y.; Gu, Z.; Ren, L.; Zhu, C.; Yu, S.; Wei, R. ZFP36 protects against oxygen-glucose deprivation/reoxygenation-induced mitochondrial fragmentation and neuronal apoptosis through inhibiting NOX4-DRP1 pathway. Brain Res. Bull. 2022, 179, 57–67. [Google Scholar] [CrossRef]
- Chiang, S.K.; Chen, S.E.; Chang, L.C. A Dual Role of Heme Oxygenase-1 in Cancer Cells. Int. J. Mol. Sci. 2018, 20, 39. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Y.; Du, T.; Yang, H.; Lei, L.; Guo, M.; Ding, H.F.; Zhang, J.; Wang, H.; Chen, X.; et al. ATF3 promotes erastin-induced ferroptosis by suppressing system Xc(.). Cell Death Differ. 2020, 27, 662–675. [Google Scholar] [CrossRef]
- Lin, Q.; Li, S.; Jiang, N.; Jin, H.; Shao, X.; Zhu, X.; Wu, J.; Zhang, M.; Zhang, Z.; Shen, J.; et al. Inhibiting NLRP3 inflammasome attenuates apoptosis in contrast-induced acute kidney injury through the upregulation of HIF1A and BNIP3-mediated mitophagy. Autophagy 2021, 17, 2975–2990. [Google Scholar] [CrossRef]
- Wu, X.; Pan, Z.; Liu, W.; Zha, S.; Song, Y.; Zhang, Q.; Hu, K. The Discovery, Validation, and Function of Hypoxia-Related Gene Biomarkers for Obstructive Sleep Apnea. Front. Med. (Lausanne) 2022, 9, 813459. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhao, Q.; Chen, L.; Luo, Y.; Shen, L.; Cao, Z.; Wang, Q. UBR3 promotes inflammation and apoptosis via DUSP1/p38 pathway in the nucleus pulposus cells of patients with intervertebral disc degeneration. Hum Cell 2022, 35, 792–802. [Google Scholar] [CrossRef] [PubMed]
- Hartl, M.; Bader, A.G.; Bister, K. Molecular targets of the oncogenic transcription factor jun. Curr. Cancer Drug Targets 2003, 3, 41–55. [Google Scholar] [CrossRef]
- Zeng, R.; Wu, H.; Qiu, X.; Zhuo, Z.; Sha, W.; Chen, H. Predicting survival and immune microenvironment in colorectal cancer: A STAT signaling-related signature. QJM 2022, 115, 596–604. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, P.C.; Castell, J.V.; Andus, T. Interleukin-6 and the acute phase response. Biochem. J. 1990, 265, 621–636. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef]
- Dong, Y.; Ming, B.; Dong, L. The Role of HMGB1 in Rheumatic Diseases. Front. Immunol. 2022, 13, 815257. [Google Scholar] [CrossRef]
- Girbl, T.; Lenn, T.; Perez, L.; Rolas, L.; Barkaway, A.; Thiriot, A.; Del Fresno, C.; Lynam, E.; Hub, E.; Thelen, M.; et al. Distinct Compartmentalization of the Chemokines CXCL1 and CXCL2 and the Atypical Receptor ACKR1 Determine Discrete Stages of Neutrophil Diapedesis. Immunity 2018, 49, 1062–1076.e6. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, P.; Wang, X.; Wang, L.; Zhu, Y.; Song, Y.; Gao, W. Celastrol mediates autophagy and apoptosis via the ROS/JNK and Akt/mTOR signaling pathways in glioma cells. J. Exp. Clin. Cancer Res. 2019, 38, 184. [Google Scholar] [CrossRef]
- Ma, L.; Zhang, R.; Li, D.; Qiao, T.; Guo, X. Fluoride regulates chondrocyte proliferation and autophagy via PI3K/AKT/mTOR signaling pathway. Chem. Biol. Interact. 2021, 349, 109659. [Google Scholar] [CrossRef]
- Chen, R.; Zhao, L.D.; Liu, H.; Li, H.H.; Ren, C.; Zhang, P.; Guo, K.T.; Zhang, H.X.; Geng, D.Q.; Zhang, C.Y. Fluoride Induces Neuroinflammation and Alters Wnt Signaling Pathway in BV2 Microglial Cells. Inflammation 2017, 40, 1123–1130. [Google Scholar] [CrossRef]
- Younis, N.S.; Ghanim, A.M.H. The Protective Role of Celastrol in Renal Ischemia-Reperfusion Injury by Activating Nrf2/HO-1, PI3K/AKT Signaling Pathways, Modulating NF-kappab Signaling Pathways, and Inhibiting ERK Phosphorylation. Cell Biochem. Biophys. 2022, 80, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.B.; Deng, D.Y.; Lai, C.S.; Hong, C.C.; Cuny, G.D.; Bouxsein, M.L.; Hong, D.W.; McManus, P.M.; Katagiri, T.; Sachidanandan, C.; et al. BMP type I receptor inhibition reduces heterotopic [corrected] ossification. Nat. Med. 2008, 14, 1363–1369. [Google Scholar] [CrossRef] [PubMed]
- Franco, R.A.G.; McKenna, E.; Robey, P.G.; Shajib, M.S.; Crawford, R.W.; Doran, M.R.; Futrega, K. Inhibition of BMP signaling with LDN 193189 can influence bone marrow stromal cell fate but does not prevent hypertrophy during chondrogenesis. Stem. Cell Reports 2022, 17, 616–632. [Google Scholar] [CrossRef]
- Liu, X.; Chen, C.; Han, D.; Zhou, W.; Cui, Y.; Tang, X.; Xiao, C.; Wang, Y.; Gao, Y. SLC7A11/GPX4 Inactivation-Mediated Ferroptosis Contributes to the Pathogenesis of Triptolide-Induced Cardiotoxicity. Oxid. Med. Cell Longev. 2022, 2022, 3192607. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Lin, X.; Huang, H.; Feng, D.; Ba, Y.; Cheng, X.; Cui, L. Sodium fluoride induces apoptosis through reactive oxygen species-mediated endoplasmic reticulum stress pathway in Sertoli cells. J. Environ. Sci. (China) 2015, 30, 81–89. [Google Scholar] [CrossRef]
- Raghunath, A.; Jeyabaskar, D.; Sundarraj, K.; Panneerselvam, L.; Perumal, E. In Silico prediction of microRNAs on fluoride induced sperm toxicity in mice. Food Chem. Toxicol. 2016, 98, 34–49. [Google Scholar] [CrossRef] [PubMed]
- Ke, L.; Zheng, X.; Sun, Y.; Ouyang, W.; Zhang, Z. Effects of Sodium Fluoride on Lipid Peroxidation and PARP, XBP-1 Expression in PC12 Cell. Biol. Trace Elem. Res. 2016, 173, 161–167. [Google Scholar] [CrossRef]
- Srivastava, S.; Flora, S.J.S. Fluoride in Drinking Water and Skeletal Fluorosis: A Review of the Global Impact. Curr. Environ. Health Rep. 2020, 7, 140–146. [Google Scholar] [CrossRef]
- Angwa, L.M.; Jiang, Y.; Pei, J.; Sun, D. Antioxidant Phytochemicals for the Prevention of Fluoride-Induced Oxidative Stress and Apoptosis: A Review. Biol. Trace Elem. Res. 2022, 200, 1418–1441. [Google Scholar] [CrossRef]
- Gupta, S.K.; Gupta, R.C.; Seth, A.K.; Gupta, A. Reversal of fluorosis in children. Acta Paediatr. Jpn. 1996, 38, 513–519. [Google Scholar] [CrossRef]
- Vandana, K.L.; Srishti Raj, B.; Desai, R. Dental Fluorosis and Periodontium: An Original Research Report of In Vitro and In Vivo Institutional Studies. Biol. Trace Elem. Res. 2021, 199, 3579–3592. [Google Scholar] [CrossRef] [PubMed]
- Shankar, P.; Khandare, A.L.; Validandi, V.; Khandare, S. Supplementation of Calcium and Fluoride-Free Water Mitigates Skeletal Fluorosis in Fluoride-Intoxicated Rats. Biol. Trace Elem. Res. 2021, 199, 2225–2237. [Google Scholar] [CrossRef]
- Gandhi, D.; Naoghare, P.K.; Bafana, A.; Kannan, K.; Sivanesan, S. Fluoride-Induced Oxidative and Inflammatory Stress in Osteosarcoma Cells: Does It Affect Bone Development Pathway? Biol. Trace Elem. Res. 2017, 175, 103–111. [Google Scholar] [CrossRef]
- Li, S.; Zhou, C.; Xu, Y.; Wang, Y.; Li, L.; Pelekos, G.; Ziebolz, D.; Schmalz, G.; Qin, Z. Similarity and Potential Relation Between Periimplantitis and Rheumatoid Arthritis on Transcriptomic Level: Results of a Bioinformatics Study. Front. Immunol. 2021, 12, 702661. [Google Scholar] [CrossRef] [PubMed]
- Zhou, N.; Bao, J. FerrDb: A manually curated resource for regulators and markers of ferroptosis and ferroptosis-disease associations. Database 2020, 2020, baaa021. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Wang, L.G.; Han, Y.; He, Q.Y. clusterProfiler: An R package for comparing biological themes among gene clusters. OMICS 2012, 16, 284–287. [Google Scholar] [CrossRef]
- Luo, W.; Brouwer, C. Pathview: An R/Bioconductor package for pathway-based data integration and visualization. Bioinformatics 2013, 29, 1830–1831. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Yin, Y.X.; Tang, Y.P.; Wei, K.L.; Pan, Z.G.; Li, K.Z.; Guo, X.W.; Hu, B.L. Diagnostic and Predictive Value of Immune-Related Genes in Crohn’s Disease. Front. Immunol. 2021, 12, 643036. [Google Scholar] [CrossRef]
- Han, Y.L.; Luo, D.; Habaxi, K.; Tayierjiang, J.; Zhao, W.; Wang, W.; Aikebaier, W.; Wang, L. COL5A2 Inhibits the TGF-beta and Wnt/beta-Catenin Signaling Pathways to Inhibit the Invasion and Metastasis of Osteosarcoma. Front. Oncol. 2022, 12, 813809. [Google Scholar] [CrossRef]
- Fabbri, C.; Pain, O.; Hagenaars, S.P.; Lewis, C.M.; Serretti, A. Transcriptome-wide association study of treatment-resistant depression and depression subtypes for drug repurposing. Neuropsychopharmacology 2021, 46, 1821–1829. [Google Scholar] [CrossRef]
- Subramanian, A.; Narayan, R.; Corsello, S.M.; Peck, D.D.; Natoli, T.E.; Lu, X.; Gould, J.; Davis, J.F.; Tubelli, A.A.; Asiedu, J.K.; et al. A Next Generation Connectivity Map: L1000 Platform and the First 1,000,000 Profiles. Cell 2017, 171, 1437–1452.e17. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Chen, L.; Chao, Z.; Chen, T.; Zhou, Y. Ferroptosis Related Genes in Ischemic and Idiopathic Cardiomyopathy: Screening for Potential Pharmacological Targets. Front. Cell Dev. Biol. 2022, 10, 817819. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Luo, D.; Zhong, N.; Li, D.; Zheng, J.; Liao, H.; Li, Z.; Lin, X.; Chen, Q.; Zhang, C.; et al. GPC2 Is a Potential Diagnostic, Immunological, and Prognostic Biomarker in Pan-Cancer. Front. Immunol. 2022, 13, 857308. [Google Scholar] [CrossRef] [PubMed]
- Thul, P.J.; Lindskog, C. The human protein atlas: A spatial map of the human proteome. Protein. Sci. 2018, 27, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Gao, R.; Zhou, Z.; Tang, X.; Lin, J.; Wang, L.; Zhou, X.; Shen, T. A network pharmacology based approach for predicting active ingredients and potential mechanism of Lianhuaqingwen capsule in treating COVID-19. Int. J. Med. Sci. 2021, 18, 1866–1876. [Google Scholar] [CrossRef] [PubMed]
- Das, L.; Shekhar, S.; Chandrani, P.; Varma, A.K. In Silico structural analysis of secretory clusterin to assess pathogenicity of mutations identified in the evolutionarily conserved regions. J. Biomol. Struct. Dyn. 2023, 41, 469–478. [Google Scholar] [CrossRef]
- Al-Hizab, F.; Kandeel, M. Mycophenolate suppresses inflammation by inhibiting prostaglandin synthases: A study of molecular and experimental drug repurposing. PeerJ 2021, 9, e11360. [Google Scholar] [CrossRef]




| Type | Genes |
|---|---|
| Driver | G6PD, PIK3CA, SCP2, ACSL4, KRAS, GOT1, HMOX1, DPP4, CHAC1, ELAVL1, HIF1A, HMGB1, ATF3, LONP1, GCLC, IL6 |
| Suppressor | PIK3CA, HMOX1, HIF1A, MT1G, FTMT, SCD, ACSL3, SESN2, JUN, CA9, LAMP2, ZFP36, CAV1, RRM2 |
| Marker | ATF3, HMOX1, CHAC1, ELAVL1, HMGB1, SESN2, DUSP1, LOC284561, TXNIP, TRIB3, SNORA16A, IL6, CXCL2, HSD17B11, SLC2A14, RRM2 |
| Score | Name | Description |
|---|---|---|
| −99.93 | Celastrol | Anti-inflammatory |
| −99.93 | LDN-193189 | Serine/threonine kinase inhibitor |
| −99.89 | XPNPEP1 | Methionyl aminopeptidase |
| −99.76 | GNB5 | WD repeat domain containing |
| −99.75 | Importazole | Importin-beta transport receptor inhibitor |
| −99.74 | LDHB | - |
| −99.66 | NUAK1 | NuaK subfamily |
| −99.63 | IFNG | Interferons |
| −99.54 | IFNB1 | Interferons |
| −99.50 | YWHAH | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, B.; Fu, X.; Du, Y.; Feng, Z.; Liu, X.; Li, Z.; Yu, F.; Zhou, G.; Ba, Y. In Silico Analysis of Ferroptosis-Related Genes and Its Implication in Drug Prediction against Fluorosis. Int. J. Mol. Sci. 2023, 24, 4221. https://doi.org/10.3390/ijms24044221
Liu B, Fu X, Du Y, Feng Z, Liu X, Li Z, Yu F, Zhou G, Ba Y. In Silico Analysis of Ferroptosis-Related Genes and Its Implication in Drug Prediction against Fluorosis. International Journal of Molecular Sciences. 2023; 24(4):4221. https://doi.org/10.3390/ijms24044221
Chicago/Turabian StyleLiu, Bin, Xiaoli Fu, Yuhui Du, Zichen Feng, Xiaoxue Liu, Zhiyuan Li, Fangfang Yu, Guoyu Zhou, and Yue Ba. 2023. "In Silico Analysis of Ferroptosis-Related Genes and Its Implication in Drug Prediction against Fluorosis" International Journal of Molecular Sciences 24, no. 4: 4221. https://doi.org/10.3390/ijms24044221
APA StyleLiu, B., Fu, X., Du, Y., Feng, Z., Liu, X., Li, Z., Yu, F., Zhou, G., & Ba, Y. (2023). In Silico Analysis of Ferroptosis-Related Genes and Its Implication in Drug Prediction against Fluorosis. International Journal of Molecular Sciences, 24(4), 4221. https://doi.org/10.3390/ijms24044221






