The Crosstalk between Intestinal Epithelial Cells and Mast Cells Is Modulated by the Probiotic Supplementation in Co-Culture Models
Abstract
1. Introduction
2. Results
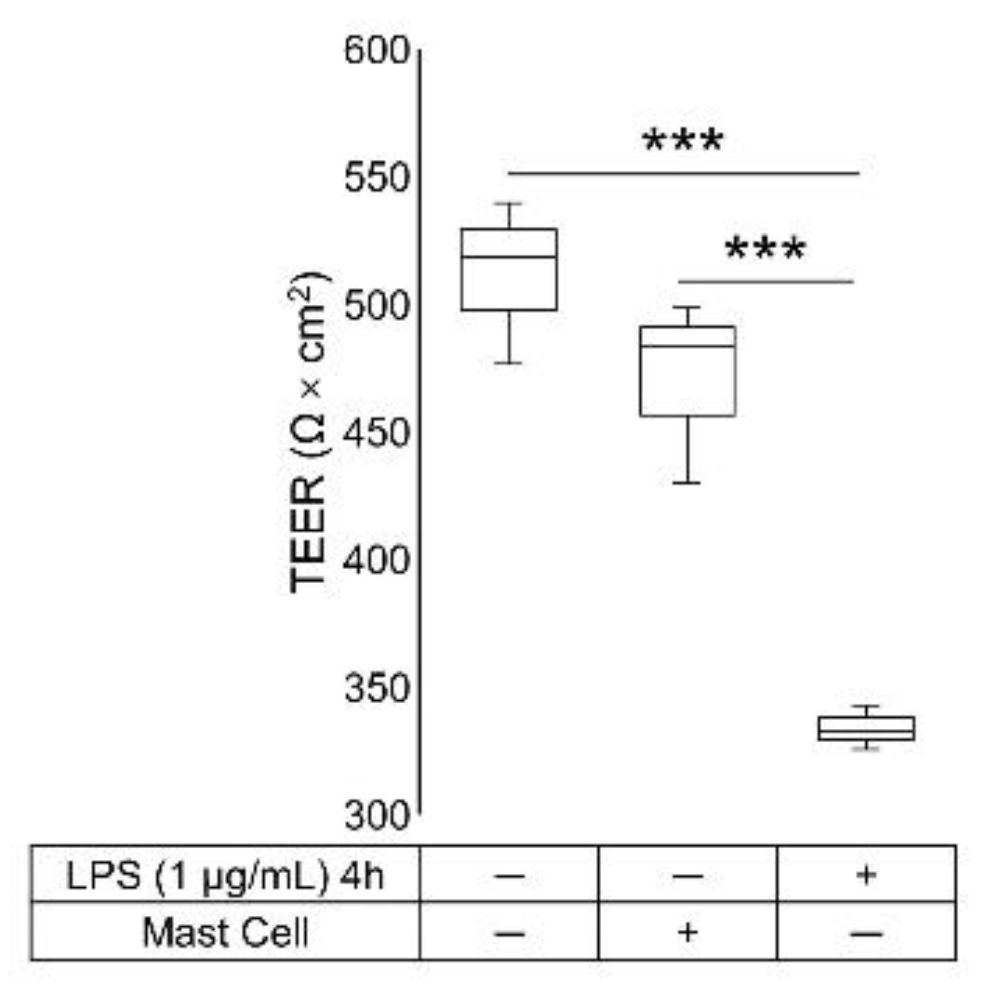
2.1. TEER Measurement
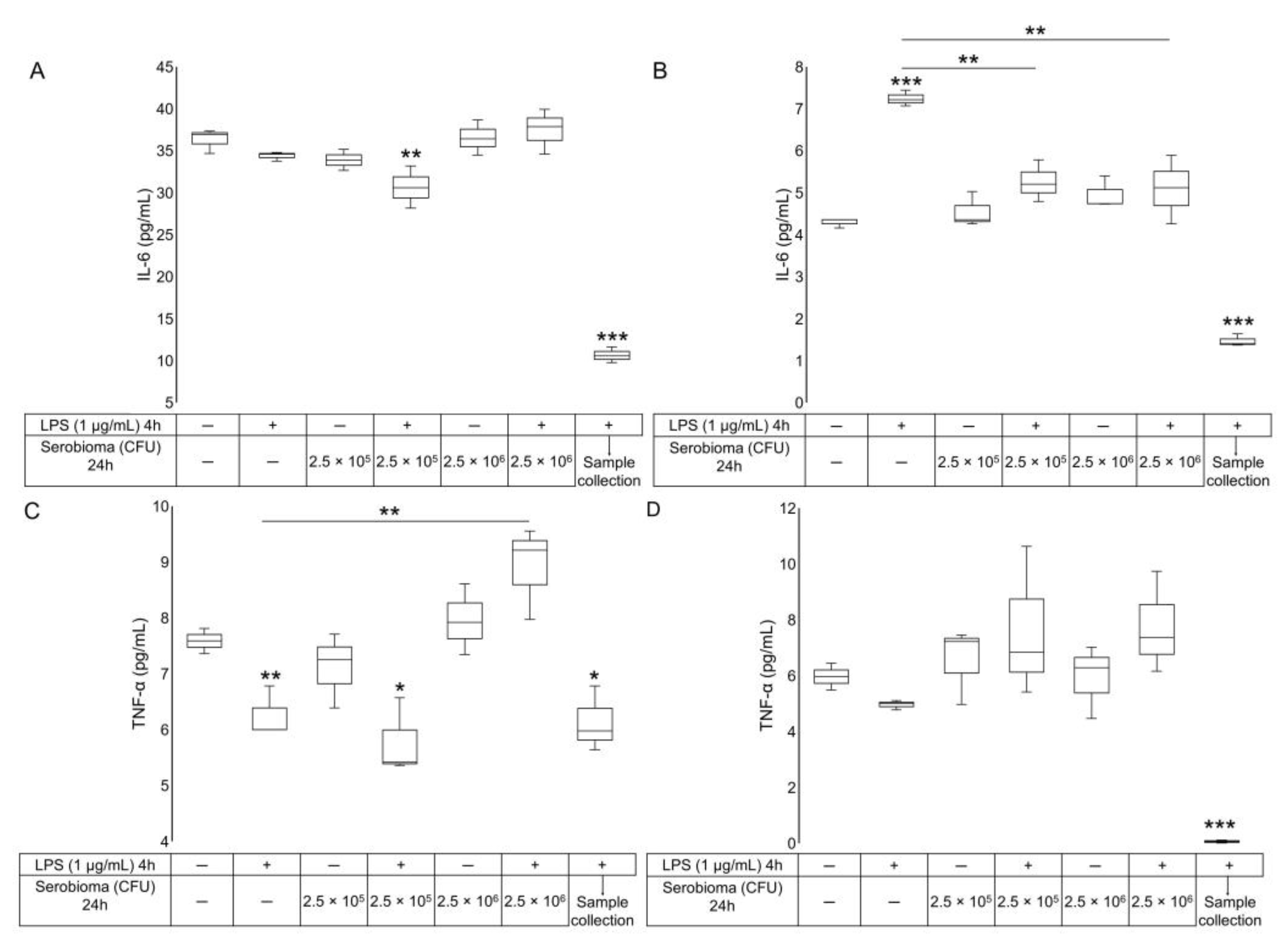
2.2. ELISA Assay
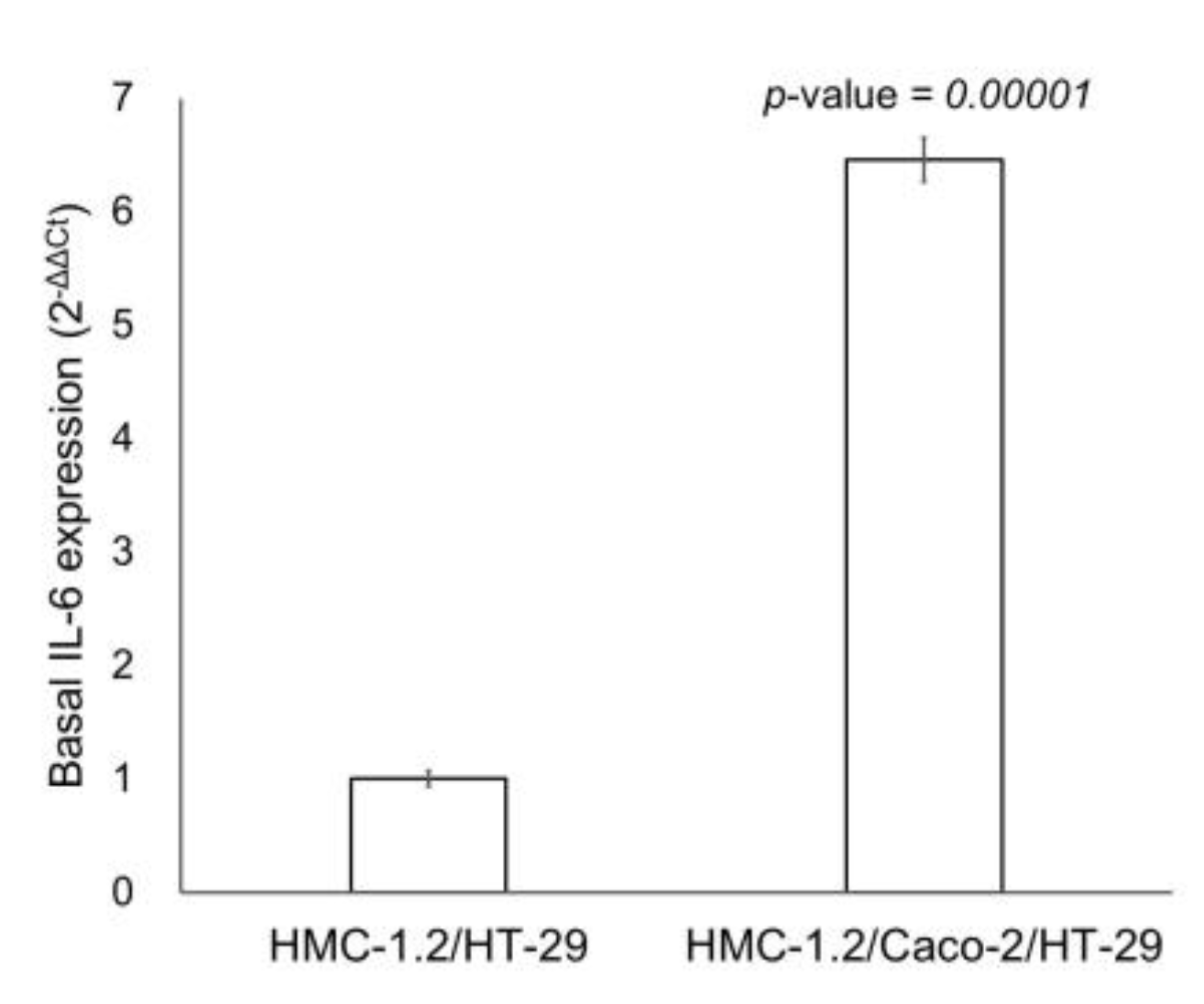
2.3. RT-qPCR
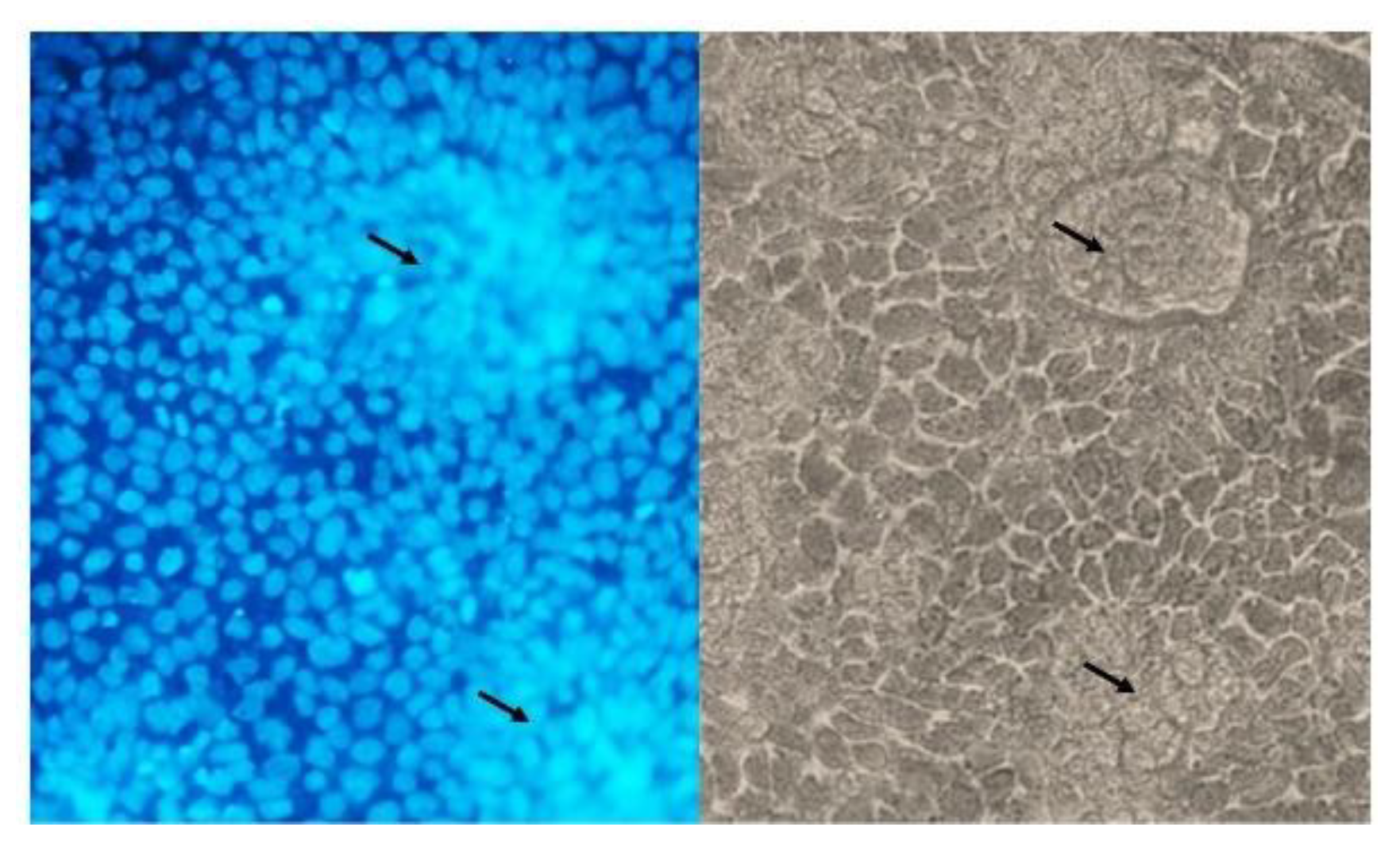
2.4. DAPI-Staining
3. Discussion
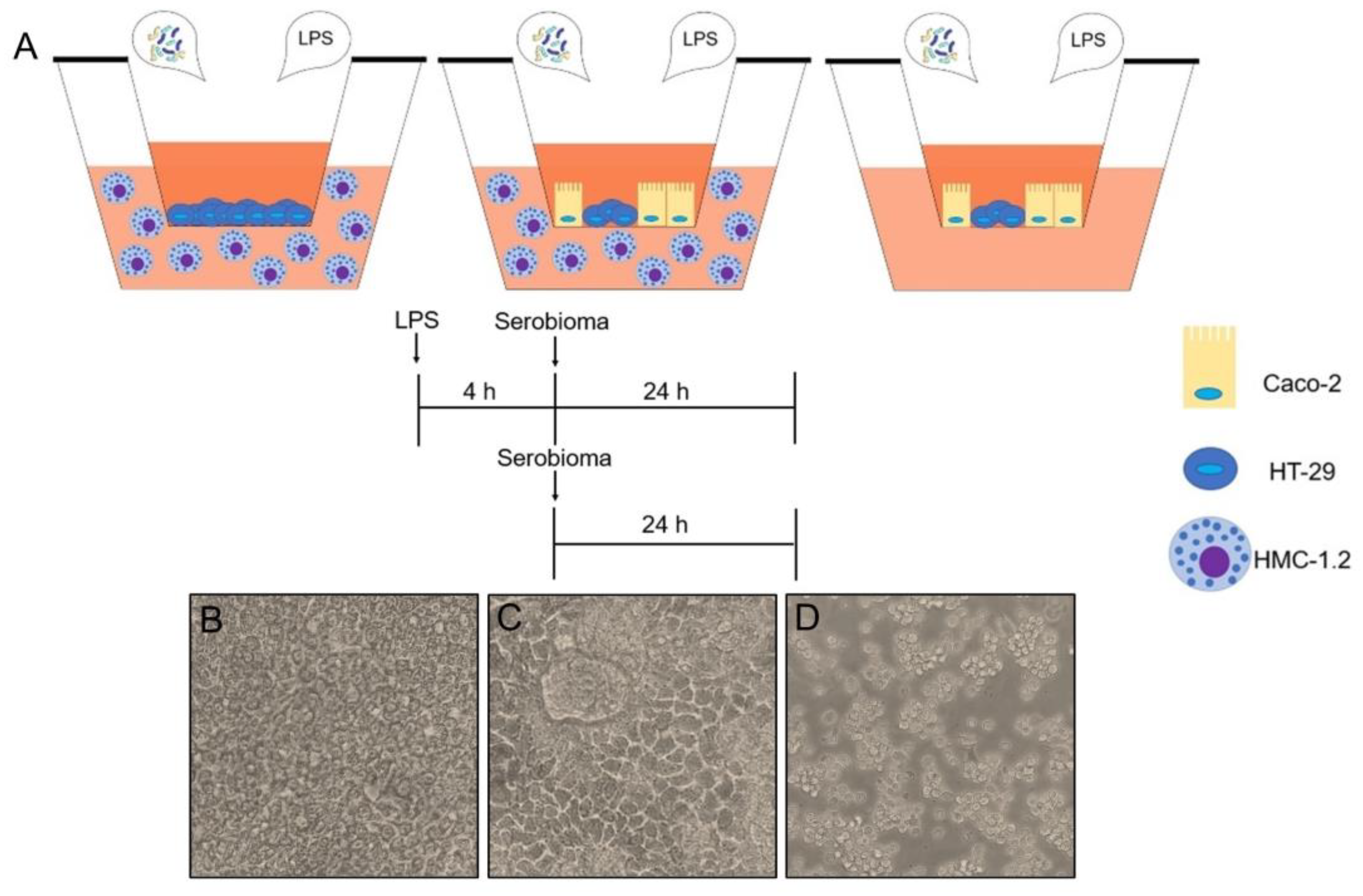
4. Materials and Methods
4.1. Cell Lines
4.2. Transwell Experimental Models
4.2.1. HT-29 Cell Layer Interfaced with HMC-1.2 Cells
4.2.2. Caco2/HT-29 Cell Layer Interfaced with HMC-1.2 Cells
4.2.3. Caco-2/HT-29 Cell Layer
4.3. Treatment with the Probiotic Formulation
4.4. TEER Measurement
4.5. ELISA Assay
4.6. RT-qPCR
4.7. DAPI Staining
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thursby, E.; Juge, N. Introduction to the Human Gut Microbiota. Biochem. J. 2017, 474, 1823–1836. [Google Scholar] [CrossRef] [PubMed]
- Ahluwalia, B.; Magnusson, M.K.; Öhman, L. Mucosal Immune System of the Gastrointestinal Tract: Maintaining Balance between the Good and the Bad. Scand. J. Gastroenterol. 2017, 52, 1185–1193. [Google Scholar] [CrossRef] [PubMed]
- Kong, S.; Zhang, Y.H.; Zhang, W. Regulation of Intestinal Epithelial Cells Properties and Functions by Amino Acids. BioMed Res. Int. 2018, 2018, 2819154. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Gutierrez, E.; Mayer, M.J.; Cotter, P.D.; Narbad, A. Gut Microbiota as a Source of Novel Antimicrobials. Gut Microbes 2019, 10, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Snoeck, V.; Goddeeris, B.; Cox, E. The Role of Enterocytes in the Intestinal Barrier Function and Antigen Uptake. Microbes Infect. 2005, 7, 997–1004. [Google Scholar] [CrossRef] [PubMed]
- Traina, G. Mast Cells in Gut and Brain and Their Potential Role as an Emerging Therapeutic Target for Neural Diseases. Front. Cell. Neurosc. 2019, 13, 345. [Google Scholar] [CrossRef]
- Traina, G. The Role of Mast Cells in the Gut and Brain. J. Integr. Neurosci. 2021, 20, 185–196. [Google Scholar] [CrossRef]
- Kitamura, Y.; Ito, A. Mast Cell-Committed Progenitors. Proc. Natl. Acad. Sci. USA 2005, 102, 11129–11130. [Google Scholar] [CrossRef]
- Dahlin, J.S.; Hallgren, J. Mast Cell Progenitors: Origin, Development and Migration to Tissues. Mol. Immunol. 2015, 63, 9–17. [Google Scholar] [CrossRef]
- Komi, D.E.A.; Wöhrl, S.; Bielory, L. Mast Cell Biology at Molecular Level: A Comprehensive Review. Clin. Rev. Allergy Immunol. 2020, 58, 342–365. [Google Scholar] [CrossRef]
- Frossi, B.; Mion, F.; Sibilano, R.; Danelli, L.; Pucillo, C.E.M. Is It Time for a New Classification of Mast Cells? What do We Know about Mast Cell Heterogeneity? Immunol. Rev. 2018, 282, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Beil, W.J.; Schulz, M.; Wefelmeyer, U. Mast Cell Granule Composition and Tissue Location—A Close Correlation. Histol. Histopathol. 2000, 15, 937–946. [Google Scholar] [CrossRef] [PubMed]
- Abraham, S.N.; St John, A.L. Mast Cell-Orchestrated Immunity to Pathogens. Nat. Rev. Immunol. 2010, 10, 440–452. [Google Scholar] [CrossRef] [PubMed]
- Supajatura, V.; Ushio, H.; Nakao, A.; Akira, S.; Okumura, K.; Ra, C.; Ogawa, H. Differential Responses of Mast Cell Toll-Like Receptors 2 and 4 in Allergy and Innate Immunity. J. Clin. Investig. 2002, 109, 1351–1359. [Google Scholar] [CrossRef] [PubMed]
- Thaiss, C.A.; Zmora, N.; Levy, M.; Elinav, E. The Microbiome and Innate Immunity. Nature 2016, 535, 65–74. [Google Scholar] [CrossRef] [PubMed]
- De Zuani, M.; Dal Secco, C.; Frossi, B. Mast Cells at the Crossroads of Microbiota and IBD. Eur. J. Immunol. 2018, 48, 1929–1937. [Google Scholar] [CrossRef]
- Toor, D.; Wasson, M.K.; Kumar, P.; Karthikeyan, G.; Kaushik, N.K.; Goel, C.; Singh, S.; Kumar, A.; Prakash, H. Dysbiosis Disrupts Gut Immune Homeostasis and Promotes Gastric Diseases. Int. J. Mol. Sci. 2019, 20, 2432. [Google Scholar] [CrossRef]
- Dominici, L.; Moretti, M.; Villarini, M.; Vannini, S.; Cenci, G.; Zampino, C.; Traina, G. In vivo Antigenotoxic Properties of a Commercial Probiotic Supplement Containing Bifidobacteria. Int. J. Probiot. Prebiot. 2011, 6, 179–186. [Google Scholar]
- Dominici, L.; Villarini, M.; Trotta, F.; Federici, E.; Moretti, G.C. and M. Protective Effects of Probiotic Lactobacillus Rhamnosus IMC501 in Mice Treated with PhIP. J. Microbiol. Biotechnol. 2014, 24, 371–378. [Google Scholar] [CrossRef]
- Persichetti, E.; De Michele, A.; Codini, M.; Traina, G. Antioxidative Capacity of Lactobacillus Fermentum LF31 Evaluated in vitro by Oxygen Radical Absorbance Capacity Assay. Nutrition 2014, 30, 936–938. [Google Scholar] [CrossRef]
- Traina, G.; Menchetti, L.; Rappa, F.; Casagrande-Proietti, P.; Barbato, O.; Leonardi, L.; Carini, F.; Piro, F.; Brecchia, G. Probiotic Mixture Supplementation in the Preventive Management of Trinitrobenzenesulfonic Acid-Induced Inflammation in a Murine Model. J. Biol. Regul. Homeost. Agents 2016, 30, 895–901. [Google Scholar] [PubMed]
- Bäckhed, F.; Fraser, C.M.; Ringel, Y.; Sanders, M.E.; Sartor, R.B.; Sherman, P.M.; Versalovic, J.; Young, V.; Finlay, B.B. Defining a Healthy Human Gut Microbiome: Current Concepts, Future Directions, and Clinical Applications. Cell Host Microbe 2012, 12, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Wouters, M.M.; Vicario, M.; Santos, J. The Role of Mast Cells in Functional GI Disorders. Gut 2016, 65, 155–168. [Google Scholar] [CrossRef]
- Sichetti, M.; De Marco, S.; Pagiotti, R.; Traina, G.; Pietrella, D. Anti-Inflammatory Effect of Multistrain Probiotic Formulation (L. Rhamnosus, B. Lactis, and B. Longum). Nutrition 2018, 53, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Pan, F.; Han, L.; Zhang, Y.; Yu, Y.; Liu, J. Optimization of Caco-2 and HT29 Co-Culture in vitro Cell Models for Permeability Studies. Int. J. Food Sci. Nutr. 2015, 66, 680–685. [Google Scholar] [CrossRef]
- Srinivasan, B.; Kolli, A.R.; Esch, M.B.; Abaci, H.E.; Shuler, M.L.; Hickman, J.J. TEER Measurement Techniques for in vitro Barrier Model Systems. J. Lab. Autom. 2015, 20, 107–126. [Google Scholar] [CrossRef]
- Nilsson, G.; Blom, T.; Kusche-Gullberg, M.; Kjellén, L.; Butterfield, J.H.; Sundström, C.; Nilsson, K.; Hellman, L. Phenotypic Characterization of the Human Mast-Cell Line HMC-1. Scand. J. Immunol. 1994, 39, 489–498. [Google Scholar] [CrossRef]
- di Vito, R.; Conte, C.; Traina, G. A Multi-Strain Probiotic Formulation Improves Intestinal Barrier Function by the Modulation of Tight and Adherent Junction Proteins. Cells 2022, 11, 2617. [Google Scholar] [CrossRef]
- He, S.; Guo, Y.; Zhao, J.; Xu, X.; Wang, N.; Liu, Q. Ferulic Acid Ameliorates Lipopolysaccharide-Induced Barrier Dysfunction via MicroRNA-200c-3p-Mediated Activation of PI3K/AKT Pathway in Caco-2 Cells. Front. Pharmacol. 2020, 11, 376. [Google Scholar] [CrossRef]
- Wei, C.-X.; Wu, J.-H.; Huang, Y.-H.; Wang, X.-Z.; Li, J.-Y. Lactobacillus Plantarum Improves LPS-Induced Caco2 Cell Line Intestinal Barrier Damage via Cyclic AMP-PKA Signaling. PLoS ONE 2022, 17, e0267831. [Google Scholar] [CrossRef]
- Guo, S.; Al-Sadi, R.; Said, H.M.; Ma, T.Y. Lipopolysaccharide Causes an Increase in Intestinal Tight Junction Permeability in vitro and in vivo by Inducing Enterocyte Membrane Expression and Localization of TLR-4 and CD14. Am. J. Pathol 2013, 182, 375–387. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Nighot, M.; Al-Sadi, R.; Alhmoud, T.; Nighot, P.; Ma, T.Y. Lipopolysaccharide Regulation of Intestinal Tight Junction Permeability is Mediated by TLR4 Signal Transduction Pathway Activation of FAK and MyD88. J. Immunol. 2015, 195, 4999–5010. [Google Scholar] [CrossRef] [PubMed]
- Conte, C.; Sichetti, M.; Traina, G. Gut–Brain Axis: Focus on Neurodegeneration and Mast Cells. Appl. Sci. 2020, 10, 1828. [Google Scholar] [CrossRef]
- Groschwitz, K.R.; Hogan, S.P. Intestinal Barrier Function: Molecular Regulation and Disease Pathogenesis. J. Allergy Clin. Immunol. 2009, 124, 3–22. [Google Scholar] [CrossRef]
- Resta-Lenert, S.; Barrett, K.E. Probiotics and Commensals Reverse TNF-α– and IFN-γ–Induced Dysfunction in Human Intestinal Epithelial Cells. Gastroenterology 2006, 130, 731–746. [Google Scholar] [CrossRef]
- Yu, Y.; Blokhuis, B.; Derks, Y.; Kumari, S.; Garssen, J.; Redegeld, F. Human Mast Cells Promote Colon Cancer Growth via Bidirectional Crosstalk: Studies in 2D and 3D Coculture Models. Oncoimmunology 2018, 7, e1504729. [Google Scholar] [CrossRef]
- Hyun, J.; Romero, L.; Riveron, R.; Flores, C.; Kanagavelu, S.; Chung, K.D.; Alonso, A.; Sotolongo, J.; Ruiz, J.; Manukyan, A.; et al. Human Intestinal Epithelial Cells Express Interleukin-10 through Toll-Like Receptor 4-Mediated Epithelial-Macrophage Crosstalk. J. Innate Immun. 2014, 7, 87–101. [Google Scholar] [CrossRef]
- Vitetta, L.; Vitetta, G.; Hall, S. Immunological Tolerance and Function: Associations between Intestinal Bacteria, Probiotics, Prebiotics, and Phages. Front. Immunol. 2018, 9, 2240. [Google Scholar] [CrossRef]
- Yao, S.; Zhao, Z.; Wang, W.; Liu, X. Bifidobacterium Longum: Protection against Inflammatory Bowel Disease. J. Immunol. Res. 2021, 2021, e8030297. [Google Scholar] [CrossRef]
- Tamaki, H.; Nakase, H.; Inoue, S.; Kawanami, C.; Itani, T.; Ohana, M.; Kusaka, T.; Uose, S.; Hisatsune, H.; Tojo, M.; et al. Efficacy of Probiotic Treatment with Bifidobacterium Longum 536 for Induction of Remission in Active Ulcerative Colitis: A Randomized, Double-Blinded, Placebo-Controlled Multicenter Trial. Dig. Endosc. 2016, 28, 67–74. [Google Scholar] [CrossRef]
- Oksaharju, A.; Kankainen, M.; Kekkonen, R.A.; Lindstedt, K.A.; Kovanen, P.T.; Korpela, R.; Miettinen, M. Probiotic Lactobacillus Rhamnosus Downregulates FCER1 and HRH4 Expression in Human Mast Cells. World J. Gastroenterol. 2011, 17, 750–759. [Google Scholar] [CrossRef]
- Forsythe, P.; Wang, B.; Khambati, I.; Kunze, W.A. Systemic Effects of Ingested Lactobacillus Rhamnosus: Inhibition of Mast Cell Membrane Potassium (IKCa) Current and Degranulation. PLoS ONE 2012, 7, e41234. [Google Scholar] [CrossRef] [PubMed]
- Foligne, B.; Zoumpopoulou, G.; Dewulf, J.; Younes, A.B.; Chareyre, F.; Sirard, J.-C.; Pot, B.; Grangette, C. A Key Role of Dendritic Cells in Probiotic Functionality. PLoS ONE 2007, 2, e313. [Google Scholar] [CrossRef]
- Kayama, H.; Takeda, K. Manipulation of Epithelial Integrity and Mucosal Immunity by Host and Microbiota-Derived Metabolites. Eur. J. Immunol. 2020, 50, 921–931. [Google Scholar] [CrossRef] [PubMed]
- Vale, G.C.; Mota, B.I.S.; Ando-Suguimoto, E.S.; Mayer, M.P.A. Effect of Probiotics Lactobacillus Acidophilus and Lacticaseibacillus Rhamnosus on Antibacterial Response Gene Transcription of Human Peripheral Monocytes. Probiot. Antimicrob. Proteins 2021. [Google Scholar] [CrossRef] [PubMed]
- Vale, G.C.; Mayer, M.P.A. Effect of Probiotic Lactobacillus rhamnosus by-Products on Gingival Epithelial Cells Challenged with Porphyromonas Gingivalis. Arch. Oral Biol. 2021, 128, 105174. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.B.; Odamaki, T.; Xiao, J. Beneficial Effects of Bifidobacterium Longum Subsp. Longum BB536 on Human Health: Modulation of Gut Microbiome as the Principal Action. J. Funct. Foods 2019, 54, 506–519. [Google Scholar] [CrossRef]
- Akatsu, H.; Iwabuchi, N.; Xiao, J.-Z.; Matsuyama, Z.; Kurihara, R.; Okuda, K.; Yamamoto, T.; Maruyama, M. Clinical Effects of Probiotic Bifidobacterium Longum BB536 on Immune Function and Intestinal Microbiota in Elderly Patients Receiving Enteral Tube Feeding. JPEN J. Parenter. Enter. Nutr. 2013, 37, 631–640. [Google Scholar] [CrossRef]
- Zhong, Y.; Wang, S.; Di, H.; Deng, Z.; Liu, J.; Wang, H. Gut Health Benefit and Application of Postbiotics in Animal Production. J. Anim. Sci. Biotechnol. 2022, 13, 38. [Google Scholar] [CrossRef]
- Kim, J.-H.; Jeun, E.-J.; Hong, C.-P.; Kim, S.-H.; Jang, M.S.; Lee, E.-J.; Moon, S.J.; Yun, C.H.; Im, S.-H.; Jeong, S.-G.; et al. Extracellular Vesicle–Derived Protein from Bifidobacterium Longum Alleviates Food Allergy through Mast Cell Suppression. J. Allergy Clin. Immunol. 2016, 137, 507–516.e8. [Google Scholar] [CrossRef]
- Li, Y.-J.; Li, J.; Dai, C. The Role of Intestinal Microbiota and Mast Cell in a Rat Model of Visceral Hypersensitivity. J. Neurogastroenterol. Motil. 2020, 26, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Moens, F.; Duysburgh, C.; van den Abbeele, P.; Morera, M.; Marzorati, M. Lactobacillus Rhamnosus GG and Saccharomyces Cerevisiae Boulardii Exert Synergistic Antipathogenic Activity in Vitro against Enterotoxigenic Escherichia coli. Benef. Microbes 2019, 10, 923–935. [Google Scholar] [CrossRef] [PubMed]
- Villarini, M.; Acito, M.; di Vito, R.; Vannini, S.; Dominici, L.; Fatigoni, C.; Pagiotti, R.; Moretti, M. Pro-Apoptotic Activity of Artichoke Leaf Extracts in Human HT-29 and RKO Colon Cancer Cells. Int. J. Environ. Res. Public Health 2021, 18, 4166. [Google Scholar] [CrossRef] [PubMed]
- Hubatsch, I.; Ragnarsson, E.G.E.; Artursson, P. Determination of Drug Permeability and Prediction of Drug Absorption in Caco-2 Monolayers. Nat. Protoc. 2007, 2, 2111–2119. [Google Scholar] [CrossRef] [PubMed]
- Taticchi, A.; Urbani, S.; Albi, E.; Servili, M.; Codini, M.; Traina, G.; Balloni, S.; Patria, F.F.; Perioli, L.; Beccari, T.; et al. In vitro Anti-Inflammatory Effects of Phenolic Compounds from Moraiolo Virgin Olive Oil (MVOO) in Brain Cells via Regulating the TLR4/NLRP3 Axis. Molecules 2019, 24, E4523. [Google Scholar] [CrossRef]

| Accession Number | Gene Name | Symbol | Product Length | Primer Sequences (F: Forward; R: Reverse) |
|---|---|---|---|---|
| NM_002046.7 | Glyceraldehyde-3- phosphate Dehydrogenase | GAPDH | 120 | F: TGACTTCAACAGCGACACCCA R: CACCCTGTTGCTGTAGCCAAA |
| NM_000600.5 | Interleukin 6 | IL-6 | 149 | F: ACTCACCTCTTCAGAACGAATTG R: CCATCTTTGGAAGGTTGAGGTT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
di Vito, R.; Di Mezza, A.; Conte, C.; Traina, G. The Crosstalk between Intestinal Epithelial Cells and Mast Cells Is Modulated by the Probiotic Supplementation in Co-Culture Models. Int. J. Mol. Sci. 2023, 24, 4157. https://doi.org/10.3390/ijms24044157
di Vito R, Di Mezza A, Conte C, Traina G. The Crosstalk between Intestinal Epithelial Cells and Mast Cells Is Modulated by the Probiotic Supplementation in Co-Culture Models. International Journal of Molecular Sciences. 2023; 24(4):4157. https://doi.org/10.3390/ijms24044157
Chicago/Turabian Styledi Vito, Raffaella, Alessia Di Mezza, Carmela Conte, and Giovanna Traina. 2023. "The Crosstalk between Intestinal Epithelial Cells and Mast Cells Is Modulated by the Probiotic Supplementation in Co-Culture Models" International Journal of Molecular Sciences 24, no. 4: 4157. https://doi.org/10.3390/ijms24044157
APA Styledi Vito, R., Di Mezza, A., Conte, C., & Traina, G. (2023). The Crosstalk between Intestinal Epithelial Cells and Mast Cells Is Modulated by the Probiotic Supplementation in Co-Culture Models. International Journal of Molecular Sciences, 24(4), 4157. https://doi.org/10.3390/ijms24044157







