Effects of Estradiol/Micronized Progesterone vs. Conjugated Equine Estrogens/Medroxyprogesterone Acetate on Breast Cancer Gene Expression in Healthy Postmenopausal Women
Abstract
1. Introduction
2. Results
2.1. Serum Hormones
2.2. Microarray
2.3. Q-PCR
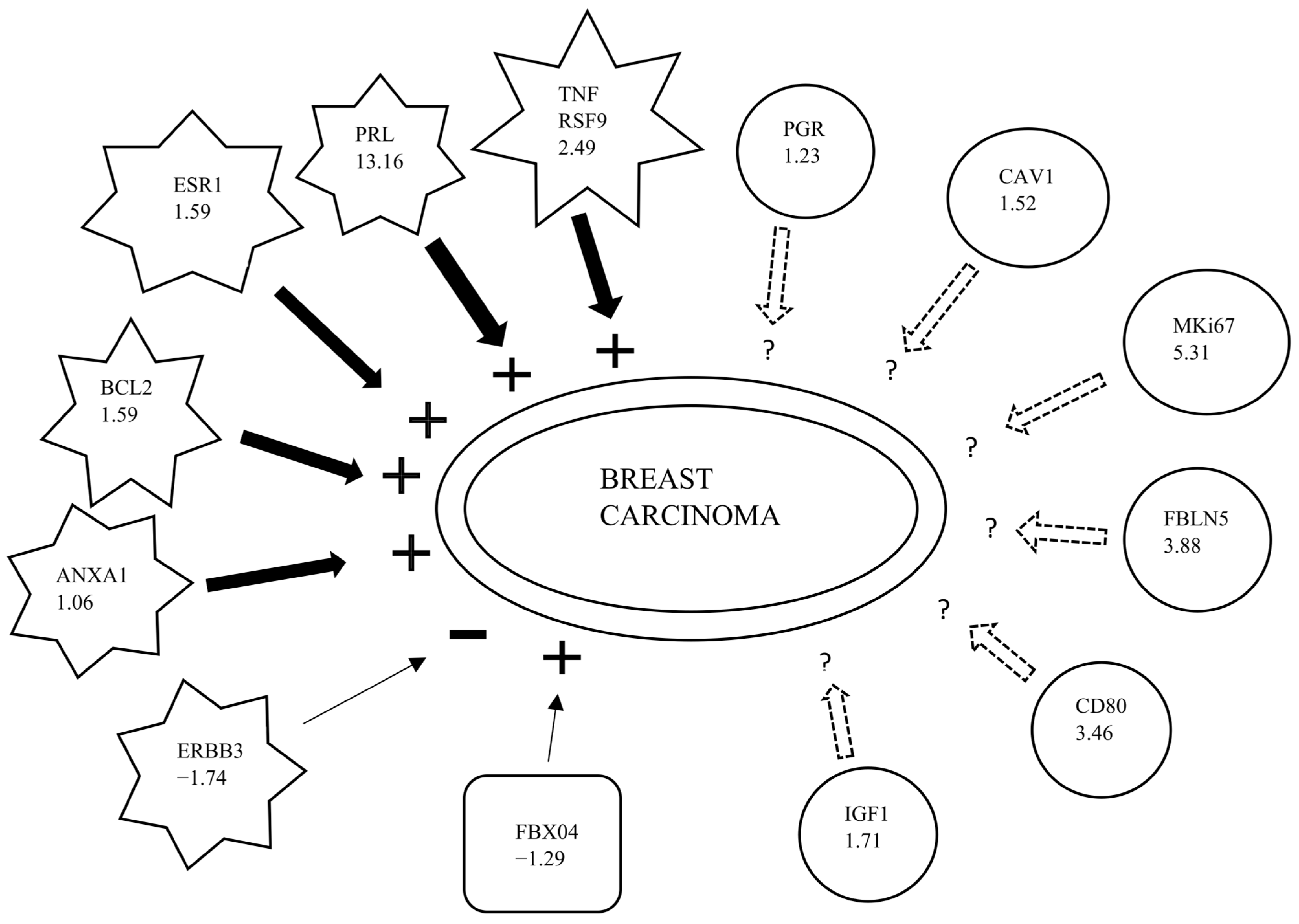
3. Discussion
4. Materials and Methods
4.1. Study Design and Patients
4.2. Analytical Methods
4.3. Randomisation and Masking
4.4. Study Medication
4.5. Biopsies
4.6. RNA Extraction, cDNA Synthesis and Microarray
4.7. Quantitative PCR (Q-PCR)
4.8. Statistical Analysis
5. Conclusions
Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rossouw, J.E.; Anderson, G.L.; Prentice, R.L.; LaCroix, A.Z.; Kooperberg, C.; Stefanick, M.L.; Jackson, R.D.; Beresford, S.A.A.; Howard, B.V.; Johnsin, K.C.; et al. Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results from the Women’s Health Initiative randomized controlled trial. JAMA 2002, 288, 321–333. [Google Scholar] [PubMed]
- Santen, R.J.; Heitjan, D.F.; Gompel, A.; Lumsden, M.A.; Pinkerton, J.A.V.; Davies, S.R.; Stuenkel, C.A. Underlying Breast Cancer Risk and Menopausal Hormone Therapy. J. Clin. Endocrinol. Metab. 2020, 105, 2299–2307. [Google Scholar] [CrossRef] [PubMed]
- Weiss, L.K.; Burkman, R.T.; Cushing-Haugen, K.L.; Voigt, L.F.; Simon, M.S.; Daling, J.R. Hormone Replacement Therapy Regimens and Breast Cancer Risk. Obstet. Gynecol. 2002, 100, 1148–1158. [Google Scholar]
- Chlebowski, R.T.; Hendrix, S.L.; Langer, R.D.; Stefanik, M.L.; Gass, M.; Lane, D.; Rodabough, R.J.; Gilligan, M.A.; Cyr, M.G.; Thomson, C.A.; et al. Influence of Estrogen Plus Progestin on Breast Cancer and Mammography in Healthy Postmenopausal Women. The Women’s Initiative Randomized Trial. JAMA 2003, 289, 3243–3353. [Google Scholar] [CrossRef] [PubMed]
- Manson, J.E.; Chlebowski, R.T.; Stefanick, M.L.; Aragaki, A.K.; Rossouw, J.E.; Prentice, R.L.; Anderson, G.; Howard, B.V.; Thomson, C.A.; LaCroix, A.Z.; et al. Menopausal hormone therapy and health outcomes during the intervention and extended post-stopping phases of the Women’s Health Initiative randomized trials. JAMA 2013, 310, 1353–1368. [Google Scholar] [CrossRef]
- Chlebowski, R.T.; Anderson, G.L.; Aaron, K.; Aragaki, A.K.; Manson, J.A.E.; Stefanick, M.L.; Pan, K.; Barrington, W.; Kuller, L.H.; Simon, M.S.; et al. Association of Menopausal Hormone Therapy With Breast Cancer Incidence and Mortality During Long-term Follow-up of the Women’s Health Initiative Randomized Clinical Trials. JAMA 2020, 324, 369–380. [Google Scholar] [CrossRef]
- Wood, C.E.; Bransetter, D.; Jacob, A.P.; Cline, M.J.; Register, T.C.; Rohrbach, K.; Huang, L.-Y.; Borgerink, H.; Dougall, W.C. Progestin effects on cell proliferation pathways in the postmenopausal mammary gland. Breast Cancer Res. 2013, 15, R62. [Google Scholar] [CrossRef]
- Murkes, D.; Conner, P.; Leifland, K.; Tani, E.; Beliard, A.; Lundström, E.; Söderqvist, G. Effects of percutaneous estradiol-oral progesterone versus oral conjugated equine estrogen-medroxyprogesterone acetate on breast cell proliferation and bcl-2 protein in healthy women. Fertil. Steril. 2011, 95, 1188–1191. [Google Scholar] [CrossRef]
- Conner, P.; Söderqvist, G.; Skoog, L.; Gräser, T.; Walter, F.; Tani, E.; Carlström, K.; von Shoultz, B. Breast cell proliferation in postmenopausal women during HRT evaluated through fine needle aspiration cytology. Breast Cancer Res. Treat. 2003, 78, 159–165. [Google Scholar] [CrossRef]
- Fournier, A.; Berrino, F.; Clavel-Chapelon, F. Unequal risks for breast cancer associated with different hormone replacement therapies: Results from the E3N cohort study. Breast Cancer Res. Treat. 2008, 108, 103–111. [Google Scholar] [CrossRef]
- Cadeau, C.; Fournier, A.; Mesrine, S.; Clavel-Chapelon, F.; Fagherazzi, G.; Boutron-Ruault, M.C. Postmenopausal breast cancer risk and interactions between body mass index, menopausal hormone therapy use, and vitamin D supplementation: Evidence from the E3N cohort. Int. J. Cancer 2016, 139, 2193–2200. [Google Scholar] [CrossRef] [PubMed]
- Trabert, B.; Sherman, M.E.; Kannan, N.; Stanczyk, F.Z. Progesterone and Breast Cancer. Endocr. Rev. 2020, 41, 320–344. [Google Scholar] [CrossRef] [PubMed]
- Leifland, K.; Lundquist, H.; Lagerstedt, U. Comparison of stereotactic fine needle aspiration cytology and core needle biopsy in 522 non-palpable breast lesions. Acta Radiol. 2003, 44, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Conner, P.; Register, T.; Skoog, L.; Tani, E.; von Schoultz, B.; Cline, M. Expression of p53 and markers for apoptosis in breast tissue during long-term hormone therapy in cynomolgus monkeys. Am. J. Gynecol. 2005, 193, 58–63. [Google Scholar] [CrossRef]
- Isaksson, E.; von Schoultz, E.; Odlind, V.; Söderqvist, G.; Csemiczky, G.; Carlström, K.; Skoog, L.; von Schoultz, B. Effects of oral contraceptives on breast proliferation. Breast Cancer Res. Treat. 2001, 65, 163–169. [Google Scholar] [CrossRef]
- Lundström, E.; Söderqvist, G.; Svane, G.; Azavedo, E.; Olovsson, M.; Skoog, L.; von Shoultz, E.; von Schoultz, B. Digitized assessment of mammographic breast density in patients who received low-dose intrauterine levonorgestrel in continuous combination with oral estradiol valerate: A pilot study. Fertil. Steril. 2006, 85, 989–995. [Google Scholar] [CrossRef]
- Sieuwerts, A.; De Napoli, G.; van Galen, A.; Kloosterboer, H.; de Weerd, V.; Zhang, H.; Martens, J.; Foekens, J.; De Geyter, G. Hormone replacement therapy dependent changes in breast cancer-related gene expression in breast tissue of healthy postmenopausal women. Mol. Oncol. 2011, 5, 504–516. [Google Scholar] [CrossRef]
- Krämer, A. Causal analysis approaches in Ingenuity Pathway Analysis. Bioinformatics 2014, 30, 523–530. [Google Scholar] [CrossRef]
- L’Hermite, M. HRT optimization, using transdermal estradiol plus micronized progesterone, a safer HRT. Climacteric 2013, 16, 1644–1653. [Google Scholar] [CrossRef]
- Dartois, L.; Fagherazzi, G.; Baglietto, L.; Boutron-Ruault, M.-C.; Delaloge, S.; Mesrine, S.; Clavel-Chapelon, F. Proportion of premenopausal and postmenopausal breast cancers attributable to known risk factors: Estimates from the E3N-EPIC cohort. Int. J. Cancer 2016, 138, 2415–2427. [Google Scholar] [CrossRef]
- Lindén-Hirschberg, A.; Tani, E.; Brismar, K.; Lundström, E. Effects of drospirenone and norethisterone acetate combined with estradiol on mammographic density and proliferation of breast epithelial cells—A prospective randomized trial. Maturitas 2019, 126, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Lundström, E.; Ivana Virijevic, I.; Söderqvist, G. Differences in breast cell proliferation between oral estradiol/norethisterone acetate, sequential conjugated equine estrogen/medroxyprogesterone acetate and oral estradiol-valerate/low-dose levo-norgestrel intrauterine system, in healthy postmenopausal women. Horm. Mol. Biol. Clin. Investig. 2020, 41, 20190051. [Google Scholar] [CrossRef]
- Engman, M.; Skoog, L.; Söderqvist, G.; Gemzell-Danielsson, K. The effect of mifepristone on breast cell proliferation in premenopausal women evaluated through fine needle aspiration cytology. Hum. Reprod 2008, 23, 2072–2079. [Google Scholar] [CrossRef]
- Isaksson, E.; Sahlin, L.; Söderqvist, G.; von Schoultz, E.; Masironi, B.; Wickman, M.; Wilking, N.; von Schoultz, B.; Skoog, L. Expression of sex steroid receptors and IGF-1 mRNA in breast tissue—Effects of hormonal treatment. J. Steroid Biochem. Mol. Biol. 1999, 70, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Hankinson, S.E.; Willett, W.C.; Colditz, G.A.; Hunter, D.J.; Michaud, D.S.; Deroo, B.; Rosner, B.; Speizer, F.E.; Pollak, M. Circulating concentrations of insulin-like growth factor-I and risk of breast cancer. Lancet 1998, 351, 1293–1296. [Google Scholar] [CrossRef]
- Key, T.J.; Appleby, P.N.; Reeves, G.K.; Roddam, A.W. The Breast Cancer Collaborative Group. Endogenous hormones and breast cancer: Insulin-like growth factor 1 (IGF-1) IGF-binding protein 3 (IGFBP-3) and breast cancer risk. Pooled individual analysis of 17 prospective studies. Lancet Oncol. 2010, 11, 530–542. [Google Scholar]
- Harris, J.R.; Lippman, M.E.; Morrow, M.; Hellman, S. Diseases of the Breast; Lippincott-Raven: Philadelphia, PA, USA, 1996. [Google Scholar]
- Wang, M.; Wu, X.; Chai, F.; Zhang, Y.; Jiang, J. Plasma prolactin and breast cancer risk: A meta-analysis. Sci. Rep. 2016, 6, 25998. [Google Scholar] [CrossRef]
- Carver, K.C.; Arendt, L.M.; Schuler, L.A. Complex prolactin cross-talk in breast cancer: New therapeutical implications. Mol. Cell Endocrinol. 2009, 307, 1–7. [Google Scholar] [CrossRef]
- Huh, S.J.; Oh, H.; Peterson, M.A.; Almendro, V.; Hu, R.; Bowden, M.; Lis, R.L.; Cotter, M.B.; Loda, M.; Barry, W.T.; et al. The proliferative activity of mammary epithelial cells in normal tissue predicts breast cancer risk in premenopausal women. Cancer Res. 2016, 76, 1926–1934. [Google Scholar] [CrossRef]
- Preston-Martin, S.; Pike, M.C.; Ross, R.K.; Jones, P.A.; Henderson, B.E. Increased cell division as a cause of human cancer. Cancer Res. 1990, 50, 7415–7421. [Google Scholar]
- Leifland, K.; Lundquist, H.; Lagerstedt, U.; Svane, G. Comparison of pre-operative simultaneous stereotactic fine needle aspiration biopsy and stereotactic core needle biopsy in ductal carcinoma in situ of the breast. Acta Radiol. 2003, 44, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR-data by the comparative method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
| CEE/MPA n = 15 | E2/P n = 15 | ||
|---|---|---|---|
| Age | Mean | 55.8 | 58.0 |
| Median | 56.0 | 58.0 | |
| IQR | 54.0–60.0 | 56.0–60.0 | |
| BMI | Mean | 25.9 | 24.9 |
| Median | 26.0 | 24.6 | |
| IQR | 24.0–28.0 | 23.3–26.9 | |
| Parity | Mean | 1.9 | 2.1 |
| Median | 2.0 | 2.0 | |
| IQR | 1.0–3.0 | 1.0–2.8 | |
| YSMP | Mean | 6.7 | 7.0 |
| Median | 6.0 | 5.0 | |
| IQR | 3.5–10.0 | 3.2–10.0 |
| Affymetrix ID | Gene Abbreviation | Full Gene Name | Taqman Assay ID |
|---|---|---|---|
| 8084710 | ADIPOQ | Adiponectin, C1Q and collagen domain containing | Hs 00605917_m1 |
| 8155849 | ANXA1 | Annexin A1 | Hs 00167549_m1 |
| 8056909 | ATF2 | Activating transcription factor 2 | Hs 01095345_m1 |
| 8023646 | BCl-2 | Apoptosis regulator Bcl 2, B-cell lymphoma 2 | Hs 00608023_m1 |
| 8135594 | CAV1 | Caveolin 1, coding caveolae protein 22 kDa | Hs 00971716_m1 |
| 8089771 | CD80 | Cluster of Differentiation 80 | Hs 00175478_m1 |
| 7956120 | ERBB3 | Receptor tyrosine-kinase erbB-3, HER3 (human epidermal growth factor) | Hs 00176538_m1 |
| 8122843 | ESR1 | Estrogen receptor 1 | Hs 00174860_m1 |
| 7980908 | FBLN5 | Fibulin 5 | Hs 00197064_m1 |
| 8105111 | FBXO4 | F box protein 4 | Hs 00254777_m1 |
| 7902227 | GADD45A | Growth arrest and DNA damage inducible α | Hs 00169255_m1 |
| 7965873 | IGF1 | Insulin-like growth factor 1 | Hs 01547656_m1 |
| 7937020 | MKI67 | Monoclonal antibody Ki 67 | Hs 01032443_m1 |
| 7951165 | PGR | Progesterone receptor | Hs 01556702_m1 |
| 8124185 | PRL | Prolactin | Hs 00168730_m1 |
| 7912145 | TNFRSF9 | Tumor necrosis factor receptor superfamily, member 9 | Hs 00155512_m1 |
| Genes | Fold Change CEE/MPA | Fold Change E2/P |
|---|---|---|
| MKi- | 14.16 * | 2.67 |
| IGF-1 | 1.83 * | 1.07 |
| PRL | −1.13 | −14.88 * |
| BCl-2 | −1.03 | −1.64 * |
| ESR 1 | −1.80 | −2.86 |
| PGR B | 2.47 | 2.02 |
| TNFSR9 | 1.03 | −2.43 |
| ANXA-1 | −1.52 | −1.22 |
| CD 80 | 2.22 | −1.56 |
| ATF 2 | 1.01 | −1.11 |
| ADIPOQ | −1.01 | −0.52 |
| GADD 45A | 1.03 | −0.89 |
| FBX 04 | −0.18 | 1.09 |
| CAV 1 | −1.16 | −1.77 |
| Fibulin 5 | 3.02 | −1.29 |
| cERB B3 | −1.70 | 1.02 |
| Affymetrix ID | Genes | Predicted Effect [a] | Fold Change Ratio | Findings [b] |
|---|---|---|---|---|
| 7937020 | PRL | Increased | 13.60 | Increases |
| 7980908 | MKi-67 | Affected | 5.307 | Affects |
| 8089771 | FBLN5 | Affected | 3.884 | Affects |
| 7912145 | CD80 | Affected | 3.456 | Affects |
| 7965873 | TNFRSF9 | Increased | 2.491 | Increases |
| 8122843 | IGF1 | Affected | 1.708 | Affects |
| 8023646 | ESR1 | Increased | 1.591 | Increases |
| 8135594 | BCL-2 | Increased | 1.587 | Increases |
| 7951165 | CAV1 | Affected | 1.518 | Affects |
| 8155849 | PGR | Affected | 1.226 | Affects |
| 8105111 | ANXA1 | Increased | 1.062 | Increases |
| 7956120 | FBXO4 | Increased | −1.290 | Decreases |
| 7956120 | ERBB3 | Decreased | −1.741 | Increases |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lalitkumar, P.G.L.; Lundström, E.; Byström, B.; Ujvari, D.; Murkes, D.; Tani, E.; Söderqvist, G. Effects of Estradiol/Micronized Progesterone vs. Conjugated Equine Estrogens/Medroxyprogesterone Acetate on Breast Cancer Gene Expression in Healthy Postmenopausal Women. Int. J. Mol. Sci. 2023, 24, 4123. https://doi.org/10.3390/ijms24044123
Lalitkumar PGL, Lundström E, Byström B, Ujvari D, Murkes D, Tani E, Söderqvist G. Effects of Estradiol/Micronized Progesterone vs. Conjugated Equine Estrogens/Medroxyprogesterone Acetate on Breast Cancer Gene Expression in Healthy Postmenopausal Women. International Journal of Molecular Sciences. 2023; 24(4):4123. https://doi.org/10.3390/ijms24044123
Chicago/Turabian StyleLalitkumar, Parameswaran Grace Luther, Eva Lundström, Birgitta Byström, Dorina Ujvari, Daniel Murkes, Edneia Tani, and Gunnar Söderqvist. 2023. "Effects of Estradiol/Micronized Progesterone vs. Conjugated Equine Estrogens/Medroxyprogesterone Acetate on Breast Cancer Gene Expression in Healthy Postmenopausal Women" International Journal of Molecular Sciences 24, no. 4: 4123. https://doi.org/10.3390/ijms24044123
APA StyleLalitkumar, P. G. L., Lundström, E., Byström, B., Ujvari, D., Murkes, D., Tani, E., & Söderqvist, G. (2023). Effects of Estradiol/Micronized Progesterone vs. Conjugated Equine Estrogens/Medroxyprogesterone Acetate on Breast Cancer Gene Expression in Healthy Postmenopausal Women. International Journal of Molecular Sciences, 24(4), 4123. https://doi.org/10.3390/ijms24044123






