Polysaccharides’ Structures and Functions in Biofilm Architecture of Antimicrobial-Resistant (AMR) Pathogens
Abstract
1. Introduction
2. Biofilm Polysaccharides
2.1. PNAG (PIA)
2.2. Alginate, Psl, Pel
2.3. Galactosaminogalactans and Galactomannans
2.4. Other Polysaccharides
3. Characterization of Exopolysaccharides
3.1. Protein-Based Methods
3.2. Chromatographic Techniques
3.3. Nuclear Magnetic Resonance
3.4. Colorimetric Assays
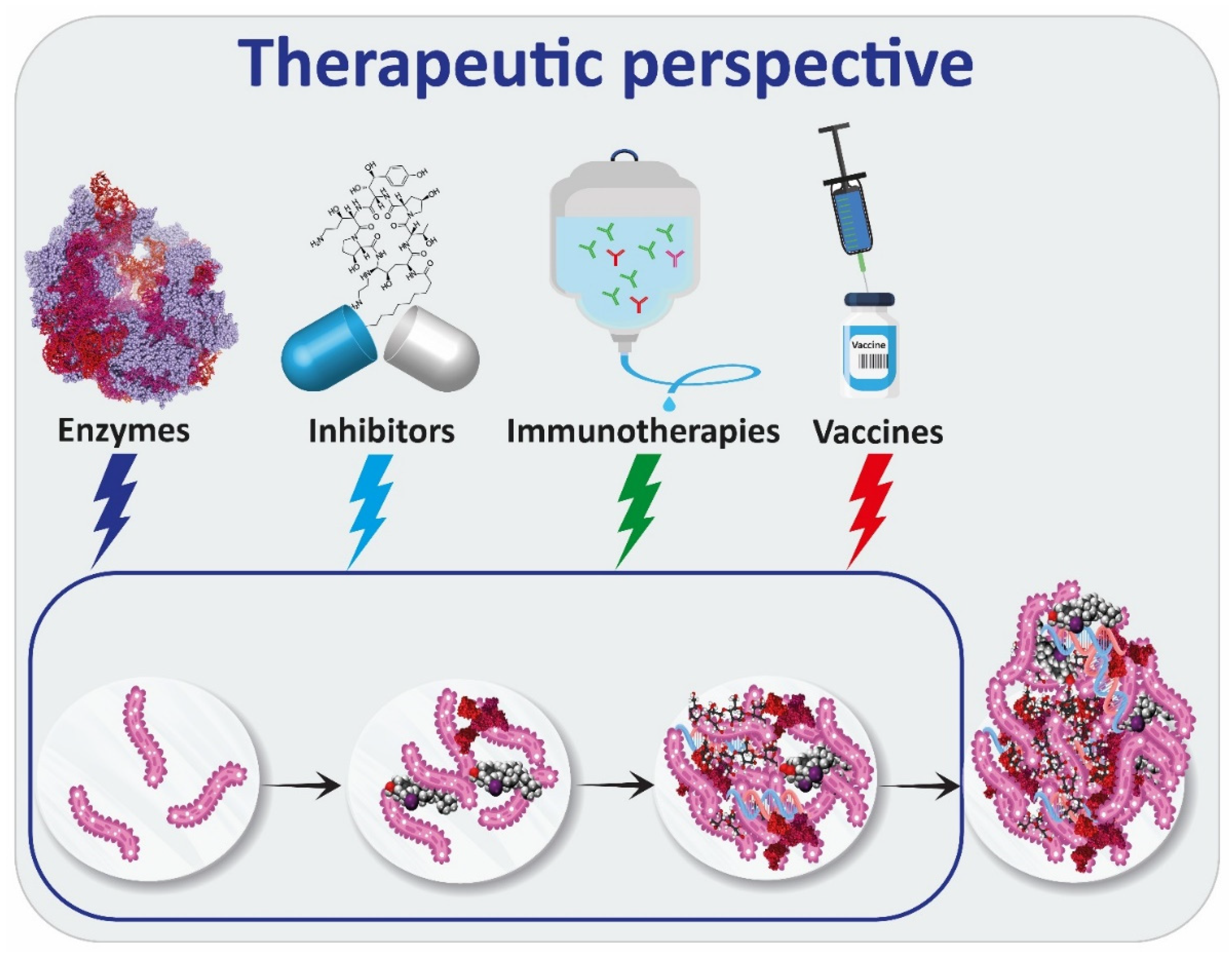
4. Therapies Targeting Exopolysaccharides
4.1. Enzymes
4.2. Inhibitors of Exopolysaccharides’ Production
4.3. Immunotherapies
4.4. Vaccines
5. Future Perspectives
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bjarnsholt, T. Introduction to Biofilms. In Biofilm Infections; Springer: Berlin/Heidelberg, Germany, 2011; p. 1e9. [Google Scholar]
- Mei, L.; Wang, X.; Yin, Y.; Tang, G.; Wang, C. Conservative Production of Galactosaminogalactan in Metarhizium Is Responsible for Appressorium Mucilage Production and Topical Infection of Insect Hosts. PLoS Pathog. 2021, 17, 1–25. [Google Scholar] [CrossRef]
- Chen, M.; Yu, Q.; Sun, H. Novel Strategies for the Prevention and Treatment of Biofilm Related Infections. Int. J. Mol. Sci. 2013, 14, 18488–18501. [Google Scholar] [CrossRef] [PubMed]
- Santajit, S.; Indrawattana, N. Mechanisms of Antimicrobial Resistance in ESKAPE Pathogens. BioMed Res. Int. 2016, 2016, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, M.H.; Idris, A.L.; Fan, X.; Guo, Y.; Yu, Y.; Jin, X.; Qiu, J.; Guan, X.; Huang, T. Beyond Risk: Bacterial Biofilms and Their Regulating Approaches. Front. Microbiol. 2020, 11, 928. [Google Scholar] [CrossRef]
- Sauer, K.; Stoodley, P.; Goeres, D.M.; Hall-Stoodley, L.; Burmølle, M.; Stewart, P.S.; Bjarnsholt, T. The Biofilm Life Cycle: Expanding the Conceptual Model of Biofilm Formation. Nat. Rev. Microbiol. 2022, 20, 608–620. [Google Scholar] [CrossRef]
- Flemming, H.C.; Neu, T.R.; Wozniak, D.J. The EPS Matrix: The “House of Biofilm Cells. J. Bacteriol. 2007, 189, 7945–7947. [Google Scholar] [CrossRef] [PubMed]
- Jefferson, K.K. What Drives Bacteria to Produce a Biofilm? FEMS Microbiol. Lett. 2004, 236, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Bjarnsholt, T. The Role of Bacterial Biofilms in Chronic Infections. APMIS. Suppl. 2013, 121, 1–51. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Sarkar, S.; Das, B.; Bhattacharjee, S.; Tribedi, P. Biofilm, Pathogenesis and Prevention—A Journey to Break the Wall: A Review. Arch. Microbiol. 2016, 198, 1–15. [Google Scholar] [CrossRef]
- Hall, C.W.; Mah, T.F. Molecular Mechanisms of Biofilm-Based Antibiotic Resistance and Tolerance in Pathogenic Bacteria. FEMS Microbiol. Rev. 2017, 41, 276–301. [Google Scholar] [CrossRef]
- Fux, C.A.; Stoodley, P.; Hall-Stoodley, L.; Costerton, J.W. Bacterial Biofilms: A Diagnostic and Therapeutic Challenge. Expert Rev. Anti. Infect. Ther. 2003, 1, 667–683. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Mah, T.F. Involvement of a Novel Efflux System in Biofilm-Specific Resistance to Antibiotics. J. Bacteriol. 2008, 190, 4447–4452. [Google Scholar] [CrossRef] [PubMed]
- Adam, B.; Baillie, G.S.; Douglas, L.J. Mixed Species Biofilms of Candida Albicans and Staphylococcus Epidermidis. J. Med. Microbiol. 2002, 51, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Joo, H.-S.; Otto, M. Molecular Basis of In-Vivo Biofilm Formation by Bacterial Pathogens. Chem. Biol. 2013, 19, 1503–1513. [Google Scholar] [CrossRef]
- Drago, L.; Toscano, M. Biofilm Formation and the Biological Response. In Management of Periprosthetic Joint Infections (PJIs); Arts, J.C., Geurts, J., Eds.; Woodhead Publishing: Sawstons, UK, 2017; pp. 25–39. [Google Scholar]
- Ruhal, R.; Kataria, R. Biofilm Patterns in Gram-Positive and Gram-Negative Bacteria. Microbiol. Res. 2021, 251, 126829. [Google Scholar] [CrossRef]
- Flemming, H.-C.; Wingender, J. The Biofilm Matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef]
- Maira-Litrán, T.; Kropec, A.; Abeygunawardana, C.; Joyce, J.; Mark, G.; Goldmann, D.A.; Pier, G.B. Immunochemical Properties of the Staphylococcal Poly-N-Acetylglucosamine Surface Polysaccharide. Infect. Immun. 2002, 70, 4433–4440. [Google Scholar] [CrossRef]
- Mack, D.; Nedelmann, M.; Krokotsch, A.; Schwarzkopf, A.; Heesemann, J.; Laufs, R. Characterization of Transposon Mutants of Biofilm-Producing Staphylococcus Epidermidis Impaired in the Accumulative Phase of Biofilm Production: Genetic Identification of a Hexosamine-Containing Polysaccharide Intercellular Adhesin. Infect. Immun. 1994, 62, 3244–3253. [Google Scholar] [CrossRef]
- Kropec, A.; Maira-Litran, T.; Jefferson, K.K.; Grout, M.; Cramton, S.E.; Götz, F.; Goldmann, D.A.; Pier, G.B. Poly-N-Acetylglucosamine Production in Staphylococcus Aureus Is Essential for Virulence in Murine Models of Systemic Infection. Infect. Immun. 2005, 73, 6868–6876. [Google Scholar] [CrossRef]
- Vuong, C.; Kocianova, S.; Voyich, J.M.; Yao, Y.; Fischer, E.R.; DeLeo, F.R.; Otto, M. A Crucial Role for Exopolysaccharide Modification in Bacterial Biofilm Formation, Immune Evasion, and Virulence. J. Biol. Chem. 2004, 279, 54881–54886. [Google Scholar] [CrossRef]
- Wang, X.; Preston, J.F.; Romeo, T. The PgaABCD Locus of Escherichia Coli Promotes the Synthesis of a Polysaccharide Adhesin Required for Biofilm Formation. J. Bacteriol. 2004, 186, 2724–2734. [Google Scholar] [CrossRef] [PubMed]
- Yakandawala, N.; Gawande, P.V.; LoVetri, K.; Cardona, S.T.; Romeo, T.; Nitz, M.; Madhyastha, S. Characterization of the Poly-β-1,6-N-Acetylglucosamine Polysaccharide Component of Burkholderia Biofilms. Appl. Environ. Microbiol. 2011, 77, 8303–8309. [Google Scholar] [CrossRef] [PubMed]
- Bentancor, L.V.; O’malley, J.M.; Bozkurt-Guzel, C.; Pier, G.B.; Maira-Litrán, T. Poly-n-Acetyl-β-(1-6)-Glucosamine Is a Target for Protective Immunity against Acinetobacter Baumannii Infections. Infect. Immun. 2012, 80, 651–656. [Google Scholar] [CrossRef]
- Chen, K.M.; Chiang, M.K.; Wang, M.; Ho, H.C.; Lu, M.C.; Lai, Y.C. The Role of PgaC in Klebsiella Pneumoniae Virulence and Biofilm Formation. Microb. Pathog. 2014, 77, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Roux, D.; Cywes-Bentley, C.; Zhang, Y.F.; Pons, S.; Konkol, M.; Kearns, D.B.; Little, D.J.; Howell, P.L.; Skurnik, D.; Pier, G.B. Identification of Poly-N-Acetylglucosamine as a Major Polysaccharide Component of the Bacillus Subtilis Biofilm Matrix. J. Biol. Chem. 2015, 290, 19261–19272. [Google Scholar] [CrossRef]
- Cerca, N.; Maira-Litrán, T.; Jefferson, K.K.; Grout, M.; Goldmann, D.A.; Pier, G.B. Protection against Escherichia Coli Infection by Antibody to the Staphylococcus Aureus Poly-N-Acetylglucosamine Surface Polysaccharide. Proc. Natl. Acad. Sci. USA 2007, 104, 7528–7533. [Google Scholar] [CrossRef] [PubMed]
- Cywes-Bentley, C.; Skurnik, D.; Zaidi, T.; Roux, D.; DeOliveira, R.B.; Garrett, W.S.; Lu, X.; O’Malley, J.; Kinzel, K.; Zaidi, T.; et al. Antibody to a Conserved Antigenic Target Is Protective against Diverse Prokaryotic and Eukaryotic Pathogens. Proc. Natl. Acad. Sci. USA 2013, 110, E2209–E2218. [Google Scholar] [CrossRef] [PubMed]
- Cramton, S.E.; Gerke, C.; Schnell, N.F.; Nichols, W.W.; Götz, F. The Intercellular Adhesion (Ica) Locus Is Present in Staphylococcus Aureus and Is Required for Biofilm Formation. Infect. Immun. 1999, 67, 5427–5433. [Google Scholar] [CrossRef]
- Götz, F. Staphylococcus and Biofilms. Mol. Microbiol. 2002, 43, 1367–1378. [Google Scholar] [CrossRef]
- Rupp, M.E.; Ulphani, J.S.; Fey, P.D.; Dietrich, M. Characterization of Staphylococcus Epidermidis Polysaccharide Intercellular Adhesin/Hemagglutinin in the Pathogenesis of Intravascular Catheter-Associated Infection in a Rat Model. J. Infect. Dis. 1999, 183, 1038–1042. [Google Scholar] [CrossRef]
- Olson, M.E.; Garvin, K.L.; Fey, P.D.; Rupp, M.E. Adherence of Staphylococcus Epidermidis to Biomaterials Is Augmented by PIA. Clin. Orthop. Relat. Res. 2006, 451, 21–24. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.T.T.; Nguyen, T.H.; Otto, M. The Staphylococcal Exopolysaccharide PIA—Biosynthesis and Role in Biofilm Formation, Colonization, and Infection. Comput. Struct. Biotechnol. J. 2020, 18, 3324–3334. [Google Scholar] [CrossRef] [PubMed]
- Rupp, M.E.; Fey, P.D.; Heilmann, C.; Götz, F. Characterization of the Importance of Staphylococcus Epidermidis Autolysin and Polysaccharide Intercellular Adhesin in the Pathogenesis of Intravascular Catheter-Associated Infection in a Rat Model. J. Infect. Dis. 2001, 183, 1038–1042. [Google Scholar] [CrossRef] [PubMed]
- Schommer, N.N.; Christner, M.; Hentschke, M.; Ruckdeschel, K.; Aepfelbacher, M.; Rohde, H. Staphylococcus Epidermidis Uses Distinct Mechanisms of Biofilm Formation to Interfere with Phagocytosis and Activation of Mouse Macrophage-like Cells 774A.1. Infect. Immun. 2011, 79, 2267–2276. [Google Scholar] [CrossRef] [PubMed]
- Otto, M. Staphylococcal Infections: Mechanisms of Biofilm Maturation and Detachment as Critical Determinants of Pathogenicity. Annu. Rev. Med. 2013, 64, 175–188. [Google Scholar] [CrossRef]
- Kaplan, J.B.; Ragunath, C.; Ramasubbu, N.; Fine, D.H. Detachment of Actinobacillus Actinomycetemcomitans Biofilm Cells by an Endogenous β-Hexosaminidase Activity. J. Bacteriol. 2003, 185, 4693–4698. [Google Scholar] [CrossRef]
- Franklin, M.J.; Nivens, D.E.; Weadge, J.T.; Lynne Howell, P. Biosynthesis of the Pseudomonas Aeruginosa Extracellular Polysaccharides, Alginate, Pel, and Psl. Front. Microbiol. 2011, 2, 167. [Google Scholar] [CrossRef]
- Ghafoor, A.; Hay, I.D.; Rehm, B.H.A. Role of Exopolysaccharides in Pseudomonas Aeruginosa Biofilm Formation and Architecture. Appl. Environ. Microbiol. 2011, 77, 5238–5246. [Google Scholar] [CrossRef]
- Byrd, M.S.; Pang, B.; Hong, W.; Waligora, E.A.; Juneau, R.A.; Armbruster, C.E.; Weimer, K.E.D.; Murrah, K.; Mann, E.E.; Lu, H.; et al. Direct Evaluation of Pseudomonas Aeruginosa Biofilm Mediators in a Chronic Infection Model. Infect. Immun. 2011, 79, 3087–3095. [Google Scholar] [CrossRef]
- Jennings, L.K.; Dreifus, J.E.; Reichhardt, C.; Storek, K.M.; Secor, P.R.; Wozniak, D.J.; Hisert, K.B.; Parsek, M.R. Pseudomonas Aeruginosa Aggregates in Cystic Fibrosis Sputum Produce Exopolysaccharides That Likely Impede Current Therapies. Cell Rep. 2021, 34, 108782. [Google Scholar] [CrossRef]
- Wozniak, D.J.; Wyckoff, T.J.O.; Starkey, M.; Keyser, R.; Azadi, P.; O’Toole, G.A.; Parsek, M.R. Alginate Is Not a Significant Component of the Extracellular Polysaccharide Matrix of PA14 and PAO1 Pseudomonas Aeruginosa Biofilms. Proc. Natl. Acad. Sci. USA 2003, 100, 7907–7912. [Google Scholar] [CrossRef] [PubMed]
- Jackson, K.D.; Starkey, M.; Kremer, S.; Parsek, M.R.; Wozniak, D.J. Identification of Psl, a Locus Encoding a Potential Exopolysaccharide That Is Essential for Pseudomonas Aeruginosa PAO1 Biofilm Formation. J. Bacteriol. 2004, 186, 4466–4475. [Google Scholar] [CrossRef] [PubMed]
- Matsukawa, M.; Greenberg, E.P. Putative Exopolysaccharide Synthesis Genes Influence Pseudomonas Aeruginosa Biofilm Development. J. Bacteriol. 2004, 186, 4449–4456. [Google Scholar] [CrossRef] [PubMed]
- Byrd, M.S.; Sadovskaya, I.; Vinogradov, E.; Lu, H.; Sprinkle, A.B.; Richardson, S.H.; Ma, L.; Ralston, B.; Parsek, M.R.; Anderson, E.M.; et al. Genetic and Biochemical Analyses of the Pseudomonas Aeruginosa Psl Exopolysaccharide Reveal Overlapping Roles for Polysaccharide Synthesis Enzymes in Psl and LPS Production. Mol. Microbiol. 2009, 73, 622–638. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Wang, S.; Wang, D.; Parsek, M.R.; Wozniak, D.J. The Roles of Biofilm Matrix Polysaccharide Psl in Mucoid Pseudomonas Aeruginosa Biofilms. FEMS Immunol. Med. Microbiol. 2012, 65, 377–380. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.J.; Wozniak, D.J. Psl Produced by Mucoid Pseudomonas Aeruginosa Contributes to the Establishment of Biofilms and Immune Evasion. mBio 2017, 8, e00864-17. [Google Scholar] [CrossRef]
- Wang, S.; Parsek, M.R.; Wozniak, D.J.; Ma, L.Z. A Spider Web Strategy of Type IV Pili-Mediated Migration to Build a Fibre-like Psl Polysaccharide Matrix in Pseudomonas Aeruginosa Biofilms. Environ. Microbiol. 2013, 15, 2238–2253. [Google Scholar] [CrossRef]
- Ma, L.; Conover, M.; Lu, H.; Parsek, M.R.; Bayles, K.; Wozniak, D.J. Assembly and Development of the Pseudomonas Aeruginosa Biofilm Matrix. PLoS Pathog. 2009, 5, e1000354. [Google Scholar] [CrossRef]
- Staudinger, B.J.; Muller, J.F.; Halldórsson, S.; Boles, B.; Angermeyer, A.; Nguyen, D.; Rosen, H.; Baldursson, Ó.; Gottfreðsson, M.; Guðmundsson, G.H.; et al. Conditions Associated with the Cystic Fibrosis Defect Promote Chronic Pseudomonas Aeruginosa Infection. Am. J. Respir. Crit. Care Med. 2014, 189, 812–824. [Google Scholar] [CrossRef]
- Irie, Y.; Borlee, B.R.; O’Connor, J.R.; Hill, P.J.; Harwood, C.S.; Wozniak, D.J.; Parsek, M.R. Self-Produced Exopolysaccharide Is a Signal That Stimulates Biofilm Formation in Pseudomonas Aeruginosa. Proc. Natl. Acad. Sci. USA 2012, 109, 20632–20636. [Google Scholar] [CrossRef]
- Ma, L.; Jackson, K.D.; Landry, R.M.; Parsek, M.R.; Wozniak, D.J. Analysis of Pseudomonas Aeruginosa Conditional Psl Variants Reveals Roles for the Psl Polysaccharide in Adhesion and Maintaining Biofilm Structure Postattachment. J. Bacteriol. 2006, 188, 8213–8221. [Google Scholar] [CrossRef] [PubMed]
- Borlee, B.R.; Goldman, A.D.; Murakami, K.; Samudrala, R.; Wozniak, D.J.; Parsek, M.R. Pseudomonas Aeruginosa Uses a Cyclic-Di-GMP-Regulated Adhesin to Reinforce the Biofilm Extracellular Matrix. Mol. Microbiol. 2010, 75, 827–842. [Google Scholar] [CrossRef] [PubMed]
- Billings, N.; Ramirez Millan, M.; Caldara, M.; Rusconi, R.; Tarasova, Y.; Stocker, R.; Ribbeck, K. The Extracellular Matrix Component Psl Provides Fast-Acting Antibiotic Defense in Pseudomonas Aeruginosa Biofilms. PLoS Pathog. 2013, 9, e1003526. [Google Scholar] [CrossRef] [PubMed]
- Mishra, M.; Byrd, M.S.; Sergeant, S.; Azad, A.K.; Parsek, M.R.; McPhail, L.; Schlesinger, L.S.; Wozniak, D.J. Pseudomonas Aeruginosa Psl Polysaccharide Reduces Neutrophil Phagocytosis and the Oxidative Response by Limiting Complement-Mediated Opsonization. Cell. Microbiol. 2012, 14, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Le Mauff, F.; Razvi, E.; Reichhardt, C.; Sivarajah, P.; Parsek, M.R.; Howell, P.L.; Sheppard, D.C. The Pel Polysaccharide Is Predominantly Composed of a Dimeric Repeat of α-1,4 Linked Galactosamine and N-Acetylgalactosamine. Commun. Biol. 2022, 5, 502. [Google Scholar] [CrossRef] [PubMed]
- Colvin, K.M.; Alnabelseya, N.; Baker, P.; Whitney, J.C.; Howell, P.L.; Parsek, M.R. PelA Deacetylase Activity Is Required for Pel Polysaccharide Synthesis in Pseudomonas Aeruginosa. J. Bacteriol. 2013, 195, 2329–2339. [Google Scholar] [CrossRef]
- Jennings, L.K.; Storek, K.M.; Ledvina, H.E.; Coulon, C.; Marmont, L.S.; Sadovskaya, I.; Secor, P.R.; Tseng, B.S.; Scian, M.; Filloux, A.; et al. Pel Is a Cationic Exopolysaccharide That Cross-Links Extracellular DNA in the Pseudomonas Aeruginosa Biofilm Matrix. Proc. Natl. Acad. Sci. USA 2015, 112, 11353–11358. [Google Scholar] [CrossRef]
- Friedman, L.; Kolter, R. Genes Involved in Matrix Formation in Pseudomonas Aeruginosa PA14 Biofilms. Mol. Microbiol. 2004, 51, 675–690. [Google Scholar] [CrossRef]
- Colvin, K.M.; Irie, Y.; Tart, C.S.; Urbano, R.; Whitney, J.C.; Ryder, C.; Howell, P.L.; Wozniak, D.J.; Parsek, M.R. The Pel and Psl Polysaccharides Provide Pseudomonas Aeruginosa Structural Redundancy within the Biofilm Matrix. Environ. Microbiol. 2012, 14, 1913–1928. [Google Scholar] [CrossRef]
- Yang, L.; Hu, Y.; Liu, Y.; Zhang, J.; Ulstrup, J.; Molin, S. Distinct Roles of Extracellular Polymeric Substances in Pseudomonas Aeruginosa Biofilm Development. Environ. Microbiol. 2011, 13, 1705–1717. [Google Scholar] [CrossRef]
- Baker, P.; Hill, P.J.; Snarr, B.D.; Alnabelseya, N.; Pestrak, M.J.; Lee, M.J.; Jennings, L.K.; Tam, J.; Melnyk, R.A.; Parsek, M.R.; et al. Exopolysaccharide Biosynthetic Glycoside Hydrolases Can Be Utilized to Disrupt and Prevent Pseudomonas Aeruginosa Biofilms. Sci. Adv. 2016, 2, e1501632. [Google Scholar] [CrossRef]
- Maunders, E.; Welch, M. Matrix Exopolysaccharides; the Sticky Side of Biofilm Formation. FEMS Microbiol. Lett. 2017, 364, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Fleming, D.; Niese, B.; Redman, W.; Vanderpool, E.; Gordon, V.; Rumbaugh, K.P. Contribution of Pseudomonas Aeruginosa Exopolysaccharides Pel and Psl to Wound Infections. Front. Cell. Infect. Microbiol. 2022, 12, 323. [Google Scholar] [CrossRef] [PubMed]
- Doggett, R.G. Incidence of Mucoid Pseudomonas Aeruginosa from Clinical Sources. Appl. Microbiol. 1969, 18, 936–937. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, S.S.; Hoiby, N.; Espersen, F.; Koch, C. Role of Alginate in Infection with Mucoid Pseudomonas Aeruginosa in Cystic Fibrosis. Thorax 1992, 47, 6–13. [Google Scholar] [CrossRef]
- Ciofu, O.; Tolker-Nielsen, T.; Jensen, P.Ø.; Wang, H.; Høiby, N. Antimicrobial Resistance, Respiratory Tract Infections and Role of Biofilms in Lung Infections in Cystic Fibrosis Patients. Adv. Drug Deliv. Rev. 2015, 85, 7–23. [Google Scholar] [CrossRef] [PubMed]
- Folkesson, A.; Jelsbak, L.; Yang, L.; Johansen, H.K.; Ciofu, O.; Høiby, N.; Molin, S. Adaptation of Pseudomonas Aeruginosa to the Cystic Fibrosis Airway: An Evolutionary Perspective. Nat. Rev. Microbiol. 2012, 10, 841–851. [Google Scholar] [CrossRef]
- Evans, L.R.; Linker, A. Production and Characterization of the Slime Polysaccharide of Pseudomonas Aeruginosa. J. Bacteriol. 1973, 116, 915–924. [Google Scholar] [CrossRef]
- Sherbrock-Cox, V.; Russell, N.J.; Gacesa, P. The Purification and Chemical Characterisation of the Alginate Present in Extracellular Material Produced by Mucoid Strains of Pseudomonas Aeruginosa. Carbohydr. Res. 1984, 135, 147–154. [Google Scholar] [CrossRef]
- Russell, N.J.; Gacesa, P. Chemistry and Biology of the Alginate of Mucoid Strains of Pseudomonas Aeruginosa in Cystic Fibrosis. Mol. Aspects Med. 1988, 10, 1–91. [Google Scholar] [CrossRef]
- Gloag, E.S.; German, G.K.; Stoodley, P.; Wozniak, D.J. Viscoelastic Properties of Pseudomonas Aeruginosa Variant Biofilms. Sci. Rep. 2018, 8, 9691. [Google Scholar] [CrossRef] [PubMed]
- Rehm, B.H.A.; Valla, S. Bacterial Alginates: Biosynthesis and Applications. Appl. Microbiol. Biotechnol. 1997, 48, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Pier, G.B.; Coleman, F.; Grout, M.; Franklin, M.; Ohman, D.E. Role of Alginate O-Acetylation in Resistance of Mucoid Pseudomonas Aeruginosa to Opsonic Phagocytosis. Infect. Immun. 2001, 69, 1895–1901. [Google Scholar] [CrossRef] [PubMed]
- Nivens, D.E.; Ohman, D.E.; Williams, J.; Franklin, M.J. Role of Alginate and Its O-Acetylation in Formation of Pseudomonas Aeruginosa Microcolonies and Biofilms. J. Bacteriol. 2001, 183, 1047–1057. [Google Scholar] [CrossRef] [PubMed]
- Hay, I.D.; Gatland, K.; Campisano, A.; Jordens, J.Z.; Rehm, B.H.A. Impact of Alginate Overproduction on Attachment and Biofilm Architecture of a Supermucoid Pseudomonas Aeruginosa Strain. Appl. Environ. Microbiol. 2009, 75, 6022–6025. [Google Scholar] [CrossRef] [PubMed]
- Tielen, P.; Strathmann, M.; Jaeger, K.E.; Flemming, H.C.; Wingender, J. Alginate Acetylation Influences Initial Surface Colonization by Mucoid Pseudomonas Aeruginosa. Microbiol. Res. 2005, 160, 165–176. [Google Scholar] [CrossRef]
- Skjåk-Bræk, G.; Zanetti, F.; Paoletti, S. Effect of Acetylation on Some Solution and Gelling Properties of Alginates. Carbohydr. Res. 1989, 185, 131–138. [Google Scholar] [CrossRef]
- Windhues, T.; Borchard, W. Effect of Acetylation on Physico-Chemical Properties of Bacterial and Algal Alginates in Physiological Sodium Chloride Solutions Investigated with Light Scattering Techniques. Carbohydr. Polym. 2003, 52, 47–52. [Google Scholar] [CrossRef]
- Govan, J.R.; Deretic, V. Microbial Pathogenesis in Cystic Fibrosis: Mucoid Pseudomonas Aeruginosa and Burkholderia Cepacia. Microbiol. Rev. 1996, 60, 539–574. [Google Scholar] [CrossRef]
- Hentzer, M.; Teitzel, G.M.; Balzer, G.J.; Heydorn, A.; Molin, S.; Givskov, M.; Parsek, M.R. Alginate Overproduction Affects Pseudomonas Aeruginosa Biofilm Structure and Function. J. Bacteriol. 2001, 183, 5395–5401. [Google Scholar] [CrossRef]
- Costerton, J.W.; Cheng, K.J.; Geesey, G.G.; Ladd, T.I.; Nickel, J.C.; Dasgupta, M.; Marrie, T.J. Bacterial Biofilms in Nature and Disease. Annu. Rev. Microbiol. 1987, 41, 435–464. [Google Scholar] [CrossRef]
- Ramphal, R.; Pier, G.B. Role of Pseudomonas Aeruginosa Mucoid Exopolysaccharide in Adherence to Tracheal Cells. Infect. Immun. 1985, 47, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Hodges, N.A.; Gordon, C.A. Protection of Pseudomonas Aeruginosa against Ciprofloxacin and Beta-Lactams by Homologous Alginate. Antimicrob. Agents Chemother. 1991, 35, 2450–2452. [Google Scholar] [CrossRef]
- Learn, D.B.; Brestel, E.P.; Seetharama, S. Hypochlorite Scavenging by Pseudomonas Aeruginosa Alginate. Infect. Immun. 1987, 55, 1813–1818. [Google Scholar] [CrossRef] [PubMed]
- Pezzoni, M.; Lemos, M.; Pizarro, R.A.; Costa, C.S. UVA as Environmental Signal for Alginate Production in Pseudomonas Aeruginosa: Role of This Polysaccharide in the Protection of Planktonic Cells and Biofilms against Lethal UVA Doses. Photochem. Photobiol. Sci. 2022, 21, 1459–1472. [Google Scholar] [CrossRef] [PubMed]
- Simpson, J.A.; Smith, S.E.; Dean, R.T. Scavenging by Alginate of Free Radicals Released by Macrophages. Free Radic. Biol. Med. 1989, 6, 347–353. [Google Scholar] [CrossRef]
- Pier, G.B. Pseudomonas Aeruginosa: A Key Problem in Cystic Fibrosis. ASM News 1998, 6, 339–347. [Google Scholar]
- Del Bino, L.; Romano, M.R. Role of Carbohydrate Antigens in Antifungal Glycoconjugate Vaccines and Immunotherapy. Drug Discov. Today Technol. 2020, 38, 45–55. [Google Scholar] [CrossRef]
- Beauvais, A.; Schmidt, C.; Guadagnini, S.; Roux, P.; Perret, E.; Henry, C.; Paris, S.; Mallet, A.; Prévost, M.C.; Latgé, J.P. An Extracellular Matrix Glues Together the Aerial-Grown Hyphae of Aspergillus Fumigatus. Cell. Microbiol. 2007, 9, 1588–1600. [Google Scholar] [CrossRef]
- Loussert, C.; Schmitt, C.; Prevost, M.-C.; Balloy, V.; Fadel, E.; Philippe, B.; Kauffmann-Lacroix, C.; Latgé, J.P.; Beauvais, A. In Vivo Biofilm Composition of Aspergillus Fumigatus. Cell. Microbiol. 2010, 12, 405–410. [Google Scholar] [CrossRef]
- Gravelat, F.N.; Beauvais, A.; Liu, H.; Lee, M.J.; Snarr, B.D.; Chen, D.; Xu, W.; Kravtsov, I.; Hoareau, C.M.Q.; Vanier, G.; et al. Aspergillus Galactosaminogalactan Mediates Adherence to Host Constituents and Conceals Hyphal β-Glucan from the Immune System. PLoS Pathog. 2013, 9, e1003575. [Google Scholar] [CrossRef] [PubMed]
- Ramage, G.; Rajendran, R.; Sherry, L.; Williams, C. Fungal Biofilm Resistance. Int. J. Microbiol. 2012, 2012, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Seidler, M.J.; Salvenmoser, S.; Müller, F.M.C. Aspergillus Fumigatus Forms Biofilms with Reduced Antifungal Drug Susceptibility on Bronchial Epithelial Cells. Antimicrob. Agents Chemother. 2008, 52, 4130–4136. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Geller, A.M.; Bamford, N.C.; Liu, H.; Gravelat, F.N.; Snarr, B.D.; Le Mauff, F.; Chabot, J.; Ralph, B.; Ostapska, H.; et al. Deacetylation of Fungal Exopolysaccharide Mediates Adhesion and Biofilm Formation. mBio 2016, 7, e00252-16. [Google Scholar] [CrossRef]
- Gresnigt, M.S.; Bozza, S.; Becker, K.L.; Joosten, L.A.B.; Abdollahi-Roodsaz, S.; van der Berg, W.B.; Dinarello, C.A.; Netea, M.G.; Fontaine, T.; De Luca, A.; et al. A Polysaccharide Virulence Factor from Aspergillus Fumigatus Elicits Anti-Inflammatory Effects through Induction of Interleukin-1 Receptor Antagonist. PLoS Pathog. 2014, 10, e1003936. [Google Scholar] [CrossRef]
- Robinet, P.; Baychelier, F.; Fontaine, T.; Picard, C.; Debré, P.; Vieillard, V.; Latgé, J.-P.; Elbim, C. A Polysaccharide Virulence Factor of a Human Fungal Pathogen Induces Neutrophil Apoptosis via NK Cells. J. Immunol. 2014, 192, 5332–5342. [Google Scholar] [CrossRef]
- Zhang, Y.; Gómez-Redondo, M.; Jiménez-Osés, G.; Arda, A.; Overkleeft, H.S.; van der Marel, G.A.; Jiménez-Barbero, J.; Codée, J.D.C. Synthesis and Structural Analysis of Aspergillus Fumigatus Galactosaminogalactans. Angew. Chemie—Int. Ed. 2020, 59, 12746–12750. [Google Scholar] [CrossRef]
- Beauvais, A.; Latgé, J. Aspergillus Biofilm In Vitro and In Vivo. Microbiol. Spectr. 2015, 3. [Google Scholar] [CrossRef]
- Silva, A.J.; Benitez, J.A. Vibrio Cholerae Biofilms and Cholera Pathogenesis. PLoS Negl. Trop. Dis. 2016, 10, e0004330. [Google Scholar] [CrossRef]
- Yildiz, F.; Fong, J.; Sadovskaya, I.; Grard, T.; Vinogradov, E. Structural Characterization of the Extracellular Polysaccharide from Vibrio Cholerae O1 El-Tor. PLoS ONE 2014, 9, e86751. [Google Scholar] [CrossRef]
- Fong, J.C.N.; Rogers, A.; Michael, A.K.; Parsley, N.C.; Cornell, W.-C.; Lin, Y.-C.; Singh, P.K.; Hartmann, R.; Drescher, K.; Vinogradov, E.; et al. Structural Dynamics of RbmA Governs Plasticity of Vibrio Cholerae Biofilms. eLife 2017, 6, e26163. [Google Scholar] [CrossRef] [PubMed]
- Kanampalliwar, A.; Singh, D.V. Extracellular DNA Builds and Interacts with Vibrio Polysaccharide in the Biofilm Matrix Formed by Vibrio Cholerae. Environ. Microbiol. Rep. 2020, 12, 594–606. [Google Scholar] [CrossRef] [PubMed]
- Krzyściak, W.; Jurczak, A.; Kościelniak, D.; Bystrowska, B.; Skalniak, A. The Virulence of Streptococcus Mutans and the Ability to Form Biofilms. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 499–515. [Google Scholar] [CrossRef] [PubMed]
- Rozen, R.; Bachrach, G.; Bronshteyn, M.; Gedalia, I.; Steinberg, D. The Role of Fructans on Dental Biofilm Formation by Streptococcus Sobrinus, Streptococcus Mutans, Streptococcus Gordonii and Actinomyces Viscosus. FEMS Microbiol. Lett. 2001, 195, 205–210. [Google Scholar] [CrossRef]
- Sharma, G.; Sharma, S.; Sharma, P.; Chandola, D.; Dang, S.; Gupta, S.; Gabrani, R. Escherichia Coli Biofilm: Development and Therapeutic Strategies. J. Appl. Microbiol. 2016, 121, 309–319. [Google Scholar] [CrossRef]
- Pando, J.M.; Karlinsey, J.E.; Lara, J.C.; Libby, S.J.; Fanga, F.C. The Rcs-Regulated Colanic Acid Capsule Maintains Membrane Potential in Salmonella Enterica Serovar Typhimurium. MBio 2017, 8, e00808-17. [Google Scholar] [CrossRef]
- Hanna, A.; Berg, M.; Stout, V.; Razatos, A. Role of Capsular Colanic Acid in Adhesion of Uropathogenic Escherichia Coli. Appl. Environ. Microbiol. 2003, 69, 4474–4481. [Google Scholar] [CrossRef]
- Danese, P.N.; Pratt, L.A.; Kolter, R. Exopolysaccharide Production Is Required for Development of Escherichia Coli K-12 Biofilm Architecture. J. Bacteriol. 2000, 182, 3593–3596. [Google Scholar] [CrossRef]
- McNamara, J.T.; Morgan, J.L.W.; Zimmer, J. A Molecular Description of Cellulose Biosynthesis. Annu. Rev. Biochem. 2015, 84, 895–921. [Google Scholar] [CrossRef]
- Sande, C.; Whitfield, C. Capsules and Extracellular Polysaccharides in Escherichia Coli and Salmonella. EcoSal Plus 2021, 9, eESP00332020. [Google Scholar] [CrossRef]
- Solomon, E.B.; Niemira, B.A.; Sapers, G.M.; Annous, B.A. Biofilm Formation, Cellulose Production, and Curli Biosynthesis by Salmonella Originating from Produce, Animal, and Clinical Sources†. J. Food Prot. 2005, 68, 906–912. [Google Scholar] [CrossRef] [PubMed]
- Thongsomboon, W.; Serra, D.O.; Possling, A.; Hadjineophytou, C.; Hengge, R.; Cegelski, L. Phosphoethanolamine Cellulose: A Naturally Produced Chemically Modified Cellulose. Science 2018, 359, 334–338. [Google Scholar] [CrossRef] [PubMed]
- Neuhaus, F.C.; Baddiley, J. A Continuum of Anionic Charge: Structures and Functions of d-Alanyl-Teichoic Acids in Gram-Positive Bacteria. Microbiol. Mol. Biol. Rev. 2003, 67, 686–723. [Google Scholar] [CrossRef] [PubMed]
- Endl, J.; Seidl, H.P.; Fiedler, F.; Schleider, K.H. Chemical Composition and Structure of Cell Wall Teichoic Acids of Staphylococci. Arch. Microbiol. 1983, 135, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Ronald Archibald, A.; Baddiley, J.; Heptinstall, S. The Alanine Ester Content and Magnesium Binding Capacity of Walls of Staphylococcus Aureus H Grown at Different PH Values. BBA—Biomembr. 1973, 291, 629–634. [Google Scholar] [CrossRef]
- Vergara-irigaray, M.; Merino, N.; Pier, G.B.; Women, B.; Medical, H. Exopolysaccharide to the Staphylococcus Aureus Cell Surface. Microbiology 2009, 154, 865–877. [Google Scholar] [CrossRef]
- Gross, M.; Cramton, S.E.; Götz, F.; Peschel, A. Key Role of Teichoic Acid Net Charge in Staphylococcus Aureus Colonization of Artificial Surfaces. Infect. Immun. 2001, 69, 3423–3426. [Google Scholar] [CrossRef]
- Crossman, L.; Dow, J.M. Biofilm Formation and Dispersal in Xanthomonas Campestris. Microbes Infect. 2004, 6, 623–629. [Google Scholar] [CrossRef]
- Bianco, M.I.; Toum, L.; Yaryura, P.M.; Mielnichuk, N.; Gudesblat, G.E.; Roeschlin, R.; Marano, M.R.; Ielpi, L.; Vojnov, A.A. Xanthan Pyruvilation Is Essential for the Virulence of Xanthomonas Campestris Pv. Campestris. Mol. Plant-Microbe Interact. 2016, 29, 688–699. [Google Scholar] [CrossRef]
- Taff, H.T.; Nett, J.E.; Zarnowski, R.; Ross, K.M.; Sanchez, H.; Cain, M.T.; Hamaker, J.; Mitchell, A.P.; Andes, D.R. A Candida Biofilm-Induced Pathway for Matrix Glucan Delivery: Implications for Drug Resistance. PLoS Pathog. 2012, 8, e1002848. [Google Scholar] [CrossRef]
- Kennedy, J.F.; Palva, P.M.G.; Corella, M.T.S.; Cavalcanti, M.S.M.; Coelho, L.C.B.B. Lectins, Versatile Proteins of Recognition: A Review. Carbohydr. Polym. 1995, 26, 219–230. [Google Scholar] [CrossRef]
- Cheah, Y.T.; Chan, D.J.C. A Methodological Review on the Characterization of Microalgal Biofilm and Its Extracellular Polymeric Substances. J. Appl. Microbiol. 2022, 132, 3490–3514. [Google Scholar] [CrossRef] [PubMed]
- Randrianjatovo-Gbalou, I.; Girbal-Neuhauser, E.; Marcato-Romain, C.-E. Quantification of Biofilm Exopolysaccharides Using an in Situ Assay with Periodic Acid–Schiff Reagent. Anal. Biochem. 2016, 500, 12–14. [Google Scholar] [CrossRef] [PubMed]
- Barranguet, C.; van Beusekom, S.; Veuger, B.; Neu, T.; Manders, E.; Sinke, J.; Admiraal, W. Studying Undisturbed Autotrophic Biofilms: Still a Technical Challenge. Aquat. Microb. Ecol. 2004, 34, 1–9. [Google Scholar] [CrossRef]
- Thomas, V.L.; Sanford, B.A.; Moreno, R.; Ramsay, M.A. Enzyme-Linked Lectinsorbent Assay Measures N -Acetyl-D-Glucosamine in Matrix of Biofilm Produced by Staphylococcus Epidermidis. Curr. Microbiol. 1997, 35, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Leriche, V.; Sibille, P.; Carpentier, B. Use of an Enzyme-Linked Lectinsorbent Assay To Monitor the Shift in Polysaccharide Composition in Bacterial Biofilms. Appl. Environ. Microbiol. 2000, 66, 1851–1856. [Google Scholar] [CrossRef]
- Strathmann, M.; Wingender, J.; Flemming, H.C. Application of Fluorescently Labelled Lectins for the Visualization and Biochemical Characterization of Polysaccharides in Biofilms of Pseudomonas Aeruginosa. J. Microbiol. Methods 2002, 50, 237–248. [Google Scholar] [CrossRef]
- Eddenden, A.; Nitz, M. Applications of an Inactive Dispersin B Probe to Monitor Biofilm Polysaccharide Production. In Methods in Enzymology; Elsevier Inc.: Amsterdam, The Netherlands, 2022; Volume 665, pp. 209–231. ISBN 9780323952675. [Google Scholar]
- Lembre, P.; Lorentz, C.; Di, P. Exopolysaccharides of the Biofilm Matrix: A Complex Biophysical World. In The Complex World of Polysaccharides; InTech: London, UK, 2012. [Google Scholar]
- Ruas-Madiedo, P.; Tuinier, R.; Kanning, M.; Zoon, P. Role of Exopolysaccharides Produced by Lactococcus Lactis Subsp. Cremoris on the Viscosity of Fermented Milks. Int. Dairy J. 2002, 12, 689–695. [Google Scholar] [CrossRef]
- Song, Y.; Ma, F.; Sun, M.; Mu, G.; Tuo, Y. The Chemical Structure Properties and Promoting Biofilm Activity of Exopolysaccharide Produced by Shigella Flexneri. Front. Microbiol. 2022, 12, 4322. [Google Scholar] [CrossRef]
- Ciucanu, I.; Kerek, F. A Simple and Rapid Method for the Permethylation of Carbohydrates. Carbohydr. Res. 1984, 131, 209–217. [Google Scholar] [CrossRef]
- Bales, P.M.; Renke, E.M.; May, S.L.; Shen, Y.; Nelson, D.C. Purification and Characterization of Biofilm-Associated EPS Exopolysaccharides from ESKAPE Organisms and Other Pathogens. PLoS ONE 2013, 8, e67950. [Google Scholar] [CrossRef]
- Faber, E.J.; Van Den Haak, M.J.; Kamerling, J.P.; Vliegenthart, J.F.G. Structure of the Exopolysaccharide Produced by Streptococcus Thermophilus S3. Carbohydr. Res. 2001, 331, 173–182. [Google Scholar] [CrossRef]
- Reichhardt, C.; Cegelski, L. Solid-State NMR for Bacterial Biofilms. Mol. Phys. 2014, 112, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Cegelski, L. Bottom-up and Top-down Solid-State NMR Approaches for Bacterial Biofilm Matrix Composition. J. Magn. Reson. 2015, 253, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.H.; Yu, H.L.; Ba, Z.Y.; Chen, J.Y.; Sun, H.G.; Han, B.Z. Sampling Methods for NMR-Based Metabolomics of Staphylococcus Aureus. Biotechnol. J. 2010, 5, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Rabinowitz, J.D. Cellular Metabolomics of Escherchia Coli. Expert Rev. Proteom. 2007, 4, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Reichhardt, C.; Joubert, L.M.; Clemons, K.V.; Stevens, D.A.; Cegelski, L. Integration of Electron Microscopy and Solid-State NMR Analysis for New Views and Compositional Parameters of Aspergillus Fumigatus Biofilms. Med. Mycol. 2019, 57, S239–S244. [Google Scholar] [CrossRef]
- Morris, D.L. Quantitative Determination of Carbohydrates With Dreywood’s Anthrone Reagent. Science 1948, 107, 254–255. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.; Hamilton, J.K.; Rebers, P.A.; Smith, F. A Colorimetric Method for the Determination of Sugars. Nature 1951, 168, 167. [Google Scholar] [CrossRef]
- Monsigny, M.; Petit, C.; Roche, A.-C. Colorimetric Determination of Neutral Sugars by a Resorcinol Sulfuric Acid Micromethod. Anal. Biochem. 1988, 175, 525–530. [Google Scholar] [CrossRef]
- Reissig, J.L.; Storminger, J.L.; Leloir, L.F. A Modified Colorimetric Method for the Estimation of N-Acetylamino Sugars. J. Biol. Chem. 1955, 217, 959–966. [Google Scholar] [CrossRef]
- Park, J.T.; Johnson, M.J. A Submicrodetermination of Glucose. J. Biol. Chem. 1949, 181, 149–151. [Google Scholar] [CrossRef]
- Kilcoyne, M.; Gerlach, J.Q.; Farrell, M.P.; Bhavanandan, V.P.; Joshi, L. Periodic Acid-Schiff’s Reagent Assay for Carbohydrates in a Microtiter Plate Format. Anal. Biochem. 2011, 416, 18–26. [Google Scholar] [CrossRef]
- Van den Berg, D.J.C.; Robijn, G.W.; Janssen, A.C.; Giuseppin, M.L.F.; Vreeker, R.; Kamerling, J.P.; Vliegenthart, J.F.G.; Ledeboer, A.M.; Verrips, C.T. Production of a Novel Extracellular Polysaccharide by Lactobacillus Sake 0-1 and Characterization of the Polysaccharide. Appl. Environ. Microbiol. 1995, 61, 2840–2844. [Google Scholar] [CrossRef]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Ruijssenaars, H.J.; Stingele, F.; Hartmans, S. Biodegradability of Food-Associated Extracellular Polysaccharides. Curr. Microbiol. 2000, 40, 194–199. [Google Scholar] [CrossRef]
- Masuko, T.; Minami, A.; Iwasaki, N.; Majima, T.; Nishimura, S.I.; Lee, Y.C. Carbohydrate Analysis by a Phenol-Sulfuric Acid Method in Microplate Format. Anal. Biochem. 2005, 339, 69–72. [Google Scholar] [CrossRef]
- Mantle, M.; Allen, A. A Colorimetric Assay for Glycoproteins Based on the Periodic Acid/Schiff Stain. Biochem. Soc. Trans. 1978, 6, 607–609. [Google Scholar] [CrossRef]
- Polasko, A.L.; Ramos, P.; Kaner, R.B.; Mahendra, S. A Multipronged Approach for Systematic in Vitro Quantification of Catheter-Associated Biofilms. J. Hazard. Mater. Lett. 2021, 2, 100032. [Google Scholar] [CrossRef]
- Fulaz, S.; Vitale, S.; Quinn, L.; Casey, E. Nanoparticle–Biofilm Interactions: The Role of the EPS Matrix. Trends Microbiol. 2019, 27, 915–926. [Google Scholar] [CrossRef]
- Flemming, H.-C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An Emergent Form of Bacterial Life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar] [CrossRef]
- Chaignon, P.; Sadovskaya, I.; Ragunah, C.; Ramasubbu, N.; Kaplan, J.B.; Jabbouri, S. Susceptibility of Staphylococcal Biofilms to Enzymatic Treatments Depends on Their Chemical Composition. Appl. Microbiol. Biotechnol. 2007, 75, 125–132. [Google Scholar] [CrossRef]
- Dobrynina, O.Y.; Bolshakova, T.N.; Umyarov, A.M.; Boksha, I.S.; Lavrova, N.V.; Grishin, A.V.; Lyashchuk, A.M.; Galushkina, Z.M.; Avetisian, L.R.; Chernukha, M.Y.; et al. Disruption of Bacterial Biofilms Using Recombinant Dispersin B. Microbiology 2015, 84, 498–501. [Google Scholar] [CrossRef]
- Lee, E.; Kim, D.-H.; Woo, Y.; Hur, H.-G.; Lim, Y. Solution Structure of Peptide AG4 Used to Form Silver Nanoparticles. Biochem. Biophys. Res. Commun. 2008, 376, 595–598. [Google Scholar] [CrossRef]
- Chen, K.-J.; Lee, C.-K. Twofold Enhanced Dispersin B Activity by N-Terminal Fusion to Silver-Binding Peptide for Biofilm Eradication. Int. J. Biol. Macromol. 2018, 118, 419–426. [Google Scholar] [CrossRef]
- Tan, Y.; Ma, S.; Liu, C.; Yu, W.; Han, F. Enhancing the Stability and Antibiofilm Activity of DspB by Immobilization on Carboxymethyl Chitosan Nanoparticles. Microbiol. Res. 2015, 178, 35–41. [Google Scholar] [CrossRef]
- Bayer, A.S.; Park, S.; Ramos, M.C.; Nast, C.C.; Eftekhar, F.; Schiller, N.L. Effects of Alginase on the Natural History and Antibiotic Therapy of Experimental Endocarditis Caused by Mucoid Pseudomonas Aeruginosa. Infect. Immun. 1992, 60, 3979–3985. [Google Scholar] [CrossRef]
- Alkawash, M.A.; Soothill, J.S.; Schiller, N.L. Alginate Lyase Enhances Antibiotic Killing of Mucoid Pseudomonas Aeruginosa in Biofilms. APMIS 2006, 114, 131–138. [Google Scholar] [CrossRef]
- Alipour, M.; Suntres, Z.E.; Omri, A. Importance of DNase and Alginate Lyase for Enhancing Free and Liposome Encapsulated Aminoglycoside Activity against Pseudomonas Aeruginosa. J. Antimicrob. Chemother. 2009, 64, 317–325. [Google Scholar] [CrossRef]
- Lamppa, J.W.; Griswold, K.E. Alginate Lyase Exhibits Catalysis-Independent Biofilm Dispersion and Antibiotic Synergy. Antimicrob. Agents Chemother. 2013, 57, 137–145. [Google Scholar] [CrossRef]
- Patel, K.K.; Tripathi, M.; Pandey, N.; Agrawal, A.K.; Gade, S.; Anjum, M.M.; Tilak, R.; Singh, S. Alginate Lyase Immobilized Chitosan Nanoparticles of Ciprofloxacin for the Improved Antimicrobial Activity against the Biofilm Associated Mucoid P. Aeruginosa Infection in Cystic Fibrosis. Int. J. Pharm. 2019, 563, 30–42. [Google Scholar] [CrossRef]
- Wan, B.; Zhu, Y.; Tao, J.; Zhu, F.; Chen, J.; Li, L.; Zhao, J.; Wang, L.; Sun, S.; Yang, Y.; et al. Alginate Lyase Guided Silver Nanocomposites for Eradicating Pseudomonas Aeruginosa from Lungs. ACS Appl. Mater. Interfaces 2020, 12, 9050–9061. [Google Scholar] [CrossRef]
- Pestrak, M.J.; Baker, P.; Dellos-Nolan, S.; Hill, P.J.; Passos da Silva, D.; Silver, H.; Lacdao, I.; Raju, D.; Parsek, M.R.; Wozniak, D.J.; et al. Treatment with the Pseudomonas Aeruginosa Glycoside Hydrolase PslG Combats Wound Infection by Improving Antibiotic Efficacy and Host Innate Immune Activity. Antimicrob. Agents Chemother. 2019, 63, e00234-19. [Google Scholar] [CrossRef]
- Szymańska, M.; Karakulska, J.; Sobolewski, P.; Kowalska, U.; Grygorcewicz, B.; Böttcher, D.; Bornscheuer, U.T.; Drozd, R. Glycoside Hydrolase (PelAh) Immobilization Prevents Pseudomonas Aeruginosa Biofilm Formation on Cellulose-Based Wound Dressing. Carbohydr. Polym. 2020, 246, 116625. [Google Scholar] [CrossRef]
- Jiang, Y.; Geng, M.; Bai, L. Targeting Biofilms Therapy: Current Research Strategies and Development Hurdles. Microorganisms 2020, 8, 1222. [Google Scholar] [CrossRef]
- Fazekas, E.; Kandra, L.; Gyémánt, G. Model for β-1,6-N-Acetylglucosamine Oligomer Hydrolysis Catalysed by DispersinB, a Biofilm Degrading Enzyme. Carbohydr. Res. 2012, 363, 7–13. [Google Scholar] [CrossRef]
- Fleming, D.; Chahin, L.; Rumbaugh, K. Glycoside Hydrolases Degrade Polymicrobial Bacterial Biofilms in Wounds. Antimicrob. Agents Chemother. 2017, 61, e01998-16. [Google Scholar] [CrossRef]
- Banar, M.; Emaneini, M.; Satarzadeh, M.; Abdellahi, N.; Beigverdi, R.; van Leeuwen, W.B.; Jabalameli, F. Evaluation of Mannosidase and Trypsin Enzymes Effects on Biofilm Production of Pseudomonas Aeruginosa Isolated from Burn Wound Infections. PLoS ONE 2016, 11, e0164622. [Google Scholar] [CrossRef]
- Kimura, Y.; Hess, D.; Sturm, A. The N-Glycans of Jack Bean Alpha-Mannosidase. Structure, Topology and Function. Eur. J. Biochem. 1999, 264, 168–175. [Google Scholar] [CrossRef]
- McCleary, B.V.; Matheson, N.K. Action Patterns and Substrate-Binding Requirements of β-d-Mannanase with Mannosaccharides and Mannan-Type Polysaccharides. Carbohydr. Res. 1983, 119, 191–219. [Google Scholar] [CrossRef]
- Bradford, C. The Use of Commercially Available Alpha-Amylase Compounds to Inhibit and Remove Staphylococcus Aureus Biofilms. Open Microbiol. J. 2011, 5, 21–31. [Google Scholar] [CrossRef]
- Millenbaugh, N.; Watters, C.; Burton, T.; Kirui, D. Enzymatic Degradation of in Vitro Staphylococcus Aureus Biofilms Supplemented with Human Plasma. Infect. Drug Resist. 2016, 9, 71–78. [Google Scholar] [CrossRef]
- Ibberson, C.B.; Parlet, C.P.; Kwiecinski, J.; Crosby, H.A.; Meyerholz, D.K.; Horswill, A.R. Hyaluronan Modulation Impacts Staphylococcus Aureus Biofilm Infection. Infect. Immun. 2016, 84, 1917–1929. [Google Scholar] [CrossRef]
- Pecharki, D.; Petersen, F.C.; Scheie, A.A. Role of Hyaluronidase in Streptococcus Intermedius Biofilm. Microbiology 2008, 154, 932–938. [Google Scholar] [CrossRef]
- Little, D.J.; Pfoh, R.; Le Mauff, F.; Bamford, N.C.; Notte, C.; Baker, P.; Guragain, M.; Robinson, H.; Pier, G.B.; Nitz, M.; et al. PgaB Orthologues Contain a Glycoside Hydrolase Domain That Cleaves Deacetylated Poly-β(1,6)-N-Acetylglucosamine and Can Disrupt Bacterial Biofilms. PLoS Pathog. 2018, 14, e1006998. [Google Scholar] [CrossRef]
- Bamford, N.C.; Le Mauff, F.; Subramanian, A.S.; Yip, P.; Millán, C.; Zhang, Y.; Zacharias, C.; Forman, A.; Nitz, M.; Codée, J.D.C.; et al. Ega3 from the Fungal Pathogen Aspergillus Fumigatus Is an Endo-α-1,4-Galactosaminidase That Disrupts Microbial Biofilms. J. Biol. Chem. 2019, 294, 13833–13849. [Google Scholar] [CrossRef]
- Le Mauff, F.; Bamford, N.C.; Alnabelseya, N.; Zhang, Y.; Baker, P.; Robinson, H.; Codée, J.D.C.; Lynne Howell, P.; Sheppard, D.C. Molecular Mechanism of Aspergillus Fumigatus Biofilm Disruption by Fungal and Bacterial Glycoside Hydrolases. J. Biol. Chem. 2019, 294, 10760–10772. [Google Scholar] [CrossRef]
- Siala, W.; Kucharíková, S.; Braem, A.; Vleugels, J.; Tulkens, P.M.; Mingeot-Leclercq, M.-P.; Van Dijck, P.; Van Bambeke, F. The Antifungal Caspofungin Increases Fluoroquinolone Activity against Staphylococcus Aureus Biofilms by Inhibiting N-Acetylglucosamine Transferase. Nat. Commun. 2016, 7, 13286. [Google Scholar] [CrossRef]
- Beeh, K.M.; Beier, J.; Esperester, A.; Paul, L.D. Antiinflammatory Properties of Ambroxol. Eur. J. Med. Res. 2008, 13, 557–562. [Google Scholar]
- Paleari, D.; Rossi, G.A.; Nicolini, G.; Olivieri, D. Ambroxol: A Multifaceted Molecule with Additional Therapeutic Potentials in Respiratory Disorders of Childhood. Expert Opin. Drug Discov. 2011, 6, 1203–1214. [Google Scholar] [CrossRef]
- Cheng, C.; Du, L.; Yu, J.; Lu, Q.; He, Y.; Ran, T. Ciprofloxacin plus Erythromycin or Ambroxol Ameliorates Endotracheal Tube-Associated Pseudomonas Aeruginosa Biofilms in a Rat Model. Pathol. Res. Pract. 2015, 211, 982–988. [Google Scholar] [CrossRef]
- Li, F.; Yu, J.; Yang, H.; Wan, Z.; Bai, D. Effects of Ambroxol on Alginate of Mature Pseudomonas Aeruginosa Biofilms. Curr. Microbiol. 2008, 57, 1–7. [Google Scholar] [CrossRef]
- Zhou, E.; Seminara, A.B.; Kim, S.-K.; Hall, C.L.; Wang, Y.; Lee, V.T. Thiol-Benzo-Triazolo-Quinazolinone Inhibits Alg44 Binding to c-Di-GMP and Reduces Alginate Production by Pseudomonas Aeruginosa. ACS Chem. Biol. 2017, 12, 3076–3085. [Google Scholar] [CrossRef]
- van Tilburg Bernardes, E.; Charron-Mazenod, L.; Reading, D.J.; Reckseidler-Zenteno, S.L.; Lewenza, S. Exopolysaccharide-Repressing Small Molecules with Antibiofilm and Antivirulence Activity against Pseudomonas Aeruginosa. Antimicrob. Agents Chemother. 2017, 61, e01997-16. [Google Scholar] [CrossRef]
- Li, H.; Mo, K.F.; Wang, Q.; Stover, C.K.; Digiandomenico, A.; Boons, G.J. Epitope Mapping of Monoclonal Antibodies Using Synthetic Oligosaccharides Uncovers Novel Aspects of Immune Recognition of the Psl Exopolysaccharide of Pseudomonas Aeruginosa. Chem.—A Eur. J. 2013, 19, 17425–17431. [Google Scholar] [CrossRef]
- Ray, V.A.; Hill, P.J.; Stover, K.C.; Roy, S.; Sen, C.K.; Yu, L.; Wozniak, D.J.; DiGiandomenico, A. Anti-Psl Targeting of Pseudomonas Aeruginosa Biofilms for Neutrophil-Mediated Disruption. Sci. Rep. 2017, 7, 16065. [Google Scholar] [CrossRef]
- DiGiandomenico, A.; Warrener, P.; Hamilton, M.; Guillard, S.; Ravn, P.; Minter, R.; Camara, M.M.; Venkatraman, V.; MacGill, R.S.; Lin, J.; et al. Identification of Broadly Protective Human Antibodies to Pseudomonas Aeruginosa Exopolysaccharide Psl by Phenotypic Screening. J. Exp. Med. 2012, 209, 1273–1287. [Google Scholar] [CrossRef]
- DiGiandomenico, A.; Keller, A.E.; Gao, C.; Rainey, G.J.; Warrener, P.; Camara, M.M.; Bonnell, J.; Fleming, R.; Bezabeh, B.; Dimasi, N.; et al. A Multifunctional Bispecific Antibody Protects against Pseudomonas Aeruginosa. Sci. Transl. Med. 2014, 6, 1–13. [Google Scholar] [CrossRef]
- Tabor, D.E.; Oganesyan, V.; Keller, A.E.; Yu, L.; McLaughlin, R.E.; Song, E.; Warrener, P.; Rosenthal, K.; Esser, M.; Qi, Y.; et al. Pseudomonas Aeruginosa PcrV and Psl, the Molecular Targets of Bispecific Antibody MEDI3902, Are Conserved Among Diverse Global Clinical Isolates. J. Infect. Dis. 2018, 218, 1983–1994. [Google Scholar] [CrossRef]
- Maira-Litrán, T.; Kropec, A.; Goldmann, D.A.; Pier, G.B. Comparative Opsonic and Protective Activities of Staphylococcus Aureus Conjugate Vaccines Containing Native or Deacetylated Staphylococcal Poly-N-Acetyl-β-(1-6)-Glucosamine. Infect. Immun. 2005, 73, 6752–6762. [Google Scholar] [CrossRef]
- Kelly-Quintos, C.; Cavacini, L.A.; Posner, M.R.; Goldmann, D.; Pier, G.B. Characterization of the Opsonic and Protective Activity against Staphylococcus Aureus of Fully Human Monoclonal Antibodies Specific for the Bacterial Surface Polysaccharide Poly- N -Acetylglucosamine. Infect. Immun. 2006, 74, 2742–2750. [Google Scholar] [CrossRef]
- Priebe, G.P.; Goldberg, J.B. Vaccines for Pseudomonas Aeruginosa: A Long and Winding Road. Expert Rev. Vaccines 2014, 13, 507–519. [Google Scholar] [CrossRef]
- Meluleni, G.J.; Grout, M.; Evans, D.J.; Pier, G.B. Mucoid Pseudomonas Aeruginosa Growing in a Biofilm in Vitro Are Killed by Opsonic Antibodies to the Mucoid Exopolysaccharide Capsule but Not by Antibodies Produced during Chronic Lung Infection in Cystic Fibrosis Patients. J. Immunol. 1995, 155, 2029–2038. [Google Scholar] [CrossRef]
- Pier, G.B.; Saunders, J.M.; Ames, P.; Edwards, M.S.; Auerbach, H.; Goldfarb, J.; Speert, D.P.; Hurwitch, S. Opsonophagocytic Killing Antibody to Pseudomonas Aeruginosa Mucoid Exopolysaccharide in Older Noncolonized Patients with Cystic Fibrosis. N. Engl. J. Med. 1987, 317, 793–798. [Google Scholar] [CrossRef]
- Pier, G.B.; DesJardin, D.; Grout, M.; Garner, C.; Bennett, S.E.; Pekoe, G.; Fuller, S.A.; Thornton, M.O.; Harkonen, W.S.; Miller, H.C. Human Immune Response to Pseudomonas Aeruginosa Mucoid Exopolysaccharide (Alginate) Vaccine. Infect. Immun. 1994, 62, 3972–3979. [Google Scholar] [CrossRef]
- Pier, G.B.; Boyer, D.; Preston, M.; Coleman, F.T.; Llosa, N.; Mueschenborn-Koglin, S.; Theilacker, C.; Goldenberg, H.; Uchin, J.; Priebe, G.P.; et al. Human Monoclonal Antibodies to Pseudomonas Aeruginosa Alginate That Protect against Infection by Both Mucoid and Nonmucoid Strains. J. Immunol. 2004, 173, 5671–5678. [Google Scholar] [CrossRef]
- Theilacker, C.; Coleman, F.T.; Mueschenborn, S.; Llosa, N.; Grout, M.; Pier, G.B. Construction and Characterization of a Pseudomonas Aeruginosa Mucoid Exopolysaccharide-Alginate Conjugate Vaccine. Infect. Immun. 2003, 71, 3875–3884. [Google Scholar] [CrossRef]
- Cryz, S.J.; Furer, E.; Que, J.U. Synthesis and Characterization of a Pseudomonas Aeruginosa Alginate-Toxin A Conjugate Vaccine. Infect. Immun. 1991, 59, 45–50. [Google Scholar] [CrossRef]
- Kashef, N.; Behzadian-Nejad, Q.; Najar-Peerayeh, S.; Mousavi-Hosseini, K.; Moazzeni, M.; Djavid, G.E. Synthesis and Characterization of Pseudomonas Aeruginosa Alginate-Tetanus Toxoid Conjugate. J. Med. Microbiol. 2006, 55, 1441–1446. [Google Scholar] [CrossRef]
- Faezi, S.; Bahrmand, A.R.; Mahdavi, M.; Siadat, S.D.; Sardari, S.; Nikokar, I.; Khanaki, K.; Mirzajani, E.; Goudarzi, G. Preparation of Pseudomonas Aeruginosa Alginate-Flagellin Immunoconjugate. Biologicals 2017, 47, 11–17. [Google Scholar] [CrossRef]
- Farjah, A.; Owlia, P.; Siadat, S.D.; Mousavi, S.F.; Ardestani, M.S.; Mohammadpour, H.K. Immunological Evaluation of an Alginate-Based Conjugate as a Vaccine Candidate against Pseudomonas Aeruginosa. Apmis 2015, 123, 175–183. [Google Scholar] [CrossRef]
- Saeid, A.; Safari Zanjani, L. Immunization against Pseudomonas Aeruginosa Using Alg-PLGA Nano-Vaccine. Iran. J. Basic Med. Sci. 2021, 24, 476–482. [Google Scholar]
- Gening, M.L.; Maira-Litrán, T.; Kropec, A.; Skurnik, D.; Grout, M.; Tsvetkov, Y.E.; Nifantiev, N.E.; Pier, G.B. Synthetic β-(1→6)-Linked N-Acetylated and Nonacetylated Oligoglucosamines Used To Produce Conjugate Vaccines for Bacterial Pathogens. Infect. Immun. 2010, 78, 764–772. [Google Scholar] [CrossRef]
- Kumar, A. House of Cellulose—A New Hideout for Drug Tolerant Mycobacterium Tuberculosis. Microb. Cell 2016, 3, 299–301. [Google Scholar] [CrossRef]
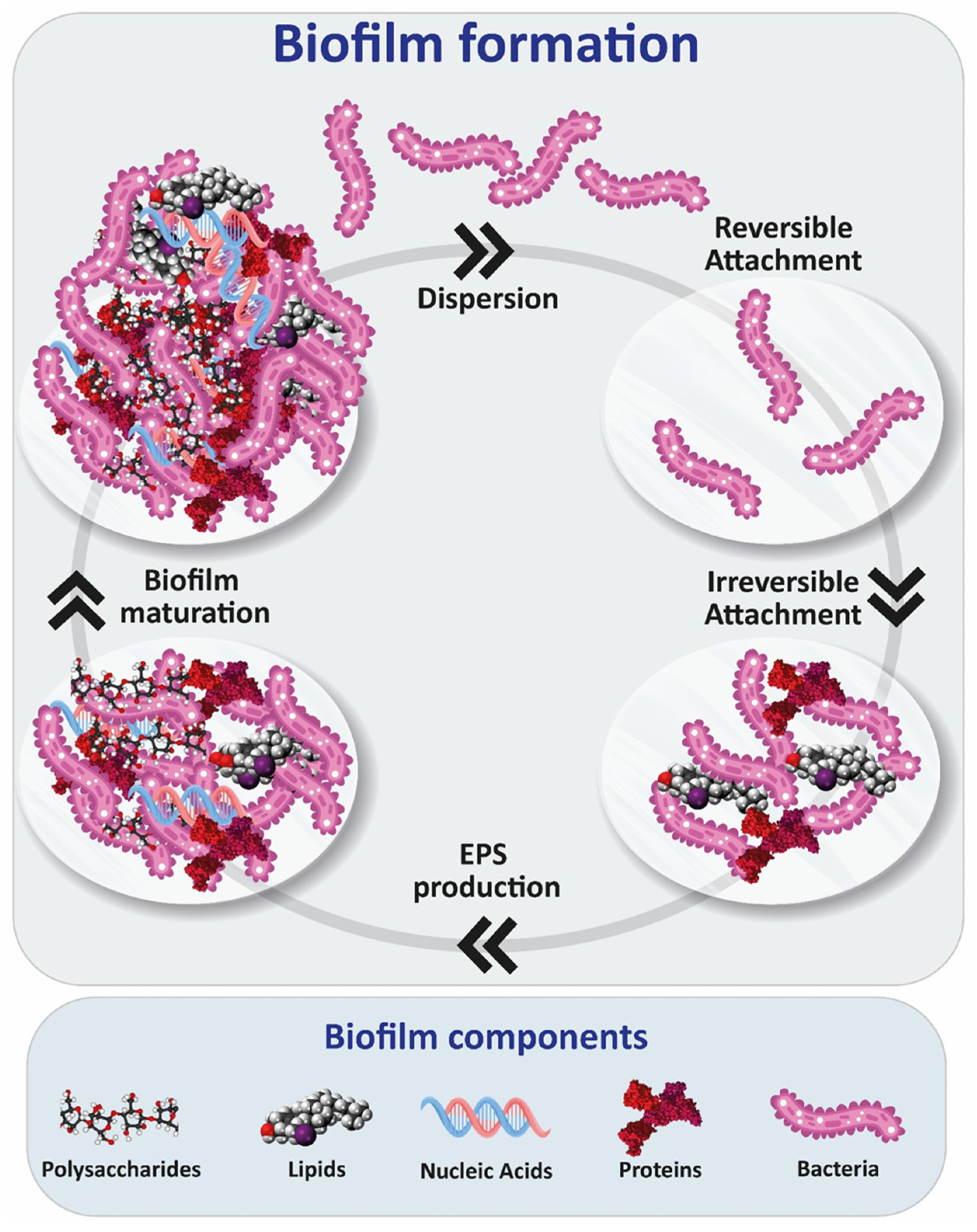
| Polysaccharide | Pathogen | Function | References |
|---|---|---|---|
| PNAG | S. aureus, S. epidermidis, E. coli, Y. pestis, A. pleuropneumoniae, A. baumannii | Attachment of bacterial cells to a surface, biofilm proliferation and maturation, final detachment | [20,21,22,23,29] |
| Alginate | P. aeruginosa | Biofilm maturation, formation of thick and highly structured biofilms with differentiated microcolonies, protection against antibiotics, resistance to oxidative stress | [39,40,41] |
| Psl | P. aeruginosa | Adhesion to surface and cell-to-cell interactions, role in aggregation of microcolonies, maintenance and maturation of biofilm through promotion of c-di-GMP production, shielding from antimicrobials and neutrophils | [39,40,41] |
| Pel | P. aeruginosa | Surface attachment, structural scaffold, confers tolerance to aminoglycoside antibiotics and to colistin | [39,40,41] |
| Galactosaminogalactans | A. fumigatus | Adherence to surface, anti-inflammatory properties | [91,92,93,94,95] |
| Galactomannans | A. fumigatus | Silence inflammation, promote the initial establishment of fungal cells in the lung | [91,92,93,94,95] |
| Glucans | A. fumigatus, S. mutans, Candida | Resistance mechanism to antifungal drugs, adhesion, agglutination mediation | [91,92,93,94,95,105,106,122] |
| Fructans | S. mutans | Adhesion | [105,106] |
| Vibrio polysaccharide (VPS) | V. cholerae | Determines biofilm architecture, cell–cell adhesion, stability | [102] |
| Colanic acid | E. coli | Attachment to abiotic surface | [107,109,110] |
| Cellulose | E. coli, Salmonella | Adherence to abiotic surface, cell-to-cell interaction, biofilm architecture | [107,112,113] |
| Wall teichoic acids (WTAs) | S. aureus, S. epidermidis | Adhesion of bacteria to surfaces and other bacterial cells | [115,118] |
| Xanthans | X. campestrispathovar campestris | Suppression of plant defenses, attachment initiation | [120,121] |
| Source | Full Name | Abbreviation | Specificity |
|---|---|---|---|
| Lectin from mushrooms | Aleuria Aurantia Lectin | AAL | Fucose; Arabinose [a] |
| Lectin from seeds | Amaranthus Caudatus Lectin | ACA/ACL | Galactose-β(1-3)-N-acetylgalactosamine [b] |
| Lectin from ground elder | Aegopodium Podagraria Agglutinin | APA/APP | N-acetylgalactosamine [c] |
| Lectin from chickpeas | Cicer Arietinum Lectin | CAA/CAL | N-glycans; N-acetylgalactosamine [b] |
| Lectin from jack-bean | Concavalin A | ConA | α-Mannose; α-Glucose [a] |
| Lectin from coral tree seeds | Erythrina Cristagalli Lectin | ECA/ECL | β-Galactose; N-acetylgalactosamine; Lactose [a] |
| Lectin from snowdrop bulbs | Galanthus Nivalis Lectin | GNA/GNL | α-(1,3)-Mannose [a] |
| Lectin from seed | Griffonia Simplicifolia Lectin I | GSL-I/BSL-i | Galactose; N-acetylgalactosamine [a] |
| Lectin from roman snail | Helix Pomatia Lectin | HPA/HPL | α-N-acetylgalactosamine [d] |
| Lectin from lima bean | Phaseolus Limensis Agglutinin | LBA | N-acetylgalactosamine-α(1,3)-Galactose [e] |
| Lectin from lentil | Lens Culinaris Agglutinin | LCA | α-Mannose; α-Glucose [a] |
| Lectin from tomatoes | Lycopersicon Esculentum Lectin | LEL/LEA | N-acetylglucosamine [a] |
| Lectin from asparagus pea | Lotus Tetragonolobus Lectin | LTL | Fucose; Arabinose [a] |
| Lectin from horseshoe crab | Limulus Polyphemus Lectin | LPA/LPL | Sialic acid; N-acetyl-d-hexosamines; D-glucuronic acid [f] |
| Lectin from winged pea | Lotus Tetragonolobus Lectin | LTL | Fucose; Arabinose [a] |
| Lectin from daffodil bulbs | Narcissus Pseudonarcissus Lectin | NPA/NPL | α-Mannose [a] |
| Lectin from bacteria | Pseudomonas Aeruginosa Galactophilic Lectin | PA-I | Galactose [g] |
| Lectin from beans | Phytohaemagglutinin | PHA | Different oligosaccharides [h] |
| Lectin from peanuts | Peanut Agglutinin | PNA | Galactose; N-acetylgalactosamine [a] |
| Lectin from peas | Pisum Sativum Agglutinin | PSA | α-Mannose; α-Glucose [a] |
| Lectin from ricinus | Ricinus Communis Agglutinin | RCA | Galactose; Lactose [a] |
| Lectin from soybeans | Soybean Agglutinin | SBA | Galactose; N-acetylgalactosamine [a] |
| Lectin from potatoes | Solanum Tuberosum Lectin | STA/STL | N-acetylgalactosamine [a] |
| Lectin from gorse | Ulex Europaeus Agglutinin I | UEA I | α-Fucose [a] |
| Lectin from fava beans | Vicia Faba Agglutinin | VFA | Mannose; Glucose [h] |
| Lectin from hairy vetch seeds | Vicia Villosa Lectin | VVA /VVL | N-acetylgalactosamine [a] |
| Lectin from Japanese Wisteria seeds | Wisteria Floribunda Lectin | WFA/WFL | N-acetylgalactosamine [a] |
| Lectin from wheat germs | Wheat Germ Agglutinin | WGA | N-acetylglucosamine; Sialic acid [a] |
| Enzyme | Bacteria Where It Acts | Summary | References |
|---|---|---|---|
| Dispersin B | S. aureus, A. actinomycetemcomitans, S. epidermidis, A. baumannii, K. pneumoniae, E. coli, Burkholderia spp., Actinobacillus Pleuropneumoniae, Yersinia Pestis, Pseudomonas fluorescens | Produced by A. actinomycetemcomitans, it degrades the polysaccharide PNAG through hydrolyzing β(1,6) glycosidic linkages. | [24,170] |
| Alginate lyase | P. aeruginosa | It degrades the exopolysaccharide Alginate, causing bacterial cell dispersal and increasing antibiotics’ efficacy and phagocytosis. | [162,164] |
| PelAh, PslGh | P. aeruginosa | They disperse mature biofilms by hydrolyzing Pel or Psl exopolysaccharides, respectively. | [63] |
| Cellulase | S. aureus and P. aeruginosa | It is produced by multiple microbes and it hydrolyzes the β(1,4) glycosidic linkage. | [171] |
| α- mannosidase | P. aeruginosa | It is believed that this acid hydrolase is involved in the turnover of N-linked glycoproteins. However, it has a cytotoxic effect on A-431 human epidermoid carcinoma cell lines. | [172,173] |
| β- mannosidase | P. aeruginosa | It hydrolyzes the terminal mannose residues, which are β(1,4) linked to oligosaccharides or glycopeptides. However, it has a cytotoxic effect on A-431 human epidermoid carcinoma cell lines. | [172,174] |
| α -amylase | V. cholerae, S. aureus and P. aeruginosa | It can derive from multiple sources and hydrolyzes α(1,4) glycosidic linkages, mediating the dispersal of mature biofilms of multiple bacterial strains. | [171,175,176] |
| Hyaluronidase | S. aureus and S. intermedius | It cleaves hyaluronic acid (HA), a component which has been found to be incorporated into the biofilms formed by multiple pathogens, causing biofilms’ dispersal. | [177,178] |
| PgaB | B. pertussis, Staphylococcus carnosus, S. epidermidis and E. coli | It disrupts PNAG-dependent biofilms through hydrolyzing PNAG, a major biofilm component of many pathogenic bacteria. | [179] |
| Ega3 | Aspergillus fumigatus and P. aeruginosa | This endo-acting α1,4-galactosaminidase disrupts biofilms formed by GAG-dependent Aspergillus fumigatus and Pel polysaccharide-dependent P. aeruginosa. | [180] |
| Sph3 | A. fumigatus | This retaining endo-α-1,4-N-acetylgalactosaminidase hydrolyzes galactosaminogalactan (GAG), a cationic polymer of α-1,4-linked galactose and partially deacetylated N-acetylgalactosamine (GalNAc), causing biofilm disruption. | [181] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balducci, E.; Papi, F.; Capialbi, D.E.; Del Bino, L. Polysaccharides’ Structures and Functions in Biofilm Architecture of Antimicrobial-Resistant (AMR) Pathogens. Int. J. Mol. Sci. 2023, 24, 4030. https://doi.org/10.3390/ijms24044030
Balducci E, Papi F, Capialbi DE, Del Bino L. Polysaccharides’ Structures and Functions in Biofilm Architecture of Antimicrobial-Resistant (AMR) Pathogens. International Journal of Molecular Sciences. 2023; 24(4):4030. https://doi.org/10.3390/ijms24044030
Chicago/Turabian StyleBalducci, Evita, Francesco Papi, Daniela Eloisa Capialbi, and Linda Del Bino. 2023. "Polysaccharides’ Structures and Functions in Biofilm Architecture of Antimicrobial-Resistant (AMR) Pathogens" International Journal of Molecular Sciences 24, no. 4: 4030. https://doi.org/10.3390/ijms24044030
APA StyleBalducci, E., Papi, F., Capialbi, D. E., & Del Bino, L. (2023). Polysaccharides’ Structures and Functions in Biofilm Architecture of Antimicrobial-Resistant (AMR) Pathogens. International Journal of Molecular Sciences, 24(4), 4030. https://doi.org/10.3390/ijms24044030






