Recent Advances in Methods for Circulating Tumor Cell Detection
Abstract
1. Introduction
2. Current Enrichment and Detection Techniques
2.1. Enrichment Techniques
2.1.1. Morphology-Based Approaches
2.1.2. Immunology-Based Approaches
2.2. CTC Detection Techniques
2.2.1. Nucleic-Acid-Based Detection Methods
2.2.2. Cytometry-Based Detection Methods
2.2.3. Microscopy-Based Detection Methods
2.3. Approaches Combining CTC Enrichment and Detection
2.4. Beyond the Detection—Studying the Biology of CTCs
3. Evolving Methods for CTC Detection and Characterization
3.1. Enrichment Techniques
3.1.1. Morphology-Based Approaches
3.1.2. Immunology-Based Approaches
3.2. Detection Techniques
3.3. Approaches Combining CTC Enrichment and Detection
3.4. Molecular Characterization Will Be Integral to Future Methods for CTC Detection
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CTCs | Circulating tumor cells |
| DTCs | Disseminated tumor cells |
| RT-PCR | Reverse transcriptase polymerase chain reaction |
| ISET | Isolation by size of epithelial tumor cells |
| CLL | Chronic lymphocytic leukemia |
| MEMS | Micro-electro-mechanical system |
| DEP-FFF | Dielectrophoretic field-flow fractionation |
| DFF | Dean flow fractionation |
| MACS | Magnetic-activated cell sorting |
| EpCAM | Epithelial cell adhesion molecule |
| HER2 | Human epidermal growth factor receptor 2 |
| CAM | Cell/collagen adhesion matrix |
| CK18 | Cytokeratin 18 |
| ICC | Immunocytochemistry |
| FC | Flow cytometry |
| ADM | Automated digital microscopy |
| FAST | Fiber-optic array scanning technology |
| FISH | Fluorescence in situ hybridization |
| EPISPOT | EPithelial ImmunoSPOT |
| CD45 | Protein tyrosine phosphatase receptor type C |
| MUC1 | Mucin 1 |
| CK19 | Cytokeratin 19 |
| PSA | Prostate-specific antigen |
| CEA | Carcinoembryonic antigen |
| EGFR | Epidermal growth factor receptor |
| PSMA | Prostate-specific membrane antigen |
| DAPI | 4′,6-diamidino-2-phenylindole |
| MS-MOFF | Multi-stage multi-orifice flow fractionation |
| STMBs | Strep-Tactin-coated magnetic beads |
| PBS | Phosphate buffered saline |
| MNPs | Magnetic nanoparticles |
| WBCs | White blood cells |
| RBCs | Red blood cells |
| MRPs | Multidrug-resistance-related proteins |
| ALDH1 | Aldehyde Dehydrogenase 1 |
| KRAS | Kirsten rat sarcoma virus, KRAS proto-oncogene |
| BRCA1 | Breast cancer suppressor gene |
| WGA | Whole-genome amplification |
| CGH | Comparative genomic hybridization |
| AI | Artificial intelligence |
| AR | Androgen receptor |
| EMT | Epithelial-mesenchymal transition |
| VOCs | Volatile organic compounds |
| PTA | Primary template-directed amplification |
| LIANTI | Linear amplification via transposon insertion |
| hMAM | Human mammaglobin |
| FACS | Fluorescence-activated cell sorting |
| BEAM | Beads, Emulsion, Amplification, Magnetics |
| CKs | Cytokeratins |
| CSF | Cerebrospinal fluid |
| 3D | Three-dimensional |
| NGS | Next-generation sequencing |
| ESA | Epithelial specific antigen |
| mRNA | Messenger ribonucleic acid |
| ssDNA | Single-stranded deoxyribonucleic acid |
| cDNA | Complementary deoxyribonucleic acid |
| CD | Cluster of differentiation |
| MALBAC | Multiple annealing and looping based amplification cycles |
| scRNA | Single-cell ribonucleic acid |
| McTNs | Microtentacles |
| ctDNA | Circulating tumor deoxyribonucleic acid |
| LC-MS | Liquid chromatography-mass spectrometry |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Shen, L.; Luo, M.; Zhang, K.; Li, J.; Yang, Q.; Zhu, F.; Zhou, D.; Zheng, S.; Chen, Y.; et al. Circulating tumor cells: Biology and clinical significance. Signal Transduct. Target. Ther. 2021, 6, 404. [Google Scholar] [CrossRef] [PubMed]
- Sai, B.; Xiang, J. Disseminated tumour cells in bone marrow are the source of cancer relapse after therapy. J. Cell. Mol. Med. 2018, 22, 5776–5786. [Google Scholar] [CrossRef]
- Borriello, L.; Coste, A.; Traub, B.; Sharma, V.P.; Karagiannis, G.S.; Lin, Y.; Wang, Y.; Ye, X.; Duran, C.L.; Chen, X.; et al. Primary tumor associated macrophages activate programs of invasion and dormancy in disseminating tumor cells. Nat. Commun. 2022, 13, 626. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.-C.; Zhang, X.-F.; Peng, J.; Li, X.-F.; Wang, A.-L.; Bie, Y.-Q.; Shi, L.-H.; Lin, M.-B.; Zhang, X.-F. Survival Mechanisms and Influence Factors of Circulating Tumor Cells. BioMed Res. Int. 2018, 2018, 6304701. [Google Scholar] [CrossRef] [PubMed]
- Castro-Giner, F.; Aceto, N. Tracking cancer progression: From circulating tumor cells to metastasis. Genome Med. 2020, 12, 31. [Google Scholar] [CrossRef] [PubMed]
- Papadaki, M.A.; Stoupis, G.; Theodoropoulos, P.A.; Mavroudis, D.; Georgoulias, V.; Agelaki, S. Circulating Tumor Cells with Stemness and Epithelial-to-Mesenchymal Transition Features Are Chemoresistant and Predictive of Poor Outcome in Metastatic Breast Cancer. Mol. Cancer Ther. 2019, 18, 437–447. [Google Scholar] [CrossRef]
- Wang, L.; Lankhorst, L.; Bernards, R. Exploiting senescence for the treatment of cancer. Nat. Rev. Cancer 2022, 22, 340–355. [Google Scholar] [CrossRef]
- Pantel, K.; Alix-Panabières, C. Liquid biopsy and minimal residual disease—Latest advances and implications for cure. Nat. Rev. Clin. Oncol. 2019, 16, 409–424. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, X.; Zhang, Y.; Liu, J. Advancing single-cell proteomics and metabolomics with microfluidic technologies. Analyst 2019, 144, 846–858. [Google Scholar] [CrossRef]
- Kaldjian, E.P.; Ramirez, A.B.; Costandy, L.; Ericson, N.G.; Malkawi, W.I.; George, T.C.; Kasi, P.M. Beyond Circulating Tumor Cell Enumeration: Cell-Based Liquid Biopsy to Assess Protein Biomarkers and Cancer Genomics Using the RareCyte® Platform. Front. Pharmacol. 2022, 13, 835727. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, R.; Zhang, L.; Guo, S. Nanomaterial-Based Immunocapture Platforms for the Recognition, Isolation, and Detection of Circulating Tumor Cells. Front. Bioeng. Biotechnol. 2022, 10, 850241. [Google Scholar] [CrossRef] [PubMed]
- Bankó, P.; Lee, S.Y.; Nagygyörgy, V.; Zrínyi, M.; Chae, C.H.; Cho, D.H.; Telekes, A. Technologies for circulating tumor cell separation from whole blood. J. Hematol. Oncol. 2019, 12, 48. [Google Scholar] [CrossRef]
- Yang, C.; Xia, B.-R.; Jin, W.-L.; Lou, G. Circulating tumor cells in precision oncology: Clinical applications in liquid biopsy and 3D organoid model. Cancer Cell Int. 2019, 19, 341. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Medina, R.; López-Tarruella, S.; del Monte-Millán, M.; Massarrah, T.; Martín, M. Technical Challenges for CTC Implementation in Breast Cancer. Cancers 2021, 13, 4619. [Google Scholar] [CrossRef] [PubMed]
- Sorolla, M.A.; Sorolla, A.; Parisi, E.; Salud, A.; Porcel, J.M. Diving into the Pleural Fluid: Liquid Biopsy for Metastatic Malignant Pleural Effusions. Cancers 2021, 13, 2798. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Li, S.; Wu, D.; Ren, H.; Ni, C.; Wang, C.; Xiang, N.; Ni, Z. High-throughput and label-free enrichment of malignant tumor cells and clusters from pleural and peritoneal effusions using inertial microfluidics. Lab Chip 2022, 22, 2097–2106. [Google Scholar] [CrossRef] [PubMed]
- Malani, R.; Fleisher, M.; Kumthekar, P.; Lin, X.; Omuro, A.; Groves, M.D.; Lin, N.U.; Melisko, M.; Lassman, A.B.; Jeyapalan, S.; et al. Cerebrospinal fluid circulating tumor cells as a quantifiable measurement of leptomeningeal metastases in patients with HER2 positive cancer. J. Neurooncol. 2020, 148, 599–606. [Google Scholar] [CrossRef]
- Eibl, R.; Schneemann, M. Liquid Biopsy and Glioblastoma. Med. Pharmacol. 2022. [Google Scholar] [CrossRef]
- Neoh, K.H.; Hassan, A.A.; Chen, A.; Sun, Y.; Liu, P.; Xu, K.-F.; Wong, A.S.T.; Han, R.P.S. Rethinking liquid biopsy: Microfluidic assays for mobile tumor cells in human body fluids. Biomaterials 2018, 150, 112–124. [Google Scholar] [CrossRef]
- Ashworth, T. A case of cancer in which cells similar to those in the tumours were seen in the blood after death. Med. J. Aust. 1869, 14, 146–149. [Google Scholar]
- Tulley, S.; Zhao, Q.; Dong, H.; Pearl, M.L.; Chen, W.-T. Vita-AssayTM Method of Enrichment and Identification of Circulating Cancer Cells/Circulating Tumor Cells (CTCs). In Breast Cancer; Cao, J., Ed.; Springer: New York, NY, USA, 2016; Volume 1406, pp. 107–119. ISBN 978-1-4939-3442-3. [Google Scholar]
- Loeian, M.S.; Mehdi Aghaei, S.; Farhadi, F.; Rai, V.; Yang, H.W.; Johnson, M.D.; Aqil, F.; Mandadi, M.; Rai, S.N.; Panchapakesan, B. Liquid biopsy using the nanotube-CTC-chip: Capture of invasive CTCs with high purity using preferential adherence in breast cancer patients. Lab Chip 2019, 19, 1899–1915. [Google Scholar] [CrossRef] [PubMed]
- Ju, J.A.; Lee, C.J.; Thompson, K.N.; Ory, E.C.; Lee, R.M.; Mathias, T.J.; Pratt, S.J.P.; Vitolo, M.I.; Jewell, C.M.; Martin, S.S. Partial thermal imidization of polyelectrolyte multilayer cell tethering surfaces (TetherChip) enables efficient cell capture and microtentacle fixation for circulating tumor cell analysis. Lab Chip 2020, 20, 2872–2888. [Google Scholar] [CrossRef] [PubMed]
- Kaldjian, E.P.; Ramirez, A.B.; Sun, Y.; Campton, D.E.; Werbin, J.L.; Varshavskaya, P.; Quarre, S.; George, T.; Madan, A.; Blau, C.A.; et al. The RareCyte® platform for next-generation analysis of circulating tumor cells: RareCyte platform CTC analysis. Cytometry 2018, 93, 1220–1225. [Google Scholar] [CrossRef] [PubMed]
- Ranc, V.; Srovnal, J.; Kvítek, L.; Hajduch, M. Discrimination of circulating tumor cells of breast cancer and colorectal cancer from normal human mononuclear cells using Raman spectroscopy. Analyst 2013, 138, 5983. [Google Scholar] [CrossRef] [PubMed]
- Vona, G.; Sabile, A.; Louha, M.; Sitruk, V.; Romana, S.; Schütze, K.; Capron, F.; Franco, D.; Pazzagli, M.; Vekemans, M.; et al. Isolation by Size of Epithelial Tumor Cells. Am. J. Pathol. 2000, 156, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.; Chen, Q.; Liu, X.; Shen, S.; Pan, Z.; Shi, C. Detection of circulating stage III–IV gastric cancer tumor cells based on isolation by size of epithelial tumor: Using the circulating tumor cell biopsy technology. Transl. Cancer Res. 2019, 8, 1342–1350. [Google Scholar] [CrossRef]
- Yang, C.; Shi, D.; Wang, S.; Wei, C.; Zhang, C.; Xiong, B. Prognostic value of pre- and post-operative circulating tumor cells detection in colorectal cancer patients treated with curative resection: A prospective cohort study based on ISET device. CMAR 2018, 10, 4135–4144. [Google Scholar] [CrossRef]
- Zheng, S.; Lin, H.; Liu, J.-Q.; Balic, M.; Datar, R.; Cote, R.J.; Tai, Y.-C. Membrane microfilter device for selective capture, electrolysis and genomic analysis of human circulating tumor cells. J. Chromatogr. A 2007, 1162, 154–161. [Google Scholar] [CrossRef]
- Laget, S.; Broncy, L.; Hormigos, K.; Dhingra, D.M.; BenMohamed, F.; Capiod, T.; Osteras, M.; Farinelli, L.; Jackson, S.; Paterlini-Bréchot, P. Technical Insights into Highly Sensitive Isolation and Molecular Characterization of Fixed and Live Circulating Tumor Cells for Early Detection of Tumor Invasion. PLoS ONE 2017, 12, e0169427. [Google Scholar] [CrossRef]
- Drucker, A.; Teh, E.M.; Kostyleva, R.; Rayson, D.; Douglas, S.; Pinto, D.M. Comparative performance of different methods for circulating tumor cell enrichment in metastatic breast cancer patients. PLoS ONE 2020, 15, e0237308. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, R.; Gertler, R.; Friederichs, J.; Fuehrer, K.; Dahm, M.; Phelps, R.; Thorban, S.; Nekarda, H.; Siewert, J.R. Comparison of two density gradient centrifugation systems for the enrichment of disseminated tumor cells in blood. Cytometry 2002, 49, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Königsberg, R.; Gneist, M.; Jahn-Kuch, D.; Pfeiler, G.; Hager, G.; Hudec, M.; Dittrich, C.; Zeillinger, R. Circulating tumor cells in metastatic colorectal cancer: Efficacy and feasibility of different enrichment methods. Cancer Lett. 2010, 293, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, M.T.; Calleja, L.R.; Chalopin, A.; Ory, B.; Heymann, D. Circulating Tumor Cells: A Review of Non–EpCAM-Based Approaches for Cell Enrichment and Isolation. Clin. Chem. 2016, 62, 571–581. [Google Scholar] [CrossRef]
- Russo, G.I.; Musso, N.; Romano, A.; Caruso, G.; Petralia, S.; Lanzanò, L.; Broggi, G.; Camarda, M. The Role of Dielectrophoresis for Cancer Diagnosis and Prognosis. Cancers 2021, 14, 198. [Google Scholar] [CrossRef]
- Gascoyne, P.R.C.; Noshari, J.; Anderson, T.J.; Becker, F.F. Isolation of rare cells from cell mixtures by dielectrophoresis. Electrophoresis 2009, 30, 1388–1398. [Google Scholar] [CrossRef]
- Kwizera, E.A.; Sun, M.; White, A.M.; Li, J.; He, X. Methods of Generating Dielectrophoretic Force for Microfluidic Manipulation of Bioparticles. ACS Biomater. Sci. Eng. 2021, 7, 2043–2063. [Google Scholar] [CrossRef]
- Torres-Castro, K.; Honrado, C.; Varhue, W.B.; Farmehini, V.; Swami, N.S. High-throughput dynamical analysis of dielectrophoretic frequency dispersion of single cells based on deflected flow streamlines. Anal. Bioanal. Chem. 2020, 412, 3847–3857. [Google Scholar] [CrossRef]
- Varmazyari, V.; Habibiyan, H.; Ghafoorifard, H.; Ebrahimi, M.; Ghafouri-Fard, S. A dielectrophoresis-based microfluidic system having double-sided optimized 3D electrodes for label-free cancer cell separation with preserving cell viability. Sci. Rep. 2022, 12, 12100. [Google Scholar] [CrossRef]
- Le Du, F.; Fujii, T.; Kida, K.; Davis, D.W.; Park, M.; Liu, D.D.; Wu, W.; Chavez-MacGregor, M.; Barcenas, C.H.; Valero, V.; et al. EpCAM-independent isolation of circulating tumor cells with epithelial-to-mesenchymal transition and cancer stem cell phenotypes using ApoStream® in patients with breast cancer treated with primary systemic therapy. PLoS ONE 2020, 15, e0229903. [Google Scholar] [CrossRef]
- Balasubramanian, P.; Kinders, R.J.; Kummar, S.; Gupta, V.; Hasegawa, D.; Menachery, A.; Lawrence, S.M.; Wang, L.; Ferry-Galow, K.; Davis, D.; et al. Antibody-independent capture of circulating tumor cells of non-epithelial origin with the ApoStream® system. PLoS ONE 2017, 12, e0175414. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.C.; Robinson, P.S.; Wagner, C.; O’Shannessy, D.J. The ParsortixTM Cell Separation System—A versatile liquid biopsy platform. Cytometry 2018, 93, 1234–1239. [Google Scholar] [CrossRef] [PubMed]
- Sollier-Christen, E.; Renier, C.; Kaplan, T.; Kfir, E.; Crouse, S.C. VTX-1 Liquid Biopsy System for Fully-Automated and Label-Free Isolation of Circulating Tumor Cells with Automated Enumeration by BioView Platform. Cytometry 2018, 93, 1240–1245. [Google Scholar] [CrossRef] [PubMed]
- Lemaire, C.A.; Liu, S.Z.; Wilkerson, C.L.; Ramani, V.C.; Barzanian, N.A.; Huang, K.-W.; Che, J.; Chiu, M.W.; Vuppalapaty, M.; Dimmick, A.M.; et al. Fast and Label-Free Isolation of Circulating Tumor Cells from Blood: From a Research Microfluidic Platform to an Automated Fluidic Instrument, VTX-1 Liquid Biopsy System. SLAS Technol. 2018, 23, 16–29. [Google Scholar] [CrossRef]
- Riahi, R.; Gogoi, P.; Sepehri, S.; Zhou, Y.; Handique, K.; Godsey, J.; Wang, Y. A novel microchannel-based device to capture and analyze circulating tumor cells (CTCs) of breast cancer. Int. J. Oncol. 2014, 44, 1870–1878. [Google Scholar] [CrossRef]
- Hou, H.W.; Warkiani, M.E.; Khoo, B.L.; Li, Z.R.; Soo, R.A.; Tan, D.S.-W.; Lim, W.-T.; Han, J.; Bhagat, A.A.S.; Lim, C.T. Isolation and retrieval of circulating tumor cells using centrifugal forces. Sci. Rep. 2013, 3, 1259. [Google Scholar] [CrossRef]
- Lee, Y.; Guan, G.; Bhagat, A.A. ClearCell® FX, a label-free microfluidics technology for enrichment of viable circulating tumor cells. Cytometry 2018, 93, 1251–1254. [Google Scholar] [CrossRef]
- Nagrath, S.; Sequist, L.V.; Maheswaran, S.; Bell, D.W.; Irimia, D.; Ulkus, L.; Smith, M.R.; Kwak, E.L.; Digumarthy, S.; Muzikansky, A.; et al. Isolation of rare circulating tumour cells in cancer patients by microchip technology. Nature 2007, 450, 1235–1239. [Google Scholar] [CrossRef]
- Lozar, T.; Jesenko, T.; Kloboves Prevodnik, V.; Cemazar, M.; Hosta, V.; Jericevic, A.; Nolde, N.; Grasic Kuhar, C. Preclinical and Clinical Evaluation of Magnetic-Activated Cell Separation Technology for CTC Isolation in Breast Cancer. Front. Oncol. 2020, 10, 554554. [Google Scholar] [CrossRef]
- Cardoso, V.F.; Francesko, A.; Ribeiro, C.; Bañobre-López, M.; Martins, P.; Lanceros-Mendez, S. Advances in Magnetic Nanoparticles for Biomedical Applications. Adv. Healthc. Mater. 2018, 7, 1700845. [Google Scholar] [CrossRef]
- Lu, N.-N.; Xie, M.; Wang, J.; Lv, S.-W.; Yi, J.-S.; Dong, W.-G.; Huang, W.-H. Biotin-Triggered Decomposable Immunomagnetic Beads for Capture and Release of Circulating Tumor Cells. ACS Appl. Mater. Interfaces 2015, 7, 8817–8826. [Google Scholar] [CrossRef]
- Chen, Y.; Tyagi, D.; Lyu, M.; Carrier, A.J.; Nganou, C.; Youden, B.; Wang, W.; Cui, S.; Servos, M.; Oakes, K.; et al. Regenerative NanoOctopus Based on Multivalent-Aptamer-Functionalized Magnetic Microparticles for Effective Cell Capture in Whole Blood. Anal. Chem. 2019, 91, 4017–4022. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wu, Z.; Sun, N.; Cao, Y.; Cai, X.; Yuan, F.; Zou, H.; Xing, C.; Pei, R. Antifouling hydrogel-coated magnetic nanoparticles for selective isolation and recovery of circulating tumor cells. J. Mater. Chem. B 2021, 9, 677–682. [Google Scholar] [CrossRef] [PubMed]
- Fan, T.; Zhao, Q.; Chen, J.J.; Chen, W.-T.; Pearl, M.L. Clinical significance of circulating tumor cells detected by an invasion assay in peripheral blood of patients with ovarian cancer. Gynecol. Oncol. 2009, 112, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Paris, P.L.; Kobayashi, Y.; Zhao, Q.; Zeng, W.; Sridharan, S.; Fan, T.; Adler, H.L.; Yera, E.R.; Zarrabi, M.H.; Zucker, S.; et al. Functional phenotyping and genotyping of circulating tumor cells from patients with castration resistant prostate cancer. Cancer Lett. 2009, 277, 164–173. [Google Scholar] [CrossRef]
- Lu, J.; Fan, T.; Zhao, Q.; Zeng, W.; Zaslavsky, E.; Chen, J.J.; Frohman, M.A.; Golightly, M.G.; Madajewicz, S.; Chen, W.-T. Isolation of circulating epithelial and tumor progenitor cells with an invasive phenotype from breast cancer patients. Int. J. Cancer 2010, 126, 669–683. [Google Scholar] [CrossRef]
- Dong, H.; Tulley, S.; Zhao, Q.; Cho, L.; Chen, D.; Pearl, M.L.; Chen, W. The propensity of invasive circulating tumor cells (iCTCs) in metastatic progression and therapeutic responsiveness. Cancer Med. 2019, 8, 3864–3874. [Google Scholar] [CrossRef]
- Dirix, L.; Buys, A.; Oeyen, S.; Peeters, D.; Liègeois, V.; Prové, A.; Rondas, D.; Vervoort, L.; Mariën, V.; Laere, S.V.; et al. Circulating tumor cell detection: A prospective comparison between CellSearch® and RareCyte® platforms in patients with progressive metastatic breast cancer. Breast Cancer Res. Treat. 2022, 193, 437–444. [Google Scholar] [CrossRef]
- Cho, H.; Chung, J.-S.; Han, K.-H. A Direct Comparison between the Lateral Magnetophoretic Microseparator and AdnaTest for Isolating Prostate Circulating Tumor Cells. Micromachines 2020, 11, 870. [Google Scholar] [CrossRef]
- Janku, F.; Srovnal, J.; Korinkova, G.; Novotny, J.; Petruzelka, L.; Power, D.; Matous, B.; Hajduch, M. Molecular detection of disseminated breast cancer cells in the bone marrow of early breast cancer patients using quantitative RT PCR for CEA. Neoplasma 2008, 55, 317–322. [Google Scholar]
- Lianidou, E.S. Gene expression profiling and DNA methylation analyses of CTCs. Mol. Oncol. 2016, 10, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Havlik, R.; Srovnal, J.; Klos, D.; Benedikova, A.; Lovecek, M.; Ghothim, M.; Cahova, D.; Neoral, C.; Hajduch, M. Occult tumour cells in peritoneal lavage are a negative prognostic factor in pancreatic cancer. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech Repub. 2013, 157, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Kruse, A.; Abdel-Azim, N.; Kim, H.N.; Ruan, Y.; Phan, V.; Ogana, H.; Wang, W.; Lee, R.; Gang, E.J.; Khazal, S.; et al. Minimal Residual Disease Detection in Acute Lymphoblastic Leukemia. IJMS 2020, 21, 1054. [Google Scholar] [CrossRef] [PubMed]
- Bustin, S.A.; Mueller, R.; Nolan, T. Parameters for Successful PCR Primer Design. In Quantitative Real-Time PCR; Biassoni, R., Raso, A., Eds.; Springer: New York, NY, USA, 2020; Volume 2065, pp. 5–22. [Google Scholar] [CrossRef]
- Zhao, S.; Yang, H.; Zhang, M.; Zhang, D.; Liu, Y.; Liu, Y.; Song, Y.; Zhang, X.; Li, H.; Ma, W.; et al. Circulating Tumor Cells (CTCs) Detected by Triple-Marker EpCAM, CK19, and hMAM RT-PCR and Their Relation to Clinical Outcome in Metastatic Breast Cancer Patients. Cell Biochem. Biophys 2013, 65, 263–273. [Google Scholar] [CrossRef]
- Ko, Y.; Grünewald, E.; Totzke, G.; Klinz, M.; Fronhoffs, S.; Gouni-Berthold, I.; Sachinidis, A.; Vetter, H. High Percentage of False-Positive Results of Cytokeratin 19 RT-PCR in Blood: A Model for the Analysis of Illegitimate Gene Expression. Oncology 2000, 59, 81–88. [Google Scholar] [CrossRef]
- Hagenbeek, A. Minimal residual disease in leukemia: State of the art 1991. Leukemia 1992, 6 (Suppl. S2), 12–16. [Google Scholar]
- Maly, V.; Maly, O.; Kolostova, K.; Bobek, V. Circulating Tumor Cells in Diagnosis and Treatment of Lung Cancer. In Vivo 2019, 33, 1027–1037. [Google Scholar] [CrossRef]
- Murray, N.; Villalon, R.; Hartmann, D.; Rodriguez, P.; Aedo, S. Improvement in the Neutrophil-Lymphocyte Ratio after Combined Fluorouracil, Leucovorina and Oxaliplatino based (FOLFOX) Chemotherapy for Stage III Colon Cancer is Associated with Improved Minimal Residual Disease and Outcome. Asian Pac. J. Cancer Prev. 2022, 23, 591–599. [Google Scholar] [CrossRef]
- Smith, B.M.; Slade, M.J.; English, J.; Graham, H.; Lüchtenborg, M.; Sinnett, H.D.; Cross, N.C.P.; Coombes, R.C. Response of Circulating Tumor Cells to Systemic Therapy in Patients With Metastatic Breast Cancer: Comparison of Quantitative Polymerase Chain Reaction and Immunocytochemical Techniques. JCO 2000, 18, 1432–1439. [Google Scholar] [CrossRef]
- Suo, Y.; Gu, Z.; Wei, X. Advances of In Vivo Flow Cytometry on Cancer Studies. Cytometry 2020, 97, 15–23. [Google Scholar] [CrossRef]
- Lopresti, A.; Malergue, F.; Bertucci, F.; Liberatoscioli, M.L.; Garnier, S.; DaCosta, Q.; Finetti, P.; Gilabert, M.; Raoul, J.L.; Birnbaum, D.; et al. Sensitive and easy screening for circulating tumor cells by flow cytometry. JCI Insight 2019, 4, e128180. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Fan, L.; Zheng, J.; Cui, R.; Liu, W.; He, Y.; Li, X.; Huang, S. Detection of circulating tumor cells in breast cancer patients utilizing multiparameter flow cytometry and assessment of the prognosis of patients in different CTCs levels. Cytometry 2010, 77A, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Ao, Z.; Liu, X. Fiber-Optic Array Scanning Technology (FAST) for Detection and Molecular Characterization of Circulating Tumor Cells. In Circulating Tumor Cells; Magbanua, M.J.M., Park, J.W., Eds.; Springer: New York, NY, USA, 2017; Volume 1634, pp. 235–246. ISBN 978-1-4939-7143-5. [Google Scholar]
- Kraeft, S.-K.; Ladanyi, A.; Galiger, K.; Herlitz, A.; Sher, A.C.; Bergsrud, D.E.; Even, G.; Brunelle, S.; Harris, L.; Salgia, R.; et al. Reliable and Sensitive Identification of Occult Tumor Cells Using the Improved Rare Event Imaging System. Clin. Cancer Res. 2004, 10, 3020–3028. [Google Scholar] [CrossRef] [PubMed]
- Somlo, G.; Lau, S.K.; Frankel, P.; Hsieh, H.B.; Liu, X.; Yang, L.; Krivacic, R.; Bruce, R.H. Multiple biomarker expression on circulating tumor cells in comparison to tumor tissues from primary and metastatic sites in patients with locally advanced/inflammatory, and stage IV breast cancer, using a novel detection technology. Breast Cancer Res. Treat. 2011, 128, 155–163. [Google Scholar] [CrossRef]
- Krivacic, R.T.; Ladanyi, A.; Curry, D.N.; Hsieh, H.B.; Kuhn, P.; Bergsrud, D.E.; Kepros, J.F.; Barbera, T.; Ho, M.Y.; Chen, L.B.; et al. A rare-cell detector for cancer. Proc. Natl. Acad. Sci. USA 2004, 101, 10501–10504. [Google Scholar] [CrossRef]
- Hillig, T.; Horn, P.; Nygaard, A.-B.; Haugaard, A.S.; Nejlund, S.; Brandslund, I.; Sölétormos, G. In Vitro detection of circulating tumor cells compared by the CytoTrack and CellSearch methods. Tumor Biol. 2015, 36, 4597–4601. [Google Scholar] [CrossRef]
- Agerbæk, M.Ø.; Bang-Christensen, S.R.; Yang, M.-H.; Clausen, T.M.; Pereira, M.A.; Sharma, S.; Ditlev, S.B.; Nielsen, M.A.; Choudhary, S.; Gustavsson, T.; et al. The VAR2CSA malaria protein efficiently retrieves circulating tumor cells in an EpCAM-independent manner. Nat. Commun. 2018, 9, 3279. [Google Scholar] [CrossRef]
- Alix-Panabières, C.; Riethdorf, S.; Pantel, K. Circulating Tumor Cells and Bone Marrow Micrometastasis. Clin. Cancer Res. 2008, 14, 5013–5021. [Google Scholar] [CrossRef]
- Alix-Panabières, C.; Vendrell, J.-P.; Slijper, M.; Pellé, O.; Barbotte, E.; Mercier, G.; Jacot, W.; Fabbro, M.; Pantel, K. Full-length cytokeratin-19 is released by human tumor cells: A potential role in metastatic progression of breast cancer. Breast Cancer Res. 2009, 11, R39. [Google Scholar] [CrossRef]
- Cayrefourcq, L.; De Roeck, A.; Garcia, C.; Stoebner, P.-E.; Fichel, F.; Garima, F.; Perriard, F.; Daures, J.-P.; Meunier, L.; Alix-Panabières, C. S100-EPISPOT: A New Tool to Detect Viable Circulating Melanoma Cells. Cells 2019, 8, 755. [Google Scholar] [CrossRef]
- Alix-Panabières, C.; Vendrell, J.-P.; Pellé, O.; Rebillard, X.; Riethdorf, S.; Müller, V.; Fabbro, M.; Pantel, K. Detection and Characterization of Putative Metastatic Precursor Cells in Cancer Patients. Clin. Chem. 2007, 53, 537–539. [Google Scholar] [CrossRef] [PubMed]
- Alix-Panabières, C.; Brouillet, J.-P.; Fabbro, M.; Yssel, H.; Rousset, T.; Maudelonde, T.; Choquet-Kastylevsky, G.; Vendrell, J.-P. Characterization and enumeration of cells secreting tumor markers in the peripheral blood of breast cancer patients. J. Immunol. Methods 2005, 299, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Zieglschmid, V.; Hollmann, C.; Gutierrez, B.; Albert, W.; Strothoff, D.; Gross, E.; Böcher, O. Combination of immunomagnetic enrichment with multiplex RT-PCR analysis for the detection of disseminated tumor cells. Anticancer Res. 2005, 25, 1803–1810. [Google Scholar] [PubMed]
- Danila, D.C.; Samoila, A.; Patel, C.; Schreiber, N.; Herkal, A.; Anand, A.; Bastos, D.; Heller, G.; Fleisher, M.; Scher, H.I. Clinical Validity of Detecting Circulating Tumor Cells by AdnaTest Assay Compared With Direct Detection of Tumor mRNA in Stabilized Whole Blood, as a Biomarker Predicting Overall Survival for Metastatic Castration-Resistant Prostate Cancer Patients. Cancer J. 2016, 22, 315–320. [Google Scholar] [CrossRef]
- Aaltonen, K.E.; Novosadová, V.; Bendahl, P.-O.; Graffman, C.; Larsson, A.-M.; Rydén, L. Molecular characterization of circulating tumor cells from patients with metastatic breast cancer reflects evolutionary changes in gene expression under the pressure of systemic therapy. Oncotarget 2017, 8, 45544–45565. [Google Scholar] [CrossRef]
- Gorges, T.M.; Stein, A.; Quidde, J.; Hauch, S.; Röck, K.; Riethdorf, S.; Joosse, S.A.; Pantel, K. Improved Detection of Circulating Tumor Cells in Metastatic Colorectal Cancer by the Combination of the CellSearch® System and the AdnaTest®. PLoS ONE 2016, 11, e0155126. [Google Scholar] [CrossRef]
- Darga, E.P.; Dolce, E.M.; Fang, F.; Kidwell, K.M.; Gersch, C.L.; Kregel, S.; Thomas, D.G.; Gill, A.; Brown, M.E.; Gross, S.; et al. PD-L1 expression on circulating tumor cells and platelets in patients with metastatic breast cancer. PLoS ONE 2021, 16, e0260124. [Google Scholar] [CrossRef]
- Chikaishi, Y.; So, T.; Takenaka, M.; Oka, S.; Hirai, A.; Iwanami, T.; Shimokawa, H.; Yoneda, K.; Nagata, Y.; Uramoto, H.; et al. Comparison of CellSearch with polymeric microfluidic devices for CTC isolation using EpCAM-negative tumor cell lines of malignant pleural mesothelioma. Cancer Res. 2014, 74, 3080. [Google Scholar] [CrossRef]
- Huebner, H.; Fasching, P.A.; Gumbrecht, W.; Jud, S.; Rauh, C.; Matzas, M.; Paulicka, P.; Friedrich, K.; Lux, M.P.; Volz, B.; et al. Filtration based assessment of CTCs and CellSearch® based assessment are both powerful predictors of prognosis for metastatic breast cancer patients. BMC Cancer 2018, 18, 204. [Google Scholar] [CrossRef]
- Nicolazzo, C.; Gradilone, A.; Loreni, F.; Raimondi, C.; Gazzaniga, P. EpCAMlow Circulating Tumor Cells: Gold in the Waste. Dis. Markers 2019, 2019, 1718920. [Google Scholar] [CrossRef]
- Riethdorf, S.; Fritsche, H.; Müller, V.; Rau, T.; Schindlbeck, C.; Rack, B.; Janni, W.; Coith, C.; Beck, K.; Jänicke, F.; et al. Detection of Circulating Tumor Cells in Peripheral Blood of Patients with Metastatic Breast Cancer: A Validation Study of the CellSearch System. Clin. Cancer Res. 2007, 13, 920–928. [Google Scholar] [CrossRef]
- Camara, O.; Jörke, C.; Hammer, U.; Egbe, A.; Rabenstein, C.; Runnebaum, I.B.; Hoeffken, K.; Pachmann, K. Monitoring circulating epithelial tumour cells (CETC) to gauge therapy: In patients with disease progression after trastuzumab persisting CETC can be eliminated by combined lapatinib treatment. J. Cancer Res. Clin. Oncol. 2009, 135, 643–647. [Google Scholar] [CrossRef] [PubMed]
- Schott, D.S.; Pizon, M.; Pachmann, U.; Pachmann, K. Sensitive detection of PD-L1 expression on circulating epithelial tumor cells (CETCs) could be a potential biomarker to select patients for treatment with PD-1/PD-L1 inhibitors in early and metastatic solid tumors. Oncotarget 2017, 8, 72755–72772. [Google Scholar] [CrossRef] [PubMed]
- Gold, M.; Pachmann, K.; Kiani, A.; Schobert, R. Monitoring of circulating epithelial tumor cells using the Maintrac® method and its potential benefit for the treatment of patients with colorectal cancer. Mol. Clin. Oncol. 2021, 15, 201. [Google Scholar] [CrossRef] [PubMed]
- Iwatsuki, M.; Kurashige, J.; Ishimoto, T.; Kosumi, K.; Baba, Y.; Sakamoto, Y.; Miyamoto, Y.; Yoshida, N.; Watanabe, M.; Baba, H. The clinical significance of circulating tumor cells in gastrointestinal cancer. J. Cancer Metastasis Treat. 2015, 1, 130. [Google Scholar] [CrossRef]
- Kanayama, M.; Kuwata, T.; Mori, M.; Nemoto, Y.; Nishizawa, N.; Oyama, R.; Matsumiya, H.; Taira, A.; Shinohara, S.; Takenaka, M.; et al. Prognostic impact of circulating tumor cells detected with the microfluidic “universal CTC-chip” for primary lung cancer. Cancer Sci. 2022, 113, 1028–1037. [Google Scholar] [CrossRef]
- Stott, S.L.; Hsu, C.-H.; Tsukrov, D.I.; Yu, M.; Miyamoto, D.T.; Waltman, B.A.; Rothenberg, S.M.; Shah, A.M.; Smas, M.E.; Korir, G.K.; et al. Isolation of circulating tumor cells using a microvortex-generating herringbone-chip. Proc. Natl. Acad. Sci. USA 2010, 107, 18392–18397. [Google Scholar] [CrossRef]
- Wang, M.; Wang, Z.; Zhang, M.; Guo, W.; Li, N.; Deng, Y.; Shi, Q. A microfluidic chip with double-sided herringbone microstructures for enhanced capture of rare tumor cells. J. Mater. Chem. B 2017, 5, 9114–9120. [Google Scholar] [CrossRef] [PubMed]
- Ozkumur, E.; Shah, A.; Ciciliano, J.; Emmink, B.; Miyamoto, D.; Brachtel, E.; Yu, M.; Chen, P.; Morgan, B.; Trautwein, J.; et al. Inertial Focusing for Tumor Antigen-Dependent and -Independent Sorting of Rare Circulating Tumor Cells. Sci. Transl. Med. 2013, 5, 179ra47. [Google Scholar] [CrossRef]
- Mishra, A.; Dubash, T.D.; Edd, J.F.; Jewett, M.K.; Garre, S.G.; Karabacak, N.M.; Rabe, D.C.; Mutlu, B.R.; Walsh, J.R.; Kapur, R.; et al. Ultrahigh-throughput magnetic sorting of large blood volumes for epitope-agnostic isolation of circulating tumor cells. Proc. Natl. Acad. Sci. USA 2020, 117, 16839–16847. [Google Scholar] [CrossRef]
- Fachin, F.; Spuhler, P.; Martel-Foley, J.M.; Edd, J.F.; Barber, T.A.; Walsh, J.; Karabacak, M.; Pai, V.; Yu, M.; Smith, K.; et al. Monolithic Chip for High-throughput Blood Cell Depletion to Sort Rare Circulating Tumor Cells. Sci. Rep. 2017, 7, 10936. [Google Scholar] [CrossRef]
- Jan, Y.J.; Chen, J.-F.; Zhu, Y.; Lu, Y.-T.; Chen, S.H.; Chung, H.; Smalley, M.; Huang, Y.-W.; Dong, J.; Chen, L.-C.; et al. NanoVelcro rare-cell assays for detection and characterization of circulating tumor cells. Adv. Drug Deliv. Rev. 2018, 125, 78–93. [Google Scholar] [CrossRef] [PubMed]
- Konczalla, L.; Wöstemeier, A.; Kemper, M.; Karstens, K.-F.; Izbicki, J.; Reeh, M. Clinical Significance of Circulating Tumor Cells in Gastrointestinal Carcinomas. Diagnostics 2020, 10, 192. [Google Scholar] [CrossRef]
- Li, Y.; Wu, S.; Bai, F. Molecular characterization of circulating tumor cells—From bench to bedside. Semin. Cell Dev. Biol. 2018, 75, 88–97. [Google Scholar] [CrossRef]
- Keller, L.; Pantel, K. Unravelling tumour heterogeneity by single-cell profiling of circulating tumour cells. Nat. Rev. Cancer 2019, 19, 553–567. [Google Scholar] [CrossRef]
- Zhou, X.; Xu, Y.; Zhu, L.; Su, Z.; Han, X.; Zhang, Z.; Huang, Y.; Liu, Q. Comparison of Multiple Displacement Amplification (MDA) and Multiple Annealing and Looping-Based Amplification Cycles (MALBAC) in Limited DNA Sequencing Based on Tube and Droplet. Micromachines 2020, 11, 645. [Google Scholar] [CrossRef] [PubMed]
- Ju, S.; Chen, C.; Zhang, J.; Xu, L.; Zhang, X.; Li, Z.; Chen, Y.; Zhou, J.; Ji, F.; Wang, L. Detection of circulating tumor cells: Opportunities and challenges. Biomark. Res. 2022, 10, 58. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Lin, X.; Hui, Y.; Wang, J.; Zhang, Q.; Kong, F. Circulating Tumor Cell Identification Based on Deep Learning. Front. Oncol. 2022, 12, 843879. [Google Scholar] [CrossRef] [PubMed]
- Nasiri, R.; Shamloo, A.; Ahadian, S.; Amirifar, L.; Akbari, J.; Goudie, M.J.; Lee, K.; Ashammakhi, N.; Dokmeci, M.R.; Di Carlo, D.; et al. Microfluidic-Based Approaches in Targeted Cell/Particle Separation Based on Physical Properties: Fundamentals and Applications. Small 2020, 16, 2000171. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhou, Y.; Qin, X.; Nair, S.; Huang, X.; Liu, Y. Label-free detection of rare circulating tumor cells by image analysis and machine learning. Sci. Rep. 2020, 10, 12226. [Google Scholar] [CrossRef]
- Lannin, T.B.; Thege, F.I.; Kirby, B.J. Comparison and optimization of machine learning methods for automated classification of circulating tumor cells: Automated Circulating Tumor Cell Image Classifiers. Cytometry 2016, 89, 922–931. [Google Scholar] [CrossRef]
- Deepcell. Available online: https://deepcell.com (accessed on 15 January 2023).
- Saucedo-Zeni, N. A novel method for the in vivo isolation of circulating tumor cells from peripheral blood of cancer patients using a functionalized and structured medical wire. Int. J. Oncol. 2012, 41, 1241–1250. [Google Scholar] [CrossRef] [PubMed]
- Dizdar, L.; Fluegen, G.; Dalum, G.; Honisch, E.; Neves, R.P.; Niederacher, D.; Neubauer, H.; Fehm, T.; Rehders, A.; Krieg, A.; et al. Detection of circulating tumor cells in colorectal cancer patients using the GILUPI CellCollector: Results from a prospective, single-center study. Mol. Oncol. 2019, 13, 1548–1558. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.-B.; Chen, M.-M.; Wang, Y.-K.; Sun, Z.-H.; Qin, Y.; Tian, S.; Dong, W.-G.; Xie, M.; Huang, W.-H. A Three-Dimensional Conductive Scaffold Microchip for Effective Capture and Recovery of Circulating Tumor Cells with High Purity. Anal. Chem. 2021, 93, 7102–7109. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Zhao, D.; Zhang, Y.; Huang, L.; Huang, H.; Guo, Q.; Zhang, W.; Hou, C.; Wang, H.; Zhang, Q.; et al. Facile synthesis of 3D hierarchical micro-/nanostructures in capillaries for efficient capture of circulating tumor cells. J. Colloid Interface Sci. 2020, 575, 108–118. [Google Scholar] [CrossRef]
- Chen, J.; Liu, C.-Y.; Wang, X.; Sweet, E.; Liu, N.; Gong, X.; Lin, L. 3D printed microfluidic devices for circulating tumor cells (CTCs) isolation. Biosens. Bioelectron. 2020, 150, 111900. [Google Scholar] [CrossRef]
- Salami, S.S.; Singhal, U.; Spratt, D.E.; Palapattu, G.S.; Hollenbeck, B.K.; Schonhoft, J.D.; Graf, R.; Louw, J.; Jendrisak, A.; Dugan, L.; et al. Circulating Tumor Cells as a Predictor of Treatment Response in Clinically Localized Prostate Cancer. JCO Precis. Oncol. 2019, 3, 00352. [Google Scholar] [CrossRef]
- Scher, H.I.; Armstrong, A.J.; Schonhoft, J.D.; Gill, A.; Zhao, J.L.; Barnett, E.; Carbone, E.; Lu, J.; Antonarakis, E.S.; Luo, J.; et al. Development and validation of circulating tumour cell enumeration (Epic Sciences) as a prognostic biomarker in men with metastatic castration-resistant prostate cancer. Eur. J. Cancer 2021, 150, 83–94. [Google Scholar] [CrossRef]
- Werner, S.L.; Graf, R.P.; Landers, M.; Valenta, D.T.; Schroeder, M.; Greene, S.B.; Bales, N.; Dittamore, R.; Marrinucci, D. Analytical Validation and Capabilities of the Epic CTC Platform: Enrichment-Free Circulating Tumour Cell Detection and Characterization. J. Circ. Biomark. 2015, 4, 3. [Google Scholar] [CrossRef]
- Epic Sciences. Available online: https://www.epicsciences.com/technology/ (accessed on 27 July 2022).
- Einoch-Amor, R.; Broza, Y.Y.; Haick, H. Detection of Single Cancer Cells in Blood with Artificially Intelligent Nanoarray. ACS Nano 2021, 15, 7744–7755. [Google Scholar] [CrossRef]
- Einoch Amor, R.; Zinger, A.; Broza, Y.Y.; Schroeder, A.; Haick, H. Artificially Intelligent Nanoarray Detects Various Cancers by Liquid Biopsy of Volatile Markers. Adv. Healthc. Mater. 2022, 11, 2200356. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.-H.; Liu, R.; Ozkaya-Ahmadov, T.; Boya, M.; Swain, B.E.; Owens, J.M.; Burentugs, E.; Bilen, M.A.; McDonald, J.F.; Sarioglu, A.F. Hybrid negative enrichment of circulating tumor cells from whole blood in a 3D-printed monolithic device. Lab Chip 2019, 19, 3427–3437. [Google Scholar] [CrossRef] [PubMed]
- Fujii, T.; Reuben, J.M.; Huo, L.; Espinosa Fernandez, J.R.; Gong, Y.; Krupa, R.; Suraneni, M.V.; Graf, R.P.; Lee, J.; Greene, S.; et al. Androgen receptor expression on circulating tumor cells in metastatic breast cancer. PLoS ONE 2017, 12, e0185231. [Google Scholar] [CrossRef] [PubMed]
- Stiefel, J.; Freese, C.; Sriram, A.; Alebrand, S.; Srinivas, N.; Sproll, C.; Wandrey, M.; Gül, D.; Hagemann, J.; Becker, J.C.; et al. Characterization of a novel microfluidic platform for the isolation of rare single cells to enable CTC analysis from head and neck squamous cell carcinoma patients. Eng. Life Sci. 2022, 22, 391–406. [Google Scholar] [CrossRef] [PubMed]
- VyCap Technology. Available online: https://www.vycap.com/technology/ctc-enumeration/ (accessed on 9 January 2023).
- Kitz, J.; Goodale, D.; Postenka, C.; Lowes, L.E.; Allan, A.L. EMT-independent detection of circulating tumor cells in human blood samples and pre-clinical mouse models of metastasis. Clin. Exp. Metastasis 2021, 38, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Schwab, F.D.; Scheidmann, M.C.; Ozimski, L.L.; Kling, A.; Armbrecht, L.; Ryser, T.; Krol, I.; Strittmatter, K.; Nguyen-Sträuli, B.D.; Jacob, F.; et al. MyCTC chip: Microfluidic-based drug screen with patient-derived tumour cells from liquid biopsies. Microsyst. Nanoeng. 2022, 8, 130. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Lin, J.; Guo, Y.; Wu, X.; Xu, Y.; Zhang, D.; Zhang, X.; Yujiao, X.; Wang, J.; Yao, C.; et al. TiO2-based Surface-Enhanced Raman Scattering bio-probe for efficient circulating tumor cell detection on microfilter. Biosens. Bioelectron. 2022, 210, 114305. [Google Scholar] [CrossRef]
- Jia, F.; Wang, Y.; Fang, Z.; Dong, J.; Shi, F.; Zhang, W.; Wang, Z.; Hu, Z. Novel Peptide-Based Magnetic Nanoparticle for Mesenchymal Circulating Tumor Cells Detection. Anal. Chem. 2021, 93, 5670–5675. [Google Scholar] [CrossRef]
- Yang, Y.; Pang, W.; Zhang, H.; Cui, W.; Jin, K.; Sun, C.; Wang, Y.; Zhang, L.; Ren, X.; Duan, X. Manipulation of single cells via a Stereo Acoustic Streaming Tunnel (SteAST). Microsyst. Nanoeng. 2022, 8, 88. [Google Scholar] [CrossRef]
- Biezuner, T.; Raz, O.; Amir, S.; Milo, L.; Adar, R.; Fried, Y.; Ainbinder, E.; Shapiro, E. Comparison of seven single cell whole genome amplification commercial kits using targeted sequencing. Sci. Rep. 2021, 11, 17171. [Google Scholar] [CrossRef]
- Gonzalez-Pena, V.; Natarajan, S.; Xia, Y.; Klein, D.; Carter, R.; Pang, Y.; Shaner, B.; Annu, K.; Putnam, D.; Chen, W.; et al. Accurate genomic variant detection in single cells with primary template-directed amplification. Proc. Natl. Acad. Sci. USA 2021, 118, e2024176118. [Google Scholar] [CrossRef]
- Chen, C.; Xing, D.; Tan, L.; Li, H.; Zhou, G.; Huang, L.; Xie, X.S. Single-cell whole-genome analyses by Linear Amplification via Transposon Insertion (LIANTI). Science 2017, 356, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Gkountela, S.; Castro-Giner, F.; Szczerba, B.M.; Vetter, M.; Landin, J.; Scherrer, R.; Krol, I.; Scheidmann, M.C.; Beisel, C.; Stirnimann, C.U.; et al. Circulating Tumor Cell Clustering Shapes DNA Methylation to Enable Metastasis Seeding. Cell 2019, 176, 98–112.e14. [Google Scholar] [CrossRef]
- Carter, L.; Rothwell, D.G.; Mesquita, B.; Smowton, C.; Leong, H.S.; Fernandez-Gutierrez, F.; Li, Y.; Burt, D.J.; Antonello, J.; Morrow, C.J.; et al. Molecular analysis of circulating tumor cells identifies distinct copy-number profiles in patients with chemosensitive and chemorefractory small-cell lung cancer. Nat. Med. 2017, 23, 114–119. [Google Scholar] [CrossRef] [PubMed]
- TRACERx Consortium; Chemi, F.; Rothwell, D.G.; McGranahan, N.; Gulati, S.; Abbosh, C.; Pearce, S.P.; Zhou, C.; Wilson, G.A.; Jamal-Hanjani, M.; et al. Pulmonary venous circulating tumor cell dissemination before tumor resection and disease relapse. Nat. Med. 2019, 25, 1534–1539. [Google Scholar] [CrossRef] [PubMed]
- Baslan, T.; Hicks, J. Unravelling biology and shifting paradigms in cancer with single-cell sequencing. Nat. Rev. Cancer 2017, 17, 557–569. [Google Scholar] [CrossRef]
- Alles, J.; Karaiskos, N.; Praktiknjo, S.D.; Grosswendt, S.; Wahle, P.; Ruffault, P.-L.; Ayoub, S.; Schreyer, L.; Boltengagen, A.; Birchmeier, C.; et al. Cell fixation and preservation for droplet-based single-cell transcriptomics. BMC Biol. 2017, 15, 44. [Google Scholar] [CrossRef]
- Cheng, Y.-H.; Chen, Y.-C.; Lin, E.; Brien, R.; Jung, S.; Chen, Y.-T.; Lee, W.; Hao, Z.; Sahoo, S.; Min Kang, H.; et al. Hydro-Seq enables contamination-free high-throughput single-cell RNA-sequencing for circulating tumor cells. Nat. Commun. 2019, 10, 2163. [Google Scholar] [CrossRef]
- Kwan, T.T.; Bardia, A.; Spring, L.M.; Giobbie-Hurder, A.; Kalinich, M.; Dubash, T.; Sundaresan, T.; Hong, X.; LiCausi, J.A.; Ho, U.; et al. A Digital RNA Signature of Circulating Tumor Cells Predicting Early Therapeutic Response in Localized and Metastatic Breast Cancer. Cancer Discov. 2018, 8, 1286–1299. [Google Scholar] [CrossRef]
- Aya-Bonilla, C.A.; Morici, M.; Hong, X.; McEvoy, A.C.; Sullivan, R.J.; Freeman, J.; Calapre, L.; Khattak, M.A.; Meniawy, T.; Millward, M.; et al. Detection and prognostic role of heterogeneous populations of melanoma circulating tumour cells. Br. J. Cancer 2020, 122, 1059–1067. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Zhuang, R.; Long, M.; Pavlovic, M.; Kang, Y.; Ilyas, A.; Asghar, W. Circulating tumor cell isolation, culture, and downstream molecular analysis. Biotechnol. Adv. 2018, 36, 1063–1078. [Google Scholar] [CrossRef]
- Ortiz, V.; Yu, M. Analyzing Circulating Tumor Cells One at a Time. Trends Cell Biol. 2018, 28, 764–775. [Google Scholar] [CrossRef]
- Abouleila, Y.; Onidani, K.; Ali, A.; Shoji, H.; Kawai, T.; Lim, C.T.; Kumar, V.; Okaya, S.; Kato, K.; Hiyama, E.; et al. Live single cell mass spectrometry reveals cancer-specific metabolic profiles of circulating tumor cells. Cancer Sci. 2019, 110, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Sinkala, E.; Sollier-Christen, E.; Renier, C.; Rosàs-Canyelles, E.; Che, J.; Heirich, K.; Duncombe, T.A.; Vlassakis, J.; Yamauchi, K.A.; Huang, H.; et al. Profiling protein expression in circulating tumour cells using microfluidic western blotting. Nat. Commun. 2017, 8, 14622. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.-Y.; Huang, M.; Wang, X.-K.; Zhu, Y.; Li, J.-S.; Wong, C.C.L.; Fang, Q. Nanoliter-Scale Oil-Air-Droplet Chip-Based Single Cell Proteomic Analysis. Anal. Chem. 2018, 90, 5430–5438. [Google Scholar] [CrossRef]
- Terekhov, S.S.; Smirnov, I.V.; Stepanova, A.V.; Bobik, T.V.; Mokrushina, Y.A.; Ponomarenko, N.A.; Belogurov, A.A.; Rubtsova, M.P.; Kartseva, O.V.; Gomzikova, M.O.; et al. Microfluidic droplet platform for ultrahigh-throughput single-cell screening of biodiversity. Proc. Natl. Acad. Sci. USA 2017, 114, 2550–2555. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lian, J.; Chen, Y.; Zhao, X.; Du, C.; Xu, Y.; Hu, H.; Rao, H.; Hong, X. Circulating Tumor Cells (CTCs): A Unique Model of Cancer Metastases and Non-invasive Biomarkers of Therapeutic Response. Front. Genet. 2021, 12, 734595. [Google Scholar] [CrossRef]
- Yang, Y.-P.; Giret, T.M.; Cote, R.J. Circulating Tumor Cells from Enumeration to Analysis: Current Challenges and Future Opportunities. Cancers 2021, 13, 2723. [Google Scholar] [CrossRef]
- Eslami, S.Z.; Cortés-Hernández, L.E.; Alix-Panabières, C. Epithelial Cell Adhesion Molecule: An Anchor to Isolate Clinically Relevant Circulating Tumor Cells. Cells 2020, 9, 1836. [Google Scholar] [CrossRef]
- Hyun, K.-A.; Koo, G.-B.; Han, H.; Sohn, J.; Choi, W.; Kim, S.-I.; Jung, H.-I.; Kim, Y.-S. Epithelial-to-mesenchymal transition leads to loss of EpCAM and different physical properties in circulating tumor cells from metastatic breast cancer. Oncotarget 2016, 7, 24677–24687. [Google Scholar] [CrossRef]
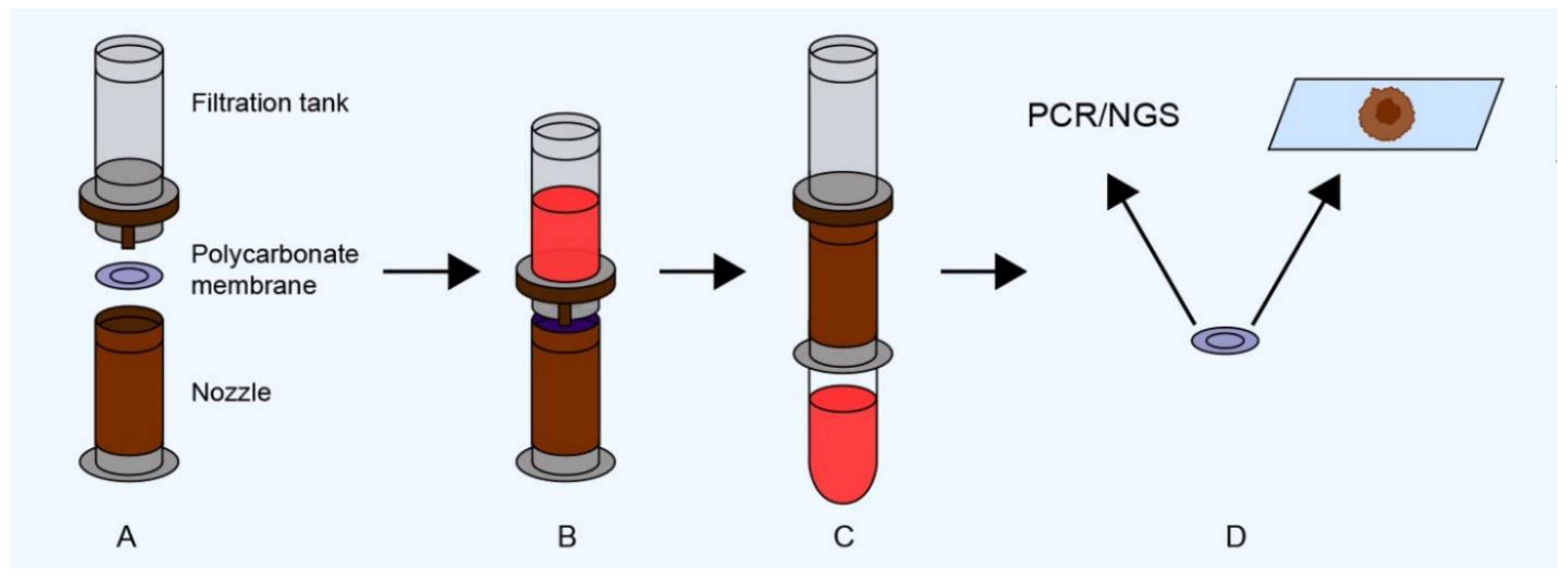
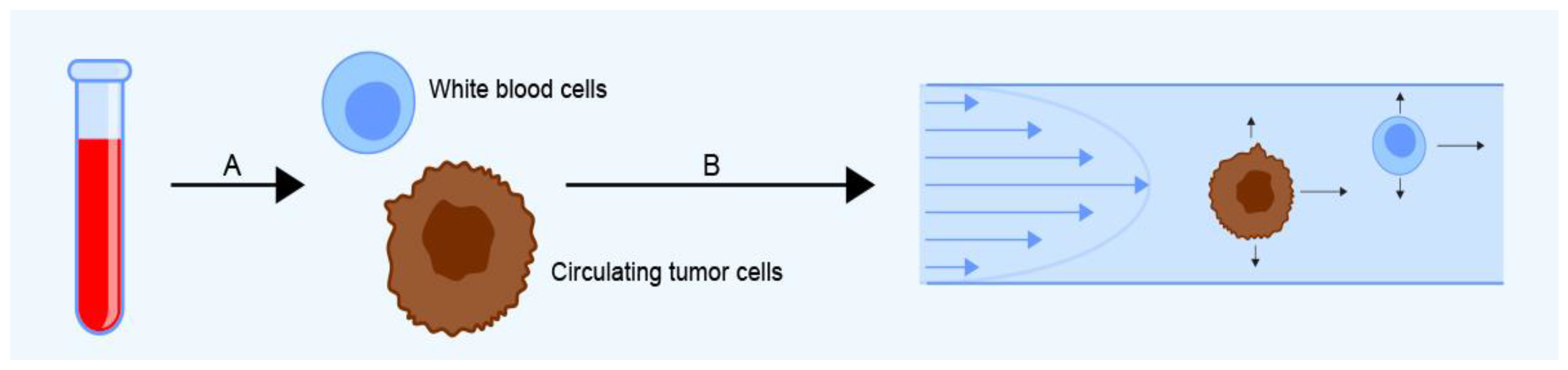
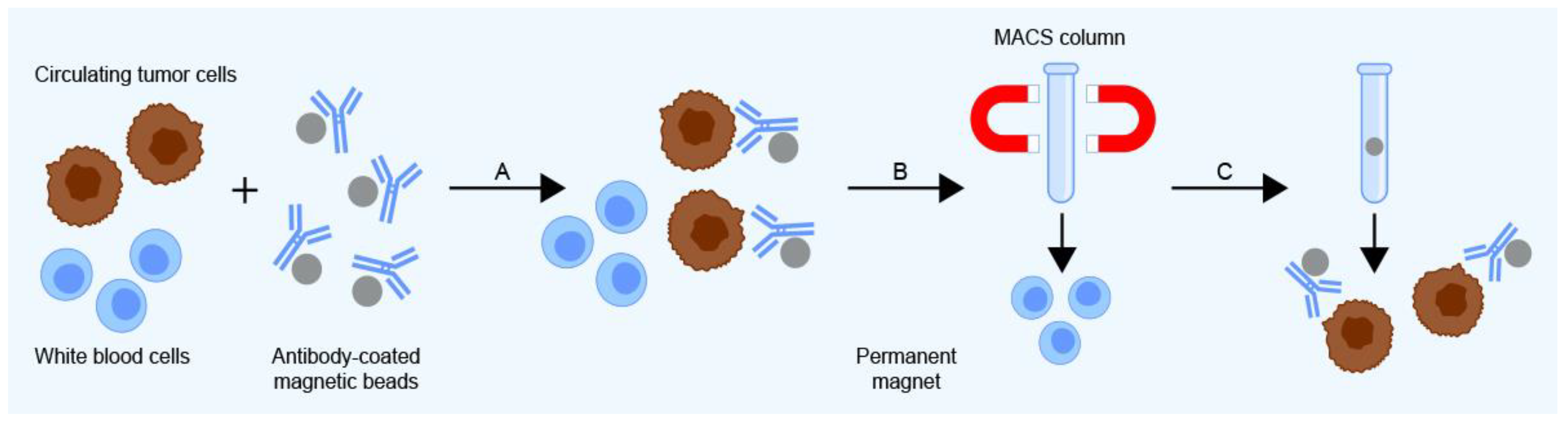

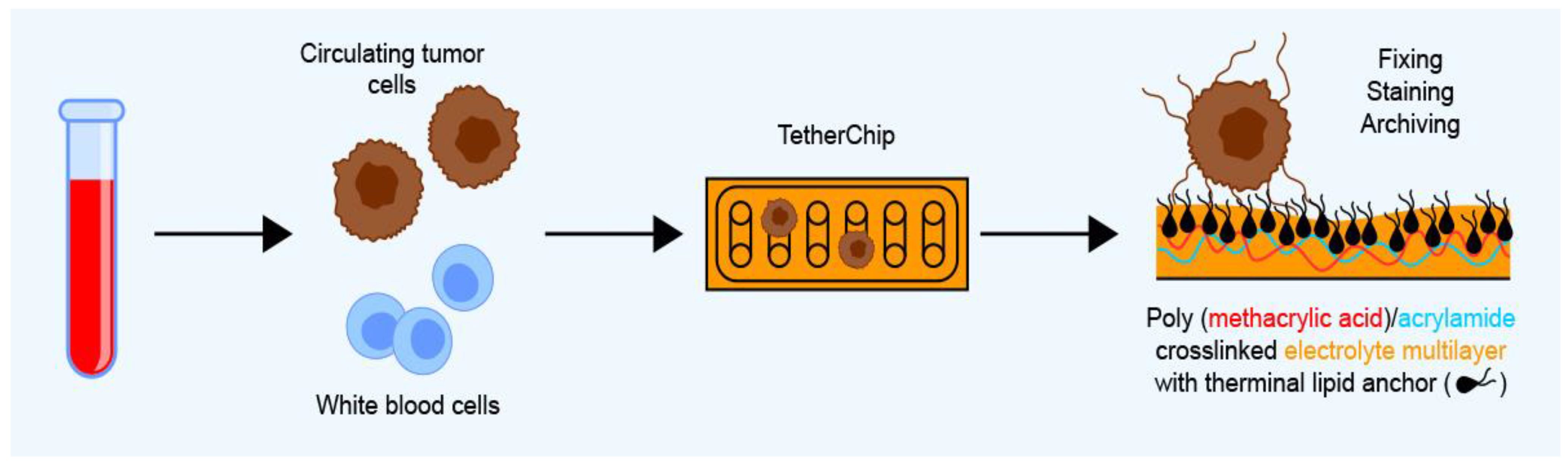
| CTC Enrichment Techniques | ||||||||
|---|---|---|---|---|---|---|---|---|
| Name | Commercially Available Formats or Providers | Mode of Enrichment | Antibodies | Material | Advantages | Disadvantages | References | |
| Morphology-based approaches | Isolation by size of epithelial tumor cells (ISET) | Screen Cell (Screen Cell, Paris, France), CTCBIOPSY® (YZYBIO Company, Wuhan, China) | Filtration by size through a polycarbonate membrane with 8-µm diameter cylindrical pores | - | Blood or other biological fluid | Simplicity, high sensitivity, possibility to detect CTCs directly on membrane or isolate them. Applicable to all tumor types; low price; fast | Results may be affected by the morphological variability of CTCs, possibly leading to false-negative responses | [28,29] |
| Micro-electro-mechanical system (MEMS) | - | Filtration by size through a parylene membrane with 10-µm diameter pores | - | Blood | Simplicity, high sensitivity, possibility to detect CTCs directly on membrane or isolate them. Applicable to all tumor types | Results may be affected by the morphological variability of CTCs, possibly leading to false-negative responses | [30] | |
| Ficoll density gradient | Ficoll-Hypaque (Cytiva, Marlborough, MA, USA), OncoQuick Assay (Hexal Gentech, Holkirchen, Germany, and Greiner Bio-One, Kremsmünster, Austria) | Density gradient centrifugation | - | Blood or bone marrow | Rapid and convenient method, provides viable cells, low price | Sensitivity is low and depends on tumor characteristics, centrifugation time, and temperature | [35] | |
| Dielectrophoretic field-flow fractionation (DEP-FFF) | ApoStream® (Apocell company, Houston, TX, USA), ParsortixTM Cell Separation System (ANGLE, Guildford, UK), VTX-1 Liquid Biopsy System (Vortex Biosciences, Pleasanton, CA, USA), JETTATM (Denovo Sciences, Yerevan, Armenia) | Separation based on the dielectric characteristics of CTCs combined with field-flow fractionation | - | Blood | Label-free, possibility to obtain viable cells that can be isolated and cultured, short processing time (~2.5 mL/h) | Possibility of dielectric interactions between cells and changes in their dielectric properties during prolonged storage | [37,40,43,44,46] | |
| Dean flow fractionation (DFF) | ClearCell® FX System (Genomax Technology, Bangkok, Thailand) | Microfluidic separation based on centrifugal force | - | Blood | Possibility to continuously collect viable CTCs, short processing time (36 mL/h) | Less efficient for small CTCs | [48] | |
| Immunology-based approaches | Magnetic-activated cell sorting (MACS) | MACS (Miltenyi Biotec, San Jose, CA, USA) | Capture by immuno-labeled magnetic microbeads using superparamagnetic nanoparticles and columns | Cytokeratin, EpCAM, EGFR, and HER2 | Blood or bone marrow | High sensitivity, enables automated separation | Expensive | [47] |
| Cell/collagen adhesion matrix (CAM) invasion assay | Vita AssayTM (Applied DNA Sciences, Stony Brook, NY, USA) | Based on CTCs’ ability to bind, invade, and ingest a CAM and express biomarkers | EpCAM, Epithelial specific antigen (ESA), and pan-CK (CKs 4, 5, 6, 8, 10, 13, and 18) | Blood | Enrichment of viable cells, which can be used to determine invasiveness and tumor progenitor phenotypes of CTCs | CAM may be present in the blood of healthy references as well as cancer cases; isolation step requires more than 12 h | [22,55] | |
| CTC Detection Techniques | ||||||||
|---|---|---|---|---|---|---|---|---|
| Name | Commercially Available Formats or Providers | Mode of Detection | Antibodies | Material | Advantages | Disadvantages | References | |
| Nucleic-acid-based detection | Reverse transcriptase polymerase chain reaction (RT-PCR) | Range of assays for selected diagnoses | Reverse transcription of CTC-specific mRNA to complementary DNA (cDNA) followed by PCR-amplification of cDNA | - | Blood, bone marrow, tissue, and other biological samples | High sensitivity | RNA instability, illegitimate expression, and false positivity; does not allow isolation of viable cells | [2,62,64] |
| Cytometry-based detection | Immunocytochemistry (ICC) | Range of assays for selected diagnoses | Antibody staining of tumor-specific antigens | Chosen based on proteins expressed in primary tumors | Blood, bone marrow, tissue, and other biological samples | Can be conjugated with automated imaging system | Limited number of cells evaluated, risks of cross-reactions with other epitopes, low sensitivity | [70,71] |
| Flow cytometry (FC) | Range of assays for selected diagnoses | Quantification of surface and intracellular antigens using antibodies conjugated with fluorescent dye | Chosen based on proteins expressed in primary tumors | Blood, bone marrow, tissue, and other biological samples | Ability to measure multiple parameters of large numbers of cells relatively quickly. Cells can be isolated for further analysis | Low sensitivity, time-consuming | [72] | |
| Automated digital microscopy (ADM) | - | Fluorescence microscopy and robotic motion control system to automate imaging | Antibodies against tumor-specific biomarkers | Blood | Identification of very rare epithelial cells in whole blood samples | Enrichment step needed, long exposure time (800 cells/s) | [75,77] | |
| Fiber-optic array scanning technology (FAST) | FASTcell™ (SRI International, Menlo Park, CA, USA) | Image analysis of immunocytochemically labeled tumor cells | Antibodies to tumor-specific biomarkers | Blood | Does not require enrichment step | Special type of cytometer needed | [75,76,77] | |
| Microscopy-based detection | Fluorescence microscopy | Range of assays for selected diagnoses, e.g., CytoTrack® (Cytotrack Aps, Lyngby, Denmark), RareCyte® (RareCyte, Inc., Seattle, WA, USA) | Optical microscopic examination of cells stained immunologically | Based on genes expressed in primary tumors, e.g., Anti-EpCAM and cytokeratins | Blood, bone marrow, tissue, other biological samples | Enrichment-free; automated microscopic imaging system; possibility of single-cell retrieval and further molecular characterization of CTCs; can be used for non-epithelial cells | Limited observation time, manual assessment necessary; long processing time; isolated cells are not viable | [11,25,79] |
| Approaches Combining CTC Enrichment and Detection | ||||||||
|---|---|---|---|---|---|---|---|---|
| Name | Commercially Available Formats or Providers | Mode of Enrichment | Mode of Detection | Antibodies | Material | Advantages | Disadvantages | References |
| EPithelial ImmunoSPOT assay (EPISPOT) | - | Negative selection using anti-CD45 immuno-magnetic beads, culture in plates pre-coated with antibodies to capture secreted protein of interest | Secreted protein spots are detected via immunological techniques and counted | Cathepsin D, MUC1, CK19, PSA | Blood | Detects only viable cells | The protein used to identify CTCs must be actively secreted, shed, or released from cells | [81,82,83] |
| AdnaTest | AdnaTest (Qiagen, Hilden, Germany) | Immuno-magnetic separation (AdnaTest Select) | Multiplex RT-PCR (AdnaTest Detect) | MUC-1, HER-2, EpCAM, CEA, EGFR, PSA, PSMA, Aldehyde dehydrogenase 1 (ALDH1) | Blood | Specific enrichment and high sensitivity | Not automated; processing time of 5 h; expensive | [86,89] |
| CellSearch system | CellSearch (Menarini Silicon Biosystems, Castel Maggiore, Italy) | Immuno-magnetic separation | Flow cytometry and immunofluorescence imaging | EpCAM, CKs 8, 18, 19 | Blood | High sensitivity, specificity, and reproducibility; semi-automated; FDA approved | Low sensitivity for cells with low EpCAM expression | [90,92,93] |
| MAINTRAC | - | Red blood cell lysis and centrifugation | Laser scanning cytometry, automated fluorescence microscopy | EpCAM | Blood | Does not clean or enrich cells, which minimizes cell damage and loss | Cannot be used for early diagnosis | [95,96,97] |
| CTC-Chip | CTC-Chip, CTC-iChip (Massachusetts General Hospital, Boston, MA, USA), NanoVelcro chip (UCLA, Los Angeles, CA, USA) | Microfluidic separation on silicon chip microposts with EpCAM antibodies | Cytokeratin antibodies and DAPI | Cytokeratin | Blood | High sensitivity; isolates viable CTCs; short processing time | Does not detect CTC-WBC clusters | [98,102,104,105] |
| Evolving Methods for CTC Detection and Characterization | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Name | Commercially Available Formats or Providers | Mode of Enrichment | Mode of Detection | Antibodies | Material | Advantages | Disadvantages | References | ||
| Enrichment techniques | Morphology-based approaches | Deepcell | Deepcell platform (Deepcell, Inc., Menlo Park, CA, USA) | Isolation of viable cells based on morphological distinction | Images are analyzed using deep learning AI | - | Blood and other body fluids | Permits cluster analysis and further molecular characterization of CTCs | Not clinically validated | [115] |
| Nanotube-CTC-chip | - | CTCs adhere to a carbon nanotube surface via filaments extending from the main body of the cell | CTCs are immunostained on-chip and analyzed using automated fluorescence microscopy | DAPI, CKs 8/18, Her2, EGFR, and anti-CD45 | Blood | Antigen- and size-independent capture | RBC lysis is necessary | [23] | ||
| TetherChip | - | CTCs are captured based on the affinity of their microtentacles for a polyelectrolyte multilayer | Immunofluorescence staining with Hoechst, WGA, and GFP followed by fluorescence microscopy analysis | - | Blood | Preserves microtentacle structure after fixation and isolation from blood; enables testing of functional phenotypes in CTCs | Only tested on cell lines at the time of writing | [24] | ||
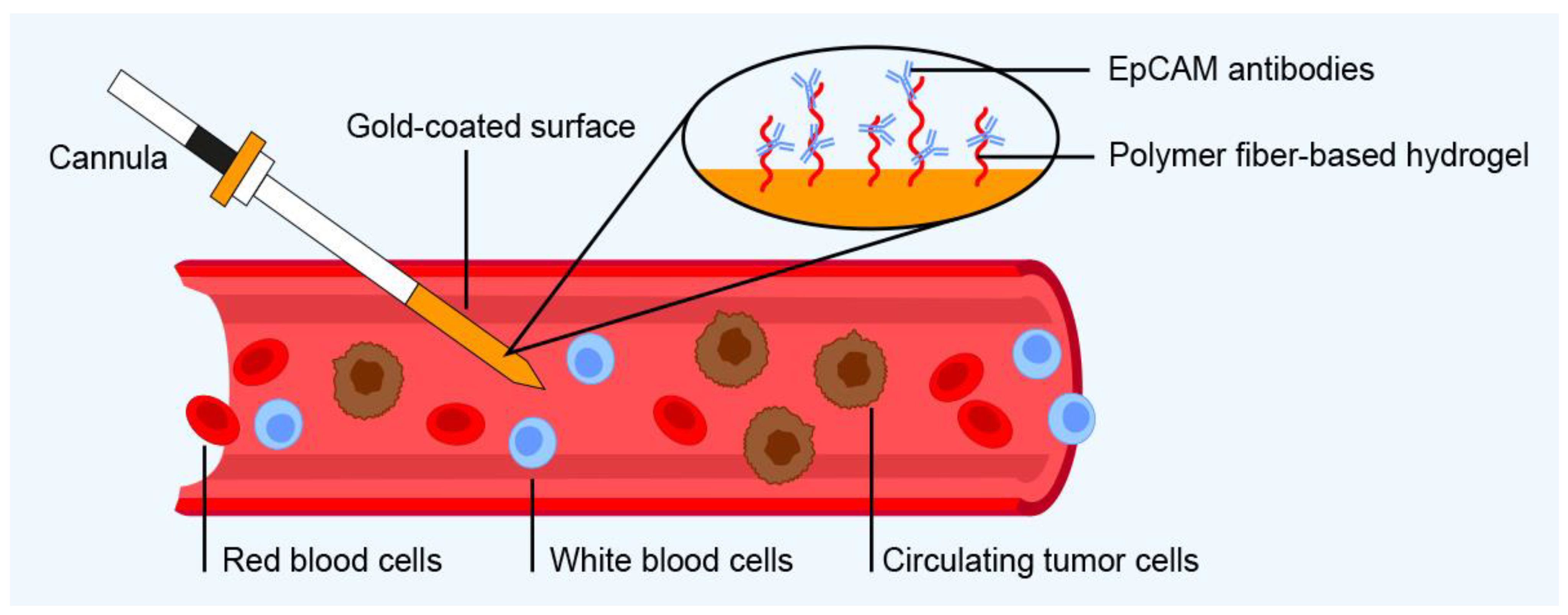
| Immunology-based approaches | GILUPI CellCollector | GILUPI CellCollector (GILUPI GmbH, Potsdam, Germany) | CTCs captured by antibodies immobilized on a hydrogel | Immunofluorescence staining and molecular analysis (e.g., PCR, sequencing, gene expression analysis) | EpCAM | Blood | Enriches CTCs directly from bloodstream rather than volume-limited blood samples; enrichment time is 30 min | Used only for enrichment of CTCs directly from patient’s bloodstream | [116,117] | |
| 3D conductive scaffold microchip | - | CTCs are captured on a 3D conductive scaffold made from porous polydimethylsiloxane with immobilized gold nanotubes (Au-NT) coated with an anti-EpCAM antibody | Immunocytochemistry using FITC-CK, PE-CD45, and DAPI | EpCAM | Blood | Captured cells can be reversibly released with high viability; high sensitivity | CTC clusters released less efficiently than single CTCs because of re-capture by the 3D scaffold | [118] | ||
| 3D nanoforest array | - | Cellular filopodia of CTCs interact with lateral branches of Zn(OH)F nanowires conjugated to an anti-EpCAM antibody | Immunofluorescence staining and fluorescence microscopy analysis | EpCAM, CD45, CK | Blood | Large binding surfaces provide many binding sites for CTC capture | Only tested on cell lines at the time of writing | [119] | ||
| 3D-printed functionalized device | - | 3D-printed channel whose inner surface was functionalized with anti-EpCAM | Confocal laser scanning microscopy | EpCAM | Blood | Microfluidic device with a large binding surface area | Only tested on cell lines at the time of writing | [120] | ||
| Detection techniques | Epic Sciences | Epic Sciences (Epic Sciences, Inc., San Diego, CA, USA) | - | Pyxis™—whole slide fluorescent scanner | Cytokeratin, CD45, DAPI, and specific antibodies | Blood | Enrichment-free; cancer profiling combining CTC technology with circulating tumor DNA (ctDNA) and immune cell analysis | Samples must be sent to the company for analysis, only for prostate and breast cancer | [121,122,124] | |
| AI nanoarray | - | Detects both cancer cells and VOCs from cancer cells and their microenvironment | Gas chromatography linked with mass spectrometry | - | Blood | High sensitivity and specificity for early detection | Only tested on cell lines and a mouse model at the time of writing | [125,126] | ||
| Approaches combining CTC enrichment and detection | 3D-printed microfluidic device | - | WBCs are captured in the device’s immunocapture channels; RBCs, platelets, and all nucleated cells migrate to a membrane micropore filter | CTCs are immunostained on-chip and analyzed using fluorescence microscopy | CD45 | Blood | Label-free negative depletion of CTCs; isolation of very small CTCs | Only tested on cell lines at the time of writing | [127] | |
| CTCelect | CTCelect system (Fraunhofer Institute for Microengineering and Microsystems, IMM, Mainz, Germany) | Combines immunomagnetic enrichment with microfluidic sorting of fluorescence-activated cells | Fluorescence microscopy | EpCAM | Blood | Fully automated; permits further molecular characterization of CTCs | Captures only single cells, not clusters | [128,129] | ||
| VyCAP | VyCAP technology (VyCap B.V., Enschede, The Netherlands) | Size-based filtration through a microsieve filter chip | Fluorescence microscopy with automated imaging system | CK, CD16, and CD45. Other cancer-specific labels can also be used (e.g., MUC-1, PDL-1) | Blood | Fully automated; filtration under low pressure, which minimizes damage to captured cells | Not clinically validated | [130,131] | ||
| MyCTC chip | - | CTCs are captured on microfluidic chip with a polydimethylsiloxane upper layer and a rigid cyclic olefin copolymer underlayer | Cultivation of captured CTCs | - | Blood or other body fluids | Label- and antigen-free; captures clusters with high efficiency | Not clinically validated | [132] | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vidlarova, M.; Rehulkova, A.; Stejskal, P.; Prokopova, A.; Slavik, H.; Hajduch, M.; Srovnal, J. Recent Advances in Methods for Circulating Tumor Cell Detection. Int. J. Mol. Sci. 2023, 24, 3902. https://doi.org/10.3390/ijms24043902
Vidlarova M, Rehulkova A, Stejskal P, Prokopova A, Slavik H, Hajduch M, Srovnal J. Recent Advances in Methods for Circulating Tumor Cell Detection. International Journal of Molecular Sciences. 2023; 24(4):3902. https://doi.org/10.3390/ijms24043902
Chicago/Turabian StyleVidlarova, Monika, Alona Rehulkova, Pavel Stejskal, Andrea Prokopova, Hanus Slavik, Marian Hajduch, and Josef Srovnal. 2023. "Recent Advances in Methods for Circulating Tumor Cell Detection" International Journal of Molecular Sciences 24, no. 4: 3902. https://doi.org/10.3390/ijms24043902
APA StyleVidlarova, M., Rehulkova, A., Stejskal, P., Prokopova, A., Slavik, H., Hajduch, M., & Srovnal, J. (2023). Recent Advances in Methods for Circulating Tumor Cell Detection. International Journal of Molecular Sciences, 24(4), 3902. https://doi.org/10.3390/ijms24043902







