Effect of Fibrillization pH on Gelation Viscoelasticity and Properties of Biofabricated Dense Collagen Matrices via Gel Aspiration-Ejection
Abstract
1. Introduction
2. Results and Discussion
2.1. Turbidity Measurements
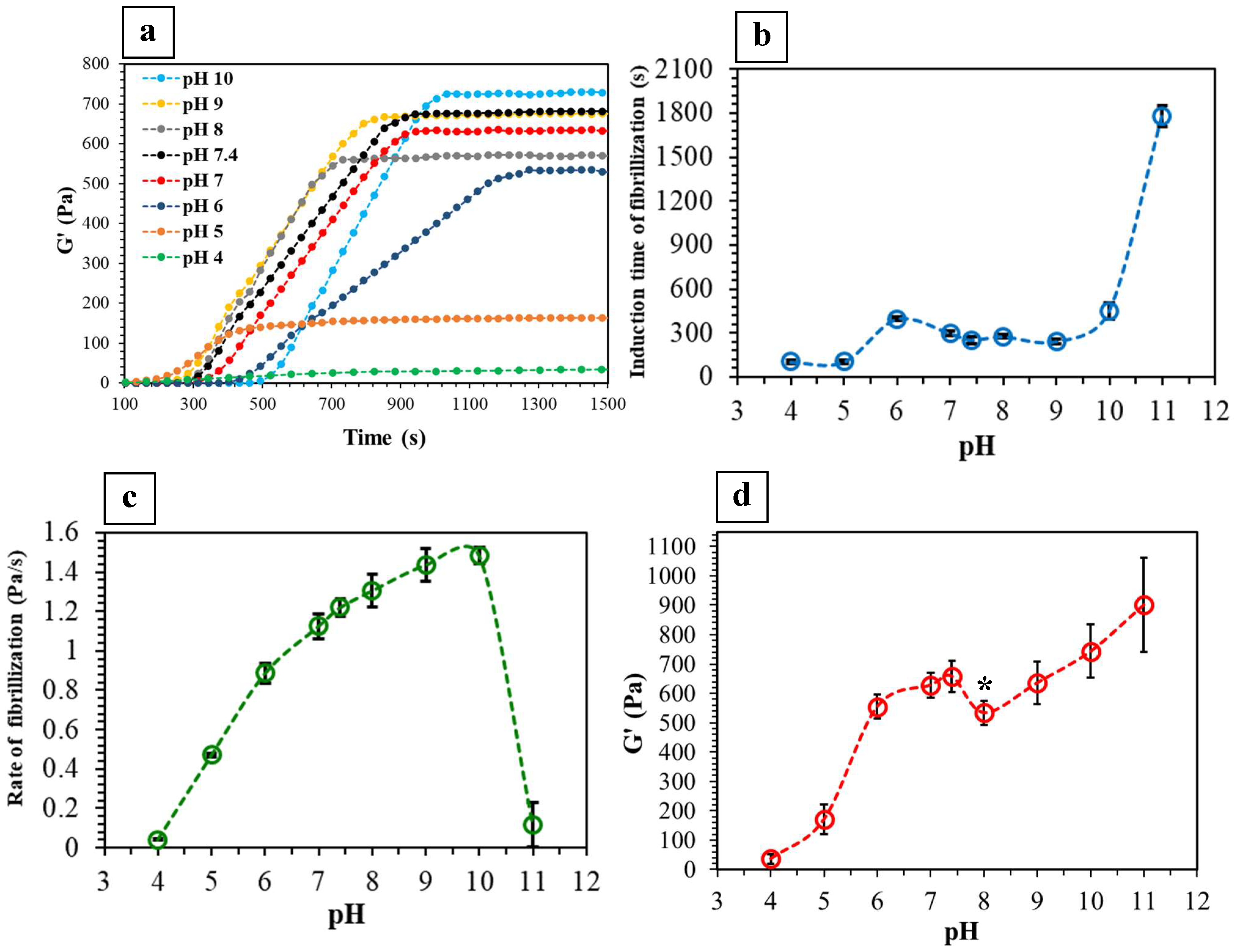
2.2. Viscoelastic Properties
2.3. GAE-Densified Collagen Gels
2.3.1. Fibril Alignment and CFD
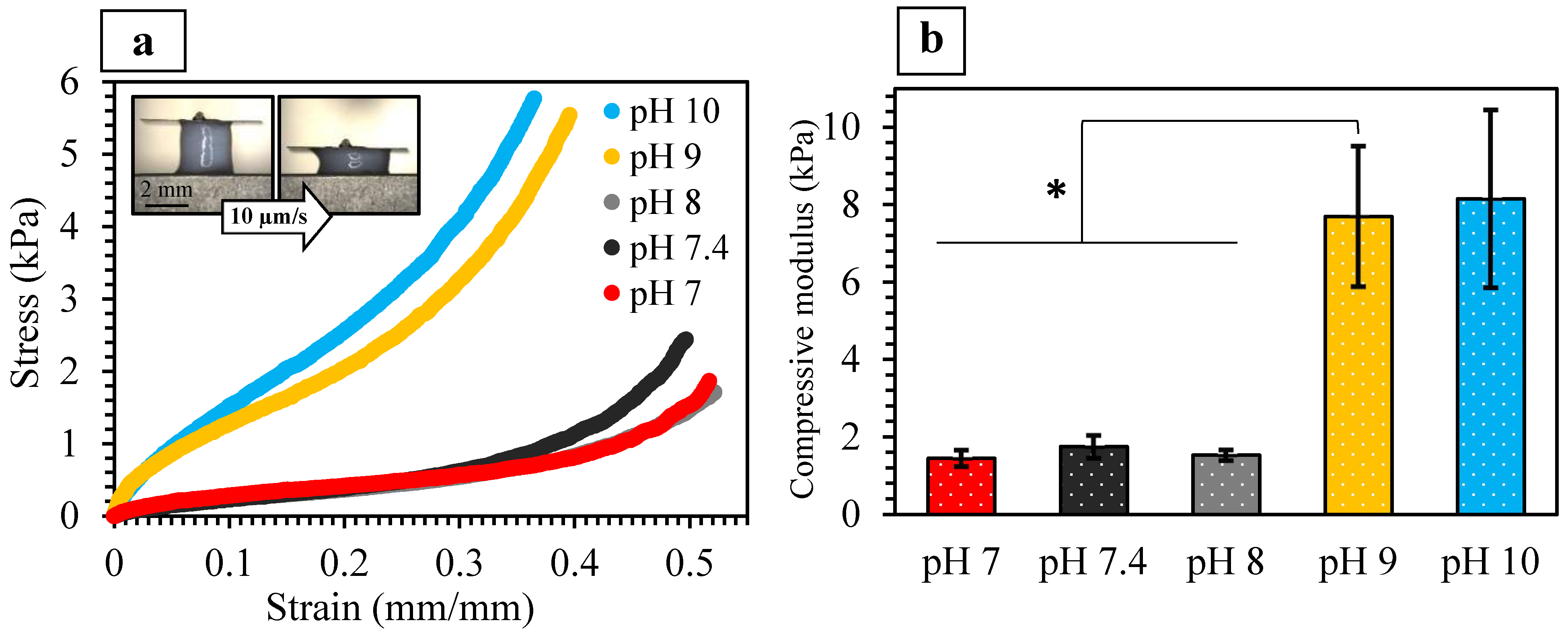
2.3.2. Mechanical Properties
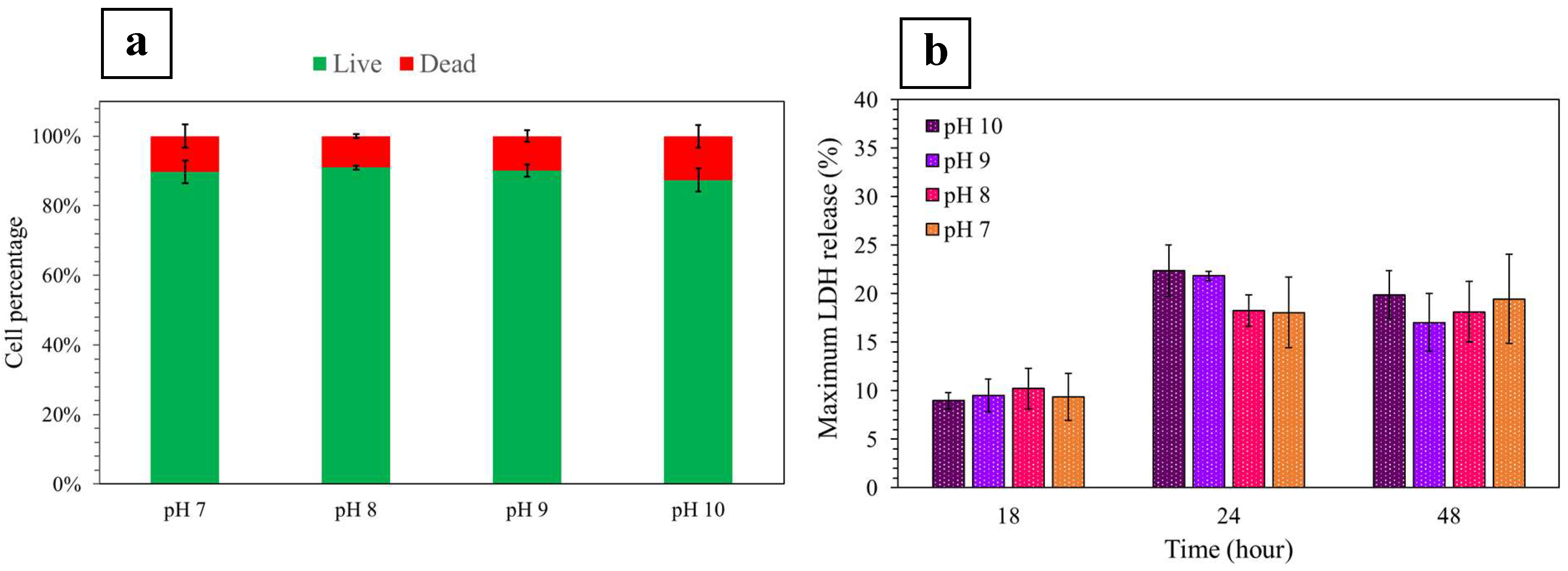
2.3.3. Seeded Fibroblast Viability and Mortality
3. Materials and Methods
3.1. Assessment of Gelation by Turbidity and Rheological Analyses
3.2. Production of the Dense Collagen Gels via GAE
3.3. Scanning Electron Microscopy (SEM)
3.4. Collagen Fibrillar Density (CFD)
3.5. Micro-Compression Testing
3.6. Seeded Cell Viability and Mortality
3.6.1. Production of Fibroblast-Seeded Dense Collagen Gels
3.6.2. Fibroblast Extraction from Cell-Seeded Gels
3.6.3. Detection of Cellular Lactate Dehydrogenase Release (LDH)
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Christiansen, D.L.; Huang, E.K.; Silver, F.H. Assembly of type I collagen: Fusion of fibril subunits and the influence of fibril diameter on mechanical properties. Matrix Biol. 2000, 19, 409–420. [Google Scholar] [CrossRef]
- Brown, R.A.; Wiseman, M.; Chuo, C.B.; Cheema, U.; Nazhat, S.N. Ultrarapid engineering of biomimetic materials and tissues: Fabrication of nano-and microstructures by plastic compression. Adv. Funct. Mater. 2005, 15, 1762–1770. [Google Scholar] [CrossRef]
- Bose, S.; Ke, D.; Sahasrabudhe, H.; Bandyopadhyay, A. Additive manufacturing of biomaterials. Prog. Mater. Sci. 2018, 93, 45–111. [Google Scholar] [CrossRef] [PubMed]
- Miri, A.K.; Muja, N.; Kamranpour, N.O.; Lepry, W.C.; Boccaccini, A.R.; Clarke, S.A.; Nazhat, S.N. Ectopic bone formation in rapidly fabricated acellular injectable dense collagen-Bioglass hybrid scaffolds via gel aspiration-ejection. Biomaterials 2016, 85, 128–141. [Google Scholar] [CrossRef]
- Ghezzi, C.E.; Marelli, B.; Muja, N.; Nazhat, S.N. Immediate production of a tubular dense collagen construct with bioinspired mechanical properties. Acta Biomater. 2012, 8, 1813–1825. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Lee, D.; Sopko, N.; Matsui, H.; Sabnekar, P.; Liu, X.; Elisseeff, J.; Schoenberg, M.P.; Pienta, K.; Bivalacqua, T.J. Biomanufacturing seamless tubular and hollow collagen scaffolds with unique design features and biomechanical properties. Adv. Healthc. Mater. 2017, 6, 1601136. [Google Scholar] [CrossRef]
- Cowin, S.C. Do liquid crystal-like flow processes occur in the supramolecular assembly of biological tissues? J. Non-Newton. Fluid Mech. 2004, 119, 155–162. [Google Scholar] [CrossRef]
- Diamantides, N.; Wang, L.; Pruiksma, T.; Siemiatkoski, J.; Dugopolski, C.; Shortkroff, S.; Kennedy, S.; Bonassar, L.J. Correlating rheological properties and printability of collagen bioinks: The effects of riboflavin photocrosslinking and pH. Biofabrication 2017, 9, 034102-15. [Google Scholar] [CrossRef]
- Piechocka, I.K.; van Oosten, A.S.; Breuls, R.G.; Koenderink, G.H. Rheology of heterotypic collagen networks. Biomacromolecules 2011, 12, 2797–2805. [Google Scholar] [CrossRef] [PubMed]
- Sung, K.E.; Su, G.; Pehlke, C.; Trier, S.M.; Eliceiri, K.W.; Keely, P.J.; Friedl, A.; Beebe, D.J. Control of 3-dimensional collagen matrix polymerization for reproducible human mammary fibroblast cell culture in microfluidic devices. Biomaterials 2009, 30, 4833–4841. [Google Scholar] [CrossRef]
- Pederson, A.W.; Ruberti, J.W.; Messersmith, P.B. Thermal assembly of a biomimetic mineral/collagen composite. Biomaterials 2003, 24, 4881–4890. [Google Scholar] [CrossRef] [PubMed]
- Rosenblatt, J.; Devereux, B.; Wallace, D.G. Injectable collagen as a pH-sensitive hydrogel. Biomaterials 1994, 15, 985–995. [Google Scholar] [CrossRef] [PubMed]
- Walters, B.D.; Stegemann, J.P. Strategies for directing the structure and function of three-dimensional collagen biomaterials across length scales. Acta Biomater. 2014, 10, 1488–1501. [Google Scholar] [CrossRef]
- Achilli, M.; Mantovani, D. Tailoring mechanical properties of collagen-based scaffolds for vascular tissue engineering: The effects of pH, temperature and ionic strength on gelation. Polymers 2010, 2, 664–680. [Google Scholar] [CrossRef]
- Rosenblatt, J.; Devereux, B.; Wallace, D.G. Effect of electrostatic forces on the dynamic rheological properties of injectable collagen biomaterials. Biomaterials 1992, 13, 878–886. [Google Scholar] [CrossRef]
- Abou Neel, E.A.; Cheema, U.; Knowles, J.C.; Brown, R.A.; Nazhat, S.N. Use of multiple unconfined compression for control of collagen gel scaffold density and mechanical properties. Soft Matter 2006, 2, 986–992. [Google Scholar] [CrossRef] [PubMed]
- Marelli, B.; Ghezzi, C.E.; Zhang, Y.L.; Rouiller, I.; Barralet, J.E.; Nazhat, S.N. Fibril formation pH controls intrafibrillar collagen biomineralization in vitro and in vivo. Biomaterials 2015, 37, 252–259. [Google Scholar] [CrossRef]
- Jiang, W.; Griffanti, G.; Tamimi, F.; McKee, M.D.; Nazhat, S.N. Multiscale structural evolution of citrate-triggered intrafibrillar and interfibrillar mineralization in dense collagen gels. J. Struct. Biol. 2020, 212, 107592. [Google Scholar] [CrossRef]
- Saldin, L.T.; Cramer, M.C.; Velankar, S.S.; White, L.J.; Badylak, S.F. Extracellular matrix hydrogels from decellularized tissues: Structure and function. Acta Biomater. 2017, 49, 1–15. [Google Scholar] [CrossRef]
- Raub, C.B.; Unruh, J.; Suresh, V.; Krasieva, T.; Lindmo, T.; Gratton, E.; Tromberg, B.J.; George, S.C. Image correlation spectroscopy of multiphoton images correlates with collagen mechanical properties. Biophys. J. 2008, 94, 2361–2373. [Google Scholar] [CrossRef]
- Nehlich, O.; Richards, M.P. Establishing collagen quality criteria for sulphur isotope analysis of archaeological bone collagen. Archaeol. Anthropol. Sci. 2009, 1, 59–75. [Google Scholar] [CrossRef]
- Chaudhuri, O. Viscoelastic hydrogels for 3D cell culture. Biomater. Sci. 2017, 5, 1480–1490. [Google Scholar] [CrossRef] [PubMed]
- Kamranpour, N.O.; Miri, A.K.; James-Bhasin, M.; Nazhat, S.N. A gel aspiration-ejection system for the controlled production and delivery of injectable dense collagen scaffolds. Biofabrication 2016, 8, 015018. [Google Scholar] [CrossRef] [PubMed]
- Griffanti, G.; Rezabeigi, E.; Li, J.; Murshed, M.; Nazhat, S.N. Rapid biofabrication of printable dense collagen bioinks of tunable properties. Adv. Funct. Mater. 2020, 30, 1903874. [Google Scholar] [CrossRef]
- Troka, I.; Griffanti, G.; Canaff, L.; Hendy, G.N.; Goltzman, D.; Nazhat, S.N. Effect of menin deletion in early osteoblast lineage on the mineralization of an in vitro 3D osteoid-like dense collagen gel matrix. Biomimetics 2022, 7, 101. [Google Scholar] [CrossRef] [PubMed]
- Novak, T.; Seelbinder, B.; Twitchell, C.M.; van Donkelaar, C.C.; Voytik-Harbin, S.L.; Neu, C.P. Mechanisms and microenvironment investigation of cellularized high density gradient collagen matrices via densification. Adv. Funct. Mater. 2016, 26, 2617–2628. [Google Scholar] [CrossRef]
- Zitnay, J.L.; Reese, S.P.; Tran, G.; Farhang, N.; Bowles, R.D.; Weiss, J.A. Fabrication of dense anisotropic collagen scaffolds using biaxial compression. Acta Biomater. 2018, 65, 76–87. [Google Scholar] [CrossRef]
- Nazhat, S.N.; Marelli, B.; Ghezzi, C.; Kamranpour, N.O. Dense Hydrogels. U.S. Patent US20180000989A1, 9 September 2017. [Google Scholar]
- Marelli, B.; Ghezzi, C.E.; James-Bhasin, M.; Nazhat, S.N. Fabrication of injectable, cellular, anisotropic collagen tissue equivalents with modular fibrillar densities. Biomaterials 2015, 37, 183–193. [Google Scholar] [CrossRef]
- Griffanti, G.; Jiang, W.; Nazhat, S.N. Bioinspired mineralization of a functionalized injectable dense collagen hydrogel through silk sericin incorporation. Biomater. Sci. 2019, 7, 1064–1077. [Google Scholar] [CrossRef]
- Sampaziotis, F.; Justin, A.W.; Tysoe, O.C.; Sawiak, S.; Godfrey, E.M.; Upponi, S.S.; Gieseck III, R.L.; De Brito, M.C.; Berntsen, N.L.; Gómez-Vázquez, M.J.; et al. Reconstruction of the mouse extrahepatic biliary tree using primary human extrahepatic cholangiocyte organoids. Nat. Med. 2017, 23, 954–967. [Google Scholar] [CrossRef]
- Loy, C.; Lainé, A.; Mantovani, D. Rotation-based technique for the rapid densification of tubular collagen gel scaffolds. Biotechnol. J. 2016, 11, 1673–1679. [Google Scholar] [CrossRef] [PubMed]
- Griffanti, G.; Nazhat, S.N. Dense fibrillar collagen-based hydrogels as functional osteoid-mimicking scaffolds. Int. Mater. Rev. 2020, 65, 502–521. [Google Scholar] [CrossRef]
- Park, H.; Nazhat, S.N.; Rosenzweig, D.H. Mechanical activation drives tenogenic differentiation of human mesenchymal stem cells in aligned dense collagen hydrogels. Biomaterials 2022, 286, 121606. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Cooke, M.E.; Lacombe, J.-G.; Weber, M.H.; Martineau, P.A.; Nazhat, S.N.; Rosenzweig, D.H. Continuous two-zone in vitro co-culture model of the enthesis. Biomed. Mater. Devices, 2022. [Google Scholar] [CrossRef]
- Muangsanit, P.; Day, A.; Dimiou, S.; Ataç, A.F.; Kayal, C.; Park, H.; Nazhat, S.N.; Phillips, J.B. Rapidly formed stable and aligned dense collagen gels seeded with Schwann cells support peripheral nerve regeneration. J. Neural Eng. 2020, 17, 046036. [Google Scholar] [CrossRef] [PubMed]
- Lian, J.; Agban, Y.; Cheong, S.; Kuchel, R.P.; Raudsepp, A.; Williams, M.A.; Rupenthal, I.D.; Henning, A.; Tilley, R.D.; Holmes, G.; et al. ZnO/PVP nanoparticles induce gelation in type I collagen. Eur. Polym. J. 2016, 75, 399–405. [Google Scholar] [CrossRef]
- Yamamura, N.; Sudo, R.; Ikeda, M.; Tanishita, K. Effects of the mechanical properties of collagen gel on the in vitro formation of microvessel networks by endothelial cells. Tissue Eng. 2007, 13, 1443–1453. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Asadi, A.; Monroe, M.R.; Douglas, E.P. pH effects on collagen fibrillogenesis in vitro: Electrostatic interactions and phosphate binding. Mater. Sci. Eng. C 2009, 29, 1643–1649. [Google Scholar] [CrossRef]
- Rezabeigi, E.; Wood-Adams, P.M.; Drew, R.A. Isothermal ternary phase diagram of the polylactic acid-dichloromethane-hexane system. Polymer 2014, 55, 3100–3106. [Google Scholar] [CrossRef]
- Wood, G.; Keech, M.K. The formation of fibrils from collagen solutions 1. The effect of experimental conditions: Kinetic and electron-microscope studies. Biochem. J. 1960, 75, 588–598. [Google Scholar] [CrossRef]
- Zhu, S.; Yuan, Q.; Yin, T.; You, J.; Gu, Z.; Xiong, S.; Hu, Y. Self-assembly of collagen-based biomaterials: Preparation, characterizations and biomedical applications. J. Mater. Chem. B 2018, 6, 2650–2676. [Google Scholar] [CrossRef]
- Koetting, M.C.; Peters, J.T.; Steichen, S.D.; Peppas, N.A. Stimulus-responsive hydrogels: Theory, modern advances, and applications. Mater. Sci. Eng. R Rep. 2015, 9, 1–49. [Google Scholar] [CrossRef] [PubMed]
- Pawelec, K.M.; Best, S.M.; Cameron, R.E. Collagen: A network for regenerative medicine. J. Mater. Chem. B 2016, 4, 6484–6496. [Google Scholar] [CrossRef] [PubMed]
- Freudenberg, U.; Behrens, S.H.; Welzel, P.B.; Müller, M.; Grimmer, M.; Salchert, K.; Taeger, T.; Schmidt, K.; Pompe, W.; Werner, C. Electrostatic interactions modulate the conformation of collagen I. Biophys. J. 2007, 92, 2108–2119. [Google Scholar] [CrossRef]
- Walimbe, T.; Calve, S.; Panitch, A.; Sivasankar, M.P. Incorporation of types I and III collagen in tunable hyaluronan hydrogels for vocal fold tissue engineering. Acta Biomater. 2019, 87, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Stavrouli, N.; Aubry, T.; Tsitsilianis, C. Rheological properties of ABA telechelic polyelectrolyte and ABA polyampholyte reversible hydrogels: A comparative study. Polymer 2008, 49, 1249–1256. [Google Scholar] [CrossRef]
- Felix, L.; Hernandez, J.; Arguelles-Monal, W.M.; Goycoolea, F.M. Kinetics of gelation and thermal sensitivity of N-Isobutyryl chitosan hydrogels. Biomacromolecules 2005, 6, 2408–2415. [Google Scholar] [CrossRef]
- Achilli, M.; Lagueux, J.; Mantovani, D. On the effects of UV-C and pH on the mechanical behavior, molecular conformation and cell viability of collagen-based scaffold for vascular tissue engineering. Macromol. Biosci. 2010, 10, 307–316. [Google Scholar] [CrossRef]
- Yapp, R.D.; Insana, M.F. pH-induced contrast in viscoelasticity imaging of biopolymers. Phys. Med. Biol. 2009, 54, 1089–1109. [Google Scholar] [CrossRef]
- Chang, M.C.; Tanaka, J. FT-IR study for hydroxyapatite/collagen nanocomposite cross-linked by glutaraldehyde. Biomaterials 2002, 23, 4811–4818. [Google Scholar] [CrossRef]
- Júnior, Z.S.S.; Botta, S.B.; Ana, P.A.; França, C.M.; Fernandes, K.P.S.; Mesquita-Ferrari, R.A.; Deana, A.; Bussadori, S.K. Effect of papain-based gel on type I collagen-spectroscopy applied for microstructural analysis. Sci. Rep. 2015, 5, 11448. [Google Scholar] [CrossRef]
- de Campos Vidal, B.; Mello, M.L.S. Collagen type I amide I band infrared spectroscopy. Micron 2011, 42, 283–289. [Google Scholar] [CrossRef]
- Franchi, M.; Raspanti, M.; Dell’Orbo, C.; Quaranta, M.; De Pasquale, V.; Ottani, V.; Ruggeri, A. Different crimp patterns in collagen fibrils relate to the subfibrillar arrangement. Connect. Tissue Res. 2008, 49, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Griffanti, G.; Fairag, R.; Rosenzweig, D.H.; Haglund, L.; Nazhat, S.N. Automated biofabrication of anisotropic dense fibrin gels accelerate osteoblastic differentiation of seeded mesenchymal stem cells. J. Mater. Res. 2021, 36, 4867–4882. [Google Scholar] [CrossRef]
- Serpooshan, V.; Julien, M.; Nguyen, O.; Wang, H.; Li, A.; Muja, N.; Henderson, J.E.; Nazhat, S.N. Reduced hydraulic permeability of three-dimensional collagen scaffolds attenuates gel contraction and promotes the growth and differentiation of mesenchymal stem cells. Acta Biomater. 2010, 6, 3978–3987. [Google Scholar] [CrossRef] [PubMed]
- Cheema, U.; Chuo, C.B.; Sarathchandra, P.; Nazhat, S.N.; Brown, R.A. Engineering functional collagen scaffolds: Cyclical loading increases material strength and fibril aggregation. Adv. Funct. Mater. 2007, 17, 2426–2431. [Google Scholar] [CrossRef]
- Yin, J.; Yan, M.; Wang, Y.; Fu, J.; Suo, H. 3D Bioprinting of low-concentration cell-laden gelatin methacrylate (GelMA) bioinks with a two-step cross-linking strategy. ACS Appl. Mater. Interfaces 2018, 10, 6849–6857. [Google Scholar] [CrossRef] [PubMed]
- Marelli, B. In Vitro Mineralization of an Osteoid-Like Dense Collagen Construct for Bone Tissue Engineering. Ph.D. Dissertation, McGill University, Montréal, QC, Canada, 2011. [Google Scholar]
- Karimizade, A.; Takallu, S.; Mirzaei, E. Evaluating the effect of pH on mechanical strength and cell compatibility of nanostructured collagen hydrogel by the plastic compression method. Nanomed. J. 2018, 5, 180–185. [Google Scholar]
- Rezabeigi, E.; Schmitt, C.; Hadj Henni, A.; Barkun, A.N.; Nazhat, S.N. In vitro evaluation of real-time viscoelastic and coagulation properties of various classes of topical hemostatic agents using a novel contactless nondestructive technology. ACS Appl. Mater. Interfaces 2022, 14, 16047–16061. [Google Scholar] [CrossRef]
- Ceccaldi, C.; Strandman, S.; Hui, E.; Montagnon, E.; Schmitt, C.; Hadj Henni, A.; Lerouge, S. Validation and application of a nondestructive and contactless method for rheological evaluation of biomaterials. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 2565–2573. [Google Scholar] [CrossRef]
- Naseri, S.; Koushki, N.; Rezabeigi, E.; Ehrlicher, A.; Nazhat, S.N. A nondestructive contactless technique to assess the viscoelasticity of blood clots in real-time. J. Mech. Behav. Biomed. Mater. 2020, 110, 103921. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rezabeigi, E.; Griffanti, G.; Nazhat, S.N. Effect of Fibrillization pH on Gelation Viscoelasticity and Properties of Biofabricated Dense Collagen Matrices via Gel Aspiration-Ejection. Int. J. Mol. Sci. 2023, 24, 3889. https://doi.org/10.3390/ijms24043889
Rezabeigi E, Griffanti G, Nazhat SN. Effect of Fibrillization pH on Gelation Viscoelasticity and Properties of Biofabricated Dense Collagen Matrices via Gel Aspiration-Ejection. International Journal of Molecular Sciences. 2023; 24(4):3889. https://doi.org/10.3390/ijms24043889
Chicago/Turabian StyleRezabeigi, Ehsan, Gabriele Griffanti, and Showan N. Nazhat. 2023. "Effect of Fibrillization pH on Gelation Viscoelasticity and Properties of Biofabricated Dense Collagen Matrices via Gel Aspiration-Ejection" International Journal of Molecular Sciences 24, no. 4: 3889. https://doi.org/10.3390/ijms24043889
APA StyleRezabeigi, E., Griffanti, G., & Nazhat, S. N. (2023). Effect of Fibrillization pH on Gelation Viscoelasticity and Properties of Biofabricated Dense Collagen Matrices via Gel Aspiration-Ejection. International Journal of Molecular Sciences, 24(4), 3889. https://doi.org/10.3390/ijms24043889




