Electrocatalytic Performance of Ethanol Oxidation on Ni and Ni/Pd Surface-Decorated Porous Structures Obtained by Molten Salts Deposition/Dissolution of Al-Ni Alloys
Abstract
:1. Introduction
2. Results and Discussion
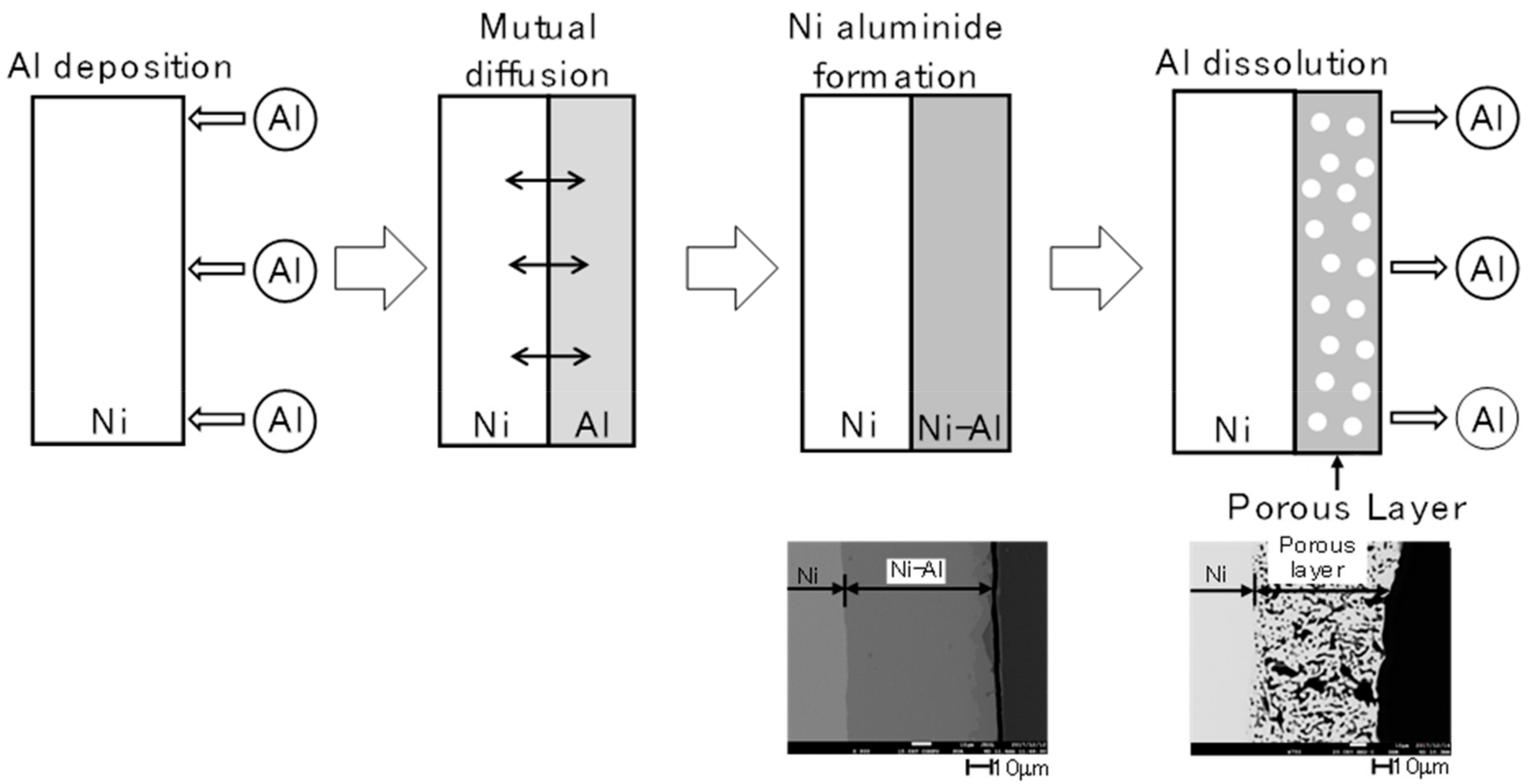
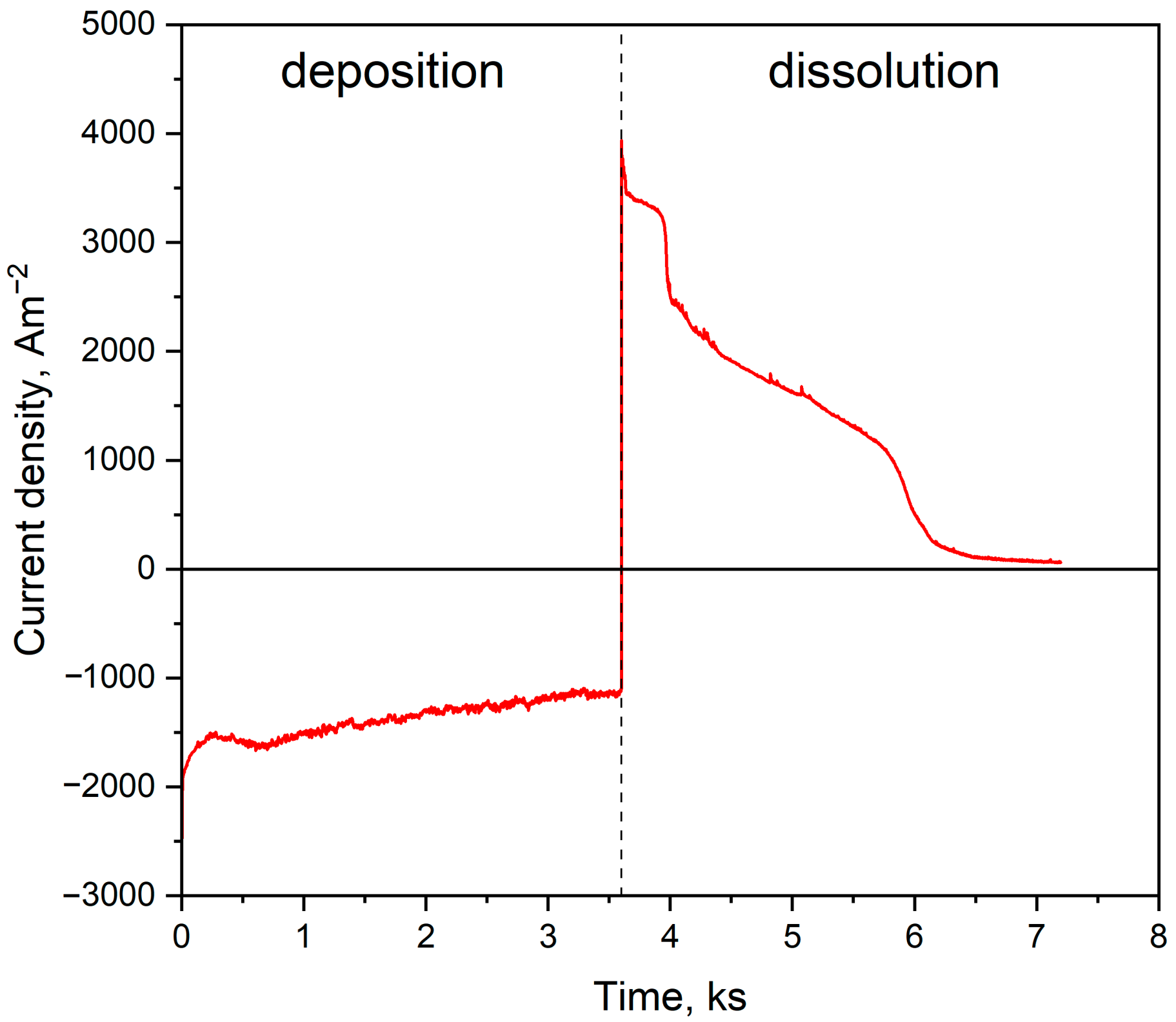
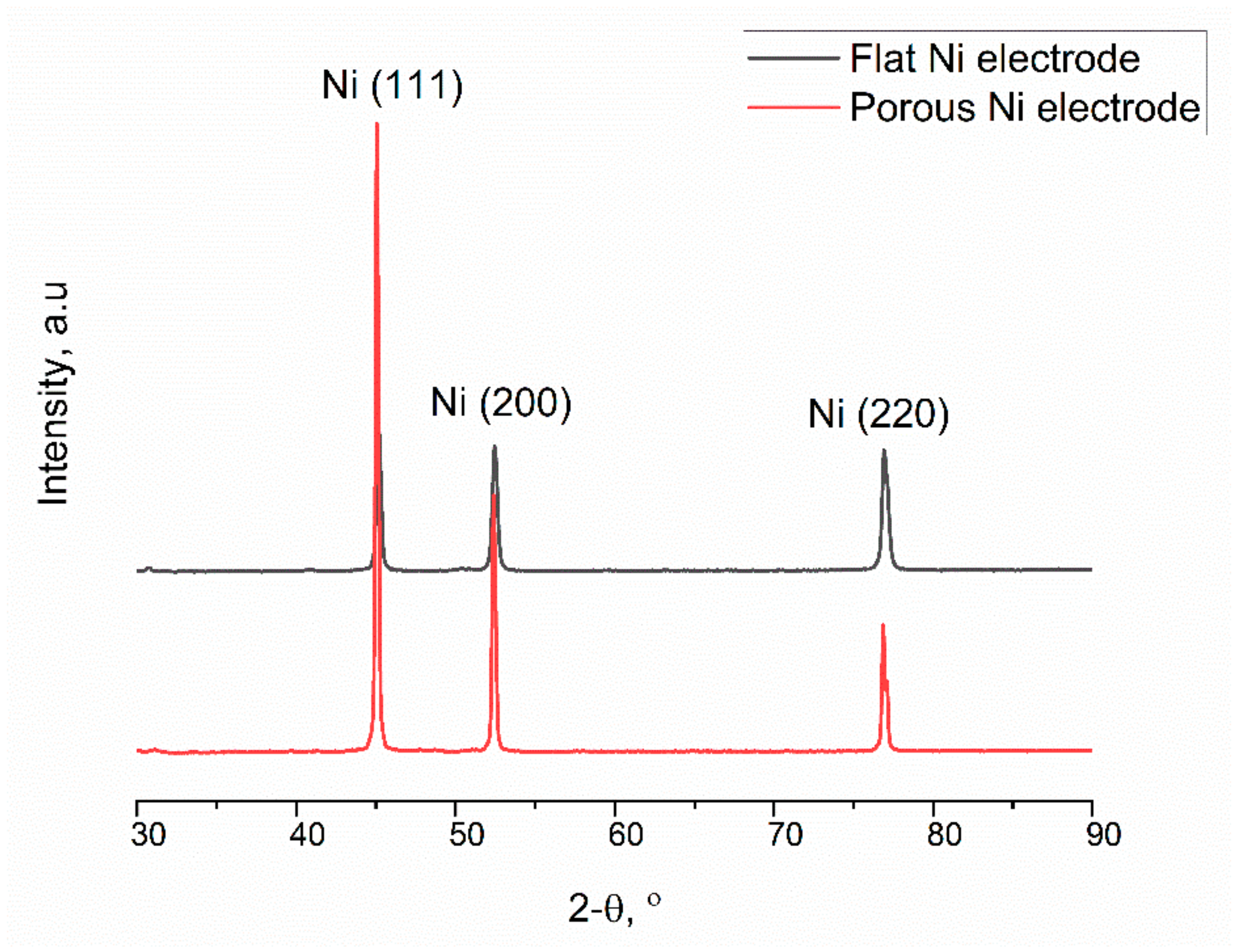
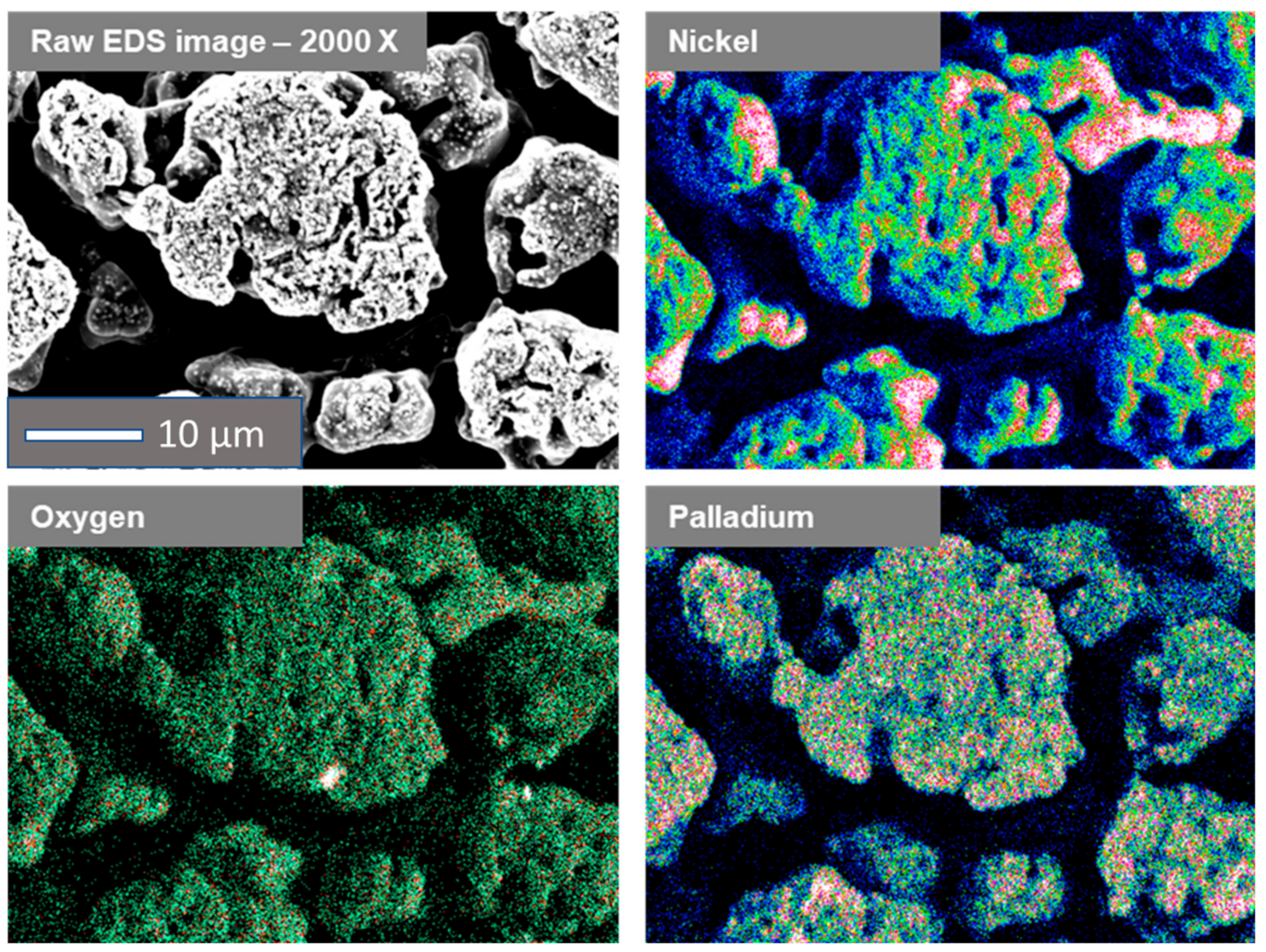
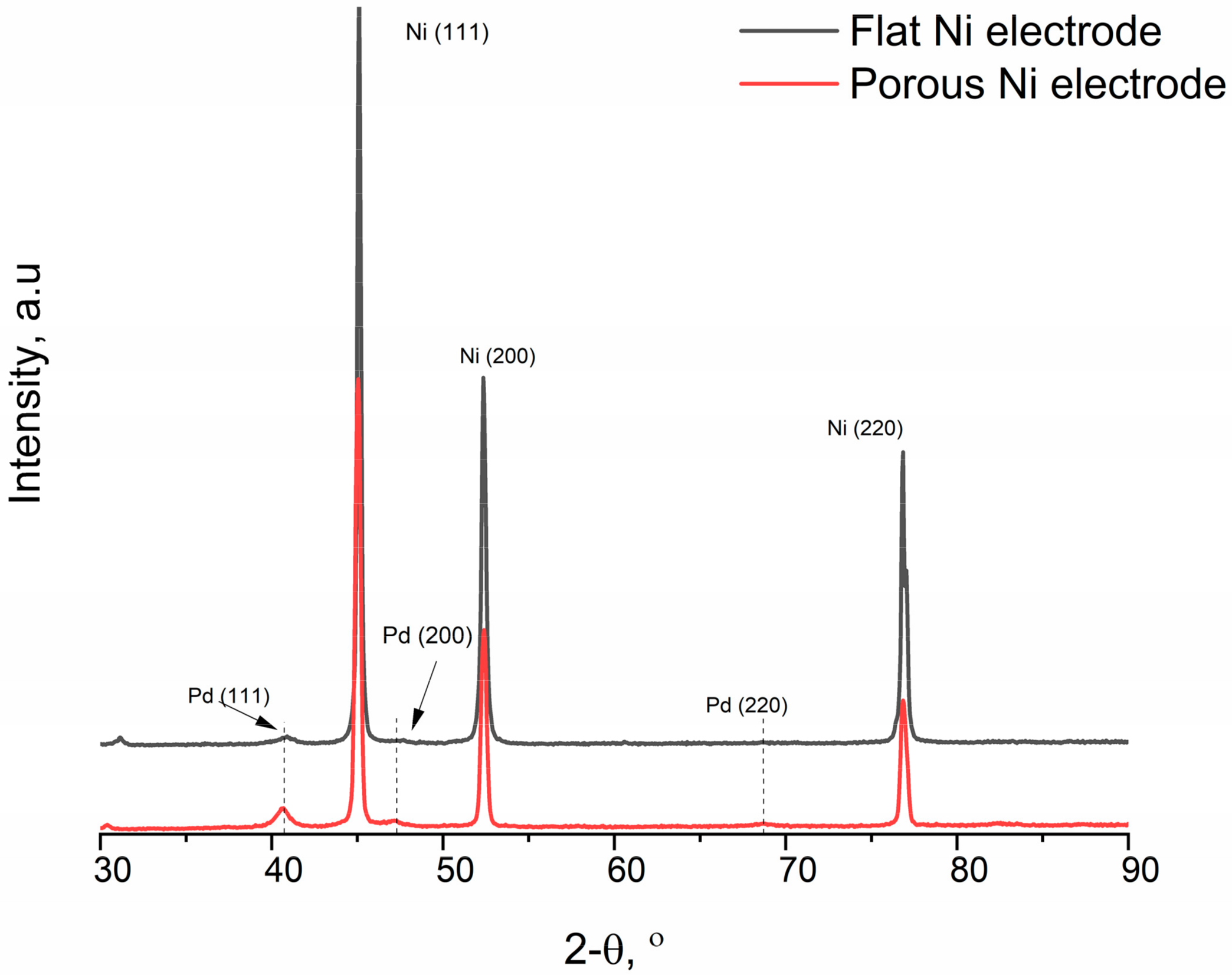
2.1. Electrodeposition and Structural Characterisation of the Porous Ni Electrodes
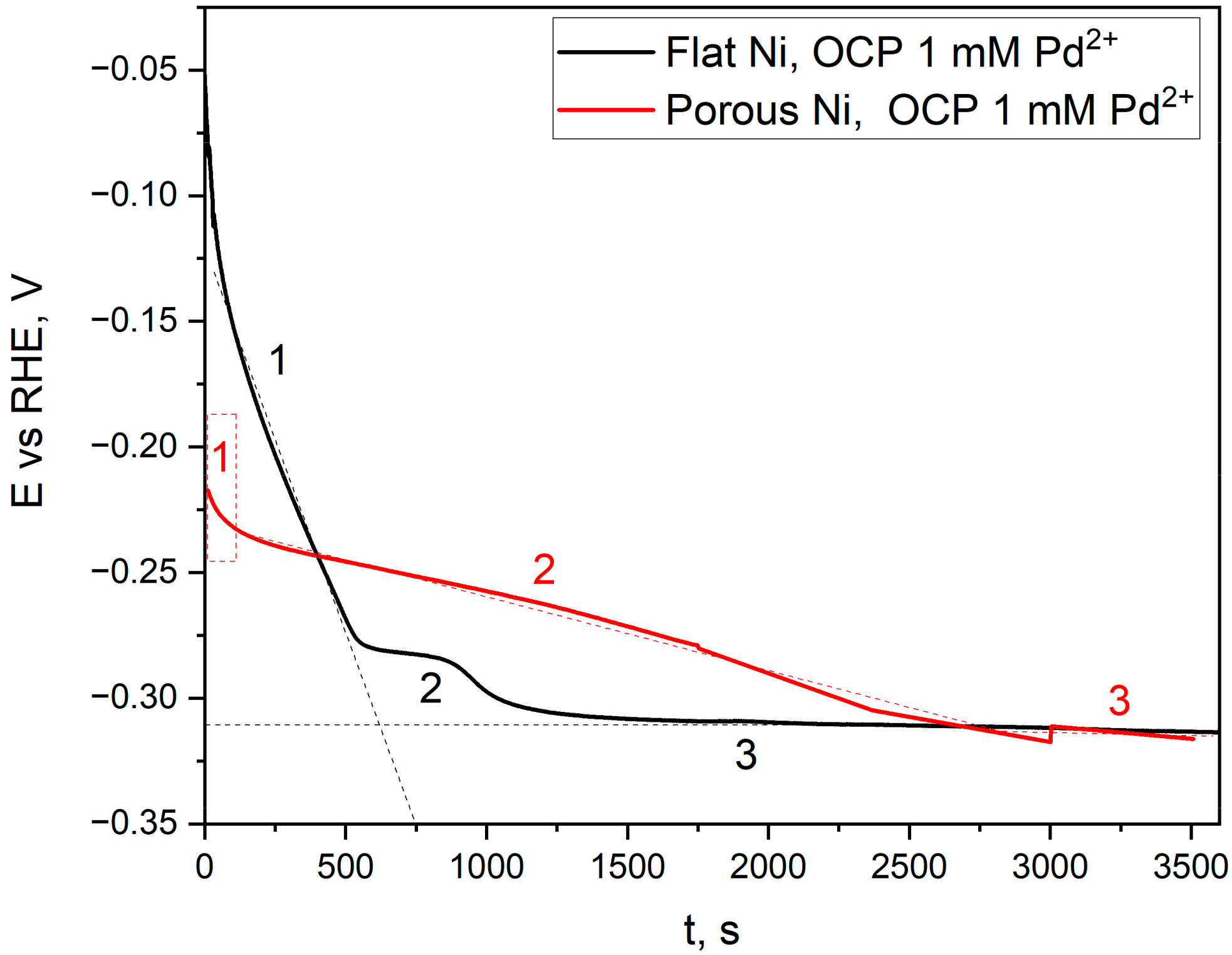
2.2. Electroless Modification of Flat and Porous Ni Electrodes by Pd2+ Ions
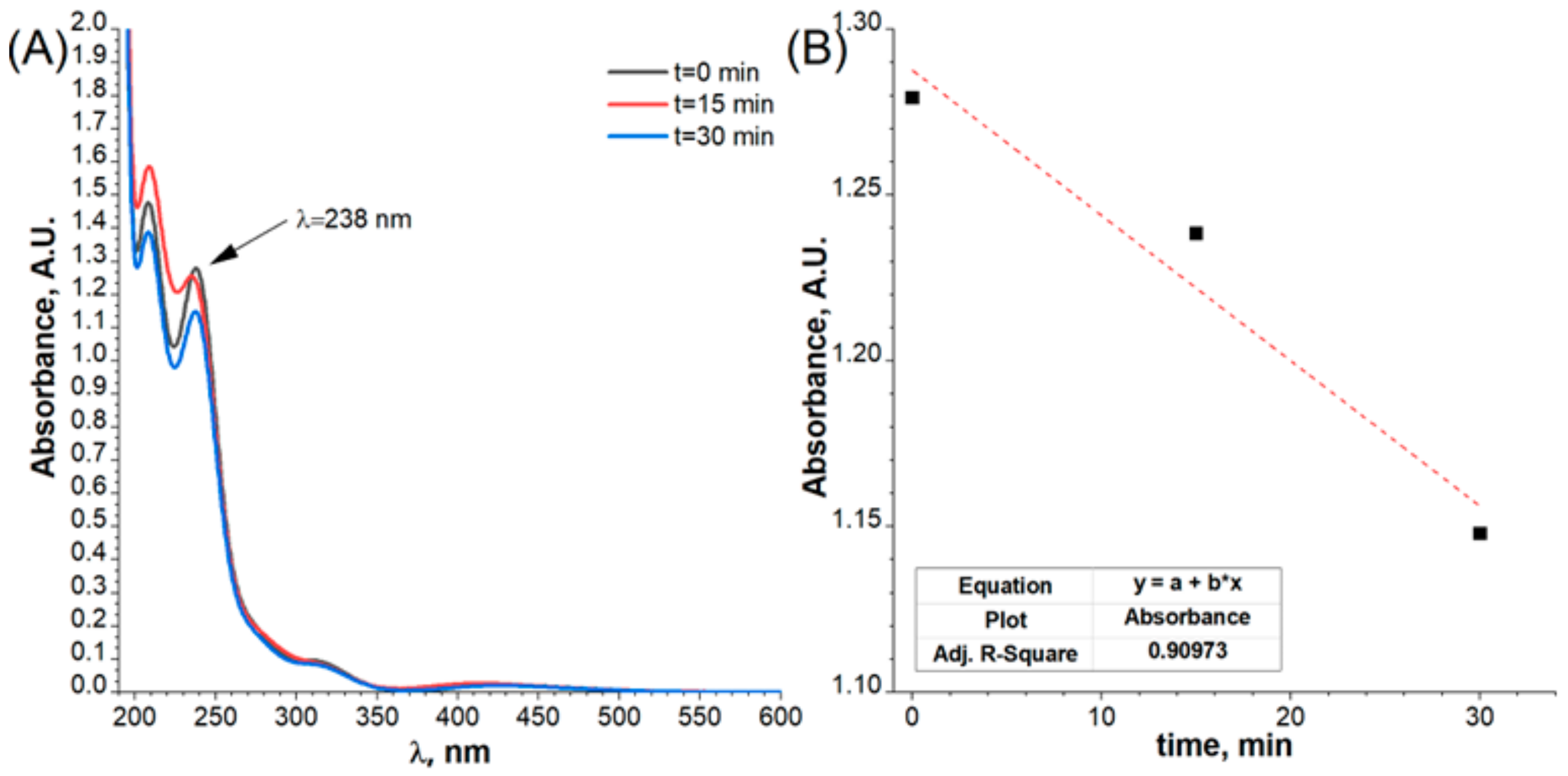
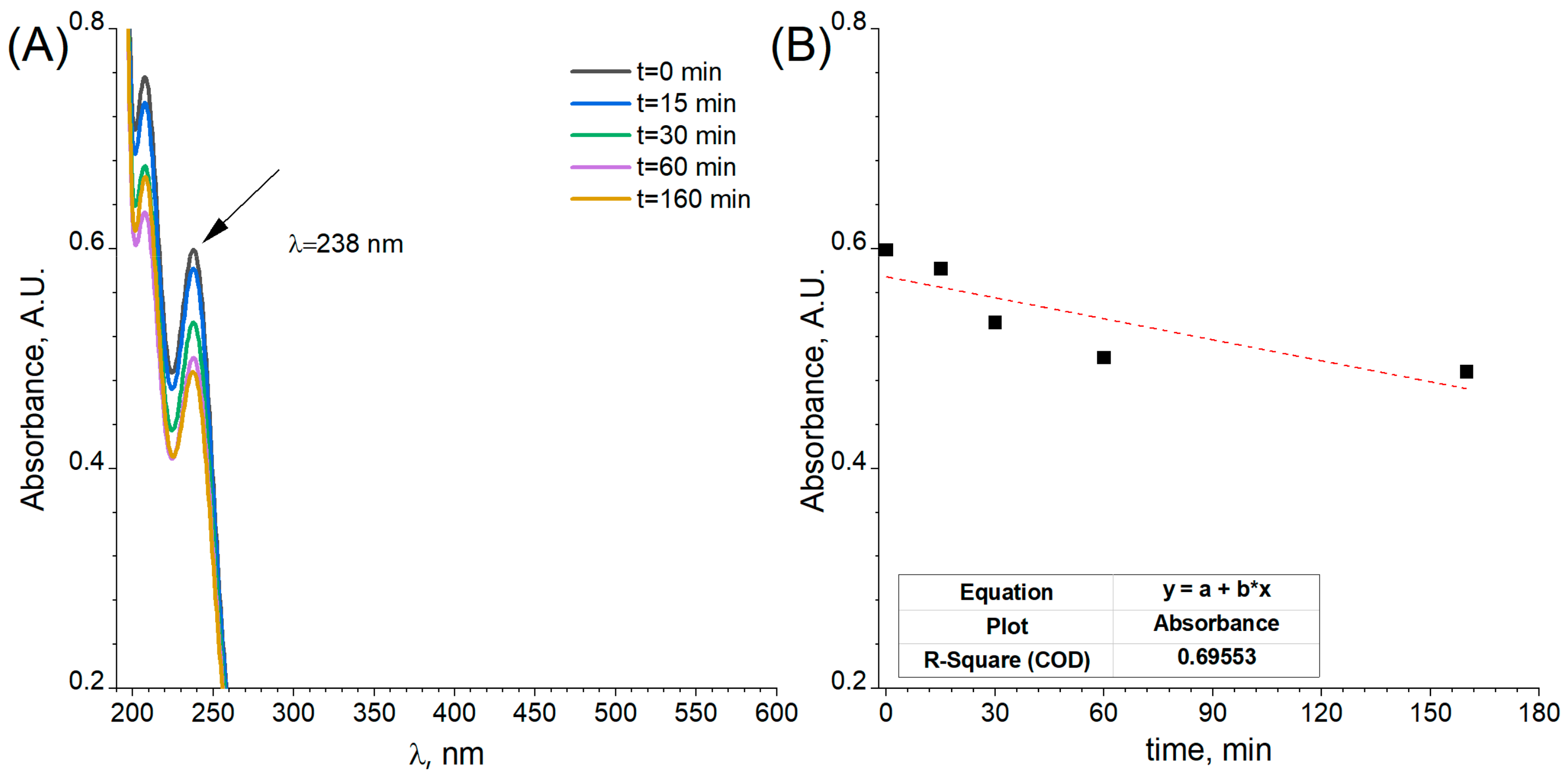
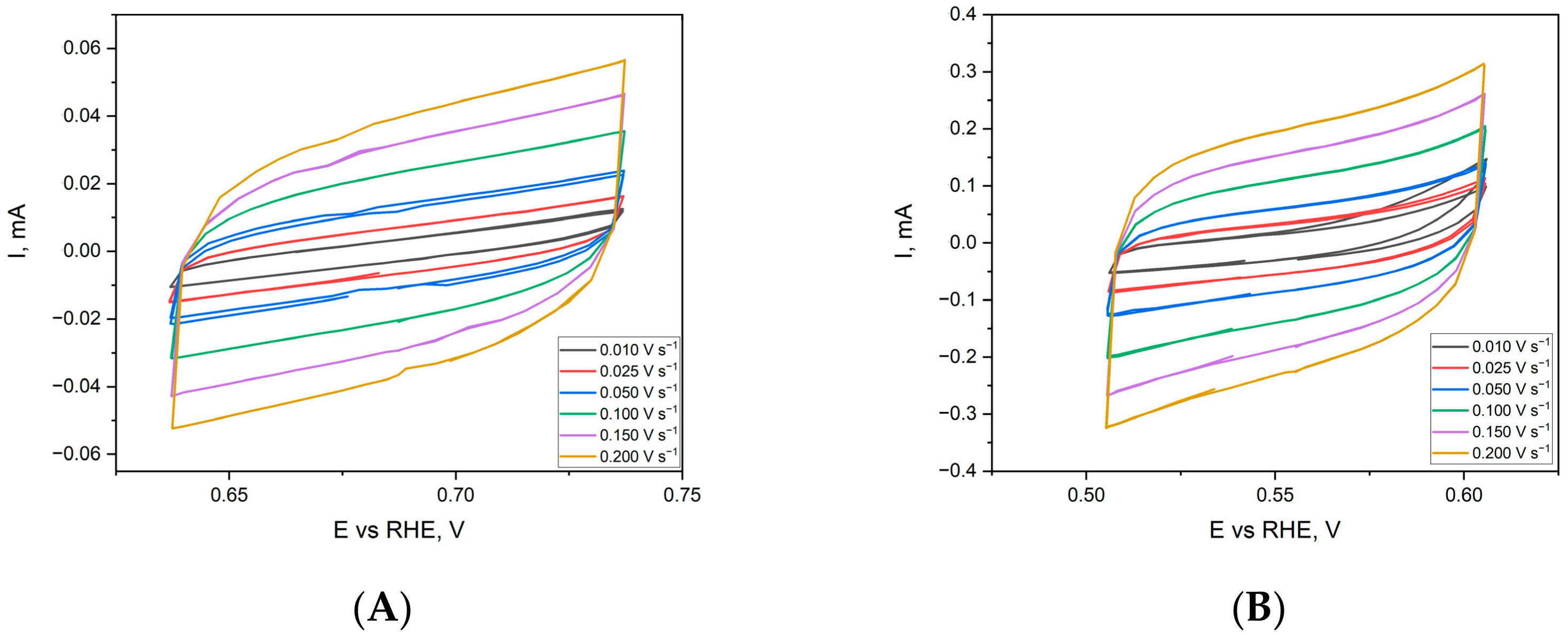
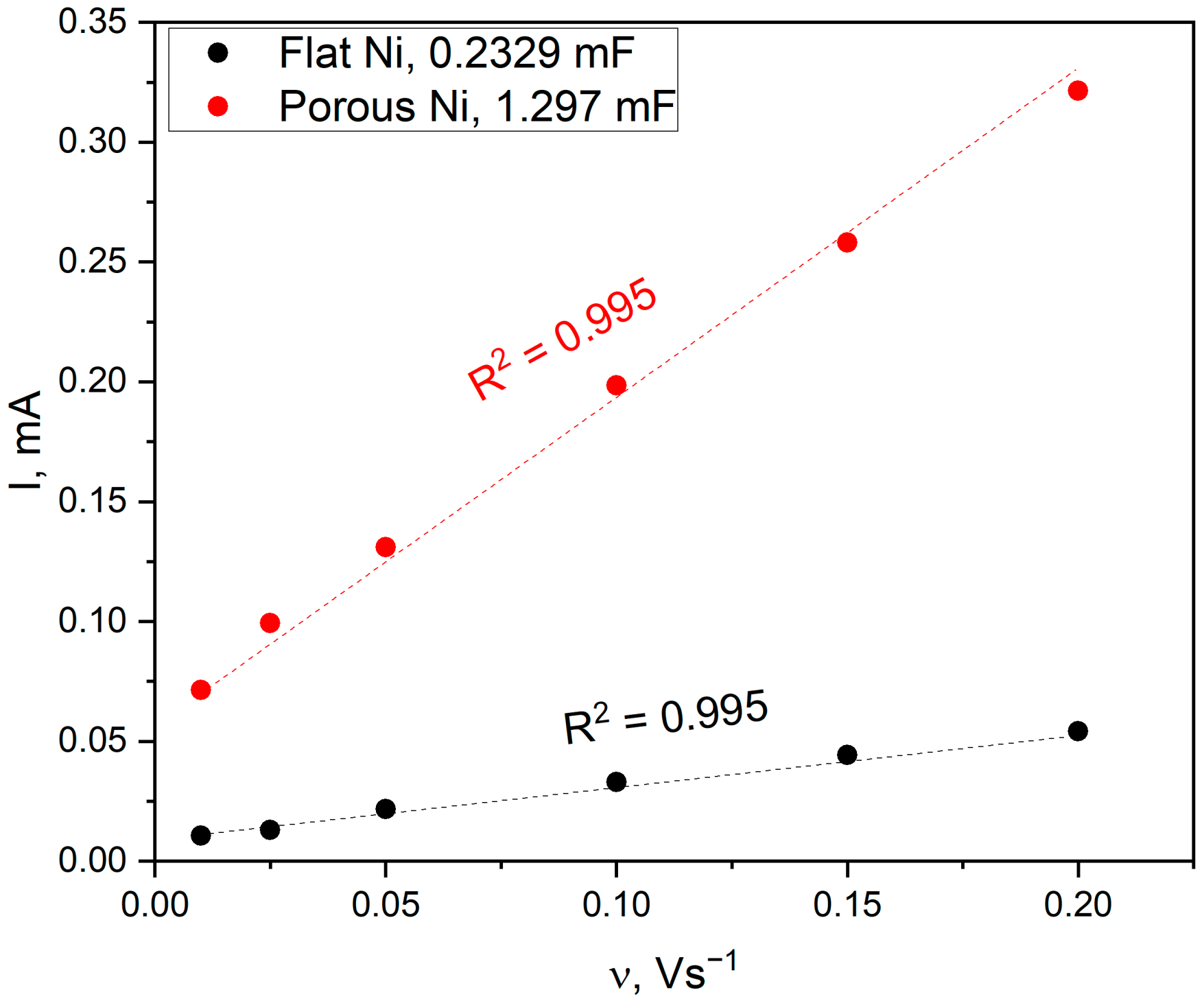
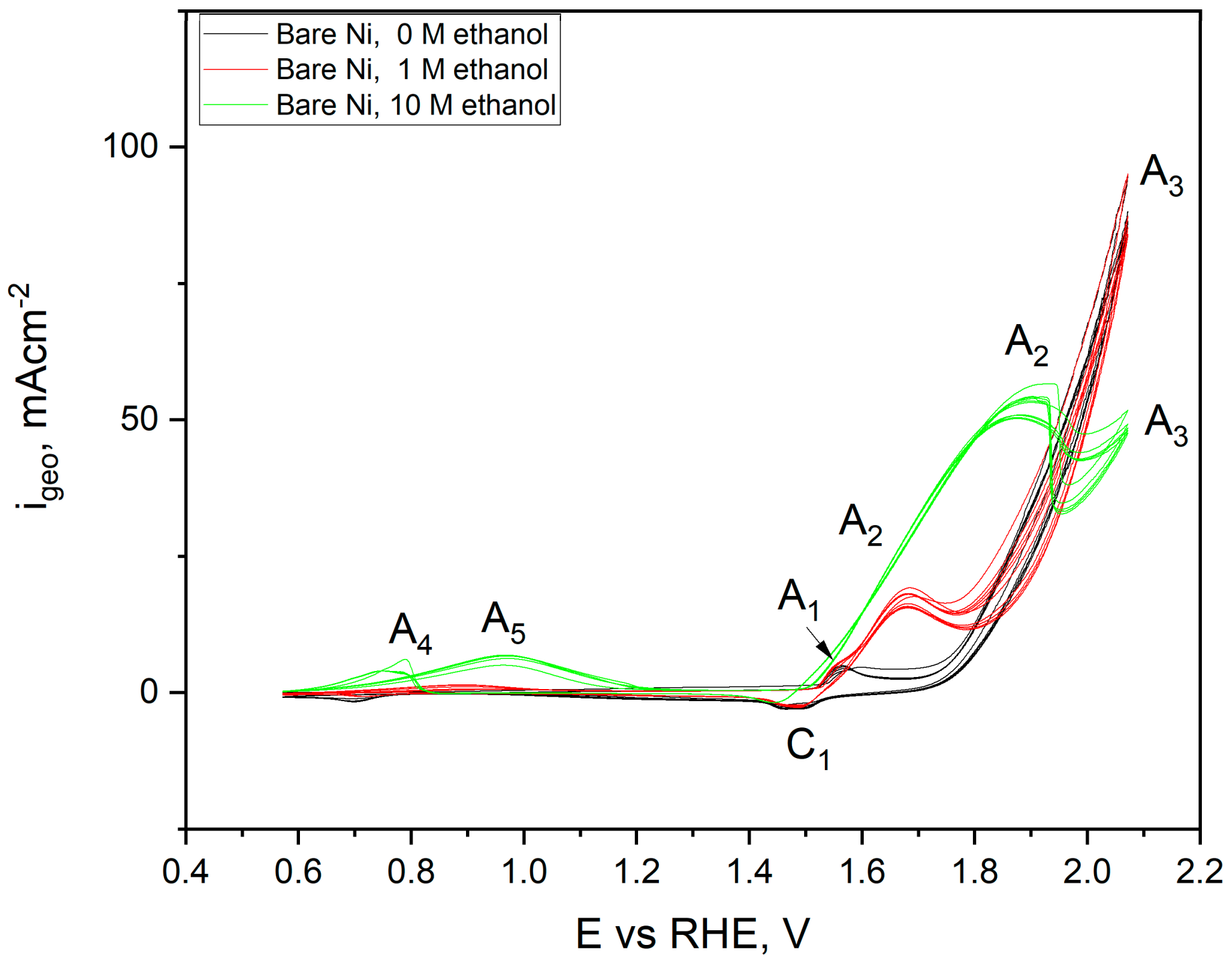
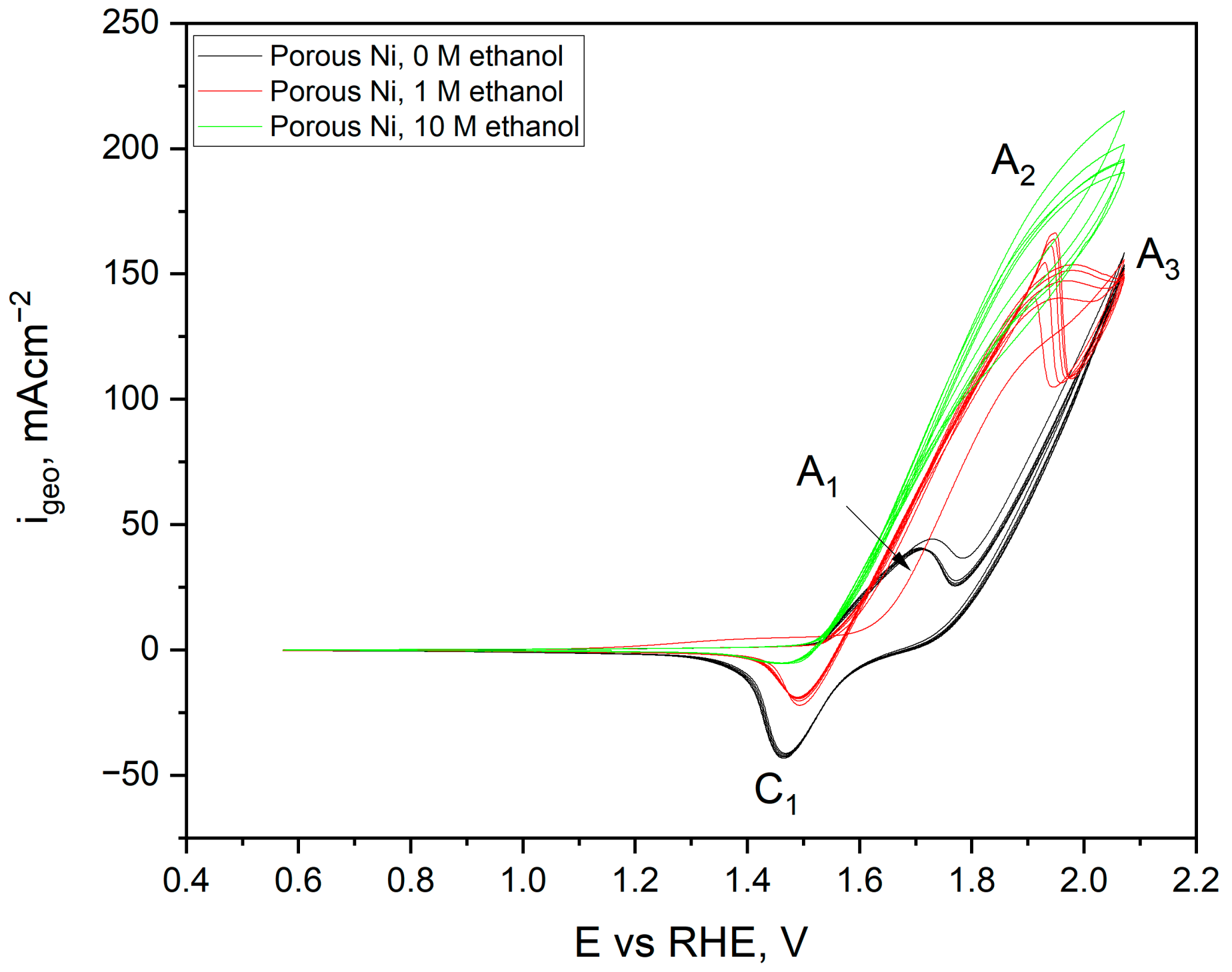
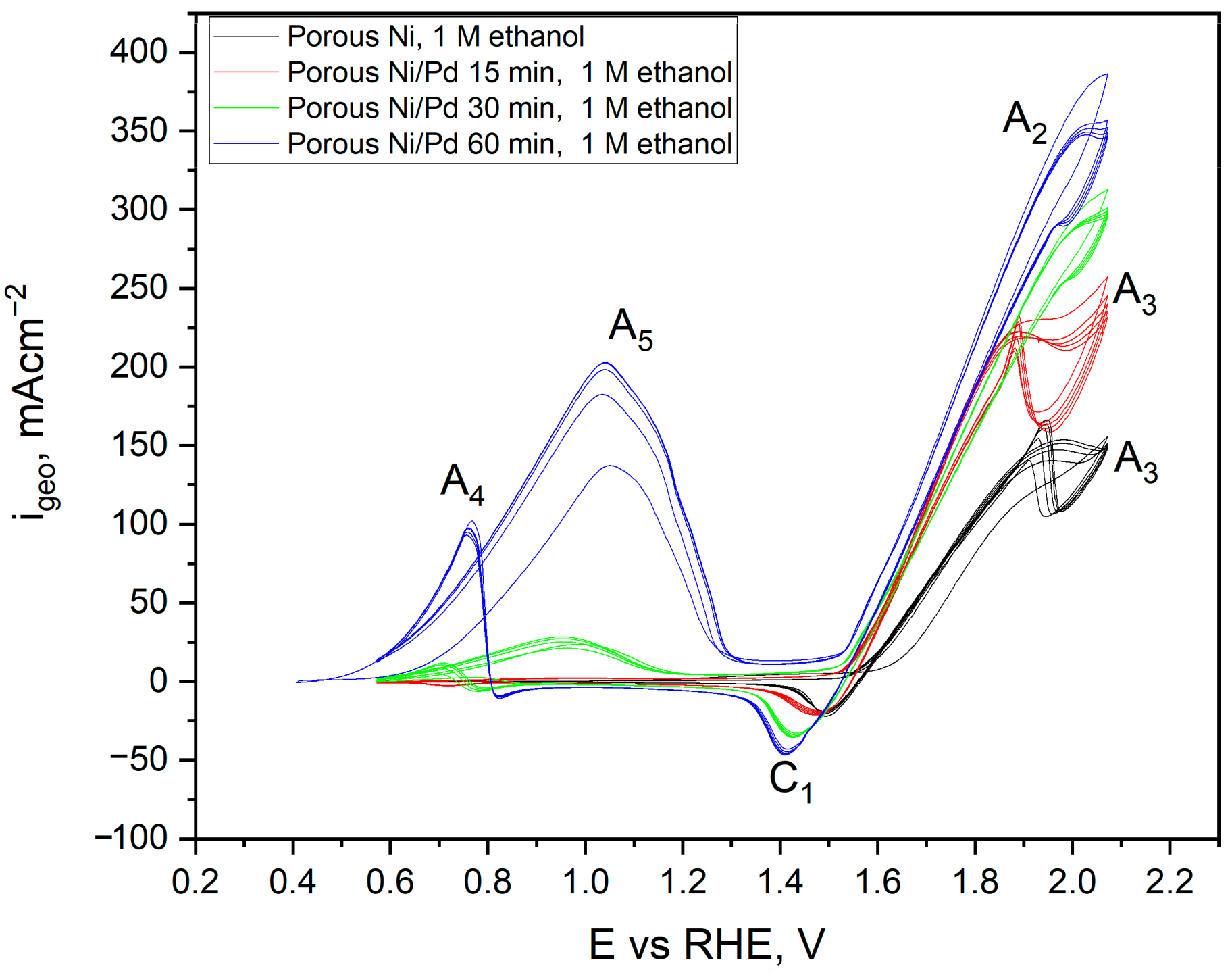
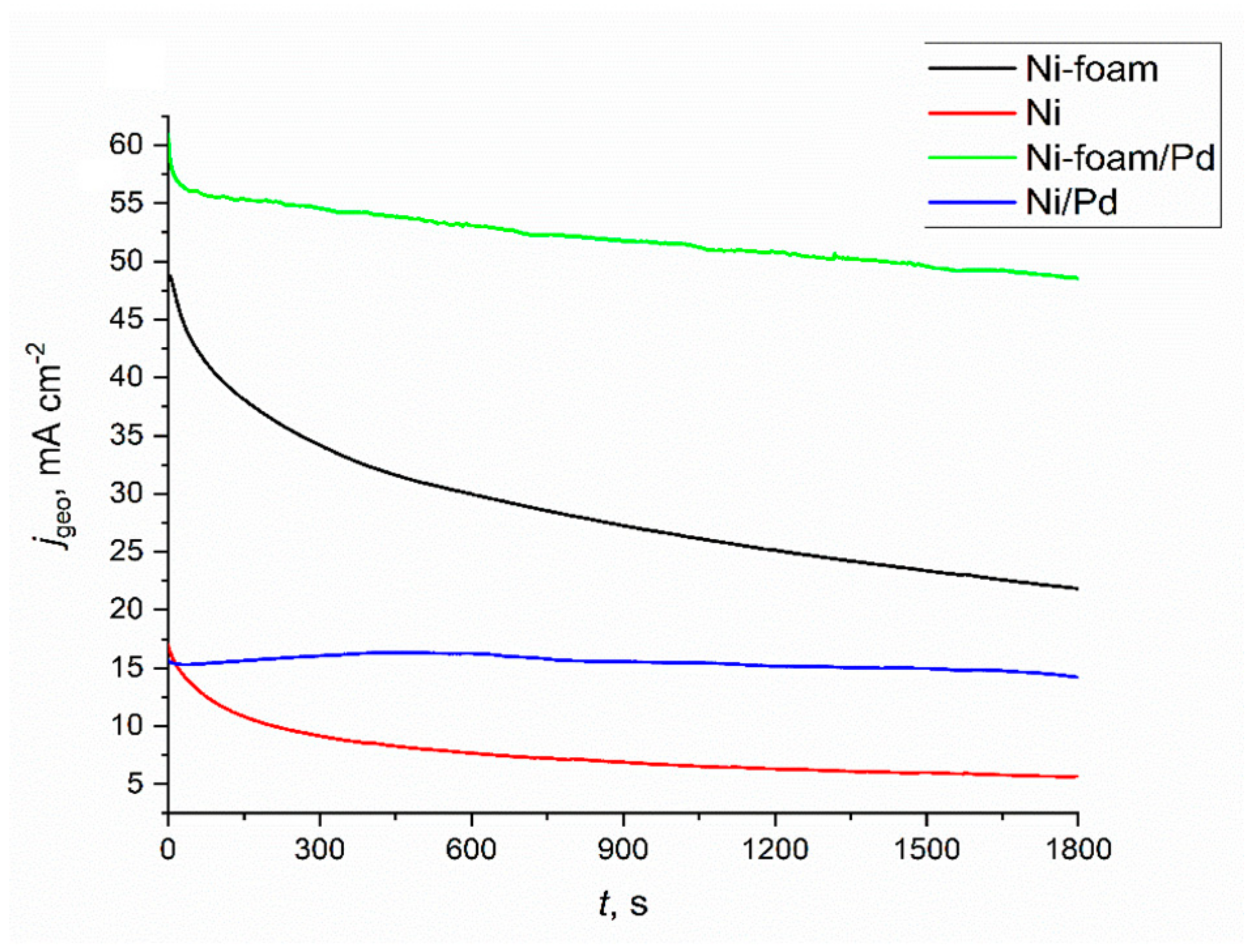
2.3. Electrochemical Characterisation and Catalytic Activity of Flat and Porous Nickel Electrodes with and without Palladium Modification
3. Materials and Methods
3.1. Preparation of Porous Electrodes
3.2. Physical Characterisation of the Electrodes
3.3. Electrochemical Measurements
- i—registered current (mA);
- ν—applied scan rate (V s−1);
- Cdl—double layer capacitance (µF cm−2).
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, R.; Zheng, M.; Pang, J.; Liu, X.; Wang, J.; Pan, X.; Wang, A.; Wang, X.; Zhang, T. Selectivity-Switchable Conversion of Cellulose to Glycols over Ni-Sn Catalysts. ACS Catal. 2016, 6, 191–201. [Google Scholar] [CrossRef]
- Izumi, Y. Modified Raney Nickel (MRNi) Catalyst: Heterogeneous Enantio-Differentiating (Asymmetric) Catalyst. Adv. Catal. 1983, 32, 215–271. [Google Scholar] [CrossRef]
- Dulle, J.; Nemeth, S.; Skorb, E.V.; Irrgang, T.; Senker, J.; Kempe, R.; Fery, A.; Andreeva, D.V. Sonochemical activation of Al/Ni hydrogenation catalyst. Adv. Funct. Mater. 2012, 22, 3128–3135. [Google Scholar] [CrossRef]
- Choquette, Y.; Brossard, L.; Ménard, H. Leaching of Raney nickel composite-coated electrodes. J. Appl. Electrochem. 1990, 20, 855–863. [Google Scholar] [CrossRef]
- Adkins, H.; Billica, H.R. The Preparation of Raney Nickel Catalysts and their Use Under Conditions Comparable with Those for Platinum and Palladium Catalysts. J. Am. Chem. Soc. 1948, 70, 695–698. [Google Scholar] [CrossRef]
- Bernäcker, C.I.; Rauscher, T.; Büttner, T.; Kieback, B.; Röntzsch, L. A Powder Metallurgy Route to Produce Raney-Nickel Electrodes for Alkaline Water Electrolysis. J. Electrochem. Soc. 2019, 166, F357–F363. [Google Scholar] [CrossRef]
- Freel, J.; Robertson, S.D.; Anderson, R.B. The structure of Raney nickel. III. The chemisorption of hydrogen and carbon monoxide. J. Catal. 1970, 18, 243–248. [Google Scholar] [CrossRef]
- Balej, J.; Divisek, J.; Schmitz, H.; Mergel, J. Preparation and properties of raney nickel electrodes on Ni-Zn base for H<inf>2</inf> and O<inf>2</inf> evolution from alkaline solutions Part I: Electrodeposition of Ni-Zn alloys from chloride solutions. J. Appl. Electrochem. 1992, 22, 705–710. [Google Scholar] [CrossRef]
- Sheela, G.; Pushpavanam, M.; Pushpavanam, S. Zinc-nickel alloy electrodeposits for water electrolysis. Int. J. Hydrog. Energy 2002, 27, 627–633. [Google Scholar] [CrossRef]
- Los, P.; Rami, A.; Lasia, A. Hydrogen evolution reaction on Ni-Al electrodes. J. Appl. Electrochem. 1993, 23, 135–140. [Google Scholar] [CrossRef]
- Cheong, A.K.; Lasia, A.; Lessard, J. Hydrogen Evolution Reaction at Composite-Coated Raney Nickel Electrodes in Aqueous and Aqueous-Methanolic Solutions. J. Electrochem. Soc. 1993, 140, 2721–2725. [Google Scholar] [CrossRef]
- Kubisztal, J.; Budniok, A.; Lasia, A. Study of the hydrogen evolution reaction on nickel-based composite coatings containing molybdenum powder. Int. J. Hydrog. Energy 2007, 32, 1211–1218. [Google Scholar] [CrossRef]
- Freel, J.; Pieters, W.J.M.; Anderson, R.B. The structure of Raney nickel. I. Pore structure. J. Catal. 1969, 14, 247–256. [Google Scholar] [CrossRef]
- Fukumoto, M.; Sugiuchi, K.; Nakajima, K. Formation of porous Ni surface by electrodeposition and dissolution in molten salt. Int. J. Hydrog. Energy 2020, 45, 28252–28259. [Google Scholar] [CrossRef]
- Nakajima, K.; Takahashi, H.; Fukumoto, M. Effect of Pt Addition on the Formation of Ni–Pt Porous Layer. Coatings 2022, 12, 1645. [Google Scholar] [CrossRef]
- Nakajima, K.; Fukumoto, M. Porous Ni–Co surface formation and analysis of hydrogen generation by gas sensor. Int. J. Hydrog. Energy 2021, 46, 26263–26271. [Google Scholar] [CrossRef]
- Sun, H.; Xu, X.; Kim, H.; Jung, W.; Zhou, W.; Shao, Z. Electrochemical Water Splitting: Bridging the Gaps between Fundamental Research and Industrial Applications. Energy Environ. Mater. 2022. [Google Scholar] [CrossRef]
- Sun, H.; Li, L.; Chen, Y.; Kim, H.; Xu, X.; Guan, D.; Hu, Z.; Zhang, L.; Shao, Z.; Jung, W. Boosting ethanol oxidation by NiOOH-CuO nano-heterostructure for energy-saving hydrogen production and biomass upgrading. Appl. Catal. B Environ. 2023, 325, 122388. [Google Scholar] [CrossRef]
- Xu, J.; Wang, B.-X.; Lyu, D.; Wang, T.; Wang, Z. In situ Raman investigation of the femtosecond laser irradiated NiFeOOH for enhanced electrochemical ethanol oxidation reaction. Int. J. Hydrog. Energy 2022, in press. [Google Scholar] [CrossRef]
- Wang, Q.; Zhu, R.; Deng, P.; Li, J.; Huang, W.; Chen, Q.; Su, Y.-Q.; Jia, C.; Liu, Z.; Kang, Z.; et al. Rhodium decorated stable platinum nickel nanowires for effective ethanol oxidation reaction. Sci. China Mater. 2023, 66, 679–685. [Google Scholar] [CrossRef]
- Kamyabi, M.A.; Jadali, S.; Alizadeh, T. Ethanol Electrooxidation on Nickel Foam Arrayed with Templated PdSn; From Catalyst Fabrication to Electrooxidation Dominance Route. ChemElectroChem 2023, 10, e202200914. [Google Scholar] [CrossRef]
- Zhang, N.; Wang, J.; Zhang, W.; Zhao, Y.; Dong, Z.; Wu, Z.; Xu, G.-R.; Wang, L. Self-supported PdNi dendrite on Ni foam for improving monohydric alcohol and polyhydric alcohols electrooxidation. Fuel 2022, 326, 125083. [Google Scholar] [CrossRef]
- Kamyabi, M.A.; Jadali, S. Rational design of PdCu nanoparticles supported on a templated Ni foam: The cooperation effect of morphology and composition for electrocatalytic oxidation of ethanol. Int. J. Hydrog. Energy 2021, 46, 39387–39403. [Google Scholar] [CrossRef]
- Sheng, S.; Song, Y.; Sha, L.; Ye, K.; Zhu, K.; Gao, Y.; Yan, J.; Wang, G.; Cao, D. Simultaneous hydrogen evolution and ethanol oxidation in alkaline medium via a self-supported bifunctional electrocatalyst of Ni-Fe phosphide/Ni foam. Appl. Surf. Sci. 2021, 561, 150080. [Google Scholar] [CrossRef]
- Shen, Y.; Lu, S.; Xu, W.; Lv, A.; Wang, Z.; Wang, H.; Liu, G.; Zhang, Y. Fabrication of Composite Material with Pd Nanoparticles and Graphene on Nickel Foam for Its Excellent Electrocatalytic Performance. Electrocatalysis 2020, 11, 522–535. [Google Scholar] [CrossRef]
- Yu, Z.; Xu, J.; Amorim, I.; Li, Y.; Liu, L. Easy preparation of multifunctional ternary PdNiP/C catalysts toward enhanced small organic molecule electro-oxidation and hydrogen evolution reactions. J. Energy Chem. 2021, 58, 256–263. [Google Scholar] [CrossRef]
- Almeida, C.V.S.; Eguiluz, K.I.B.; Salazar-Banda, G.R. Superior ethanol electrooxidation activity of Pd supported on Ni(OH)2/C. The effect of Ni(OH)2 nanosheets content. J. Electroanal. Chem. 2020, 878, 114683. [Google Scholar] [CrossRef]
- Ohtani, H.; Chen, Y.; Hasebe, M. Phase Separation of the B2 Structure Accompanied by an Ordering in Co-Al and Ni-Al Binary Systems. Mater. Trans. 2004, 45, 1489–1498. [Google Scholar] [CrossRef]
- Papaderakis, A.; Mintsouli, I.; Georgieva, J.; Sotiropoulos, S. Electrocatalysts Prepared by Galvanic Replacement. Catalysts 2017, 7, 80. [Google Scholar] [CrossRef]
- Ambrozik, S.; Rawlings, B.; Vasiljevic, N.; Dimitrov, N. Metal deposition via electroless surface limited redox replacement. Electrochem. Commun. 2014, 44, 19–22. [Google Scholar] [CrossRef]
- Skibińska, K.; Kutyła, D.; Yang, X.; Krause, L.; Marzec, M.M.; Żabiński, P. Rhodium-decorated nanoconical nickel electrode synthesis and characterization as an electrochemical active cathodic material for hydrogen production. Appl. Surf. Sci. 2022, 592, 153326. [Google Scholar] [CrossRef]
- Skibińska, K.; KutyŁa, D.; KoŁczyk, K.; Kwiecińska, A.; Kowalik, R.; Zabiński, P. Electrocatalytical properties of palladium-decorated cobalt coatings obtained by electrodeposition and galvanic displacment. Arch. Metall. Mater. 2018, 63, 1517–1521. [Google Scholar] [CrossRef]
- Alia, S.M.; Yan, Y.S.; Pivovar, B.S. Galvanic displacement as a route to highly active and durable extended surface electrocatalysts. Catal. Sci. Technol. 2014, 4, 3589–3600. [Google Scholar] [CrossRef]
- Podborska, A.; Wojnicki, M. Spectroscopic and theoretical analysis of Pd2+Cl-H2O system. J. Mol. Struct. 2017, 1128, 117–122. [Google Scholar] [CrossRef]
- Wojnicki, M.; Pacławski, K.; Rudnik, E.; Fitzner, K. Kinetics of palladium(II) chloride complex reduction in aqueous solutions using dimethylamineborane. Hydrometallurgy 2011, 110, 56–61. [Google Scholar] [CrossRef]
- McCrory, C.C.L.; Jung, S.; Ferrer, I.M.; Chatman, S.M.; Peters, J.C.; Jaramillo, T.F. Benchmarking Hydrogen Evolving Reaction and Oxygen Evolving Reaction Electrocatalysts for Solar Water Splitting Devices. J. Am. Chem. Soc. 2015, 137, 4347–4357. [Google Scholar] [CrossRef]
- Barbosa, A.F.B.; Oliveira, V.L.; van Drunen, J.; Tremiliosi-Filho, G. Ethanol electro-oxidation reaction using a polycrystalline nickel electrode in alkaline media: Temperature influence and reaction mechanism. J. Electroanal. Chem. 2015, 746, 31–38. [Google Scholar] [CrossRef]
- Real, S.G.; Barbosa, M.R.; Vilche, J.R.; Arvia, A.J. Influence of Chloride Concentration on the Active Dissolution and Passivation of Nickel Electrodes in Acid Sulfate Solutions. J. Electrochem. Soc. 1990, 137, 1696–1702. [Google Scholar] [CrossRef]
- Fleischmann, M.; Korinek, K.; Pletcher, D. The oxidation of organic compounds at a nickel anode in alkaline solution. J. Electroanal. Chem. Interfacial Electrochem. 1971, 31, 39–49. [Google Scholar] [CrossRef]
- Khan, M.; Khattak, R.; Khan, A.; Chen, Q.; Nisar, J.; Iqbal, Z.; Rashid, A.; Kamran, A.W.; Zekker, I.; Zahoor, M. Synthesis and Characterizations of PdNi Carbon Supported Nanomaterials: Studies of Electrocatalytic Activity for Oxygen Reduction in Alkaline Medium. Molecules 2021, 26, 3440. [Google Scholar] [CrossRef]
- Yaqoob, L.; Noor, T.; Iqbal, N. A comprehensive and critical review of the recent progress in electrocatalysts for the ethanol oxidation reaction. RSC Adv. 2021, 11, 16768–16804. [Google Scholar] [CrossRef] [PubMed]
- Chemchoub, S.; El Attar, A.; Oularbi, L.; Younssi, S.A.; Bentiss, F.; Jama, C.; El Rhazi, M. Electrosynthesis of eco-friendly electrocatalyst based nickel-copper bimetallic nanoparticles supported on poly-phenylenediamine with highest current density and early ethanol oxidation onset potential. Int. J. Hydrog. Energy 2022, 47, 39081–39096. [Google Scholar] [CrossRef]
- Almeida, C.V.S.; Tremiliosi-Filho, G.; Eguiluz, K.I.B.; Salazar-Banda, G.R. Improved ethanol electro-oxidation at Ni@Pd/C and Ni@PdRh/C core–shell catalysts. J. Catal. 2020, 391, 175–189. [Google Scholar] [CrossRef]
- Nguyen, M.T.X.; Nguyen, M.-K.; Pham, P.T.T.; Huynh, H.K.P.; Pham, H.H.; Vo, C.C.; Nguyen, S.T. High-performance Pd-coated Ni nanowire electrocatalysts for alkaline direct ethanol fuel cells. J. Electroanal. Chem. 2021, 888, 115180. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kutyła, D.; Nakajima, K.; Fukumoto, M.; Wojnicki, M.; Kołczyk-Siedlecka, K. Electrocatalytic Performance of Ethanol Oxidation on Ni and Ni/Pd Surface-Decorated Porous Structures Obtained by Molten Salts Deposition/Dissolution of Al-Ni Alloys. Int. J. Mol. Sci. 2023, 24, 3836. https://doi.org/10.3390/ijms24043836
Kutyła D, Nakajima K, Fukumoto M, Wojnicki M, Kołczyk-Siedlecka K. Electrocatalytic Performance of Ethanol Oxidation on Ni and Ni/Pd Surface-Decorated Porous Structures Obtained by Molten Salts Deposition/Dissolution of Al-Ni Alloys. International Journal of Molecular Sciences. 2023; 24(4):3836. https://doi.org/10.3390/ijms24043836
Chicago/Turabian StyleKutyła, Dawid, Kano Nakajima, Michihisa Fukumoto, Marek Wojnicki, and Karolina Kołczyk-Siedlecka. 2023. "Electrocatalytic Performance of Ethanol Oxidation on Ni and Ni/Pd Surface-Decorated Porous Structures Obtained by Molten Salts Deposition/Dissolution of Al-Ni Alloys" International Journal of Molecular Sciences 24, no. 4: 3836. https://doi.org/10.3390/ijms24043836
APA StyleKutyła, D., Nakajima, K., Fukumoto, M., Wojnicki, M., & Kołczyk-Siedlecka, K. (2023). Electrocatalytic Performance of Ethanol Oxidation on Ni and Ni/Pd Surface-Decorated Porous Structures Obtained by Molten Salts Deposition/Dissolution of Al-Ni Alloys. International Journal of Molecular Sciences, 24(4), 3836. https://doi.org/10.3390/ijms24043836







