Use of 3D Spheroid Models for the Assessment of RT Response in Head and Neck Cancer
Abstract
:1. Introduction
2. Results
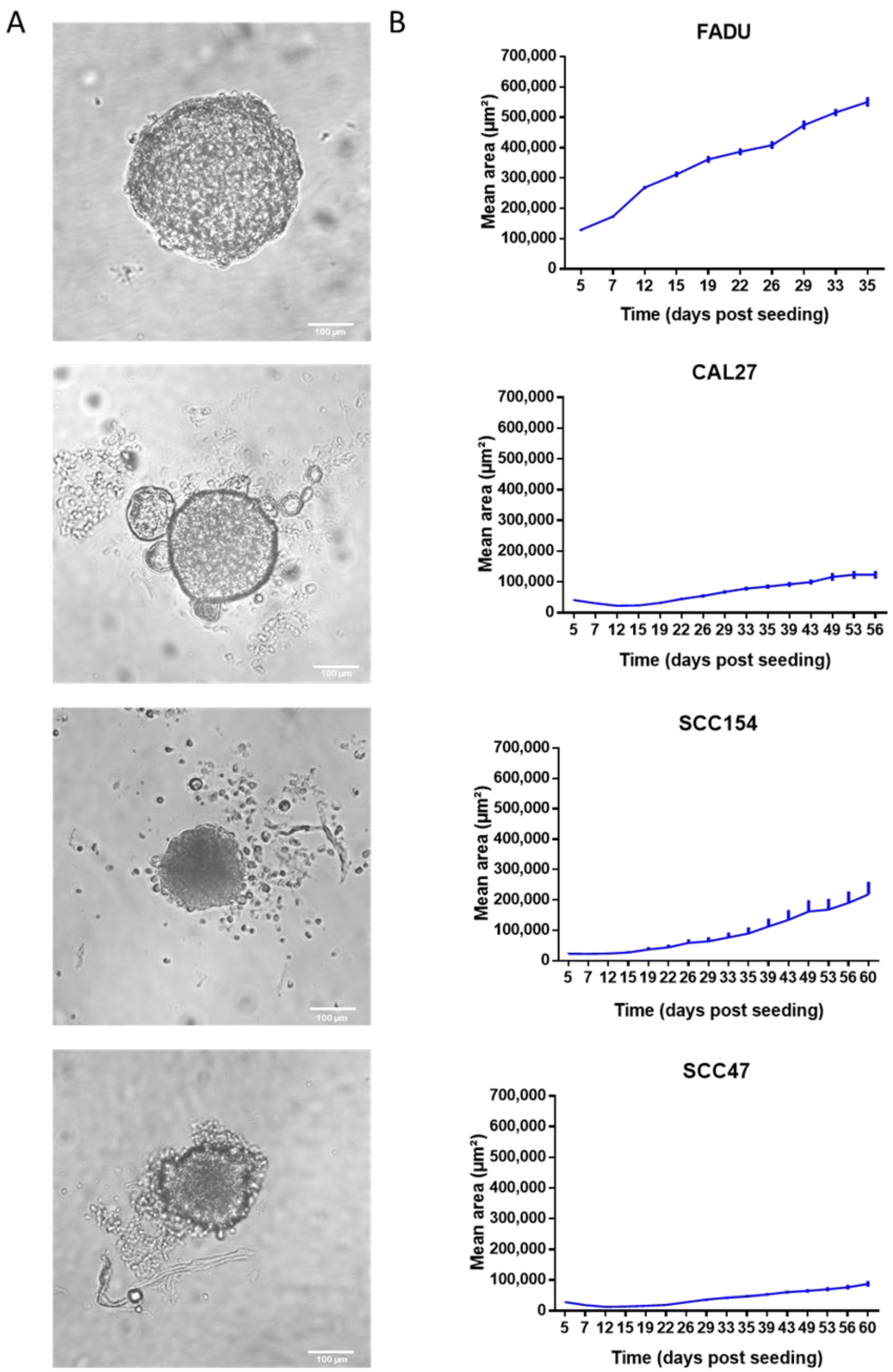
2.1. HPV-Negative Spheroids Showed a Faster Growth Rate Compared to HPV-Positive Spheroids
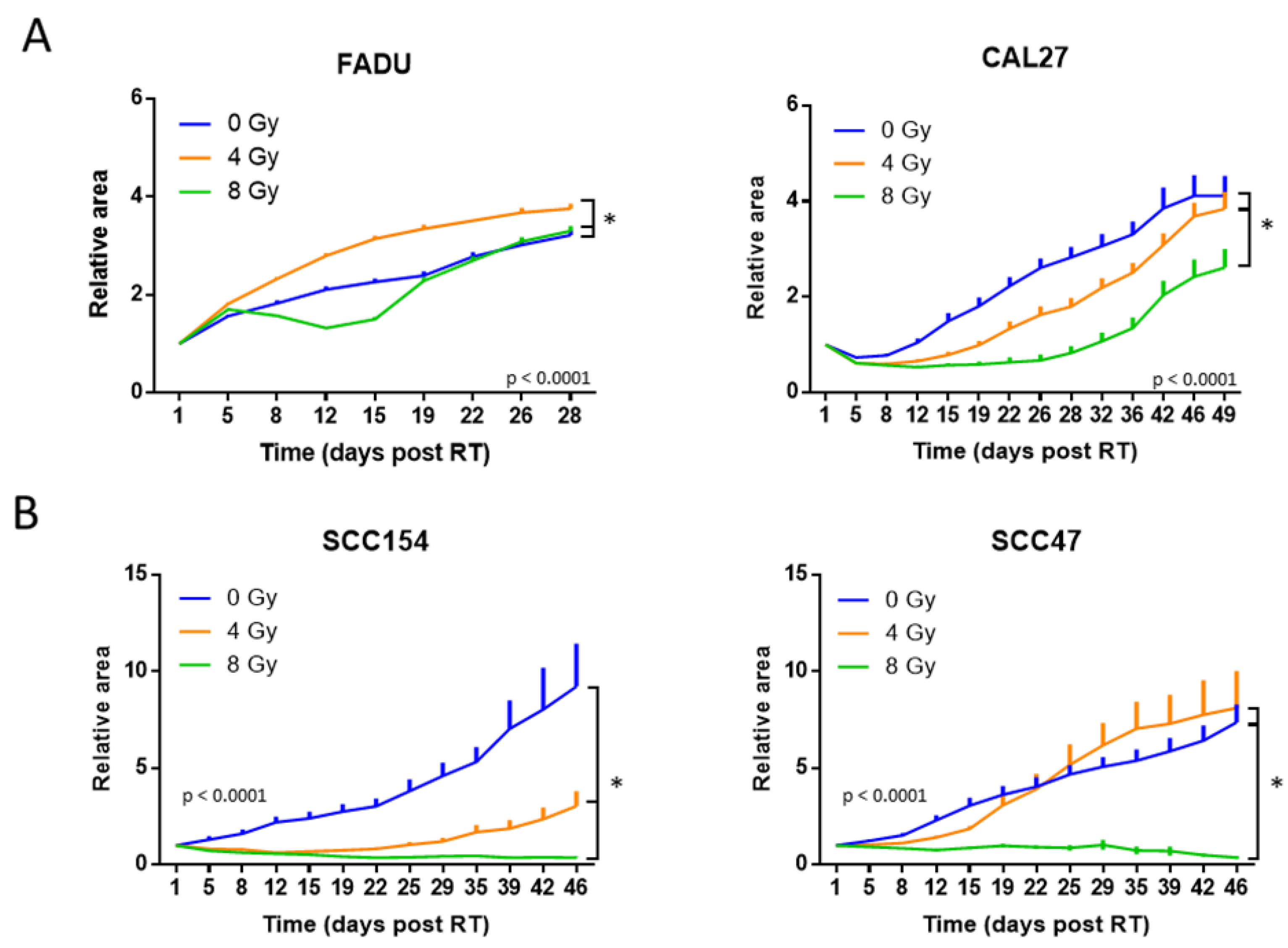
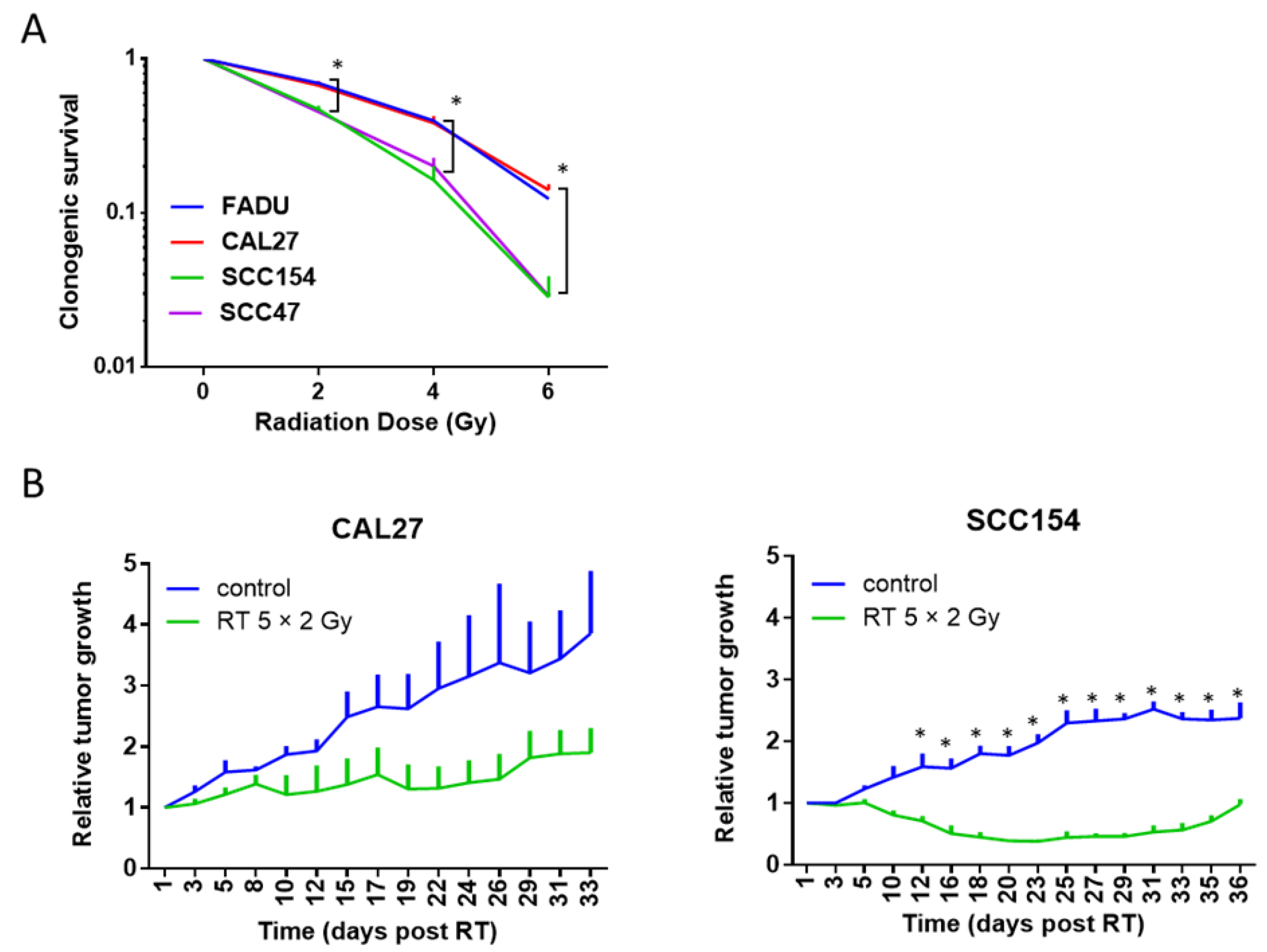
2.2. The RT Response of HPV-Positive and HPV-Negative 3D Spheroids Correlated with 2D Clonogenic Assays
2.3. The RT Response of HPV-Positive and HPV-Negative 3D Spheroids Correlated with In Vivo Data
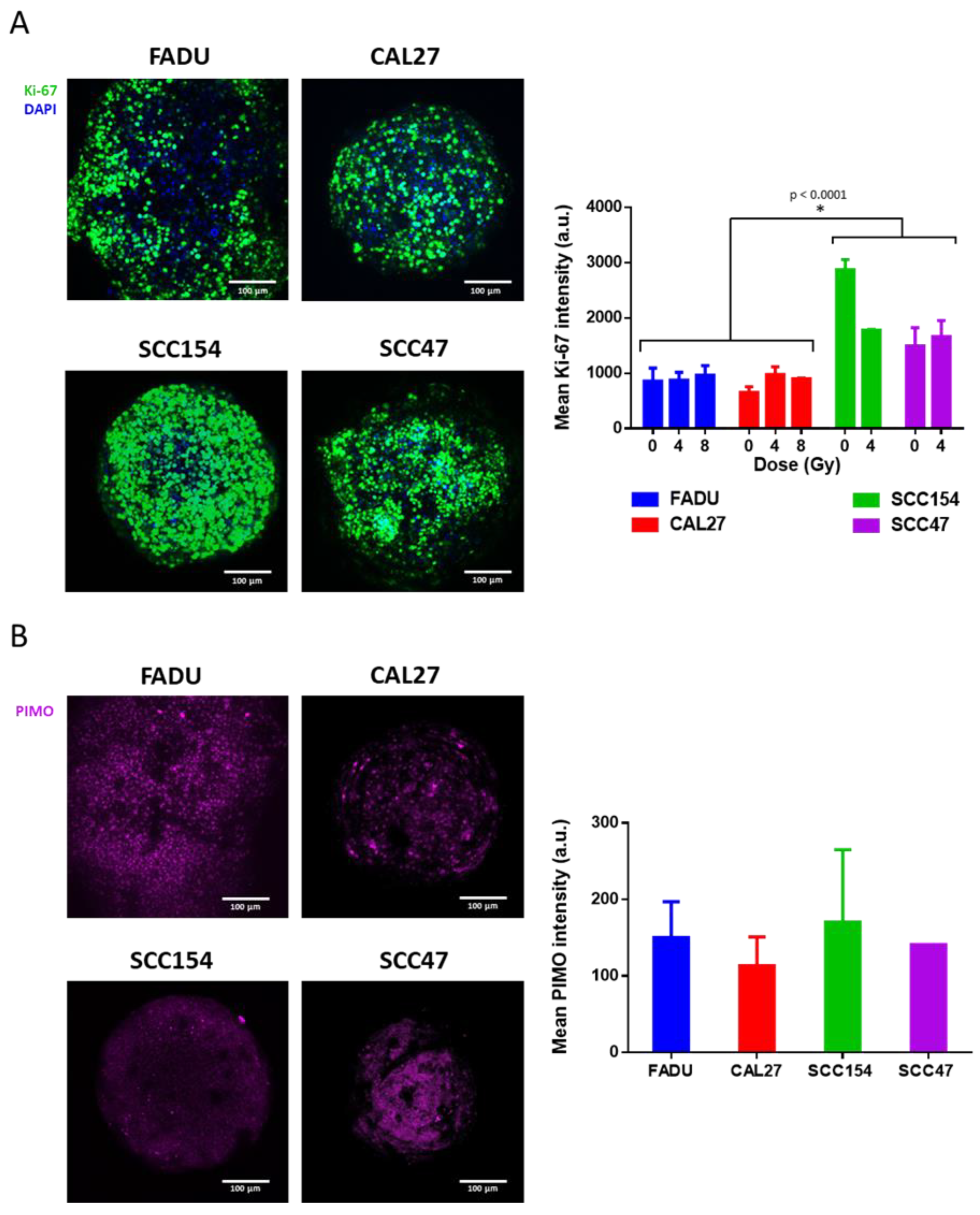
2.4. Use of 3D Spheroids as a Preclinical Model to Study Mechanisms Underlying RT Response
3. Discussion
4. Materials and Methods
4.1. Cell Lines and Reagents
4.2. Clonogenic Assay
4.3. Spheroids
4.4. In Vivo Xenograft Models
4.5. Immunofluorescence
4.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Johnson, D.E.; Burtness, B.; Leemans, C.R.; Lui, V.W.Y.; Bauman, J.E.; Grandis, J.R. Head and Neck Squamous Cell Carcinoma. Nat. Rev. Dis. Prim. 2020, 6, 92. [Google Scholar] [CrossRef] [PubMed]
- Machiels, J.P.; René Leemans, C.; Golusinski, W.; Grau, C.; Licitra, L.; Gregoire, V. Reprint of “Squamous Cell Carcinoma of the Oral Cavity, Larynx, Oropharynx and Hypopharynx: EHNS-ESMO-ESTRO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up”. Oral Oncol. 2020, 31, 1462–1475. [Google Scholar] [CrossRef]
- Gottgens, E.-L.; Ostheimer, C.; Span, P.N.; Bussink, J.; Hammond, E.M. HPV, Hypoxia and Radiation Response in Head and Neck Cancer. Br. J. Radiol. 2019, 92, 20189005. [Google Scholar] [CrossRef]
- Bamps, M.; Dok, R.; Nuyts, S. The DNA Damage Response Is Differentially Involved in HPV-Positive and HPV-Negative Radioresistant Head and Neck Squamous Cell Carcinoma. Cancers 2021, 13, 3717. [Google Scholar] [CrossRef] [PubMed]
- Powell, S.F.; Vu, L.; Spanos, W.C.; Pyeon, D. The Key Differences between Human Papillomavirus-Positive and-Negative Head and Neck Cancers: Biological and Clinical Implications. Cancers 2021, 13, 5206. [Google Scholar] [CrossRef] [PubMed]
- Baumann, M.; Krause, M.; Overgaard, J.; Debus, J.; Bentzen, S.M.; Daartz, J.; Richter, C.; Zips, D.; Bortfeld, T. Radiation Oncology in the Era of Precision Medicine. Nat. Rev. Cancer 2016, 16, 234–249. [Google Scholar] [CrossRef]
- Begg, A.C.; Stewart, F.A.; Vens, C. Strategies to Improve Radiotherapy with Targeted Drugs. Nat. Rev. Cancer 2011, 11, 239–253. [Google Scholar] [CrossRef]
- Dok, R.; Bamps, M.; Glorieux, M.; Zhao, P.; Sablina, A.; Nuyts, S. Radiosensitization Approaches for HPV-Positive and HPV-Negative Head and Neck Squamous Carcinomas. Int. J. Cancer 2020, 146, 1075–1085. [Google Scholar] [CrossRef]
- Alsahafi, E.; Begg, K.; Amelio, I.; Raulf, N.; Lucarelli, P.; Sauter, T.; Tavassoli, M. Clinical Update on Head and Neck Cancer: Molecular Biology and Ongoing Challenges. Cell Death Dis. 2019, 10, 540. [Google Scholar] [CrossRef]
- Ravichandran, A.; Clegg, J.; Adams, M.N.; Hampson, M.; Fielding, A.; Bray, L.J. 3D Breast Tumor Models for Radiobiology Applications. Cancers 2021, 13, 5714. [Google Scholar] [CrossRef]
- Brodeur, M.N.; Simeone, K.; Leclerc-Deslauniers, K.; Fleury, H.; Carmona, E.; Provencher, D.M.; Mes-Masson, A.M. Carboplatin Response in Preclinical Models for Ovarian Cancer: Comparison of 2D Monolayers, Spheroids, Ex Vivo Tumors and in Vivo Models. Sci. Rep. 2021, 11, 18183. [Google Scholar] [CrossRef] [PubMed]
- Bonartsev, A.P.; Lei, B.; Kholina, M.S.; Menshikh, K.A.; Svyatoslavov, D.S.; Samoylova, S.I.; Sinelnikov, M.Y.; Voinova, V.V.; Shaitan, K.V.; Kirpichnikov, M.P.; et al. Models of Head and Neck Squamous Cell Carcinoma Using Bioengineering Approaches. Crit. Rev. Oncol. Hematol. 2022, 175, 103724. [Google Scholar] [CrossRef] [PubMed]
- Pinto, B.; Henriques, A.C.; Silva, P.M.A.; Bousbaa, H. Three-Dimensional Spheroids as in Vitro Preclinical Models for Cancer Research. Pharmaceutics 2020, 12, 1186. [Google Scholar] [CrossRef] [PubMed]
- Alghuwainem, A.; Alshareeda, A.T.; Alsowayan, B. Scaffold-Free 3-D Cell Sheet Technique Bridges the Gap between 2-D Cell Culture and Animal Models. Int. J. Mol. Sci. 2019, 20, 4926. [Google Scholar] [CrossRef]
- Kadletz, L.; Heiduschka, G.; Domayer, J.; Schmid, R.; Enzenhofer, E.; Thurnher, D. Evaluation of Spheroid Head and Neck Squamous Cell Carcinoma Cell Models in Comparison to Monolayer Cultures. Oncol. Lett. 2015, 10, 1281–1286. [Google Scholar] [CrossRef]
- Anton, D.; Burckel, H.; Josset, E.; Noel, G. Three-Dimensional Cell Culture: A Breakthrough in Vivo. Int. J. Mol. Sci. 2015, 16, 5517–5527. [Google Scholar] [CrossRef]
- Dok, R.; Nuyts, S. HPV Positive Head and Neck Cancers: Molecular Pathogenesis and Evolving Treatment Strategies. Cancers 2016, 8, 41. [Google Scholar] [CrossRef]
- Zhang, M.; Rose, B.; Lee, C.S.; Hong, A.M. In Vitro 3-Dimensional Tumor Model for Radiosensitivity of HPV Positive OSCC Cell Lines. Cancer Biol. Ther. 2015, 16, 1231–1240. [Google Scholar] [CrossRef]
- Vincent-Chong, V.K.; Seshadri, M. Development and Radiation Response Assessment in a Novel Syngeneic Mouse Model of Tongue Cancer: 2D Culture, 3D Organoids and Orthotopic Allografts. Cancers 2020, 12, 579. [Google Scholar] [CrossRef]
- Ayuso, J.M.; Vitek, R.; Swick, A.D.; Skala, M.C.; Wisinski, K.B.; Kimple, R.J.; Lambert, P.F.; Beebe, D.J. Effects of Culture Method on Response to EGFR Therapy in Head and Neck Squamous Cell Carcinoma Cells. Sci. Rep. 2019, 9, 12480. [Google Scholar] [CrossRef]
- Storch, K.; Eke, I.; Borgmann, K.; Krause, M.; Richter, C.; Becker, K.; Schröck, E.; Cordes, N. Three-Dimensional Cell Growth Confers Radioresistance by Chromatin Density Modification. Cancer Res. 2010, 70, 3925–3934. [Google Scholar] [CrossRef] [PubMed]
- Vitti, E.T.; Kacperek, A.; Parsons, J.L. Targeting DNA Double-Strand Break Repair Enhances Radiosensitivity of HPV-Positive and HPV-Negative Head and Neck Squamous Cell Carcinoma to Photons and Protons. Cancers 2020, 12, 1490. [Google Scholar] [CrossRef] [PubMed]
- Kimple, R.J.; Smith, M.A.; Blitzer, G.C.; Torres, A.D.; Martin, J.A.; Yang, R.Z.; Peet, C.R.; Lorenz, L.D.; Nickel, K.P.; Klingelhutz, A.J.; et al. Enhanced Radiation Sensitivity in HPV-Positive Head and Neck Cancer. Cancer Res. 2013, 73, 4791–4800. [Google Scholar] [CrossRef] [PubMed]
- Rieckmann, T.; Tribius, S.; Grob, T.J.; Meyer, F.; Busch, C.-J.; Petersen, C.; Dikomey, E.; Kriegs, M. HNSCC Cell Lines Positive for HPV and P16 Possess Higher Cellular Radiosensitivity Due to an Impaired DSB Repair Capacity. Radiother. Oncol. 2013, 107, 242–246. [Google Scholar] [CrossRef]
- Arenz, A.; Ziemann, F.; Mayer, C.; Wittig, A.; Dreffke, K.; Preising, S.; Wagner, S.; Klussmann, J.P.; Engenhart-Cabillic, R.; Wittekindt, C. Increased Radiosensitivity of HPV-Positive Head and Neck Cancer Cell Lines Due to Cell Cycle Dysregulation and Induction of Apoptosis. Strahlenther. Onkol. 2014, 190, 839–846. [Google Scholar] [CrossRef]
- Ang, K.K.; Harris, J.; Wheeler, R. Human Papillomavirus and Survival of Patients with Oropharyngeal Cancer. N. Engl. J. Med. 2010, 363, 24–35. [Google Scholar] [CrossRef]
- Xue, G.; Ren, Z.; Grabham, P.W.; Chen, Y.; Zhu, J.; Du, Y.; Pan, D.; Li, X.; Hu, B. Reprogramming Mediated Radio-Resistance of 3D-Grown Cancer Cells. J. Radiat. Res. 2015, 56, 656–662. [Google Scholar] [CrossRef]
- Sowa, M.B.; Chrisler, W.B.; Zens, K.D.; Ashjian, E.J.; Opresko, L.K. Three-Dimensional Culture Conditions Lead to Decreased Radiation Induced Cytotoxicity in Human Mammary Epithelial Cells. Mutat. Res. Fundam. Mol. Mech. Mutagen 2010, 687, 78–83. [Google Scholar] [CrossRef]
- Olive, P.L.; Durand, R.E. Drug and Radiation Resistance in Spheroids: Cell Contact and Kinetics. Cancer Metastasis Rev. 1994, 13, 121–138. [Google Scholar] [CrossRef]
- Robert Grimes, D.; Partridge, M. A Mechanistic Investigation of the Oxygen Fixation Hypothesis and Oxygen Enhancement Ratio. Biomed. Phys. Eng. Express 2015, 1, 045209. [Google Scholar] [CrossRef] [PubMed]
- McMahon, S.J.; Prise, K.M. A Mechanistic DNA Repair and Survival Model (Medras): Applications to Intrinsic Radiosensitivity, Relative Biological Effectiveness and Dose-Rate. Front. Oncol. 2021, 11, 689112. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Hyeon, D.Y.; Hwang, D. Single-Cell Multiomics: Technologies and Data Analysis Methods. Exp. Mol. Med. 2020, 52, 1428–1442. [Google Scholar] [CrossRef] [PubMed]
- Grünwald, B.T.; Devisme, A.; Andrieux, G.; Vyas, F.; Aliar, K.; McCloskey, C.W.; Macklin, A.; Jang, G.H.; Denroche, R.; Romero, J.M.; et al. Spatially Confined Sub-Tumor Microenvironments in Pancreatic Cancer. Cell 2021, 184, 5577–5592. [Google Scholar] [CrossRef] [PubMed]
- Jerby-Arnon, L.; Shah, P.; Cuoco, M.S.; Rodman, C.; Su, M.J. A Cancer Cell Program Promotes T Cell Exclusion and Resistance to Checkpoint Blockade. Cell 2018, 175, 984–997. [Google Scholar] [CrossRef] [PubMed]
- Avraham-Davidi, I.; Mages, S.; Klughammer, J. Integrative Single Cell and Spatial Transcriptomics of Colorectal Cancer Reveals Multicellular Functional Units That Support Tumor Progression. bioRxiv 2022. [Google Scholar] [CrossRef]
- Anastasov, N.; Höfig, I.; Radulović, V.; Ströbel, S.; Salomon, M.; Lichtenberg, J.; Rothenaigner, I.; Hadian, K.; Kelm, J.M.; Thirion, C.; et al. A 3D-Microtissue-Based Phenotypic Screening of Radiation Resistant Tumor Cells with Synchronized Chemotherapeutic Treatment. BMC Cancer 2015, 15, 466. [Google Scholar] [CrossRef] [PubMed]
- Upreti, M.; Jamshidi-Parsian, A.; Koonce, N.A.; Webber, J.S.; Sharma, S.K.; Asea, A.A.A.; Mader, M.J.; Griffin, R.J. Tumor-Endothelial Cell Three-Dimensional Spheroids: New Aspects to Enhance Radiation and Drug Therapeutics. Transl. Oncol. 2011, 4, 365–376. [Google Scholar] [CrossRef]
- Sethi, P.; Jyoti, A.; Swindell, E.P.; Chan, R.; Langner, U.W. 3D Tumor Tissue Analogs and Their Orthotopic Implants for Understanding Tumor-Targeting of Microenvironment-Responsive Nanosized Chemotherapy and Radiation. Nanomedicine 2015, 11, 2013–2023. [Google Scholar] [CrossRef]
- Brix, N.; Samaga, D.; Belka, C.; Zitzelsberger, H.; Lauber, K. Analysis of Clonogenic Growth in Vitro. Nat. Protoc. 2021, 16, 4963–4991. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wegge, M.; Dok, R.; Dubois, L.J.; Nuyts, S. Use of 3D Spheroid Models for the Assessment of RT Response in Head and Neck Cancer. Int. J. Mol. Sci. 2023, 24, 3763. https://doi.org/10.3390/ijms24043763
Wegge M, Dok R, Dubois LJ, Nuyts S. Use of 3D Spheroid Models for the Assessment of RT Response in Head and Neck Cancer. International Journal of Molecular Sciences. 2023; 24(4):3763. https://doi.org/10.3390/ijms24043763
Chicago/Turabian StyleWegge, Marilyn, Rüveyda Dok, Ludwig J. Dubois, and Sandra Nuyts. 2023. "Use of 3D Spheroid Models for the Assessment of RT Response in Head and Neck Cancer" International Journal of Molecular Sciences 24, no. 4: 3763. https://doi.org/10.3390/ijms24043763
APA StyleWegge, M., Dok, R., Dubois, L. J., & Nuyts, S. (2023). Use of 3D Spheroid Models for the Assessment of RT Response in Head and Neck Cancer. International Journal of Molecular Sciences, 24(4), 3763. https://doi.org/10.3390/ijms24043763







