Dimeric Product of Peroxy Radical Self-Reaction Probed with VUV Photoionization Mass Spectrometry and Theoretical Calculations: The Case of C2H5OOC2H5
Abstract
1. Introduction
2. Results and Discussion
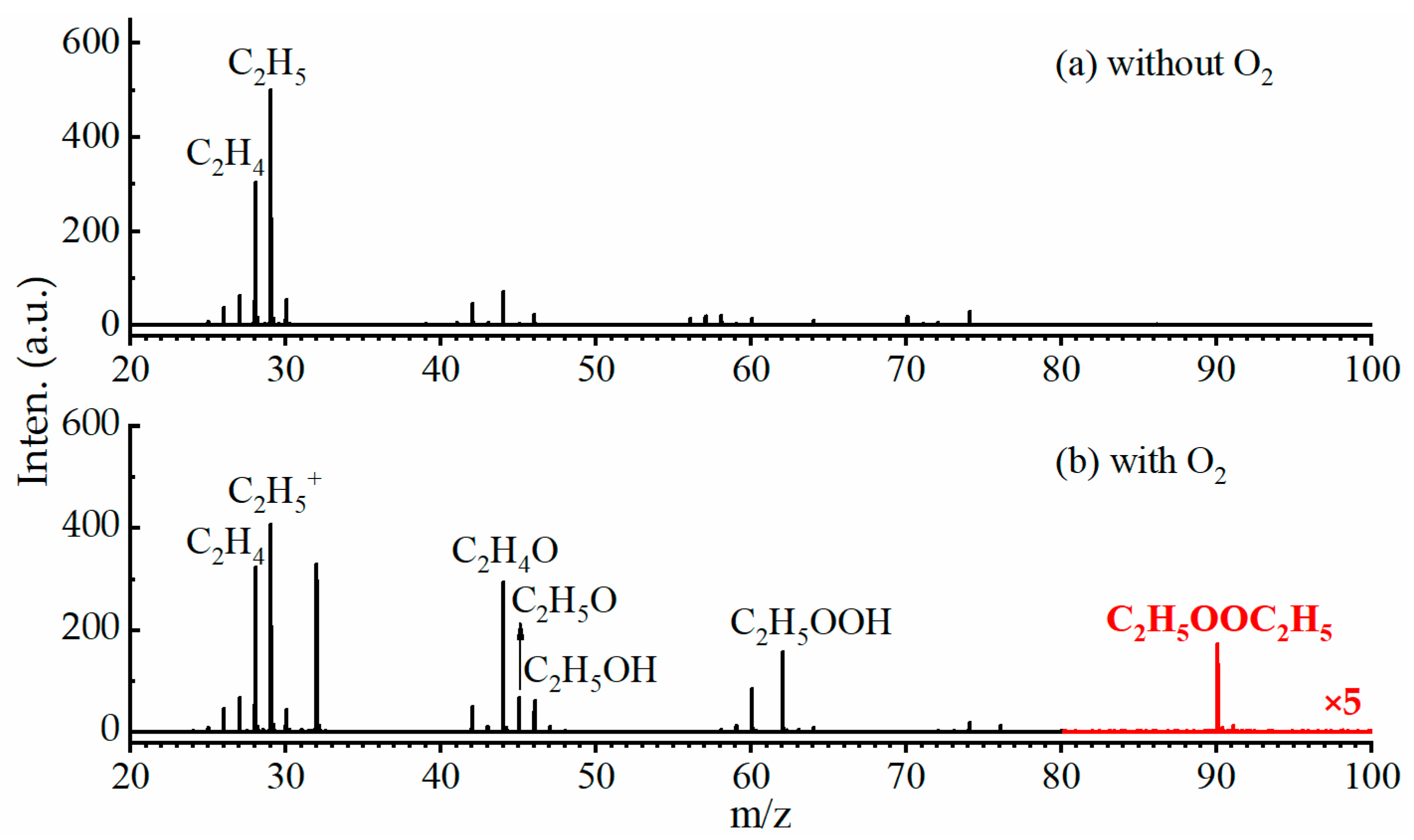
2.1. VUV Lamp Photoionization Mass Spectra
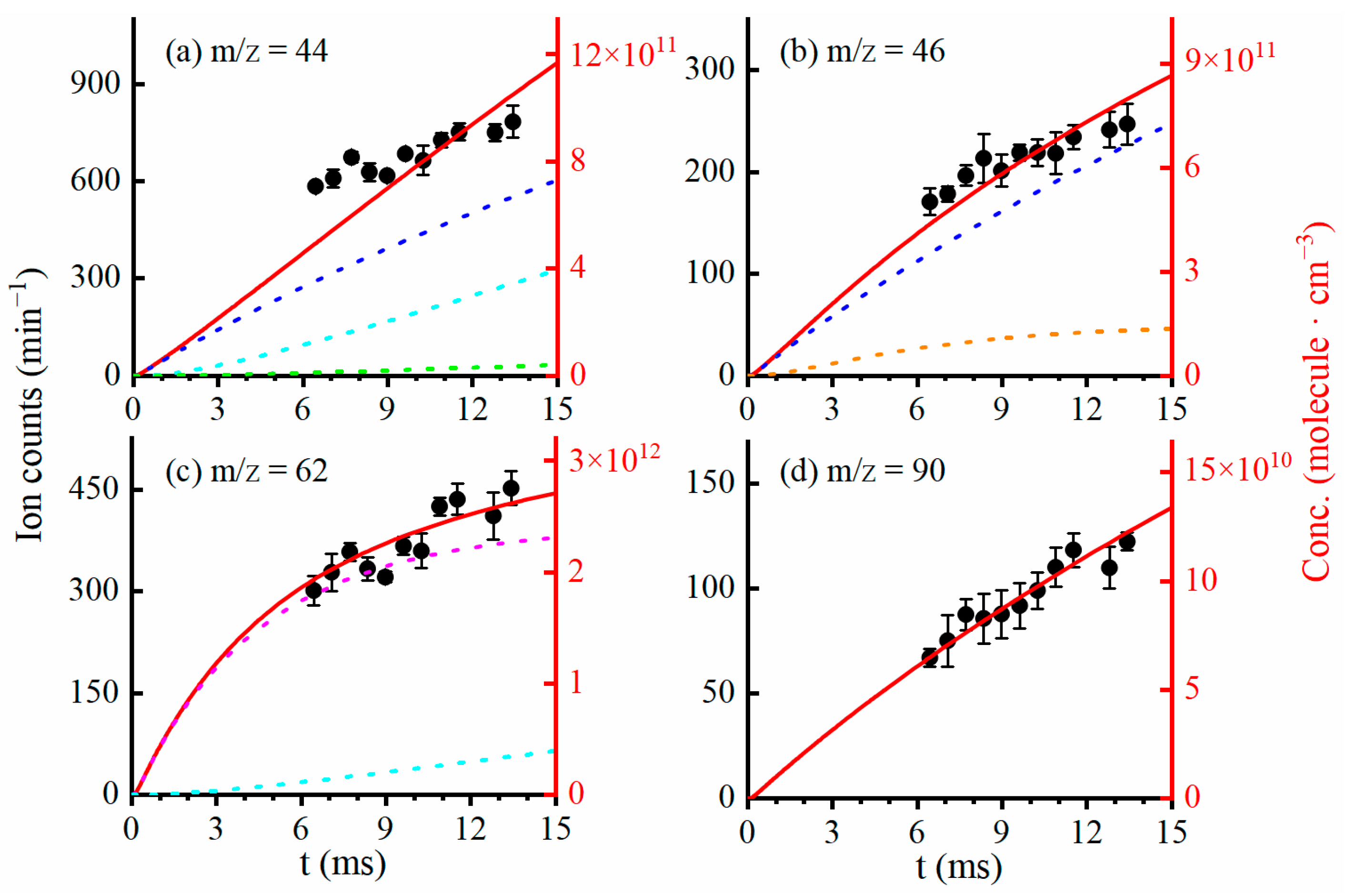
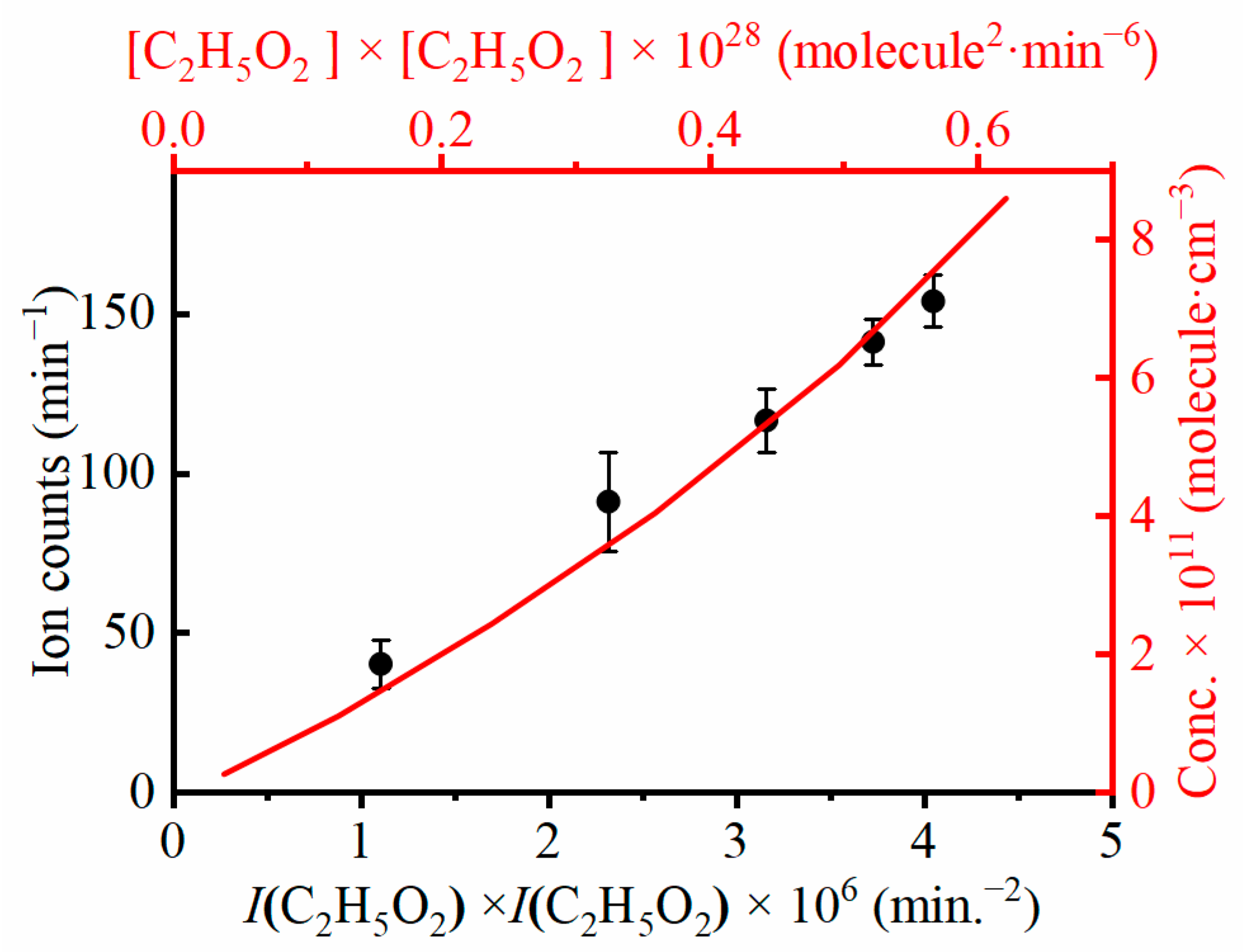
2.2. Kinetics and Reaction Mechanism
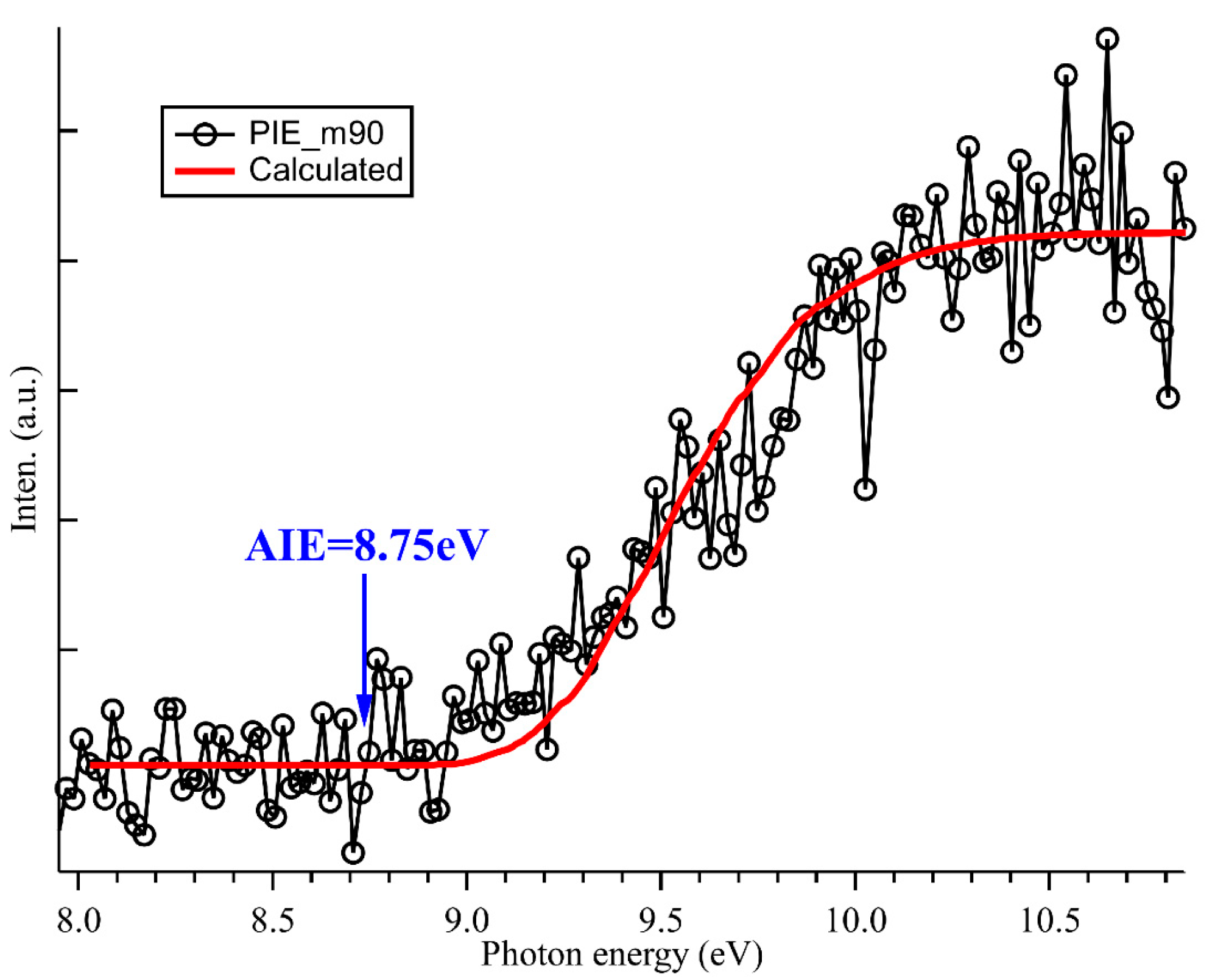
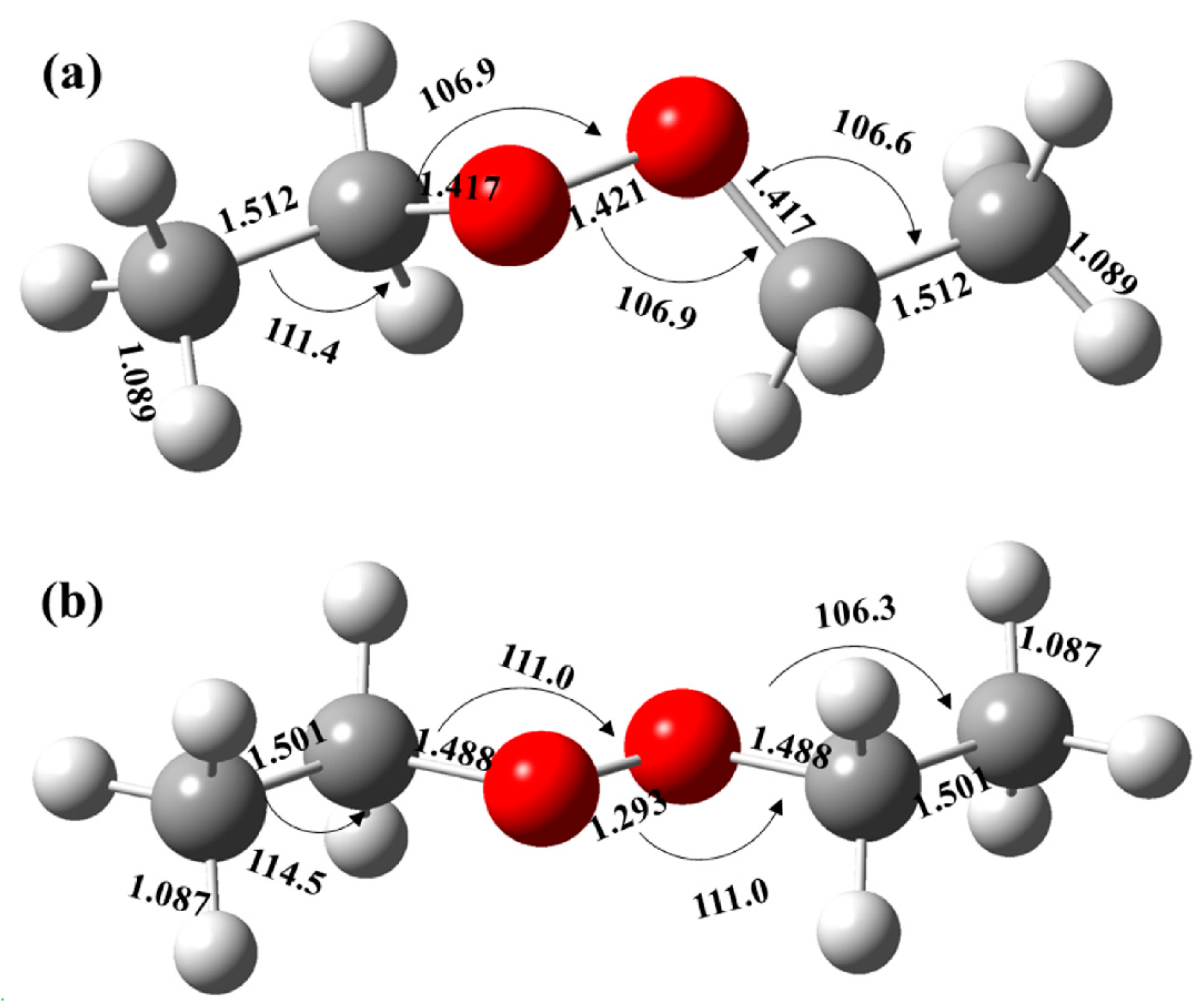
2.3. Photoionization Spectrum and Structure
2.4. Potential Energy Surface of Self-Reaction
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Orlando, J.J.; Tyndall, G.S. Laboratory studies of organic peroxy radical chemistry: An overview with emphasis on recent issues of atmospheric significance. Chem. Soc. Rev. 2012, 41, 6294–6317. [Google Scholar] [CrossRef] [PubMed]
- Jenkin, M.E.; Valorso, R.; Aumont, B.; Rickard, A.R. Estimation of rate coefficients and branching ratios for reactions of organic peroxy radicals for use in automated mechanism construction. Atmos. Chem. Phys. 2019, 19, 7691–7717. [Google Scholar] [CrossRef]
- Ziemann, P.J.; Atkinson, R. Kinetics, products, and mechanisms of secondary organic aerosol formation. Chem. Soc. Rev. 2012, 41, 6582–6605. [Google Scholar] [CrossRef]
- Berndt, T.; Scholz, W.; Mentler, B.; Fischer, L.; Herrmann, H.; Kulmala, M.; Hansel, A. Accretion product formation from self- and cross-reactions of RO2 radicals in the atmosphere. Angew. Chem. Int. Ed. 2018, 57, 3820–3824. [Google Scholar] [CrossRef] [PubMed]
- Noziere, B.; Vereecken, L. Direct Observation of Aliphatic Peroxy Radical Autoxidation and Water Effects: An Experimental and Theoretical Study. Angew. Chem. Int. Ed. 2019, 58, 13976–13982. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, F.; Kurten, T.; Riva, M.; Mohr, C.; Rissanen, M.P.; Roldin, P.; Berndt, T.; Crounse, J.D.; Wennberg, P.O.; Mentel, T.F.; et al. Highly oxygenated organic molecules (HOM) from gas-phase autoxidation involving peroxy radicals: A key contributor to atmospheric aerosol. Chem. Rev. 2019, 119, 3472–3509. [Google Scholar] [CrossRef]
- Noziere, B.; Kalberer, M.; Claeys, M.; Allan, J.; D’Anna, B.; Decesari, S.; Finessi, E.; Glasius, M.; Grgić, I.; Hamilton, J.F.; et al. The Molecular Identification of Organic Compounds in the Atmosphere: State of the Art and Challenges. Chem. Rev. 2015, 115, 3919–3983. [Google Scholar] [CrossRef]
- Tomaz, S.; Wang, D.; Zabalegui, N.; Li, D.; Lamkaddam, H.; Bachmeier, F.; Vogel, A.; Monge, M.E.; Perrier, S.; Baltensperger, U.; et al. Structures and reactivity of peroxy radicals and dimeric products revealed by online tandem mass spectrometry. Nat. Commun. 2021, 12, 300. [Google Scholar] [CrossRef]
- Su, Y.-T.; Lin, H.-Y.; Putikam, R.; Matsui, H.; Lin, M.C.; Lee, Y.-P. Extremely rapid self-reaction of the simplest Criegee intermediate CH2OO and its implications in atmospheric chemistry. Nat. Chem. 2014, 6, 477–483. [Google Scholar] [CrossRef]
- IUPAC. Available online: http://iupac.pole-ether.fr (accessed on 1 December 2022).
- Whalley, L.K.; Blitz, M.A.; Desservettaz, M.; Seakins, P.W.; Heard, D.E. Reporting the sensitivity of laser-induced fluorescence instruments used for HO2 detection to an interference from RO2 radicals and introducing a novel approach that enables HO2 and certain RO2 types to be selectively measured. Atmos. Meas. Tech. 2013, 6, 3425–3440. [Google Scholar] [CrossRef]
- Li, S.; Lu, K.; Ma, X.; Yang, X.; Chen, S.; Zhang, Y. Field measurement of the organic peroxy radicals by the low-pressure reactor plus laser-induced fluorescence spectroscopy. Chin. Chem. Lett. 2020, 31, 2799–2802. [Google Scholar] [CrossRef]
- Wu, R.; Li, J.; Hao, Y.; Li, Y.; Zeng, L.; Xie, S. Evolution process and sources of ambient volatile organic compounds during a severe haze event in Beijing, China. Sci. Total Environ. 2016, 560, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Wang, W.; Zhang, T.; Chen, L.; Du, Y.; Li, C.; Lü, J. Theoretical study on the mechanism and kinetics for the self-reaction of C2H5O2 radicals. J. Phys. Chem. A 2012, 116, 4610–4620. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.; Gryn’ova, G.; Ingold, K.U.; Coote, M.L. Why are sec-alkylperoxyl bimolecular self-reactions orders of magnitude faster than the analogous reactions of tert-alkylperoxyls? The unanticipated role of CH hydrogen bond donation. Phys. Chem. Chem. Phys. 2016, 18, 23673–23679. [Google Scholar] [CrossRef]
- Valiev, R.R.; Hasan, G.; Salo, V.-T.; Kubečka, J.; Kurten, T. Intersystem crossings drive atmospheric gas-phase dimer formation. J. Phys. Chem. A 2019, 123, 6596–6604. [Google Scholar] [CrossRef]
- Hasan, G.; Salo, V.-T.; Valiev, R.R.; Kubecka, J.; Kurtén, T. Comparing reaction routes for 3(RO···OR′) intermediates formed in peroxy radical self- and cross-reactions. J. Phys. Chem. A 2020, 124, 8305–8320. [Google Scholar] [CrossRef]
- Li, H.; Almeida, T.G.; Luo, Y.; Zhao, J.; Palm, B.B.; Daub, C.D.; Huang, W.; Mohr, C.; Krechmer, J.E.; Kurtén, T.; et al. Fragmentation inside proton-transfer-reaction-based mass spectrometers limits the detection of ROOR and ROOH peroxides. Atmos. Meas. Tech. 2022, 15, 1811–1827. [Google Scholar] [CrossRef]
- Weaver, J.; Meagher, J.; Shortridge, R.; Heicklen, J. Oxidation of acetyl radicals. J. Photochem. 1975, 4, 341–360. [Google Scholar] [CrossRef]
- Niki, H.; Maker, P.D.; Savage, C.M.; Breitenbach, L.P. Fourier-transform infrared studies of the self-reaction of CH3O2 radicals. J. Phys. Chem. 1981, 85, 877–881. [Google Scholar] [CrossRef]
- Niki, H.; Maker, P.D.; Savage, C.M.; Breitenbach, L.P. Fourier-transform infrared studies of the self-reaction of C2H5O2 radicals. J. Phys. Chem. 1982, 86, 3825–3829. [Google Scholar] [CrossRef]
- Wallington, T.J.; Gierczak, C.A.; Ball, J.C.; Japar, S.M. Fourier transform infrared study of the self reaction of C2H5O2 radicals in air at 295 K. Int. J. Chem. Kinet. 1989, 21, 1077–1089. [Google Scholar] [CrossRef]
- Anastasi, C.; Waddington, D.J.; Woolley, A. Reactions of oxygenated radicals in the gas phase. Part 10.—Self-reactions of ethylperoxy radicals. J. Chem. Soc. Faraday Trans. I 1983, 79, 505–516. [Google Scholar] [CrossRef]
- Atkinson, R.; Baulch, D.L.; Cox, R.A.; Crowley, J.N.; Hampson, R.F.; Hynes, R.G.; Jenkin, M.E.; Rossi, M.J.; Troe, J.; Subcommittee, I. Evaluated kinetic and photochemical data for atmospheric chemistry: Volume II-gas phase reactions of organic species. Atmos. Chem. Phys. 2006, 6, 3625–4055. [Google Scholar] [CrossRef]
- Noell, A.C.; Alconcel, L.S.; Robichaud, D.J.; Okumura, M.; Sander, S.P. Near-infrared kinetic spectroscopy of the HO2 and C2H5O2 self-reactions and cross reactions. J. Phys. Chem. A 2010, 114, 6983–6995. [Google Scholar] [CrossRef]
- Shamas, M.; Assali, M.; Zhang, C.; Tang, X.; Zhang, W.; Pillier, L.; Schoemaecker, C.; Fittschen, C. Rate constant and branching ratio for the reactions of the ethyl peroxy radical with itself and with the ethoxy radical. ACS Earth Space Chem. 2022, 6, 181–188. [Google Scholar] [CrossRef]
- Wen, Z.; Tang, X.; Wang, C.; Fittschen, C.; Wang, T.; Zhang, C.; Yang, J.; Pan, Y.; Liu, F.; Zhang, W. A vacuum ultraviolet photoionization time-of-flight mass spectrometer with high sensitivity for study of gas-phase radical reaction in a flow tube. Int. J. Chem. Kinet. 2019, 51, 178–188. [Google Scholar] [CrossRef]
- Wen, Z.; Tang, X.; Fittschen, C.; Zhang, C.; Wang, T.; Wang, C.; Gu, X.; Zhang, W. Online analysis of gas-phase radical reactions using vacuum ultraviolet lamp photoionization and time-of-flight mass spectrometry. Rev. Sci. Instrum. 2020, 91, 043201. [Google Scholar] [CrossRef]
- Bodi, A.; Hemberger, P.; Gerber, T.; Sztaray, B. A new double imaging velocity focusing coincidence experiment: i2PEPICO. Rev. Sci. Instrum. 2012, 83, 083105. [Google Scholar] [CrossRef]
- Sztaray, B.; Voronova, K.; Torma, K.G.; Covert, K.J.; Bodi, A.; Hemberger, P.; Gerber, T.; Osborn, D.L. CRF-PEPICO: Double velocity map imaging photoelectron photoion coincidence spectroscopy for reaction kinetics studies. J. Chem. Phys. 2017, 147, 013944. [Google Scholar] [CrossRef]
- Wen, Z.; Yue, H.; Zhang, Y.; Lin, X.; Ma, Z.; Zhang, W.; Wang, Z.; Zhang, C.; Fittschen, C.; Tang, X. Self-reaction of C2H5O2 and its cross-reaction with HO2 studied with vacuum ultraviolet photoionization mass spectrometry. Chem. Phys. Lett. 2022, 806, 140034. [Google Scholar] [CrossRef]
- Tang, X.; Lin, X.; Garcia, G.A.; Loison, J.-C.; Gouid, Z.; Abdallah, H.H.; Fittschen, C.; Hochlaf, M.; Gu, X.; Zhang, W.; et al. Identifying isomers of peroxy radicals in the gas phase: 1-C3H7O2vs. 2-C3H7O2. Chem. Commun. 2020, 56, 15525–15528. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.; Lin, X.; Tang, X.; Long, B.; Wang, C.; Zhang, C.; Fittschen, C.; Yang, J.; Gu, X.; Zhang, W. Vacuum ultraviolet photochemistry of the conformers of the ethyl peroxy radical. Phys. Chem. Chem. Phys. 2021, 23, 22096–22102. [Google Scholar] [CrossRef] [PubMed]
- Meloni, G.; Zou, P.; Klippenstein, S.J.; Ahmed, M.; Leone, S.R.; Taatjes, C.A.; Osborn, D.L. Energy-resolved photoionization of alkylperoxy radicals and the stability of their cations. J. Am. Chem. Soc. 2006, 128, 13559–13567. [Google Scholar] [CrossRef]
- Tang, X.; Lin, X.; Garcia, G.A.; Loison, J.-C.; Fittschen, C.; Röder, A.; Schleier, D.; Gu, X.; Zhang, W.; Nahon, L. Threshold photoelectron spectroscopy of the HO2 radical. J. Chem. Phys. 2020, 153, 124306. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Shamas, M.; Assali, M.; Tang, X.; Zhang, W.; Pillier, L.; Schoemaecker, C.; Fittschen, C. Absolute absorption cross-section of the à ← electronic transition of the ethyl peroxy radical and rate constant of its cross reaction with HO2. Photonics 2021, 8, 296. [Google Scholar] [CrossRef]
- Persky, A. The temperature dependence of the rate constant for the reaction F + C2H6. Chem. Phys. Lett. 2003, 380, 286–291. [Google Scholar] [CrossRef]
- Fernandes, R.X.; Luther, K.; Marowsky, G.; Rissanen, M.P.; Timonen, R.; Troe, J. Experimental and modeling study of the temperature and pressure dependence of the reaction C2H5 + O2 (+ M) → C2H5O2 (+ M). J. Phys. Chem. A. 2015, 119, 7263–7269. [Google Scholar] [CrossRef]
- Dobis, O.; Benson, S.W. Temperature coefficients of the rates of chlorine atom reactions with C2H6, C2H5, and C2H4. The rates of disproportionation and recombination of ethyl radicals. J. Am. Chem. Soc. 2002, 113, 6377–6386. [Google Scholar] [CrossRef]
- Shafir, E.V.; Slagle, I.R.; Knyazev, V.D. Kinetics of the self-reaction of C2H5 radicals. J. Phys. Chem. A. 2003, 107, 6804–6813. [Google Scholar] [CrossRef]
- Fittschen, C.; Frenzel, A.; Imrik, K.; Devolder, P. Rate constants for the reactions of C2H5O, i-C3H7O, and n-C3H7O with NO and O2 as a function of temperature. Int. J. Chem. Kinet. 1999, 31, 860–866. [Google Scholar] [CrossRef]
- Assaf, E.; Schoemaecker, C.; Vereecken, L.; Fittschen, C. The reaction of fluorine atoms with methanol: Yield of CH3O/CH2OH and rate constant of the reactions CH3O + CH3O and CH3O + HO2. Phys. Chem. Chem. Phys. 2018, 20, 10660–10670. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, R.; Baulch, D.L.; Cox, R.A.; Crowley, J.N.; Hampson, R.F.; Hynes, R.G.; Jenkin, M.E.; Rossi, M.J.; Troe, J. Evaluated kinetic and photochemical data for atmospheric chemistry: Volume I-gas phase reactions of OX, HOX, NOX and SOX species. Atmos. Chem. Phys. 2004, 4, 1461–1738. [Google Scholar] [CrossRef]
- Werner, H.J.; Knowles, P.J.; Knizia, G.; Manby, F.R.; Schütz, M.; Celani, P.; Györffy, W.; Kats, D.; Korona, T.; Lindh, R.; et al. MOLPRO Version 2015, a Package of ab Initio Programs; University of Cardiff Chemistry Consultants (UC3): Cardiff, Wales, UK, 2015. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Rev. C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Bodi, A.; Sztaray, B.; Baer, T.; Johnson, M.; Gerber, T. Data acquisition schemes for continuous two-particle time-of-flight coincidence experiments. Rev. Sci. Instrum. 2007, 78, 084102. [Google Scholar] [CrossRef] [PubMed]

| Lists | Reactions | Rate Constants a | Ref. |
|---|---|---|---|
| 1 | C2H6 + F → C2H5 + HF | 1.07 × 10−10 | [37] |
| 2 | C2H5 + O2 + M → C2H5O2 + M | 4.80 × 10−12 | [38] |
| 3 | C2H5 +O2 → C2H4 + HO2 | 4.00 × 10−13 | [26] |
| 4 | C2H5 + C2H5 → C4H10 | 1.42 × 10−11 | [39] |
| 5 | C2H5 + C2H5 → C2H4 + C2H6 | 3.90 × 10−12 | [40] |
| 6 | C2H5O2 + C2H5O2 → 2C2H5O + O2 | 3.10 × 10−14 | [26] |
| 7 | C2H5O2 + C2H5O2 → C2H5OH + CH3CHO + O2 | 6.0 × 10−14 | [26] |
| 8 | C2H5O2 + C2H5O2 → C2H5OOC2H5 + O2 | 1.1 × 10−14 | This work |
| 9 | C2H5O2 + HO2 → C2H5OOH + O2 | 6.20 × 10−12 | [36] |
| 10 | C2H5O + C2H5O2 → CH3CHO + C2H5OOH | 7 × 10−12 | [26] |
| 11 | C2H5O + O2 → CH3CHO + HO2 | 8.00 × 10−15 | [41] |
| 12 | C2H5O + HO2 → C2H5OH + O2 | 1.1 × 10−10 | [42] |
| 13 | C2H5O2 → diffusion | 5 b | This work |
| 14 | HO2 → diffusion | 10 b | This work |
| 15 | HO2 + HO2 → H2O2 + O2 | 1.7 × 10−12 | [43] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yue, H.; Zhang, C.; Lin, X.; Wen, Z.; Zhang, W.; Mostafa, S.; Luo, P.-L.; Zhang, Z.; Hemberger, P.; Fittschen, C.; et al. Dimeric Product of Peroxy Radical Self-Reaction Probed with VUV Photoionization Mass Spectrometry and Theoretical Calculations: The Case of C2H5OOC2H5. Int. J. Mol. Sci. 2023, 24, 3731. https://doi.org/10.3390/ijms24043731
Yue H, Zhang C, Lin X, Wen Z, Zhang W, Mostafa S, Luo P-L, Zhang Z, Hemberger P, Fittschen C, et al. Dimeric Product of Peroxy Radical Self-Reaction Probed with VUV Photoionization Mass Spectrometry and Theoretical Calculations: The Case of C2H5OOC2H5. International Journal of Molecular Sciences. 2023; 24(4):3731. https://doi.org/10.3390/ijms24043731
Chicago/Turabian StyleYue, Hao, Cuihong Zhang, Xiaoxiao Lin, Zuoying Wen, Weijun Zhang, Sabah Mostafa, Pei-Ling Luo, Zihao Zhang, Patrick Hemberger, Christa Fittschen, and et al. 2023. "Dimeric Product of Peroxy Radical Self-Reaction Probed with VUV Photoionization Mass Spectrometry and Theoretical Calculations: The Case of C2H5OOC2H5" International Journal of Molecular Sciences 24, no. 4: 3731. https://doi.org/10.3390/ijms24043731
APA StyleYue, H., Zhang, C., Lin, X., Wen, Z., Zhang, W., Mostafa, S., Luo, P.-L., Zhang, Z., Hemberger, P., Fittschen, C., & Tang, X. (2023). Dimeric Product of Peroxy Radical Self-Reaction Probed with VUV Photoionization Mass Spectrometry and Theoretical Calculations: The Case of C2H5OOC2H5. International Journal of Molecular Sciences, 24(4), 3731. https://doi.org/10.3390/ijms24043731








