An Insight into the Combined Toxicity of 3,4-Dichloroaniline with Two-Dimensional Nanomaterials: From Classical Mixture Theory to Structure-Activity Relationship
Abstract
:1. Introduction
2. Results and Discussion
2.1. Combined Toxicity of DCA with TDNMs
2.2. Types of Joint Toxic Action of DCA and TDNMs
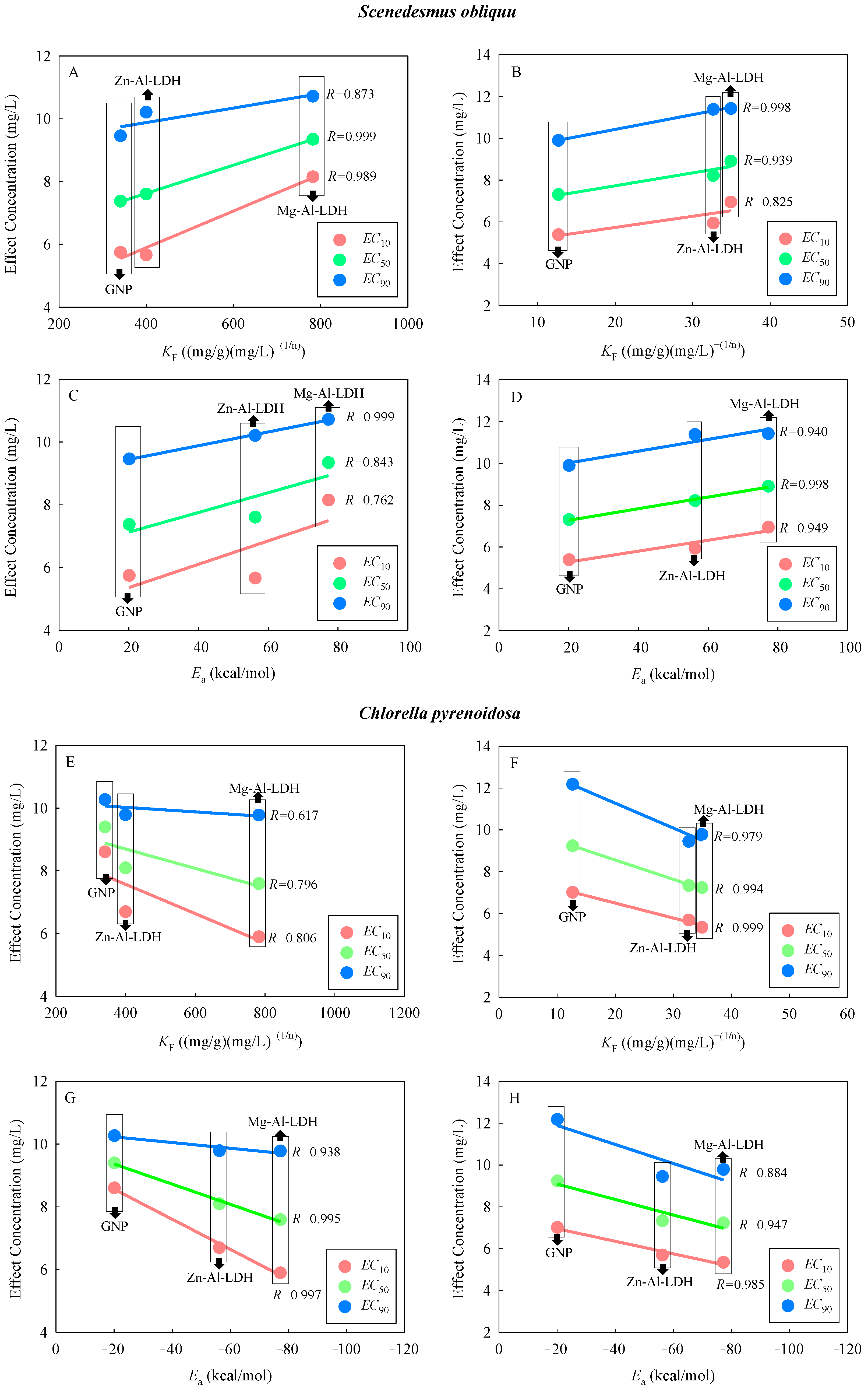
2.3. Correlation between Interaction Parameters and Combined Toxicity
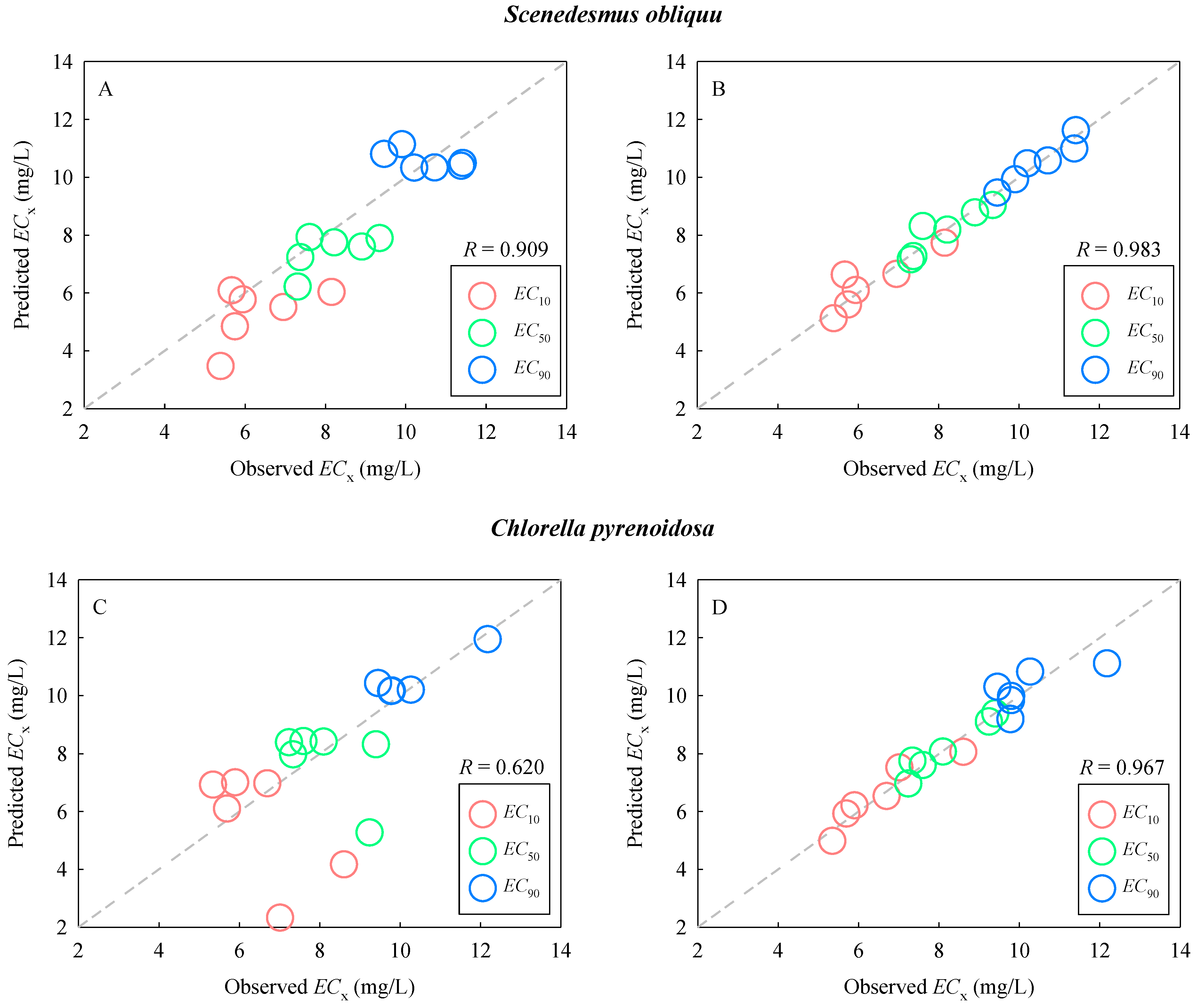
2.4. Prediction of Combined Toxicity of DCA and TDNMs
3. Materials and Methods
3.1. Test Materials, Test Species, and Test Medium
3.2. Bioassay
3.3. Concentration-Response Relationship
3.4. Assessment and Prediction of Combined Toxicity
3.5. Adsorption Experiments and Molecular Simulation
3.6. Development of a Parametric Prediction Model for ECx
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tschiche, H.R.; Bierkandt, F.S.; Creutzenberg, O.; Fessard, V.; Franz, R.; Greiner, R.; Gruber-Traub, C.; Haas, K.H.; Haase, A.; Hartwig, A.; et al. Analytical and toxicological aspects of nanomaterials in different product groups: Challenges and opportunities. NanoImpact 2022, 28, 100416. [Google Scholar] [CrossRef] [PubMed]
- Bakand, S.; Hayes, A. Toxicological considerations, toxicity assessment, and risk management of inhaled nanoparticles. Int. J. Mol. Sci. 2016, 17, 929. [Google Scholar] [CrossRef]
- Chen, P.; Huang, J.; Rao, L.; Zhu, W.; Yu, Y.; Xiao, F.; Yu, H.; Wu, Y.; Hu, R.; Liu, X.; et al. Environmental effects of nanoparticles on the ecological succession of gut microbiota across zebrafish development. Sci. Total Environ. 2022, 806, 150963. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Wang, Z.; Peijnenburg, W.J.G.M.; Vijver, M.G. Review and prospects on the ecotoxicity of mixtures of nanoparticles and hybrid nanomaterials. Environ. Sci. Technol. 2022, 56, 15238–15250. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, F.; Wang, D.-G. Predicting joint toxicity of chemicals by incorporating a weighted descriptor into a mixture model: Cases for binary antibiotics and binary nanoparticles. Ecotoxicol. Environ. Saf. 2022, 236, 113472. [Google Scholar] [CrossRef]
- Gonçalves, J.M.; Beckmann, C.; Bebianno, M.J. Assessing the effects of the cytostatic drug 5-Fluorouracil alone and in a mixture of emerging contaminants on the mussel Mytilus galloprovincialis. Chemosphere 2022, 305, 135462. [Google Scholar] [CrossRef]
- Breisch, M.; Loza, K.; Pappert, K.; Rostek, A.; Rurainsky, C.; Tschulik, K.; Heggen, M.; Epple, M.; Tiller, J.C.; Schildhauer, T.A.; et al. Enhanced dissolution of silver nanoparticles in a physical mixture with platinum nanoparticles based on the sacrificial anode effect. Nanotechnology 2020, 31, 055703. [Google Scholar] [CrossRef]
- Haghighat, F.; Kim, Y.; Sourinejad, I.; Yu, I.J.; Johari, S.A. Titanium dioxide nanoparticles affect the toxicity of silver nanoparticles in common carp (Cyprinus carpio). Chemosphere 2021, 262, 127805. [Google Scholar] [CrossRef]
- Manzoor, N.; Ali, L.; Ahmed, T.; Rizwan, M.; Ali, S.; Shahid, M.S.; Schulin, R.; Liu, Y.; Wang, G. Silicon oxide nanoparticles alleviate chromium toxicity in wheat (Triticum aestivum L.). Environ. Pollut. 2022, 315, 120391. [Google Scholar] [CrossRef]
- Sayadi, M.H.; Pavlaki, M.D.; Martins, R.; Mansouri, B.; Tyler, C.R.; Kharkan, J.; Shekari, H. Bioaccumulation and toxicokinetics of zinc oxide nanoparticles (ZnO NPs) co-exposed with graphene nanosheets (GNs) in the blackfish (Capoeta fusca). Chemosphere 2021, 269, 128689. [Google Scholar] [CrossRef]
- Martinez, D.S.T.; Ellis, L.-J.; Silva, G.; Petry, R.; Medeiros, A.M.Z.; Davoudi, H.H.; Papadiamantis, A.G.; Fazzio, A.; Afantitis, A.; Melagraki, G.; et al. Daphnia magna and mixture toxicity with nanomaterials—Current status and perspectives in data-driven risk prediction. Nano Today 2022, 43, 101430. [Google Scholar] [CrossRef]
- Iswarya, V.; Bhuvaneshwari, M.; Chandrasekaran, N.; Mukherjee, A. Trophic transfer potential of two different crystalline phases of TiO2 NPs from Chlorella sp. to Ceriodaphnia dubia. Aquat. Toxicol. 2018, 197, 89–97. [Google Scholar] [CrossRef]
- Lai, R.W.S.; Zhou, G.J.; Kang, H.M.; Jeong, C.B.; Djurišić, A.B.; Lee, J.S.; Leung, K.M.Y. Contrasting toxicity of polystyrene nanoplastics to the rotifer Brachionus koreanus in the presence of zinc oxide nanoparticles and zinc ions. Aquat. Toxicol. 2022, 253, 106332. [Google Scholar] [CrossRef]
- Martín-de-Lucía, I.; Gonçalves, S.F.; Leganés, F.; Fernández-Piñas, F.; Rosal, R.; Loureiro, S. Combined toxicity of graphite-diamond nanoparticles and thiabendazole to Daphnia magna. Sci. Total Environ. 2019, 688, 1145–1154. [Google Scholar] [CrossRef]
- Kar, S.; Pathakoti, K.; Leszczynska, D.; Tchounwou, P.B.; Leszczynski, J. In vitro and in silico study of mixtures cytotoxicity of metal oxide nanoparticles to Escherichia coli: A mechanistic approach. Nanotoxicology 2022, 16, 566–579. [Google Scholar] [CrossRef]
- Trinh, T.X.; Kim, J. Status quo in data availability and predictive models of nano-mixture toxicity. Nanomaterials 2021, 11, 124. [Google Scholar] [CrossRef]
- Huo, J.-B.; Yu, G. Layered double hydroxides derived from MIL-88A(Fe) as an efficient adsorbent for enhanced removal of lead (II) from water. Int. J. Mol. Sci. 2022, 23, 14556. [Google Scholar] [CrossRef]
- Rout, D.R.; Jena, H.M.; Baigenzhenov, O.; Hosseini-Bandegharaei, A. Graphene-based materials for effective adsorption of organic and inorganic pollutants: A critical and comprehensive review. Sci. Total Environ. 2022, 160871. [Google Scholar] [CrossRef]
- Da Gama, B.M.V.; Selvasembian, R.; Giannakoudakis, D.A.; Triantafyllidis, K.S.; McKay, G.; Meili, L. Layered double hydroxides as rising-star adsorbents for water purification: A brief discussion. Molecules 2022, 27, 4900. [Google Scholar] [CrossRef]
- Kanjwal, M.A.; Ghaferi, A.A. Graphene incorporated electrospun nanofiber for electrochemical sensing and biomedical applications: A critical review. Sensors 2022, 22, 8661. [Google Scholar] [CrossRef]
- Rodrigues, A.D.; Dos Santos Montanholi, A.; Shimabukuro, A.A.; Yonekawa, M.K.A.; Cassemiro, N.S.; Silva, D.B.; Marchetti, C.R.; Weirich, C.E.; Beatriz, A.; Zanoelo, F.F.; et al. N-acetylation of toxic aromatic amines by fungi: Strain screening, cytotoxicity and genotoxicity evaluation, and application in bioremediation of 3,4-dichloroaniline. J. Hazard. Mater. 2023, 441, 129887. [Google Scholar] [CrossRef] [PubMed]
- Hansen, B.H.; Farkas, J.; Piarulli, S.; Vicario, S.; Kvæstad, B.; Williamson, D.R.; Sørensen, L.; Davies, E.J.; Nordtug, T. Atlantic cod (Gadus morhua) embryos are highly sensitive to short-term 3,4-dichloroaniline exposure. Toxicol. Rep. 2021, 8, 1754–1761. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Thiagarajan, V.; Chandrasekaran, N.; Ravindran, B.; Mukherjee, A. Nanoplastics enhance the toxic effects of titanium dioxide nanoparticle in freshwater algae Scenedesmus obliquus. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2022, 256, 109305. [Google Scholar] [CrossRef] [PubMed]
- Kumari, R.; Barsainya, M.; Singh, D.P. Biogenic synthesis of silver nanoparticle by using secondary metabolites from Pseudomonas aeruginosa DM1 and its anti-algal effect on Chlorella vulgaris and Chlorella pyrenoidosa. Environ. Sci. Pollut. Res. Int. 2017, 24, 4645–4654. [Google Scholar] [CrossRef] [PubMed]
- Hottes, E.; da Silva, C.O.; Bauerfeldt, G.F.; Castro, R.N.; de Lima, J.H.C.; Camargo, L.P.; Dall’Antonia, L.H.; Herbst, M.H. Efficient removal of glyphosate from aqueous solutions by adsorption on Mg-Al-layered double oxides: Thermodynamic, kinetic, and mechanistic investigation. Environ. Sci. Pollut. Res Int. 2022, 29, 83698–83710. [Google Scholar] [CrossRef]
- Salih, E.Y.; Ramizy, A.; Aldaghri, O.; Sabri, M.F.M.; Madkhali, N.; Alinad, T.; Ibnaouf, K.H.; Eisa, M.H. Rapid synthesis of hexagonal-shaped Zn(Al)O-MMO nanorods for dye-sensitized solar cell Using Zn/Al-LDH as precursor. Nanomaterials 2022, 12, 1477. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, F.; Vijver, M.G.; Peijnenburg, W.J.G.M. Graphene nanoplatelets and reduced graphene oxide elevate the microalgal cytotoxicity of nano-zirconium oxide. Chemosphere 2021, 276, 130015. [Google Scholar] [CrossRef]
- Wang, P.; Zhao, L.; Huang, Y.; Qian, W.; Zhu, X.; Wang, Z.; Cai, Z. Combined toxicity of nano-TiO2 and Cd2+ to Scenedesmus obliquus: Effects at different concentration ratios. J. Hazard. Mater. 2021, 418, 126354. [Google Scholar] [CrossRef]
- Ding, T.; Lin, K.; Chen, J.; Hu, Q.; Yang, B.; Li, J.; Gan, J. Causes and mechanisms on the toxicity of layered double hydroxide (LDH) to green algae Scenedesmus quadricauda. Sci. Total Environ. 2018, 635, 1004–1011. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, Z.; White, J.C.; Xing, B. Graphene in the aquatic environment: Adsorption, dispersion, toxicity and transformation. Environ. Sci. Technol. 2014, 48, 9995–10009. [Google Scholar] [CrossRef]
- Barranger, A.; Rance, G.A.; Aminot, Y.; Dallas, L.J.; Sforzini, S.; Weston, N.J.; Lodge, R.W.; Banni, M.; Arlt, V.M.; Moore, M.N.; et al. An integrated approach to determine interactive genotoxic and global gene expression effects of multiwalled carbon nanotubes (MWCNTs) and benzoapyrene (BaP) on marine mussels: Evidence of reverse ‘Trojan Horse’ effects. Nanotoxicology 2019, 13, 1324–1343. [Google Scholar] [CrossRef]
- Mallakpour, S.; Azadi, E.; Dinari, M. Removal of cationic and anionic dyes using Ca-alginate and Zn-Al layered double hydroxide/metal-organic framework. Carbohydr. Polym. 2023, 301, 120362. [Google Scholar] [CrossRef]
- Ling-Xi, Z.; Shu-E, S.; Du, N.; Wan-Guo, H.O. A sorbent concentration-dependent Langmuir isotherm. Acta Phys. Chim. Sin. 2012, 28, 2905–2910. [Google Scholar] [CrossRef]
- Nille, O.S.; Patel, R.S.; Borate, B.Y.; Babar, S.S.; Kolekar, G.B.; Gore, A.H. One-step in-situ sustainable synthesis of magnetic carbon nanocomposite from corn comb (MCCC): Agricultural biomass valorisation for pollutant abatement in wastewater. Environ. Sci. Pollut. Res. Int. 2022; in press. [Google Scholar] [CrossRef]
- Saha Chowdhury, S.; Bera, B.; De, S. Adsorptive remediation of aqueous inorganic mercury with surfactant enhanced bismuth sulfide nanoparticles. Environ. Res. 2023, 219, 115145. [Google Scholar] [CrossRef]
- Backhaus, T.; Altenburger, R.; Boedeker, W.; Faust, M.; Scholze, M.; Grimme, L.H. Predictability of the toxicity of a multiple mixture of dissimilarly acting chemicals to Vibrio fischeri. Environ. Toxicol. Chem. 2000, 19, 2348–2356. [Google Scholar] [CrossRef]
- Altenburger, R.; Backhaus, T.; Boedeker, W.; Faust, M.; Scholze, M.; Grimme, L.H. Predictability of the toxicity of multiple chemical mixtures to Vibrio fischeri: Mixtures composed of similarly acting chemicals. Environ. Toxicol. Chem. 2000, 19, 2341–2347. [Google Scholar] [CrossRef]
- OECD Guidelines for the Testing of Chemicals. In Proceedings of the OECD Freshwater Alga and Cyanobacteria, Growth Inhibition Test No. 201, Paris, France, 28 July 2011; Available online: http://www.oecd.org (accessed on 12 December 2022).
- Abbott, W. A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 1925, 18, 265–267. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, L.; Shi, H.; He, J. Highly enantioselective and efficient asymmetric epoxidation catalysts: Inorganic nanosheets modified with α-amino acids as ligands. Angew. Chem. 2011, 123, 9337–9342. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, F.; Wang, S.; Peijnenburg, W.J.G.M. Assessment and prediction of joint algal toxicity of binary mixtures of graphene and ionic liquids. Chemosphere 2017, 185, 681–689. [Google Scholar] [CrossRef]
- Santos, S.G.; Santana, J.V.; Maia, F.F.; Lemos, V.; Freire, V.N.; Caetano, E.W.S.; Cavada, B.S.; Albuquerque, E.L. Adsorption of ascorbic acid on the C60 fullerene. J. Phys. Chem. B 2008, 112, 14267–14272. [Google Scholar] [CrossRef]


| Studied Systems | Effect Concentrations (mg/L) | |||||
|---|---|---|---|---|---|---|
| EC 10 | EC 50 | EC 90 | ||||
| S. obliquu | C. pyrenoidosa | S. obliquu | C. pyrenoidosa | S. obliquu | C. pyrenoidosa | |
| DCA | 6.10 [6.04–6.14] | 7.01 [6.84–7.07] | 7.94 [7.85–8.03] | 8.44 [8.25–8.62] | 10.33 [10.03–10.67] | 10.16 [9.63–10.87] |
| +Mg-Al-LDH (0.5) | 8.15 [8.05–8.22] | 5.90 [5.64–5.98] | 9.35 [9.29–9.41] | 7.59 [7.34–7.84] | 10.72 [10.50–10.99] | 9.78 [9.01–10.91] |
| +Mg-Al-LDH (5) | 6.95 [6.81–7.05] | 5.34 [4.92–5.46] | 8.91 [8.81–9.00] | 7.23 [6.90–7.57] | 11.42 [11.02–11.90] | 9.79 [8.72–11.64] |
| +Zn-Al-LDH (0.5) | 5.67 [5.57–5.73] | 6.70 [6.59–6.73] | 7.61 [7.49–7.72] | 8.10 [7.92–8.27] | 10.21 [9.80–10.70] | 9.79 [9.33–10.39] |
| +Zn-Al-LDH (5) | 5.94 [5.87–5.99] | 5.69 [5.58–5.76] | 8.22 [8.15–8.29] | 7.34 [7.22–7.45] | 11.38 [11.08–11.70] | 9.45 [9.05–9.94] |
| +GNP (0.5) | 5.75 [5.65–5.81] | 8.61 [8.48–8.65] | 7.37 [7.27–7.48] | 9.40 [9.29–9.51] | 9.46 [9.10–9.89] | 10.27 [9.98–10.68] |
| +GNP (5) | 5.39 [5.32–5.44] | 7.01 [6.56–7.24] | 7.31 [7.22–7.31] | 9.24 [9.04–9.44] | 9.90 [9.57–10.29] | 12.18 [11.29–13.59] |
| Studied Systems | Isotherm-Freundlich | Complex Configuration | Simulated Adsorption Energies | ||
|---|---|---|---|---|---|
| K F (mg/g)(mg/L) − (1/n) | 1/n | R2 | E a (kcal/mol) | ||
| Mg-Al-LDH (0.5) | 782.35 | 0.35 | 0.91 | 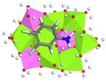 | −77.26 |
| Mg-Al-LDH (5) | 34.92 | 0.79 | 0.99 | ||
| Zn-Al-LDH (0.5) | 399.67 | 0.46 | 0.88 | 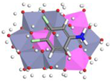 | −56.24 |
| Zn-Al-LDH (5) | 32.67 | 0.90 | 0.99 | ||
| GNP (0.5) | 340.80 | 0.31 | 0.96 | 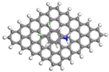 | −20.11 |
| GNP (5) | 12.68 | 0.50 | 0.84 | ||
| S. obliquu | |
| Model 1 | EC10 = 6.005 + 0.418 · KF − 0.645 · Ea N = 6, R2 = 0.762, RMSE = 0.683, Q2CUM = 0.493 |
| Model 2 | EC50 = 9.552 + 0.121 · KF − 0.861 · Ea N = 6, R2 = 0. 822, RMSE = 0.473, Q2CUM = 0.629 |
| Model 3 | EC90 = 9.339 − 0.001 · KF − 0.030 · Ea N = 6, R2 = 0.911, RMSE = 0.310, Q2CUM = 0.819 |
| C. pyrenoidosa | |
| Model 4 | EC10 = 5.500 + 0.421 · KF + 0.981 · Ea N = 6, R2 = 0.877, RMSE = 0.660, Q2CUM = 0.630 |
| Model 5 | EC50 = 8.528 − 0.272 · KF + 1.022 · Ea N = 6, R2 = 0.941, RMSE = 0.302, Q2CUM = 0.880 |
| Model 6 | EC90 = 10.210 − 0.264 · KF + 0.568 · Ea N = 6, R2 = 0.487, RMSE = 0.865, Q2CUM = 0.102 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Yu, L. An Insight into the Combined Toxicity of 3,4-Dichloroaniline with Two-Dimensional Nanomaterials: From Classical Mixture Theory to Structure-Activity Relationship. Int. J. Mol. Sci. 2023, 24, 3723. https://doi.org/10.3390/ijms24043723
Wang Z, Yu L. An Insight into the Combined Toxicity of 3,4-Dichloroaniline with Two-Dimensional Nanomaterials: From Classical Mixture Theory to Structure-Activity Relationship. International Journal of Molecular Sciences. 2023; 24(4):3723. https://doi.org/10.3390/ijms24043723
Chicago/Turabian StyleWang, Zhuang, and Le Yu. 2023. "An Insight into the Combined Toxicity of 3,4-Dichloroaniline with Two-Dimensional Nanomaterials: From Classical Mixture Theory to Structure-Activity Relationship" International Journal of Molecular Sciences 24, no. 4: 3723. https://doi.org/10.3390/ijms24043723
APA StyleWang, Z., & Yu, L. (2023). An Insight into the Combined Toxicity of 3,4-Dichloroaniline with Two-Dimensional Nanomaterials: From Classical Mixture Theory to Structure-Activity Relationship. International Journal of Molecular Sciences, 24(4), 3723. https://doi.org/10.3390/ijms24043723






