Wolfram Syndrome 1: A Pediatrician’s and Pediatric Endocrinologist’s Perspective
Abstract
1. Introduction
2. Methods
3. Results
4. Discussion
5. Main Clinical Features
5.1. Diabetes Mellitus
5.2. Diabetes Insipidus (DI)
5.3. Optic Atrophy
5.4. Deafness
6. Less Frequent Non-Endocrine Features
6.1. Neurological and Autonomic Complications
6.2. Psychiatric Abnormalities
6.3. Urological Abnormalities
7. Less Frequent Endocrine Features
8. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wolfram, D.J.; Wagener, H.P. Diabetes Mellitus and Simple Optic Atrophy among Siblings Report on Four Cases. Mayo Clin. Proc. 1938, 13, 715–718. [Google Scholar]
- Urano, F. Wolfram Syndrome: Diagnosis, Management, and Treatment. Curr. Diab. Rep. 2016, 16, 1–8. [Google Scholar] [CrossRef]
- Inoue, H.; Tanizawa, Y.; Wasson, J.; Behn, P.; Kalidas, K.; Bernal-Mizrachi, E.; Mueckler, M.; Marshall, H.; Donis-Keller, H.; Crock, P.; et al. A gene encoding a transmembrane protein is mutated in patients with diabetes mellitus and optic atrophy (Wolfram syndrome). Nat. Genet. 1998, 20, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, S.G.; Ishigaki, S.; Oslowski, C.M.; Lu, S.; Lipson, K.L.; Ghosh, R.; Hayashi, E.; Ishihara, H.; Oka, Y.; Permutt, M.A.; et al. Urano1,5Wolfram syndrome 1 gene negatively regulates ER stress signaling in rodent and human cells. J. Clin. Invest. 2010, 120, 744–755. [Google Scholar] [CrossRef]
- Eiberg, H.; Hansen, L.; Kjer, B.; Hansen, T.; Pedersen, O.; Bille, M.; Rosenberg, T.; Tranebjærg, L. Autosomal dominant optic atrophy associated with hearing impairment and impaired glucose regulation caused by a missense mutation in the WFS1 gene. J. Med. Genet. 2006, 43, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Rendtorff, N.D.; Lodahl, M.; Boulahbel, H.; Johansen, I.R.; Pandya, A.; Welch, K.O.; Norris, V.W.; Arnos, K.S.; Bitner-Glindzicz, M.; Emery, S.B.; et al. Identification of p.A684V missense mutation in the WFS1 gene as a frequent cause of autosomal dominant optic atrophy and hearing impairment. Am. J. Med. Genet. 2011, 155, 1298–1313. [Google Scholar] [CrossRef]
- Hansen, L.; Eiberg, H.; Barrett, T.; Bek, T.; Kjærsgaard, P.; Tranebjærg, L.; Rosenberg, T. Mutation analysis of the WFS1 gene in seven Danish Wolfram syndrome families; four new mutations identified. Eur. J. Hum. Genet. 2005, 13, 1275–1284. [Google Scholar] [CrossRef] [PubMed]
- Amr, S.; Heisey, C.; Zhang, M.; Xia, X.J.; Shows, K.H.; Ajlouni, K.; Pandya, A.; Satin, L.S.; El-Shanti, H.; Shiang, R. A homozygous mutation in a novel zinc-finger protein, ERIS, is responsible for Wolfram syndrome 2. Am. J. Hum. Genet. 2007, 81, 673–683. [Google Scholar] [CrossRef]
- Mozzillo, E.; Delvecchio, M.; Carella, M.; Grandone, E.; Palumbo, P.; Salina, A.; Aloi, C.; Buono, B.; Izzo, A.; D’Annunzio, G.; et al. A novel CISD2 intragenic deletion, optic neuropathy and platelet aggregation defect in Wolfram syndrome type 2. BMC Med. Genet. 2014, 15, 88. [Google Scholar] [CrossRef]
- Kumar, S. Wolfram syndrome: Important implications for pediatricians and pediatric endocrinologists. Pediatr. Diabetes 2010, 11, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, K.; Tanabe, K.; Inoue, H.; Okuya, S.; Ohta, Y.; Akiyama, M.; Taguchi, A.; Kora, Y.; Okayama, N.; Yamada, Y.; et al. Wolfram syndrome in the Japanese population; molecular analysis of WFS1 gene and characterization of clinical features. PLoS ONE 2014, 9, e106906. [Google Scholar] [CrossRef] [PubMed]
- Fraser, F.C.; Gunn, T. Diabetes mellitus, diabetes insipidus, and optic atrophy. An autosomal recessive syndrome. J. Med. Genet. 1977, 14, 190–193. [Google Scholar] [CrossRef] [PubMed]
- Medlej, R.; Wasson, J.; Baz, P.; Azar, S.; Salti, I.; Loiselet, J.; Permutt, A.; Halaby, G. Diabetes mellitus and optic atrophy: A study of Wolfram syndrome in the Lebanese population. J. Clin. Endocrinol. Metab. 2004, 89, 1656–1661. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, F.; Salzano, G.; Di Bella, C.; Aversa, T.; Pugliatti, F.; Cara, S.; Valenzise, M.; De Luca, F.; Rigoli, L. Phenotypical and genotypical expression of Wolfram syndrome in 12 patients from a Sicilian district where this syndrome might not be so infrequent as generally expected. J. Endocrinol. Invest. 2014, 37, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Zmyslowska, A.; Borowiec, M.; Fichna, P.; Iwaniszewska, B.; Majkowska, L.; Pietrzak, I.; Szalecki, M.; Szypowska, A.; Mlynarski, W. Delayed recognition of Wolfram syndrome frequently misdiagnosed as type 1 diabetes with early chronic complications. Exp. Clin. Endocrinol. Diabetes 2014, 122, 35–38. [Google Scholar] [CrossRef] [PubMed]
- Barrett, T.G.; Bundey, S.E.; Macleod, A.F. Neurodegeneration and diabetes: UK nationwide study of Wolfram (DIDMOAD) syndrome. Lancet 1995, 346, 1458–1463. [Google Scholar] [CrossRef] [PubMed]
- Zmyslowska, A.; Borowiec, M.; Fendler, W.; Jarosz-Chobot, P.; Mysśliwiec, M.; Szadkowska, A.; Młynarski, W. The prevalence of Wolfram syndrome in a paediatric population with diabetes. Endokrynol. Pol. 2014, 65, 295–297. [Google Scholar]
- Rigoli, L.; Bramanti, P.; di Bella, C.; de Luca, F. Genetic and clinical aspects of Wolfram syndrome 1, a severe neurodegenerative disease. Pediatr. Res. 2018, 83, 921–929. [Google Scholar] [CrossRef]
- Pallotta, M.T.; Tascini, G.; Crispoldi, R.; Orabona, C.; Mondanelli, G.; Grohmann, U.; Esposito, S. Wolfram syndrome, a rare neurodegenerative disease: From pathogenesis to future treatment perspectives. J. Transl. Med. 2019, 17, 238. [Google Scholar] [CrossRef]
- Rigoli, L.; Caruso, V.; Salzano, G.; Lombardo, F. Wolfram Syndrome 1: From Genetics to Therapy. Int. J. Environ. Res. Public Health 2022, 19, 3225. [Google Scholar] [CrossRef] [PubMed]
- Rigoli, L.; Lombardo, F.; Di Bella, C. Wolfram syndrome and WFS1 gene. Clin. Genet. 2011, 79, 103–117. [Google Scholar] [CrossRef] [PubMed]
- Niu, Z.; Feng, Y.; Hu, Z.; Li, J.; Sun, J.; Chen, H.; He, C.; Wang, X.; Jiang, L.; Liu, Y.; et al. Exome sequencing identifies a novel missense mutation of WFS1 as the cause of non-syndromic low-frequency hearing loss in a Chinese family. Int. J. Pediatr. Otorhinolaryngol. 2017, 100, 1–7. [Google Scholar] [CrossRef]
- Schwarz, D.S.; Blower, M.D. The endoplasmic reticulum: Structure, function and response to cellular signaling. Cell. Mol. Life Sci. 2016, 73, 79. [Google Scholar] [CrossRef]
- Gardner, B.M.; Walter, P. Unfolded proteins are Ire1-activating ligands that directly induce the unfolded protein response. Science 2011, 333, 1891–1894. [Google Scholar] [CrossRef]
- Riahi, Y.; Israeli, T.; Cerasi, E.; Leibowitz, G. Effects of proinsulin misfolding on β-cell dynamics, differentiation and function in diabetes. Diabetes Obes. Metab. 2018, 20, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Lipson, K.L.; Fonseca, S.G.; Ishigaki, S.; Nguyen, L.X.; Foss, E.; Bortell, R.; Rossini, A.A.; Urano, F. Regulation of insulin biosynthesis in pancreatic beta cells by an endoplasmic reticulum-resident protein kinase IRE1. Cell Metab. 2006, 4, 245–254. [Google Scholar] [CrossRef]
- Haze, K.; Yoshida, H.; Yanagi, H.; Yura, T.; Mori, K. Mammalian transcription factor ATF6 is synthesized as a transmembrane protein and activated by proteolysis in response to endoplasmic reticulum stress. Mol. Biol. Cell 1999, 10, 3787–3799. [Google Scholar] [CrossRef]
- Ye, J.; Rawson, R.B.; Komuro, R.; Chen, X.; Davé, U.P.; Prywes, R.; Brown, M.S.; Goldstein, J.L. ER stress induces cleavage of membrane-bound ATF6 by the same proteases that process SREBPs. Mol. Cell 2000, 6, 1355–1364. [Google Scholar] [CrossRef]
- Delprat, B.; Maurice, T.; Delettre, C. Wolfram syndrome: MAMs’ connection. Cell Death Dis. 2018, 9, 1–13. [Google Scholar] [CrossRef]
- Oslowski, C.M.; Urano, F. The binary switch that controls the life and death decisions of ER stressed β cells. Curr. Opin. Cell Biol. 2011, 23, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Cardozo, A.K.; Ortis, F.; Storling, J.; Feng, Y.H.; Rasschaert, J.; Tonnesen, M.; Van Eylen, F.; Mandrup-Poulsen, T.; Herchuelz, A.; Eizirik, D.L. Cytokines downregulate the sarcoendoplasmic reticulum pump Ca2+ ATPase 2b and deplete endoplasmic reticulum Ca2+, leading to induction of endoplasmic reticulum stress in pancreatic beta-cells. Diabetes 2005, 54, 452–461. [Google Scholar] [CrossRef] [PubMed]
- Takei, D.; Ishihara, H.; Yamaguchi, S.; Yamada, T.; Tamura, A.; Katagiri, H.; Maruyama, Y.; Oka, Y. WFS1 protein modulates the free Ca (2+) concentration in the endoplasmic reticulum. FEBS Lett. 2006, 580, 5635–5640. [Google Scholar] [CrossRef]
- Zatyka, M.; da Silva Xavier, G.; Bellomo, E.A.; Leadbeater, W.; Astuti, D.; Smith, J.; Michelangeli, F.; Rutter, G.A.; Barrett, T.G. Sarco(endo)plasmic reticulum ATPase is a molecular partner of Wolfram syndrome 1 protein, which negatively regulates its expression. Hum. Mol. Genet. 2015, 24, 814–827. [Google Scholar] [CrossRef]
- Delprat, B.; Rieusset, J.; Cile Delettre, C. Defective Endoplasmic Reticulum–Mitochondria Connection Is a Hallmark of Wolfram Syndrome. Contact 2019, 2, 251525641984740. [Google Scholar] [CrossRef]
- Cagalinec, M.; Liiv, M.; Hodurova, Z.; Hickey, M.A.; Vaarmann, A.; Mandel, M.; Zeb, A.; Choubey, V.; Kuum, M.; Safiulina, D.; et al. Role of Mitochondrial Dynamics in Neuronal Development: Mechanism for Wolfram Syndrome. PLoS Biol. 2016, 14, e1002511. [Google Scholar] [CrossRef]
- De Heredia, M.L.; Clèries, R.; Nunes, V. Genotypic classification of patients with Wolfram syndrome: Insights into the natural history of the disease and correlation with phenotype. Genet. Med. 2013, 15, 497–506. [Google Scholar] [CrossRef]
- Chaussenot, A.; Bannwarth, S.; Rouzier, C.; Vialettes, B.; Mkadem, S.A.E.; Chabrol, B.; Cano, A.; Labauge, P.; Paquis-Flucklinger, V. Neurologic features and genotype-phenotype correlation in Wolfram syndrome. Ann. Neurol. 2011, 69, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.J.A.; Crock, P.A.; King, B.R.; Meldrum, C.J.; Scott, R.J. Phenotype-genotype correlations in a series of wolfram syndrome families. Diabetes Care. 2004, 27, 2003–2009. [Google Scholar] [CrossRef]
- Rigoli, L.; Aloi, C.; Salina, A.; Di Bella, C.; Salzano, G.; Caruso, R.; Mazzon, E.; Maghnie, M.; Patti, G.; D’Annunzio, G.; et al. Wolfram syndrome 1 in the Italian population: Genotype–phenotype correlations. Pediatr. Res. 2019, 87, 456–462. [Google Scholar] [CrossRef]
- Kinsley, B.T.; Swift, M.; Dumont, R.H.; Swift, R.G. Morbidity and mortality in the Wolfram syndrome. Diabetes Care 1995, 18, 1566–1570. [Google Scholar] [CrossRef]
- Lombardo, F.; Chiurazzi, P.; Hörtnagel, K.; Arrigo, T.; Valenzise, M.; Meitinger, T.; Messina, Μ.F.; Salzano, G.; Barberi, I.; De Luca, F. Clinical picture, evolution and peculiar molecular findings in a very large pedigree with Wolfram syndrome. J. Pediatr. Endocrinol. Metab. 2005, 18, 1391–1397. [Google Scholar] [CrossRef]
- Chaussenot, A.; Rouzier, C.; Quere, M.; Plutino, M.; Ait-El-Mkadem, S.; Bannwarth, S.; Barth, M.; Dollfus, H.; Charles, P.; Nicolino, M.; et al. Mutation update and uncommon phenotypes in a French cohort of 96 patients with WFS1-related disorders. Clin Genet. 2015, 87, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Vendrell, J.; Ercilla, G.; Faundez, A.; Soler, S.; Gutierrez, C.; Gomis, R.; Vilardell, E.; Richart, C. Analysis of the contribution of the HLA system to the inheritance in the Wolfram syndrome. Diabetes Res. Clin. Pract. 1994, 22, 175–180. [Google Scholar] [CrossRef]
- Morikawa, S.; Blacher, L.; Onwumere, C.; Urano, F. Loss of Function of WFS1 Causes ER Stress-Mediated Inflammation in Pancreatic Beta-Cells. Front. Endocrinol. 2022, 13, 849204. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, S.G.; Fukuma, M.; Lipson, K.L.; Nguyen, L.X.; Allen, J.R.; Oka, Y.; Urano, F. WFS1 is a novel component of the unfolded protein response and maintains homeostasis of the endoplasmic reticulum in pancreatic beta-cells. J. Biol. Chem. 2005, 280, 39609–39615. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, S.G.; Burcin, M.; Gromada, J.; Urano, F. Endoplasmic reticulum stress in beta-cells and development of diabetes. Curr. Opin. Pharmacol. 2009, 9, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Cano, A.; Molines, L.; Valéro, R.; Simonin, G.; Paquis-Flucklinger, V.; Vialettes, B.; the French Group of Wolfram Syndrome. Microvascular diabetes complications in Wolfram syndrome (diabetes insipidus, diabetes mellitus, optic atrophy, and deafness [DIDMOAD]): An age- and duration-matched comparison with common type 1 diabetes. Diabetes Care 2007, 30, 2327–2330. [Google Scholar] [CrossRef]
- Zmyslowska, A.; Fendler, W.; Szadkowska, A.; Borowiec, M.; Mysliwiec, M.; Baranowska-Jazwiecka, A.; Buraczewska, M.; Fulmanska-Anders, M.; Mianowska, B.; Pietrzak, I.; et al. Glycemic variability in patients with Wolfram syndrome is lower than in type 1 diabetes. Acta Diabetol. 2015, 52, 1057. [Google Scholar] [CrossRef]
- Nakamura, A.; Shimizu, C.; Nagai, S.; Taniguchi, S.; Umetsu, M.; Atsumi, T.; Wada, N.; Yoshioka, N.; Ono, Y.; Tanizawa, Y.; et al. A novel mutation of WFS1 gene in a Japanese man of Wolfram syndrome with positive diabetes-related antibodies. Diabetes Res. Clin. Pract. 2006, 73, 215–217. [Google Scholar] [CrossRef]
- Sobhani, M.; Tabatabaiefar, M.A.; Ghafouri-Fard, S.; Rajab, A.; Hojat, A.; Kajbafzadeh, A.M.; Noori-Daloii, M.R. Clinical and genetic analysis of two wolfram syndrome families with high occurrence of wolfram syndrome and diabetes type II: A case report. BMC Med. Genet. 2020, 21, 1–8. [Google Scholar] [CrossRef]
- Morris, A.P.; Voight, B.F.; Teslovich, T.M.; Ferreira, T.; Segrè, A.V.; Steinthorsdottir, V.; Strawbridge, R.J.; Khan, H.; Grallert, H.; Mahajan, A.; et al. Large-scale association analysis provides insights into the genetic architecture and pathophysiology of type 2 diabetes. Nat. Genet. 2012, 44, 981–990. [Google Scholar] [PubMed]
- Hoekel, J.; Chisholm, S.A.; Al-Lozi, A.; Hershey, T.; Earhart, G.; Hullar, T.; Tychsen, L.; Washington University Wolfram Study Group. Ophthalmologic correlates of disease severity in children and adolescents with Wolfram syndrome. J. AAPOS Off. Publ. Am. Assoc. Pediatr. Ophthalmol. Strabismus 2014, 18, 461. [Google Scholar] [CrossRef] [PubMed]
- Dhalla, M.S.; Desai, U.R.; Zuckerbrod, D.S. Pigmentary maculopathy in a patient with Wolfram syndrome. Can. J. Ophthalmol. 2006, 41, 38–40. [Google Scholar] [CrossRef] [PubMed]
- Zmyslowska, A.; Waszczykowska, A.; Baranska, D.; Stawiski, K.; Borowiec, M.; Jurowski, P.; Fendler, W.; Mlynarski, W. Optical coherence tomography and magnetic resonance imaging visual pathway evaluation in Wolfram syndrome. Dev. Med. Child. Neurol. 2019, 61, 359–365. [Google Scholar] [CrossRef]
- Zmyslowska, A.; Fendler, W.; Waszczykowska, A.; Niwald, A.; Borowiec, M.; Jurowski, P.; Mlynarski, W. Retinal thickness as a marker of disease progression in longitudinal observation of patients with Wolfram syndrome. Acta Diabetol. 2017, 54, 1019–1024. [Google Scholar] [CrossRef]
- Pennings, R.J.E.; Huygen, P.L.M.; Van Den Ouweland, J.M.W.; Cryns, K.; Dikkeschei, L.D.; Van Camp, G.; Cremers, C.W.R.J. Sex-related hearing impairment in Wolfram syndrome patients identified by inactivating WFS1 mutations. Audiol. Neurootol. 2004, 9, 51–62. [Google Scholar] [CrossRef]
- Karzon, R.; Narayanan, A.; Chen, L.; Lieu, J.E.C.; Hershey, T. Longitudinal hearing loss in Wolfram syndrome. Orphanet J. Rare Dis. 2018, 13, 102. [Google Scholar] [CrossRef] [PubMed]
- Kovacs-Nagy, R.; Elek, Z.; Szekely, A.; Nanasi, T.; Sasvari-Szekely, M.; Ronai, Z. Association of aggression with a novel microRNA binding site polymorphism in the wolframin gene. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2013, 162, 404–412. [Google Scholar] [CrossRef]
- Lodha, S.; Das, L.; Ramchandani, G.D.; Bhansali, A. A case of young diabetes and parasuicide. BMJ Case Rep. 2018, 2018, 2018225839. [Google Scholar] [CrossRef]
- Swift, M.; Swift, R.G. Psychiatric disorders and mutations at the Wolfram syndrome locus. Biol. Psychiatry 2000, 47, 787–793. [Google Scholar] [CrossRef]
- Yuca, S.A.; Rendtorff, N.D.; Boulahbel, H.; Lodahl, M.; Tranebjærg, L.; Cesur, Y.; Dogan, M.; Yilmaz, C.; Akgun, C.; Acikgoz, M. Rapidly progressive renal disease as part of Wolfram syndrome in a large inbred Turkish family due to a novel WFS1 mutation (p.Leu511Pro). Eur. J. Med. Genet. 2012, 55, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Korkmaz, H.A. A case of Wolfram syndrome with chronic renal failure. Ann. Pediatr. Endocrinol. Metab. 2018, 23, 166. [Google Scholar] [CrossRef] [PubMed]
- Boutzios, G.; Livadas, S.; Marinakis, E.; Opie, N.; Economou, F.; Diamanti-Kandarakis, E. Endocrine and metabolic aspects of the Wolfram syndrome. Endocrine 2011, 40, 10–13. [Google Scholar] [CrossRef]
- Simsek, E.; Simsek, T.; Tekgül, S.; Hosal, S.; Seyrantepe, V.; Aktan, G. Wolfram (DIDMOAD) syndrome: A multidisciplinary clinical study in nine Turkish patients and review of the literature. Acta Paediatr. 2003, 92, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Lou Frances, G.; Soto De Ruiz, S.; López-Madrazo Hernández, M.J.; Macipe Costa, R.; Rodríguez Rigual, M. [Wolfram syndrome. Clinical and genetic study in two families]. An. Pediatr. 2008, 68, 54–57. [Google Scholar] [CrossRef]
- Jodoin, A.; Marchandl, M.; Beltrand, J. Wolfram syndrome in a young woman with associated hypergonadotropic hypogonadism—A case report. J Pediatr. Endocrinol. Metab. 2022, 35, 1552–1555. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, M.; Boehmer, H.V.; Zumbach, M.; Borcea, V.; Grauer, A.; Kasperk, C.; Heilmann, P.; Ziegler, R.; Wahl, P.; Nawroth, P.P. The Wolfram syndrome: Diabetes mellitus, hypacusis, optic atrophy and short stature in STH deficiency. Dtsch. Med. Wochenschr. 1997, 122, 86–90. [Google Scholar] [CrossRef]
- Reschke, F.; Rohayem, J.; Maffei, P.; Dassie, F.; Schwandt, A.; de Beaufort, C.; Toni, S.; Szypowska, A.; Cardona-Hernandez, R.; Datz, N.; et al. Collaboration for rare diabetes: Understanding new treatment options for Wolfram syndrome. Endocrine 2021, 71, 626–633. [Google Scholar] [CrossRef]
- Toppings, N.B.; McMillan, J.M.; Au, P.Y.B.; Suchowersky, O.; Donovan, L.E. Wolfram Syndrome: A Case Report and Review of Clinical Manifestations, Genetics Pathophysiology, and Potential Therapies. Case Rep. Endocrinol. 2022, 2018, 1–8. [Google Scholar] [CrossRef]
- Tarcin, G.; Turan, H.; Dagdeviren Cakir, A.; Ozer, Y.; Aykut, A.; Alpman Durmaz, A.; Ercan, O.; Evliyaoglu, O. Different clinical entities of the same mutation: A case report of three sisters with Wolfram syndrome and efficacy of dipeptidyl peptidase-4 inhibitor therapy. J. Pediatr. Endocrinol. Metab. 2021, 34, 1049–1053. [Google Scholar] [CrossRef]
- Tamborlane, W.V.; Barrientos-Pérez, M.; Fainberg, U.; Frimer-Larsen, H.; Hafez, M.; Hale, P.M.; Jalaludin, M.Y.; Kovarenko, M.; Libman, I.; Lynch, L.L.; et al. Liraglutide in Children and Adolescents with Type 2 Diabetes. N. Engl. J. Med. 2019, 381, 637–646. [Google Scholar] [CrossRef]
- Cheng, D.; Yang, S.; Zhao, X.; Wang, G. The Role of Glucagon-Like Peptide-1 Receptor Agonists (GLP-1 RA) in Diabetes-Related Neurodegenerative Diseases. Drug Des. Devel. Ther. 2022, 16, 665–684. [Google Scholar] [CrossRef]
- Frontino, G.; Raouf, T.; Canarutto, D.; Tirelli, E.; Di Tonno, R.; Rigamonti, A.; Cascavilla, M.L.; Baldoli, C.; Scotti, R.; Leocani, L.; et al. Case Report: Off-Label Liraglutide Use in Children With Wolfram Syndrome Type 1: Extensive Characterization of Four Patients. Front. Pediatr. 2021, 9, 755365. [Google Scholar] [CrossRef]
- Szentesi, P.; Collet, C.; Sárközi, S.; Szegedi, C.; Jona, I.; Jacquemond, V.; Kovács, L.; Csernoch, L. Effects of dantrolene on steps of excitation-contraction coupling in mammalian skeletal muscle fibers. J. Gen. Physiol. 2001, 118, 355–375. [Google Scholar] [CrossRef] [PubMed]
- Abreu, D.; Stone, S.I.; Pearson, T.S.; Bucelli, R.C.; Simpson, A.N.; Hurst, S.; Brown, C.M.; Kries, K.; Onwumere, C.; Guet, H.; et al. A phase Ib/IIa clinical trial of dantrolene sodium in patients with Wolfram syndrome. JCI Insight 2021, 6, e145188. [Google Scholar]
- Toots, M.; Reimets, R.; Plaas, M.; Vasar, E. Muscarinic Agonist Ameliorates Insulin Secretion in Wfs1-Deficient Mice. Can. J. Diabetes 2019, 43, 115–120. [Google Scholar] [CrossRef]
- Li, Z.; Wu, F.; Zhang, X.; Chai, Y.; Chen, D.; Yang, Y.; Xu, K.; Yin, J.; Li, R.; Shi, S.; et al. Valproate Attenuates Endoplasmic Reticulum Stress-Induced Apoptosis in SH-SY5Y Cells via the AKT/GSK3β Signaling Pathway. Int. J. Mol. Sci. 2017, 18, 315. [Google Scholar] [CrossRef] [PubMed]
- Batjargal, K.; Tajima, T.; Jimbo, E.F.; Yamagata, T. Effect of 4-phenylbutyrate and valproate on dominant mutations of WFS1 gene in Wolfram syndrome. J. Endocrinol. Investig. 2020, 43, 1317–1325. [Google Scholar] [CrossRef]
- Efficacy and Safety Trial of Sodium Valproate, in Paediatric and Adult Patients With Wolfram Syndrome. Available online: https://www.clinicaltrials.gov/ct2/home (accessed on 28 December 2022).
- Iafusco, D.; Zanfardino, A.; Piscopo, A.; Curto, S.; Troncone, A.; Chianese, A.; Rollato, A.S.; Testa, V.; Lafusco, F.; Maione, G.; et al. Metabolic Treatment of Wolfram Syndrome. Int. J. Environ. Res. Public. Health. 2022, 19, 2755. [Google Scholar] [CrossRef]
- Hamel, C.; Jagodzinska, J.; Bonner-Wersinger, D.; Koks, S.; Seveno, M.; Delettre, C. Advances in gene therapy for Wolfram syndrome. Acta Ophthalmol. 2017, 95. [Google Scholar] [CrossRef]
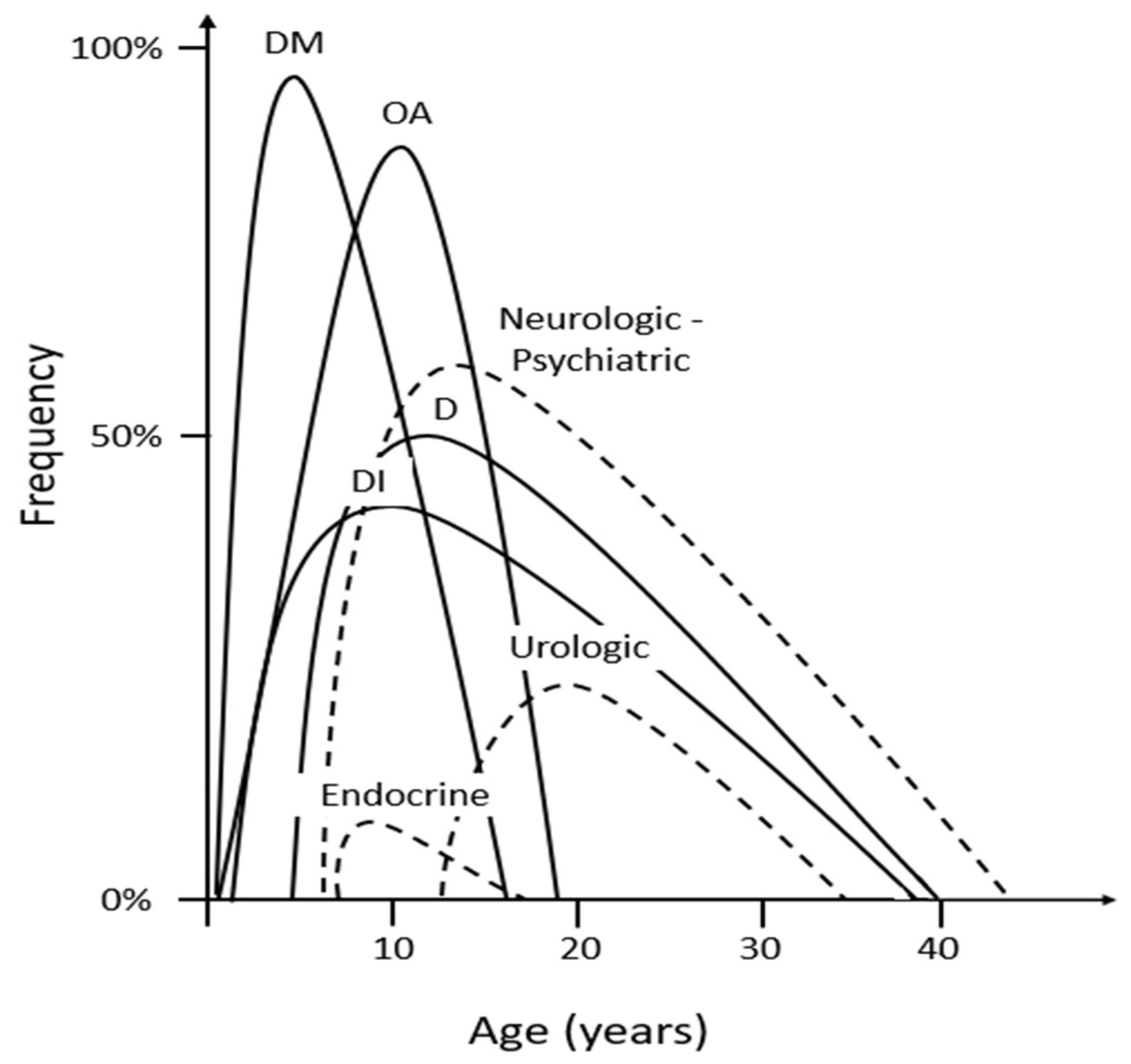
| Clinical Features | Average Age at Diagnosis (Range) | Percentage of Patients with the Specific Feature |
|---|---|---|
| Major endocrine features | ||
| Diabetes mellitus | 6 years (3 weeks–16 years) | 98.2% |
| Central diabetes insipidus | 14 years (3 months–40 years) | 37.7% |
| Major non-endocrine features | ||
| Optic atrophy | 11 years (6 weeks–19 years) | 82.1% |
| Sensorineural hearing loss | 12.5 years (5–39 years) | 48.2% |
| Secondary endocrine features | 8 years (7–9 years) | 6.6% |
| Hypogonadism | Puberty | 5% |
| Delayed menarche in females | Puberty | <1% |
| Deficient GH secretion | Childhood | 3% |
| Corticotropin deficiency | Childhood | 1.3% |
| Secondary non-endocrine features | ||
| Neurological and autonomic disorders | 16 years (5–44 years) | 17.1% |
| Psychiatric symptoms | 20.5 years (17–23 years) | 44.4% |
| Urinary tract complications | 20 years (13–33 years) | 19.4% |
| Characteristic | Wolfram Syndrome Diabetes | Type 1 Diabetes Mellitus |
|---|---|---|
| Ketoacidosis at presentation | 3% | Up to 1/3 of patients |
| Presence of other clinical features of WS1 | Yes | No |
| HLA subtype | Almost half with HLA-DR2 | Mainly HLA-DR3 and HLA-DR4 |
| Presence of insulin autoantibodies | Very rarely (coexistence with T1DM?) | >90% |
| Insulin requirement | Lower doses | Large basal and bolus doses |
| Remission (honeymoon) period | Longer | Weeks to months |
| Diabetic retinopathy | 35% | 90% |
| Diabetic nephropathy | 8% | 27% |
| Median age of death | 30–40 years | 60–70 years |
| Cause of death | Neurological disorder, urological abnormalities | Macrovascular complications (myocardial infarction, stroke) |
| Next pregnancy recurrence risk | 25% (autosomal recessive) | 3–6% |
| Compound | Mechanism of Action | Status of Use | References |
|---|---|---|---|
| Subcutaneous Insulin | Substitutes pancreatic insulin | Main therapy option in children with WS1 diabetes | [80] |
| Liraglutide | Glucagon-like peptide-1 (GLP-1) receptor agonist. It increases cyclic AMP stimulating the glucose dependent release of insulin and interferes with the ER unfolded protein response | Recently approved for use in children >12 years with type 2 diabetes. A single study in children with WS1 diabetes | [71,73] |
| Linagliptin | A Dipeptidyl Peptidase 4 (DPP-4) inhibitor, an enzyme that degrades the incretin hormones GLP-1 and glucose-dependent insulinotropic polypeptide (GIP). | Not approved for patients <18 years. A single study on its use (added to insulin regimen) in an adolescent with WS1 diabetes | [70] |
| Pioglitazone | Inhibits inositol triphosphate (IP3R)-mediated release of Ca2+ from the ER | Not approved for patients <18 years. Has been tried only in adults with WS1 diabetes | [18] |
| Dantrolene | A skeletal muscle relaxant, seems to block ryanodine receptors on the ER, reducing cytosolic calcium efflux and preserving ER integrity thus preventing β-cell apoptosis | Clinical trial with adult and pediatric WS1 patients receiving dantrolene for 6 months | [75] |
| Carbachol | A muscarinic receptor 2 agonist which potentiates glucose-stimulated insulin secretion | A single study in mice showed improved insulin secretion after carbachol administration | [76] |
| Sodium valproate | Considered to increase production of protein p21, which prevents cell apoptosis. It is also thought to increase wolframin production | A phase II randomized, double-blind, placebo-controlled trial in WS1 patients aged ≥5 years is in progress | [79] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serbis, A.; Rallis, D.; Giapros, V.; Galli-Tsinopoulou, A.; Siomou, E. Wolfram Syndrome 1: A Pediatrician’s and Pediatric Endocrinologist’s Perspective. Int. J. Mol. Sci. 2023, 24, 3690. https://doi.org/10.3390/ijms24043690
Serbis A, Rallis D, Giapros V, Galli-Tsinopoulou A, Siomou E. Wolfram Syndrome 1: A Pediatrician’s and Pediatric Endocrinologist’s Perspective. International Journal of Molecular Sciences. 2023; 24(4):3690. https://doi.org/10.3390/ijms24043690
Chicago/Turabian StyleSerbis, Anastasios, Dimitrios Rallis, Vasileios Giapros, Assimina Galli-Tsinopoulou, and Ekaterini Siomou. 2023. "Wolfram Syndrome 1: A Pediatrician’s and Pediatric Endocrinologist’s Perspective" International Journal of Molecular Sciences 24, no. 4: 3690. https://doi.org/10.3390/ijms24043690
APA StyleSerbis, A., Rallis, D., Giapros, V., Galli-Tsinopoulou, A., & Siomou, E. (2023). Wolfram Syndrome 1: A Pediatrician’s and Pediatric Endocrinologist’s Perspective. International Journal of Molecular Sciences, 24(4), 3690. https://doi.org/10.3390/ijms24043690








