Rose Bengal Induced Photothrombosis in CAM Integrated Human Split Skin Grafts—A Feasibility Study
Abstract
:1. Introduction
2. Results
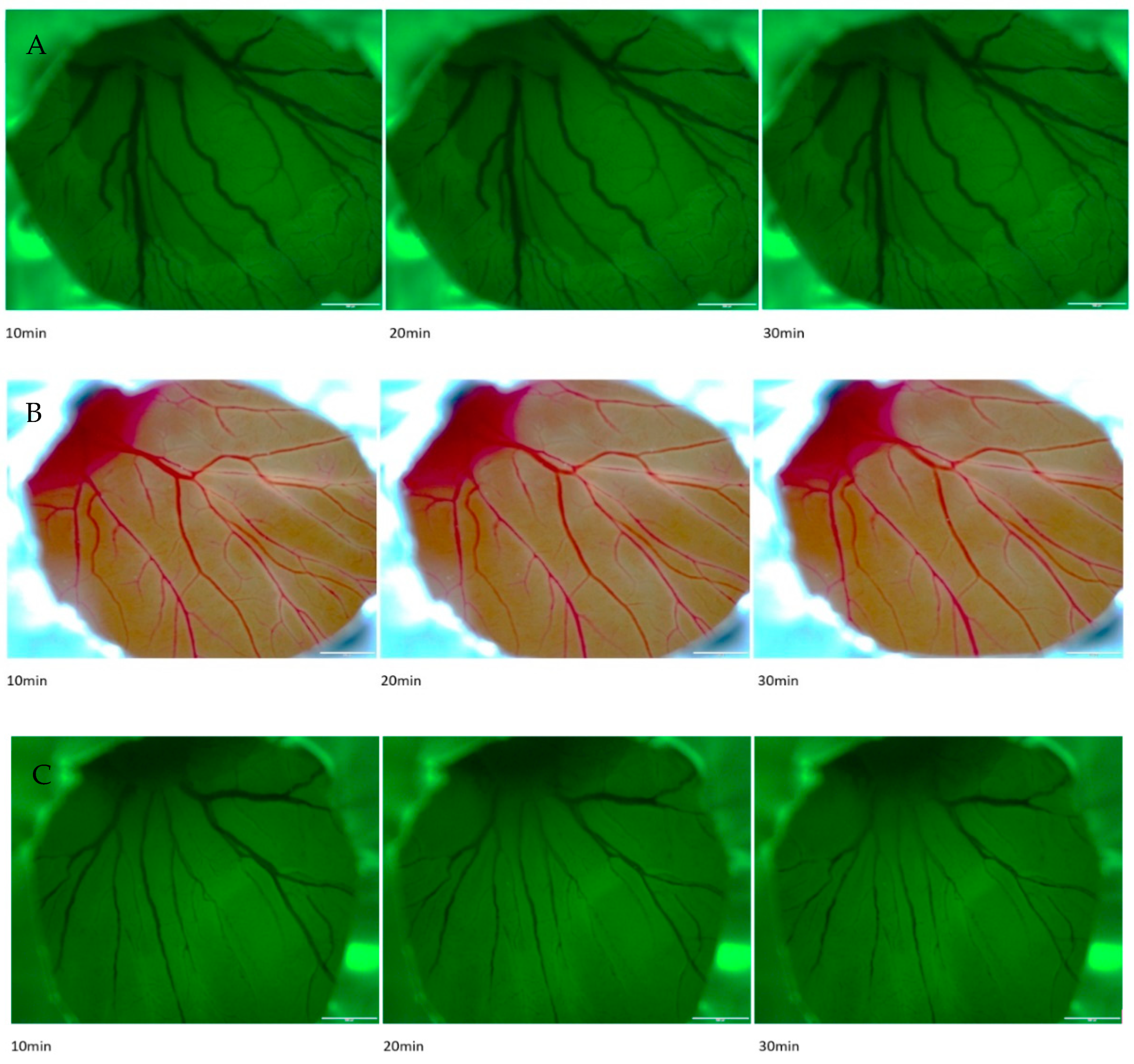
2.1. Effects of RB in 5-Day-Old Chick Embryos
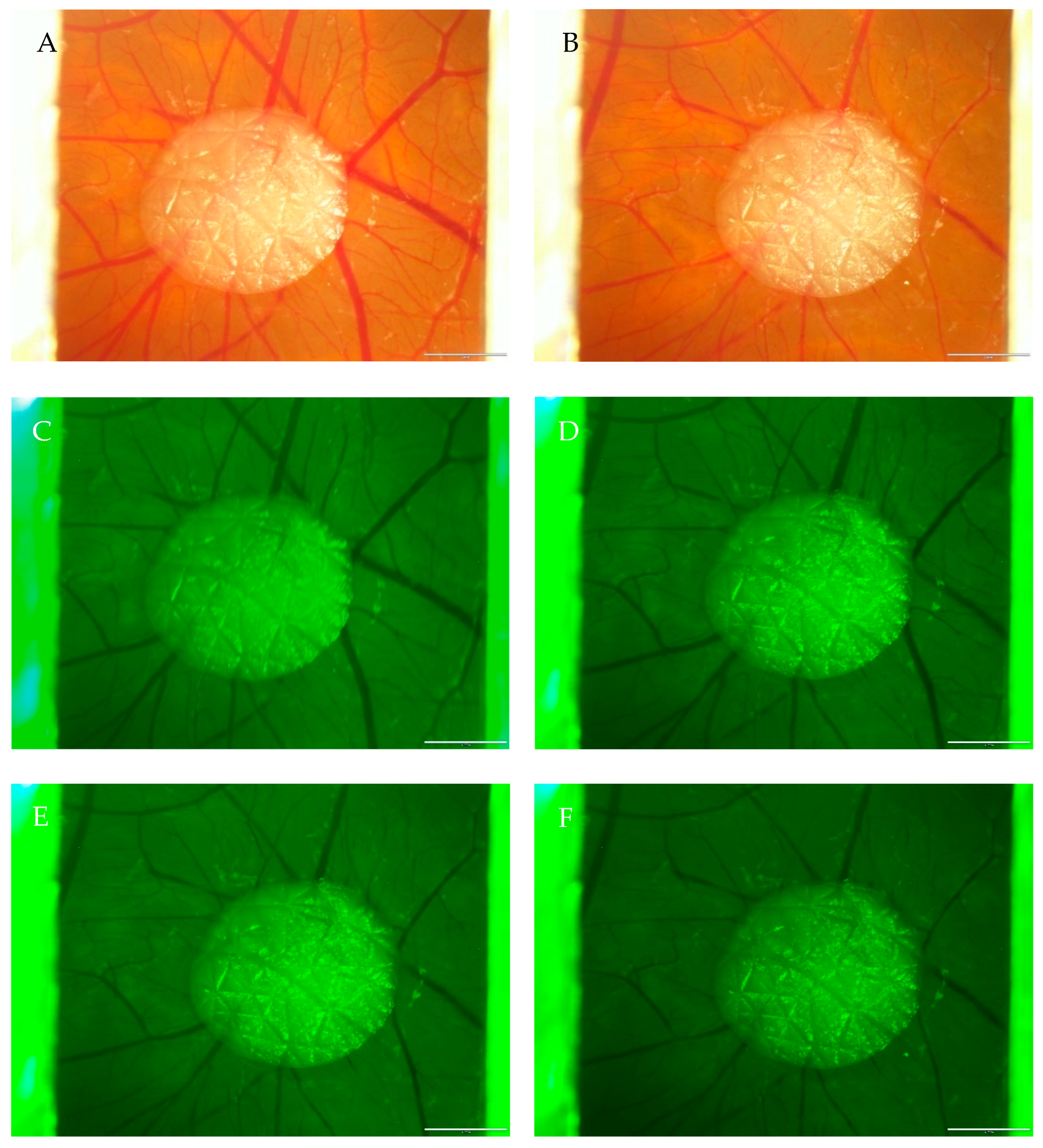
2.2. Effects of Rose Bengal with Illumination in CAM
3. Discussion
4. Materials and Methods
- A solution of Rose Bengal in phosphate-buffered saline with a concentration of 50 mg/mL was prepared.
- 3 µL of the solution was drawn up a pipette and transferred into an Eppendorf tube.
- A glass capillary was taken and put with the bottom end in the Eppendorf tube so that the solution moved up the capillary by cohesion and adhesion.
- The filled glass capillary was the injection system, and the ideal blood vessel with a diameter of about 40–80 µm was chosen.
- The next steps had to be performed under the microscope. With the help of a tweezer, the vessel was carefully lifted and fixed while the vessel was punctured with the glass capillary.
- The solution was slowly injected by blowing air into the injection system.
- To stop bleeding, oxidized cellulose was used.
- It took about 5 s until the solution of Rose Bengal was distributed in the whole vascular system of the chicken embryo and CAM.
- A cold light illuminator with a wavelength of about 549 nm—the absorption peak of Rose Bengal—and 120 W power was used for photo-activation.
- The chosen vessel was then illuminated for 10 to 30 min.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Heyer, K.; Herberger, K.; Protz, K.; Glaeske, G.; Augustin, M. Epidemiology of chronic wounds in Germany: Analysis of statutory health insurance data. Wound Repair. Regen. Off Publ. Wound Heal. Soc. Eur. Tissue Repair. Soc. 2016, 24, 434–442. [Google Scholar] [CrossRef]
- Kunzi-Rapp, K.; Rück, A.; Kaufmann, R. Characterization of the chick chorioallantoic membrane model as a short-term in vivo system for human skin. Arch. Dermatol. Res. 1999, 291, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Jiménez, I.; Hulsart-Billstrom, G.; Lanham, S.A.; Janeczek, A.A.; Kontouli, N.; Kanczler, J.M.; Oreffo, R.O. The chorioallantoic membrane (CAM) assay for the study of human bone regeneration: A refinement animal model for tissue engineering. Sci. Rep. 2016, 6, 32168. [Google Scholar] [CrossRef] [PubMed]
- Dohle, D.S.; Pasa, S.D.; Gustmann, S.; Laub, M.; Wissler, J.H.; Jennissen, H.P.; Dünker, N. Chick ex ovo culture and ex ovo CAM assay: How it really works. J. Vis. Exp. JoVE 2009, 33, e1620. [Google Scholar]
- Redmond, R.W.; Kochevar, I.E. Review: Medical Applications of Rose Bengal- and Riboflavin-Photosensitized Protein Crosslinking. Photochem. Photobiol. 2019, 95, 1097–1115. [Google Scholar] [CrossRef] [PubMed]
- Talley Watts, L.; Zheng, W.; Garling, R.J.; Frohlich, V.C.; Lechleiter, J.D. Rose Bengal Photothrombosis by Confocal Optical Imaging In Vivo: A Model of Single Vessel Stroke. J. Vis. Exp. JoVE 2015, 100, e52794. [Google Scholar]
- Watson, B.D.; Dietrich, W.D.; Busto, R.; Wachtel, M.S.; Ginsberg, M.D. Induction of reproducible brain infarction by photochemically initiated thrombosis. Ann. Neurol. 1985, 17, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Schroeter, M.; Jander, S.; Stoll, G. Non-invasive induction of focal cerebral ischemia in mice by photothrombosis of cortical microvessels: Characterization of inflammatory responses. J. Neurosci. Methods 2002, 117, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, K.A.; Dyck, R.H. Induction of reproducible focal ischemic lesions in neonatal mice by photothrombosis. Dev. Neurosci. 2005, 27, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Piao, M.S.; Lee, J.-K.; Jang, J.-W.; Kim, S.-H.; Kim, H.-S. A mouse model of photochemically induced spinal cord injury. J. Korean Neurosurg. Soc. 2009, 46, 479–483. [Google Scholar] [CrossRef] [PubMed]
- Deryugina, E.I.; Quigley, J.P. Chapter 2. Chick embryo chorioallantoic membrane models to quantify angiogenesis induced by inflammatory and tumor cells or purified effector molecules. Methods Enzymol. 2008, 444, 21–41. [Google Scholar] [PubMed]
- Lee, Y.C.; Park, C.K.; Kim, M.S.; Kim, J.H. In vitro study for staining and toxicity of rose bengal on cultured bovine corneal endothelial cells. Cornea 1996, 15, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Labat-gest, V.; Tomasi, S. Photothrombotic ischemia: A minimally invasive and reproducible photochemical cortical lesion model for mouse stroke studies. J. Vis. Exp. JoVE 2013, 76, e50370. [Google Scholar]
- Winter, R.; Dungel, P.; Reischies FM, J.; Rohringer, S.; Slezak, P.; Smolle, C.; Schicho, K. Photobiomodulation (PBM) promotes angiogenesis in-vitro and in chick embryo chorioallantoic membrane model. Sci Rep. 2018, 8, 17080. [Google Scholar] [CrossRef] [PubMed]

| CAM | Measured Vessel | Diameter in Pixels (0 min) | Diameter in Pixels (10 min) | Ratio (Before/After) |
|---|---|---|---|---|
| CAM 1 | vessel nr. 1 | 46.04 | 34.06 | 1/0.74 |
| vessel nr. 2 | 37.01 | 19.42 | 1/0.52 | |
| vessel nr. 3 | 43.00 | 23.09 | 1/0.54 | |
| vessel nr. 4 | 64.08 | 52.08 | 1/0.81 | |
| CAM 2 | vessel nr. 1 | 19.31 | 11.18 | 1/0.58 |
| vessel nr. 2 | 34.53 | 22.20 | 1/0.64 | |
| vessel nr. 3 | 20.12 | 11.18 | 1/0.56 | |
| vessel nr. 4 | 41.87 | 36.24 | 1/0.87 | |
| CAM 3 | vessel nr. 1 | 46.53 | 30.41 | 1/0.65 |
| vessel nr. 2 | 42.20 | 12.08 | 1/0.29 | |
| vessel nr. 3 | 38.08 | 13.60 | 1/0.36 | |
| vessel nr. 4 | 30.41 | 18.44 | 1/0.61 | |
| CAM 4 | vessel nr. 1 | 16.12 | 11.40 | 1/0.71 |
| vessel nr. 2 | 14.56 | 07.00 | 1/0.48 | |
| vessel nr. 3 | 16.00 | 11.18 | 1/0.70 | |
| vessel nr. 4 | 14.14 | 10.20 | 1/0.72 | |
| CAM 5 | vessel nr. 1 | 56.08 | 49.19 | 1/0.88 |
| vessel nr. 2 | 37.12 | 23.35 | 1/0.63 | |
| vessel nr. 3 | 18.44 | 12.00 | 1/0.65 | |
| vessel nr. 4 | 37.66 | 27.17 | 1/0.72 | |
| CAM 6 | vessel nr. 1 | 42.94 | 33.12 | 1/0.77 |
| vessel nr. 2 | 18.36 | 13.93 | 1/0.76 | |
| vessel nr. 3 | 32.45 | 25.24 | 1/0.78 | |
| vessel nr. 4 | 36.40 | 24.84 | 1/0.68 |
| Maximum of Vessel Lumen Reduction | Minimum of Vessel Lumen Reduction | Arithmetic Mean of Vessel Lumen Reduction | Number of Vessels Measured |
|---|---|---|---|
| 71.37% | |||
| 12.29% | 34.84% | 24 | |
| Before Treatment | After Treatment | |
|---|---|---|
| Mean | ||
| 33.48 | 22.19 | |
| Variance | 189.53 | 148.50 |
| Observations | 24 | 24 |
| Pearson correlation | 0.88 | |
| Hypothetical difference of the mean | 0 | |
| Degrees of freedom (df) | 23 | |
| T-statistics | 8.45 | |
| p-value (bilaterally) | 0.0000000165 | |
| Critical t-value bilaterally | 2.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kornhäusel, G.; Smolle, C.; Kreuzer, K.; Kamolz, L.-P.; Ghaffari-Tabrizi-Wizsy, N. Rose Bengal Induced Photothrombosis in CAM Integrated Human Split Skin Grafts—A Feasibility Study. Int. J. Mol. Sci. 2023, 24, 3689. https://doi.org/10.3390/ijms24043689
Kornhäusel G, Smolle C, Kreuzer K, Kamolz L-P, Ghaffari-Tabrizi-Wizsy N. Rose Bengal Induced Photothrombosis in CAM Integrated Human Split Skin Grafts—A Feasibility Study. International Journal of Molecular Sciences. 2023; 24(4):3689. https://doi.org/10.3390/ijms24043689
Chicago/Turabian StyleKornhäusel, Georg, Christian Smolle, Kathrin Kreuzer, Lars-Peter Kamolz, and Nassim Ghaffari-Tabrizi-Wizsy. 2023. "Rose Bengal Induced Photothrombosis in CAM Integrated Human Split Skin Grafts—A Feasibility Study" International Journal of Molecular Sciences 24, no. 4: 3689. https://doi.org/10.3390/ijms24043689
APA StyleKornhäusel, G., Smolle, C., Kreuzer, K., Kamolz, L.-P., & Ghaffari-Tabrizi-Wizsy, N. (2023). Rose Bengal Induced Photothrombosis in CAM Integrated Human Split Skin Grafts—A Feasibility Study. International Journal of Molecular Sciences, 24(4), 3689. https://doi.org/10.3390/ijms24043689







