Enhanced Resistance to Fungal and Bacterial Diseases Due to Overexpression of BSR1, a Rice RLCK, in Sugarcane, Tomato, and Torenia
Abstract
1. Introduction
2. Results
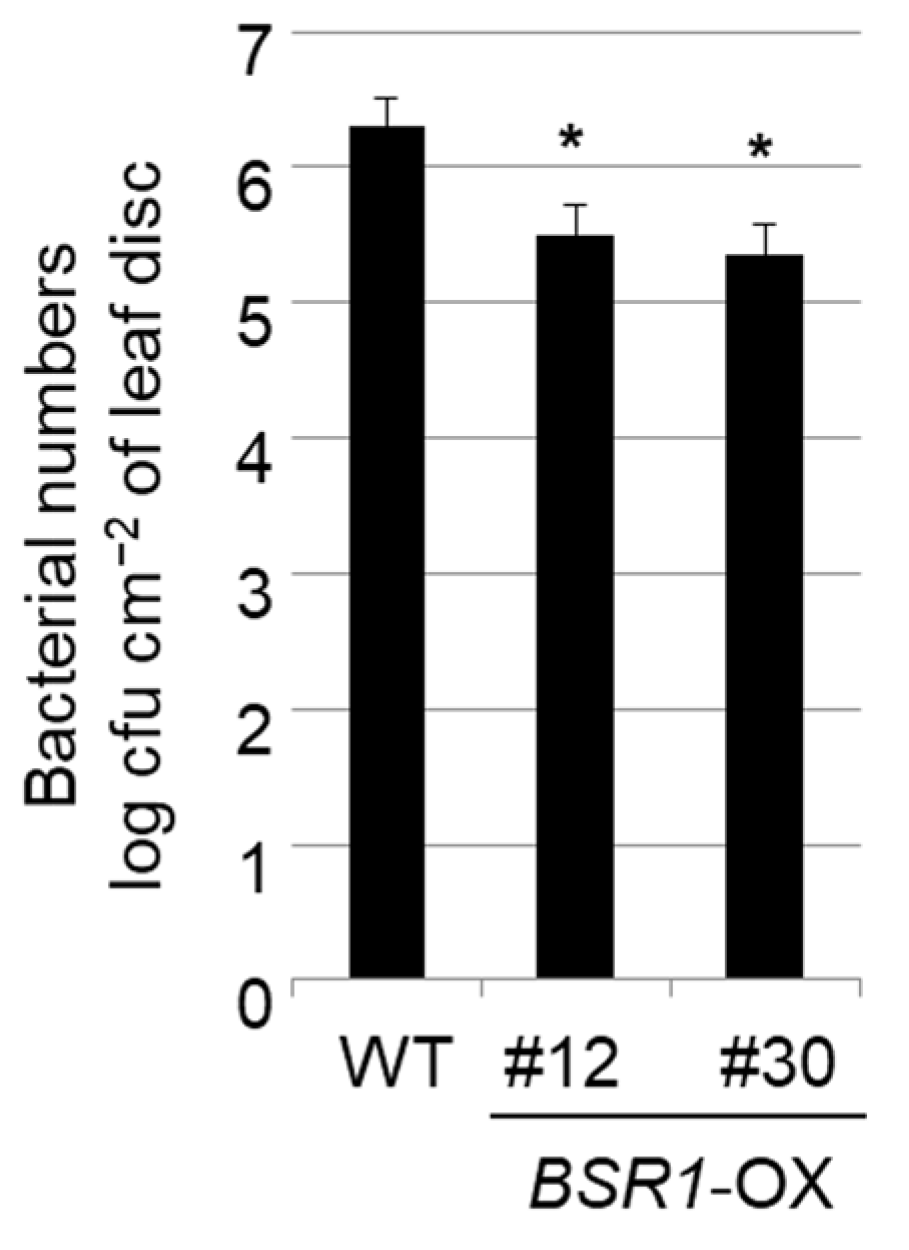
2.1. BSR1 Overexpression Conferred Bacterial Pst DC3000 Resistance to Dicot Tomato
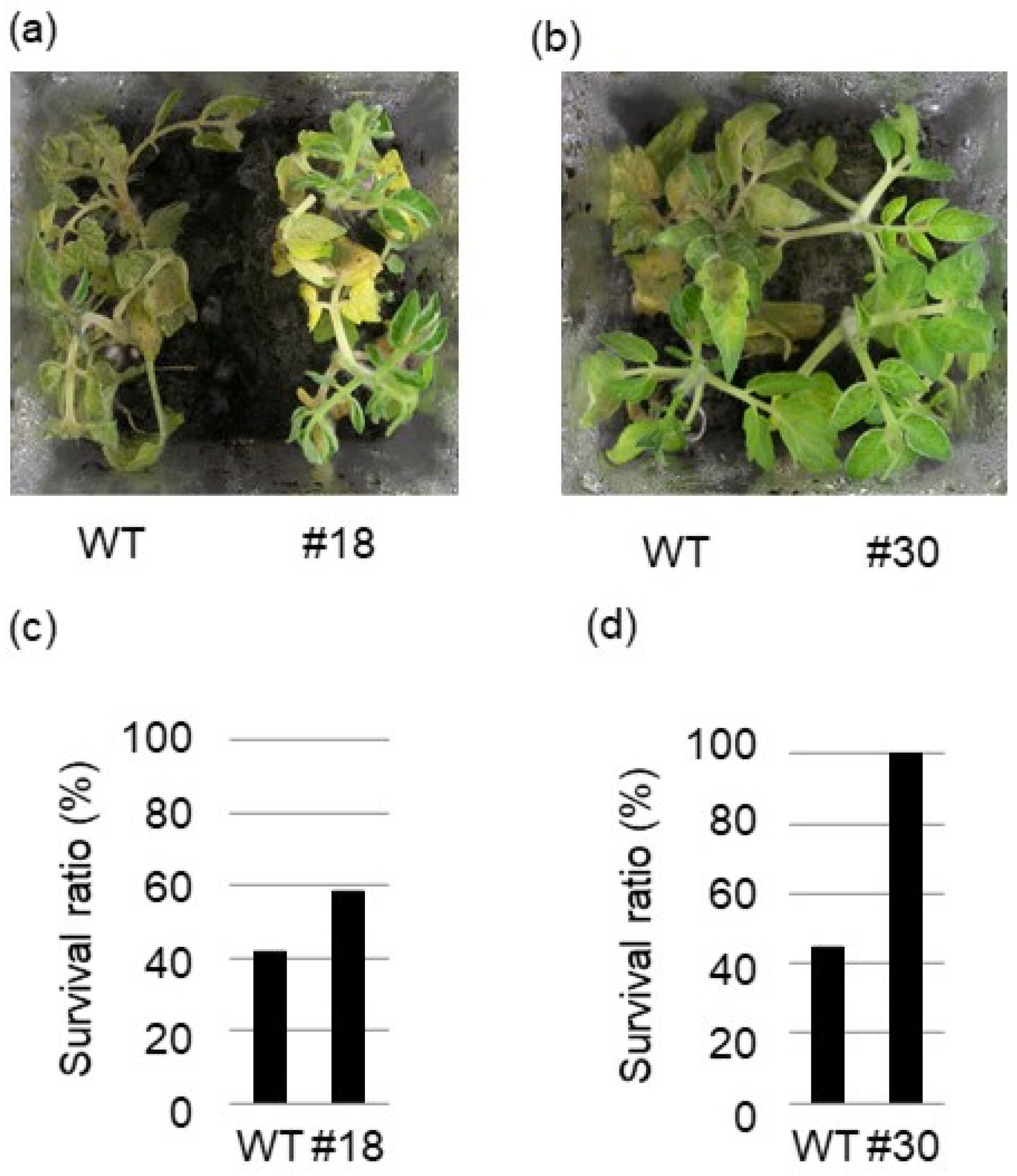
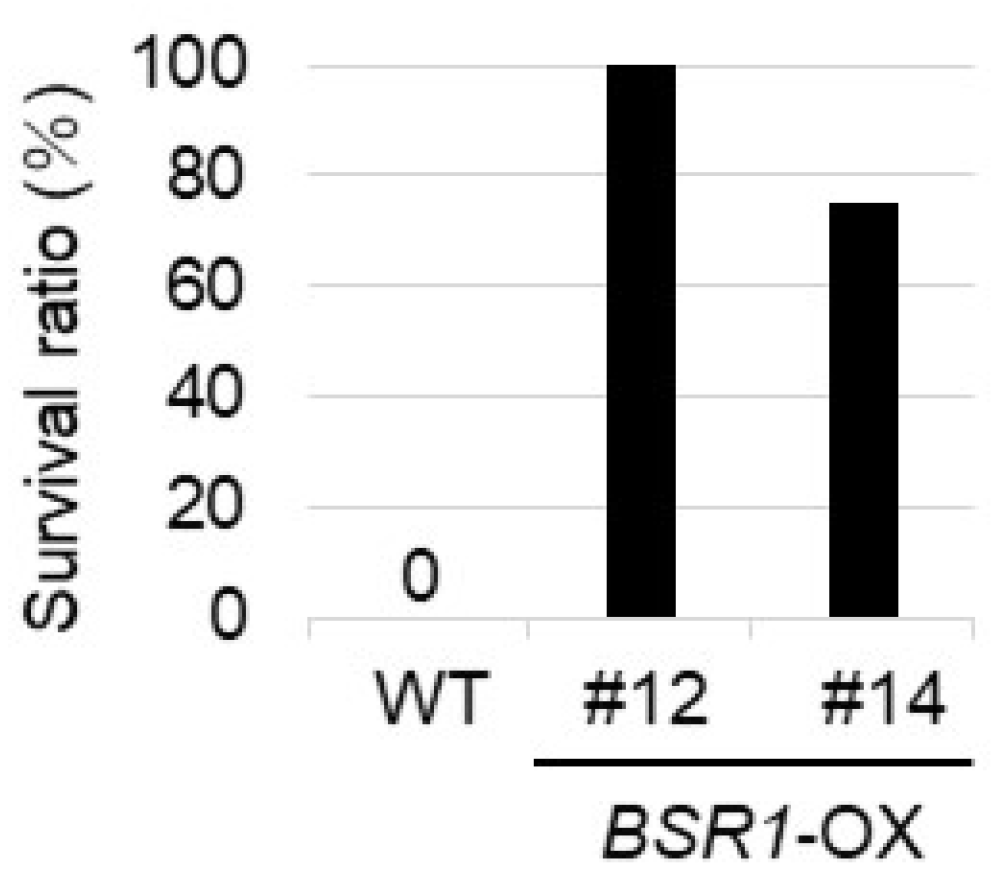
2.2. BSR1 Overexpression Conferred Fungal R. solani Resistance to Arabidopsis and Tomato
2.3. Resistance to R. solani in BSR1-OX Torenia
2.4. BSR1 Overexpression Conferred S. scitamineum Resistance to Monocot Sugarcane
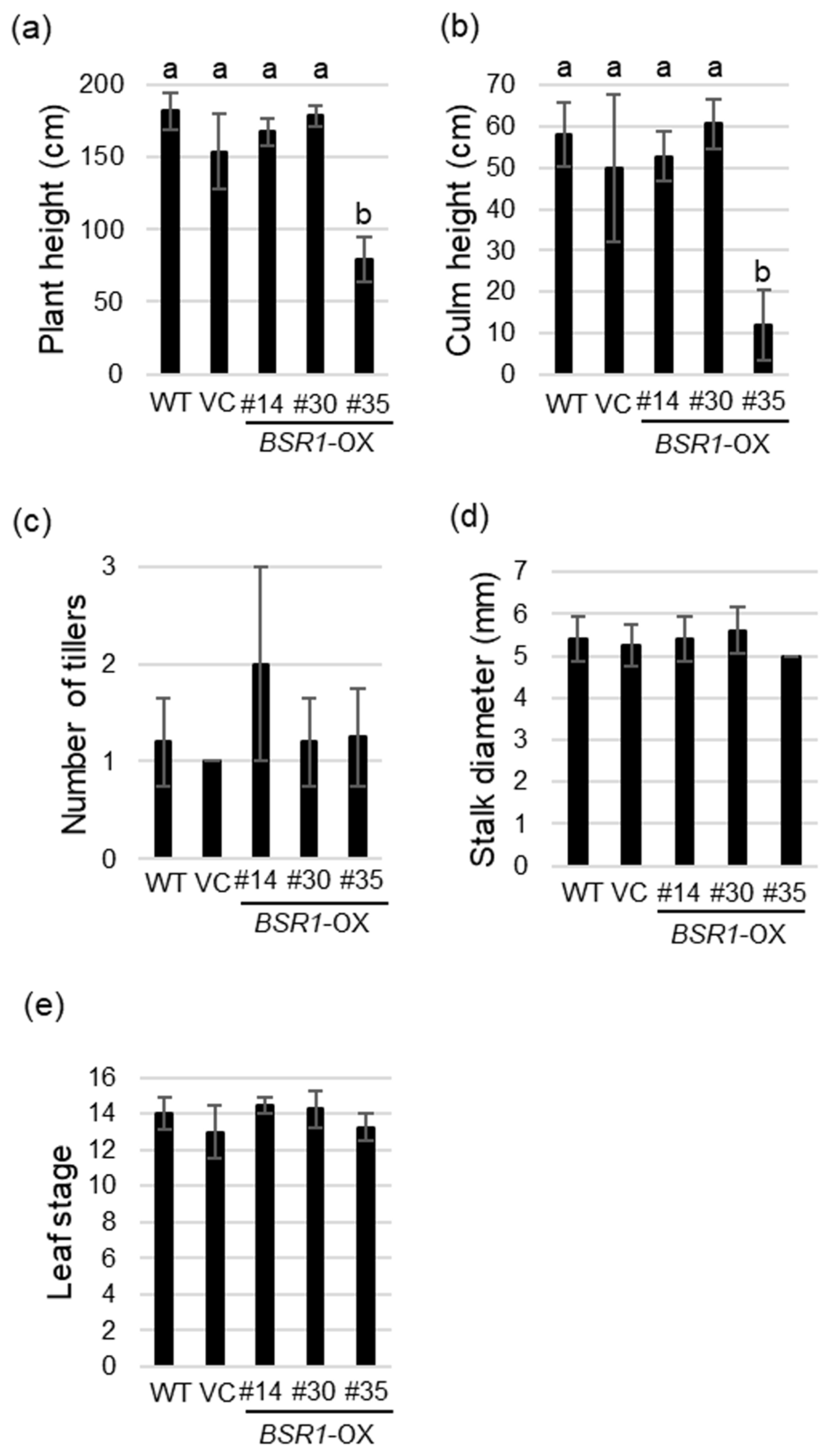
2.5. Morphological Traits and Growth of BSR1-OX Plants
3. Discussion
3.1. Broad-Spectrum Disease Resistance by BSR1 Overexpression in Sugarcane, Tomato, and Torenia
3.2. Broad-Spectrum Disease-Resistant Mechanism by BSR1 Overexpression in Sugarcane, Tomato, and Torenia
3.3. Toward Applications for Generating Broad-Spectrum Disease-Resistant Crops
4. Materials and Methods
4.1. Plant Materials and Culture
4.2. Plasmid Construction and Transformation
4.3. RNA Extraction and Quantitative Real-Time -PCR Analysis
4.4. Protein Extraction and Western Blot Analysis
4.5. Pathogens and Pathogen Cultures
4.6. Bacterial and Fungal Pathogen Resistance Assay in Arabidopsis, Tomato, Torenia, and Sugarcane
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hane, J.K.; Anderson, J.P.; Williams, A.H.; Sperschneider, J.; Singh, K.B. Genome sequencing and comparative genomics of the broad host-range pathogen Rhizoctonia solani AG8. PLoS Genet. 2014, 10, e1004281. [Google Scholar] [CrossRef]
- Gondal, A.S.; Rauf, A.; Naz, F. Anastomosis Groups of Rhizoctonia solani associated with tomato foot rot in Pothohar Region of Pakistan. Sci. Rep. 2019, 9, 3910. [Google Scholar] [CrossRef] [PubMed]
- Bartz, F.E.; Cubeta, M.A.; Toda, T.; Naito, S.; Ivors, K.L. An In Planta Method for Assessing the Role of Basidiospores in Rhizoctonia Foliar Disease of Tomato. Plant Dis. 2010, 94, 515–520. [Google Scholar] [CrossRef]
- Chen, C.X.; Wu, Y.F.; Gong, H.H.; Lin, Y.J.; Chen, C.Y. First Report of Binucleate Rhizoctonia AG-L Causing Root and Stem Rot of Wishbone Flower (Torenia fournieri) in Taiwan. Plant Dis. 2021, 105, 3304. [Google Scholar] [CrossRef]
- Jiang, S.B.; Yang, Q.Y.; Lin, B.R.; Zhang, J.X.; Shen, H.F.; Pu, X.M.; Sun, D.Y.; Bai, Y.B.; Tang, Z.Q. Occurrence of Root and Stem Rot Caused by Rhizoctonia solani AG-4 HGI on Torenia fournieri in China. Plant Dis. 2022, 106, 2266. [Google Scholar] [CrossRef]
- Que, Y.; Xu, L.; Wu, Q.; Liu, Y.; Ling, H.; Zhang, Y.; Guo, J.; Su, Y.; Chen, J.; Wang, S.; et al. Genome sequencing of Sporisorium scitamineum provides insights into the pathogenic mechanisms of sugarcane smut. BMC Genom. 2014, 15, 996. [Google Scholar] [CrossRef] [PubMed]
- Comstock, J.C.; Ferreira, S.A.; Tew, T.L. Hawaii’s approach to control of sugarcane smut. Plant Dis. 1983, 67, 452–457. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, X.; Tang, H.; Zhang, Q.; Hua, X.; Ma, X.; Zhu, F.; Jones, T.; Zhu, X.; Bowers, J.; et al. Allele-defined genome of the autopolyploid sugarcane Saccharum spontaneum L. Nat. Genet. 2018, 50, 1565–1573. [Google Scholar] [CrossRef] [PubMed]
- Dubouzet, J.G.; Maeda, S.; Sugano, S.; Ohtake, M.; Hayashi, N.; Ichikawa, T.; Kondou, Y.; Kuroda, H.; Horii, Y.; Matsui, M.; et al. Screening for resistance against Pseudomonas syringae in rice-FOX Arabidopsis lines identified a putative receptor-like cytoplasmic kinase gene that confers resistance to major bacterial and fungal pathogens in Arabidopsis and rice. Plant Biotechnol. J. 2011, 9, 466–485. [Google Scholar] [CrossRef]
- Maeda, S.; Dubouzet, J.G.; Kondou, Y.; Jikumaru, Y.; Seo, S.; Oda, K.; Matsui, M.; Hirochika, H.; Mori, M. The rice CYP78A gene BSR2 confers resistance to Rhizoctonia solani and affects seed size and growth in Arabidopsis and rice. Sci. Rep. 2019, 9, 587. [Google Scholar] [CrossRef]
- Maeda, S.; Hayashi, N.; Sasaya, T.; Mori, M. Overexpression of BSR1 confers broad-spectrum resistance against two bacterial diseases and two major fungal diseases in rice. Breed. Sci. 2016, 66, 396–406. [Google Scholar] [CrossRef] [PubMed]
- Maeda, S.; Yokotani, N.; Oda, K.; Mori, M. Enhanced resistance to fungal and bacterial diseases in tomato and Arabidopsis expressing BSR2 from rice. Plant Cell Rep. 2020, 39, 1493–1503. [Google Scholar] [CrossRef] [PubMed]
- Maeda, S.; Sasaki, K.; Kaku, H.; Kanda, Y.; Ohtsubo, N.; Mori, M. Overexpression of Rice BSR2 Confers Disease Resistance and Induces Enlarged Flowers in Torenia fournieri Lind. Int. J. Mol. Sci. 2022, 23, 4735. [Google Scholar] [CrossRef]
- Carvalho, G.; Quecine, M.; Longatto, D.; Peters, L.; Almeida, J.; Shyton, T.; Silva, M.; Crestana, G.; Creste, S.; Monteiro-Vitorello, C. Sporisorium scitamineum colonisation of sugarcane genotypes susceptible and resistant to smut revealed by GFP-tagged strains. Ann. Appl. Biol. 2016, 169, 329–341. [Google Scholar] [CrossRef]
- Kanda, Y.; Yokotani, N.; Maeda, S.; Nishizawa, Y.; Kamakura, T.; Mori, M. The receptor-like cytoplasmic kinase BSR1 mediates chitin-induced defense signaling in rice cells. Biosci. Biotechnol. Biochem. 2017, 81, 1497–1502. [Google Scholar] [CrossRef]
- Kanda, Y.; Nakagawa, H.; Nishizawa, Y.; Kamakura, T.; Mori, M. Broad-Spectrum Disease Resistance Conferred by the Overexpression of Rice RLCK BSR1 Results from an Enhanced Immune Response to Multiple MAMPs. Int. J. Mol. Sci. 2019, 20, 5523. [Google Scholar] [CrossRef]
- Kanda, Y.; Nishizawa, Y.; Kamakura, T.; Mori, M. Overexpressed BSR1-Mediated Enhancement of Disease Resistance Depends on the MAMP-Recognition System. Int. J. Mol. Sci. 2020, 21, 5397. [Google Scholar] [CrossRef] [PubMed]
- Shiu, S.; Karlowski, W.; Pan, R.; Tzeng, Y.; Mayer, K.; Li, W. Comparative analysis of the receptor-like kinase family in Arabidopsis and rice. Plant Cell 2004, 16, 1220–1234. [Google Scholar] [CrossRef]
- Rao, S.; Zhou, Z.; Miao, P.; Bi, G.; Hu, M.; Wu, Y.; Feng, F.; Zhang, X.; Zhou, J. Roles of Receptor-Like Cytoplasmic Kinase VII Members in Pattern-Triggered Immune Signaling. Plant Physiol. 2018, 177, 1679–1690. [Google Scholar] [CrossRef]
- Bi, G.; Zhou, Z.; Wang, W.; Li, L.; Rao, S.; Wu, Y.; Zhang, X.; Menke, F.L.H.; Chen, S.; Zhou, J.M. Receptor-Like Cytoplasmic Kinases Directly Link Diverse Pattern Recognition Receptors to the Activation of Mitogen-Activated Protein Kinase Cascades in Arabidopsis. Plant Cell 2018, 30, 1543–1561. [Google Scholar] [CrossRef]
- Li, Y.; Xue, J.; Wang, F.Z.; Huang, X.; Gong, B.Q.; Tao, Y.; Shen, W.; Tao, K.; Yao, N.; Xiao, S.; et al. Plasma membrane-nucleo-cytoplasmic coordination of a receptor-like cytoplasmic kinase promotes EDS1-dependent plant immunity. Nat. Plants 2022, 8, 802–816. [Google Scholar] [CrossRef]
- DeFalco, T.A.; Zipfel, C. Molecular mechanisms of early plant pattern-triggered immune signaling. Mol. Cell 2021, 81, 4346. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Yamada, K.; Ishikawa, K.; Yoshimura, S.; Hayashi, N.; Uchihashi, K.; Ishihama, N.; Kishi-Kaboshi, M.; Takahashi, A.; Tsuge, S.; et al. A receptor-like cytoplasmic kinase targeted by a plant pathogen effector is directly phosphorylated by the chitin receptor and mediates rice immunity. Cell Host Microbe 2013, 13, 347–357. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Yoshimura, Y.; Nakagawa, S.; Mezaki, H.; Yoshimura, S.; Kawasaki, T. OsDRE2 contributes to chitin-triggered response through its interaction with OsRLCK185. Biosci. Biotechnol. Biochem. 2019, 83, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, G.; Zhang, C.; Zhu, P.; Dai, H.; Yu, N.; He, Z.; Xu, L.; Wang, E. OsCERK1-Mediated Chitin Perception and Immune Signaling Requires Receptor-like Cytoplasmic Kinase 185 to Activate an MAPK Cascade in Rice. Mol. Plant 2017, 10, 619–633. [Google Scholar] [CrossRef]
- Yamada, K.; Yamaguchi, K.; Yoshimura, S.; Terauchi, A.; Kawasaki, T. Conservation of Chitin-Induced MAPK Signaling Pathways in Rice and Arabidopsis. Plant Cell Physiol. 2017, 58, 993–1002. [Google Scholar] [CrossRef]
- Ao, Y.; Li, Z.; Feng, D.; Xiong, F.; Liu, J.; Li, J.F.; Wang, M.; Wang, J.; Liu, B.; Wang, H.B. OsCERK1 and OsRLCK176 play important roles in peptidoglycan and chitin signaling in rice innate immunity. Plant J. 2014, 80, 1072–1084. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Ao, Y.; Feng, D.; Liu, J.; Wang, J.; Wang, H.B.; Liu, B. OsRLCK 57, OsRLCK107 and OsRLCK118 Positively Regulate Chitin- and PGN-Induced Immunity in Rice. Rice 2017, 10, 6. [Google Scholar] [CrossRef] [PubMed]
- Huot, B.; Yao, J.; Montgomery, B.L.; He, S.Y. Growth-defense tradeoffs in plants: A balancing act to optimise fitness. Mol. Plant 2014, 7, 1267–1287. [Google Scholar] [CrossRef]
- Liu, J.; Liu, B.; Chen, S.; Gong, B.Q.; Chen, L.; Zhou, Q.; Xiong, F.; Wang, M.; Feng, D.; Li, J.F.; et al. A Tyrosine Phosphorylation Cycle Regulates Fungal Activation of a Plant Receptor Ser/Thr Kinase. Cell Host Microbe 2018, 23, 241–253.e6. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Mezaki, H.; Fujiwara, M.; Hara, Y.; Kawasaki, T. Arabidopsis ubiquitin ligase PUB12 interacts with and negatively regulates Chitin Elicitor Receptor Kinase 1 (CERK1). PLoS ONE 2017, 12, e0188886. [Google Scholar] [CrossRef]
- Chen, X.L.; Xie, X.; Wu, L.; Liu, C.; Zeng, L.; Zhou, X.; Luo, F.; Wang, G.L.; Liu, W. Proteomic Analysis of Ubiquitinated Proteins in Rice. Front. Plant Sci. 2018, 9, 1064. [Google Scholar] [CrossRef] [PubMed]
- Aida, R.; Shibata, M. Agrobacterium-Mediated Transformation of Torenia (Torenia Fournieri). Breed. Sci. 1995, 45, 71–74. [Google Scholar] [CrossRef]
- Aida, R. A protocol for transformation of Torenia. Methods Mol. Biol. 2012, 847, 267–274. [Google Scholar] [PubMed]
- Nagai, K.; Mori, Y.; Ishikawa, S.; Furuta, T.; Gamuyao, R.; Niimi, Y.; Hobo, T.; Fukuda, M.; Kojima, M.; Takebayashi, Y.; et al. Antagonistic regulation of the gibberellic acid response during stem growth in rice. Nature 2020, 584, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, W.; Oishi, H.; Ebina, M.; Takamizo, T.; Komatsu, T. Production of transgenic Italian ryegrass (Lolium multiflorum Lam.) via microprojectile bombardment of embryogenic calli. Plant Biotechnol. 2002, 19, 241–249. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Chu, C.C.; Wang, C.C.; Sun, C.S.; Hsu, C.; Yin, K.C.; Chu, C.Y.; Bi, F.Y. Establishment of an efficient medium for anther culture of rice through comparative experiments on nitrogen-sources. Sci. Sin. 1975, 18, 659–668. [Google Scholar]
- Spangenberg, G.; Wang, Z.Y.; Wu, X.L.; Nagel, J.; Iglesias, V.A.; Potrykus, I. Transgenic Tall Fescue (Festuca arundinacea) and Red Fescue (F. rubra) Plants from Microprojectile Bombardment of Embryogenic Suspension Cells. J. Plant Physiol. 1995, 145, 693–701. [Google Scholar] [CrossRef]
- Matsushita, A.; Inoue, H.; Goto, S.; Nakayama, A.; Sugano, S.; Hayashi, N.; Takatsuji, H. Nuclear ubiquitin proteasome degradation affects WRKY45 function in the rice defense program. Plant J. 2013, 73, 302–313. [Google Scholar] [CrossRef]
- Sugano, S.; Maeda, S.; Hayashi, N.; Kajiwara, H.; Inoue, H.; Jiang, C.J.; Takatsuji, H.; Mori, M. Tyrosine phosphorylation of a receptor-like cytoplasmic kinase, BSR1, plays a crucial role in resistance to multiple pathogens in rice. Plant J. 2018, 96, 1137–1147. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maeda, S.; Ackley, W.; Yokotani, N.; Sasaki, K.; Ohtsubo, N.; Oda, K.; Mori, M. Enhanced Resistance to Fungal and Bacterial Diseases Due to Overexpression of BSR1, a Rice RLCK, in Sugarcane, Tomato, and Torenia. Int. J. Mol. Sci. 2023, 24, 3644. https://doi.org/10.3390/ijms24043644
Maeda S, Ackley W, Yokotani N, Sasaki K, Ohtsubo N, Oda K, Mori M. Enhanced Resistance to Fungal and Bacterial Diseases Due to Overexpression of BSR1, a Rice RLCK, in Sugarcane, Tomato, and Torenia. International Journal of Molecular Sciences. 2023; 24(4):3644. https://doi.org/10.3390/ijms24043644
Chicago/Turabian StyleMaeda, Satoru, Wataru Ackley, Naoki Yokotani, Katsutomo Sasaki, Norihiro Ohtsubo, Kenji Oda, and Masaki Mori. 2023. "Enhanced Resistance to Fungal and Bacterial Diseases Due to Overexpression of BSR1, a Rice RLCK, in Sugarcane, Tomato, and Torenia" International Journal of Molecular Sciences 24, no. 4: 3644. https://doi.org/10.3390/ijms24043644
APA StyleMaeda, S., Ackley, W., Yokotani, N., Sasaki, K., Ohtsubo, N., Oda, K., & Mori, M. (2023). Enhanced Resistance to Fungal and Bacterial Diseases Due to Overexpression of BSR1, a Rice RLCK, in Sugarcane, Tomato, and Torenia. International Journal of Molecular Sciences, 24(4), 3644. https://doi.org/10.3390/ijms24043644




