Ectopic Expression of PvHMA2.1 Enhances Cadmium Tolerance in Arabidopsis thaliana
Abstract
1. Introduction
2. Results
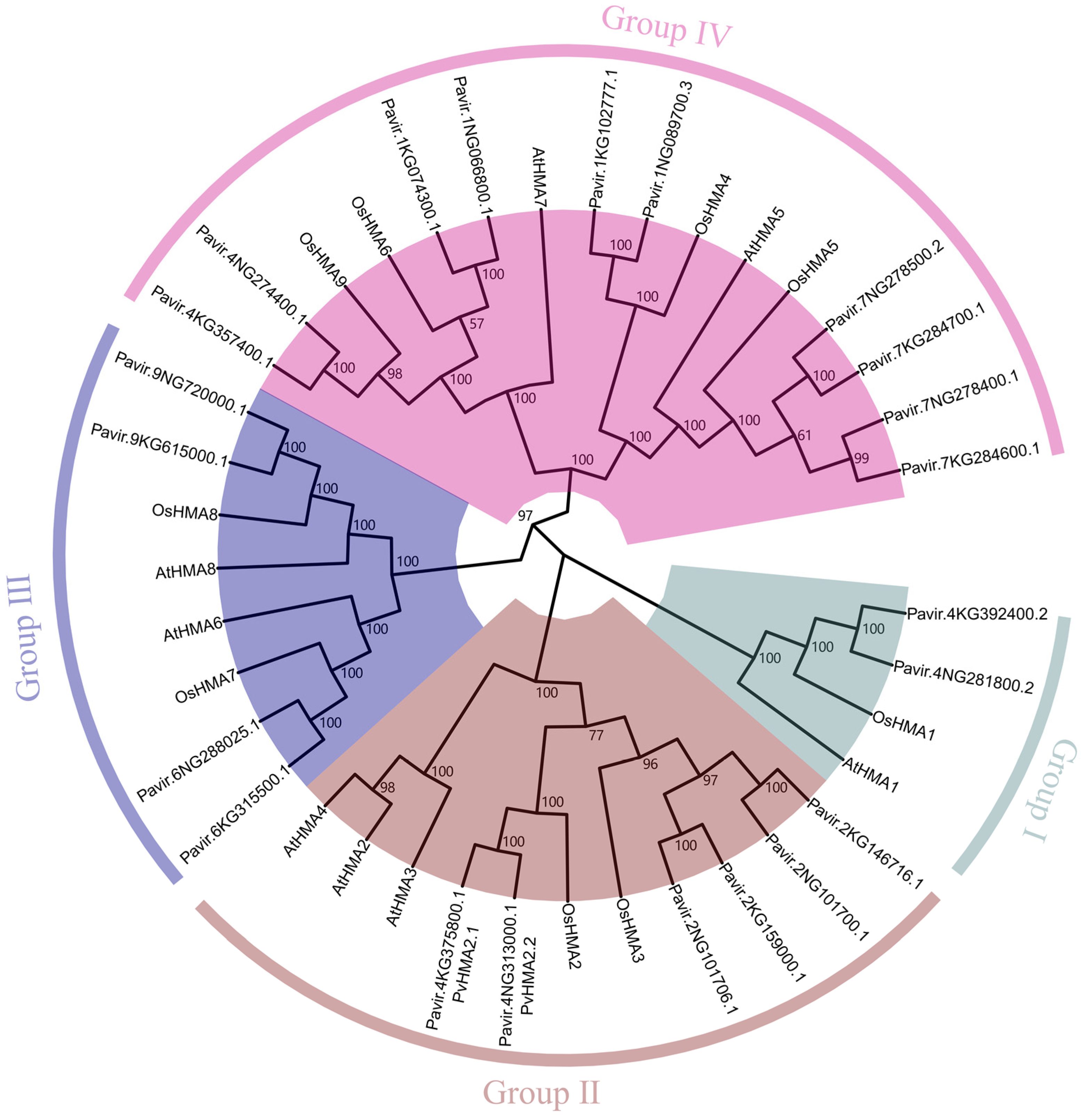
2.1. Phylogenetic Analysis of HMA Family
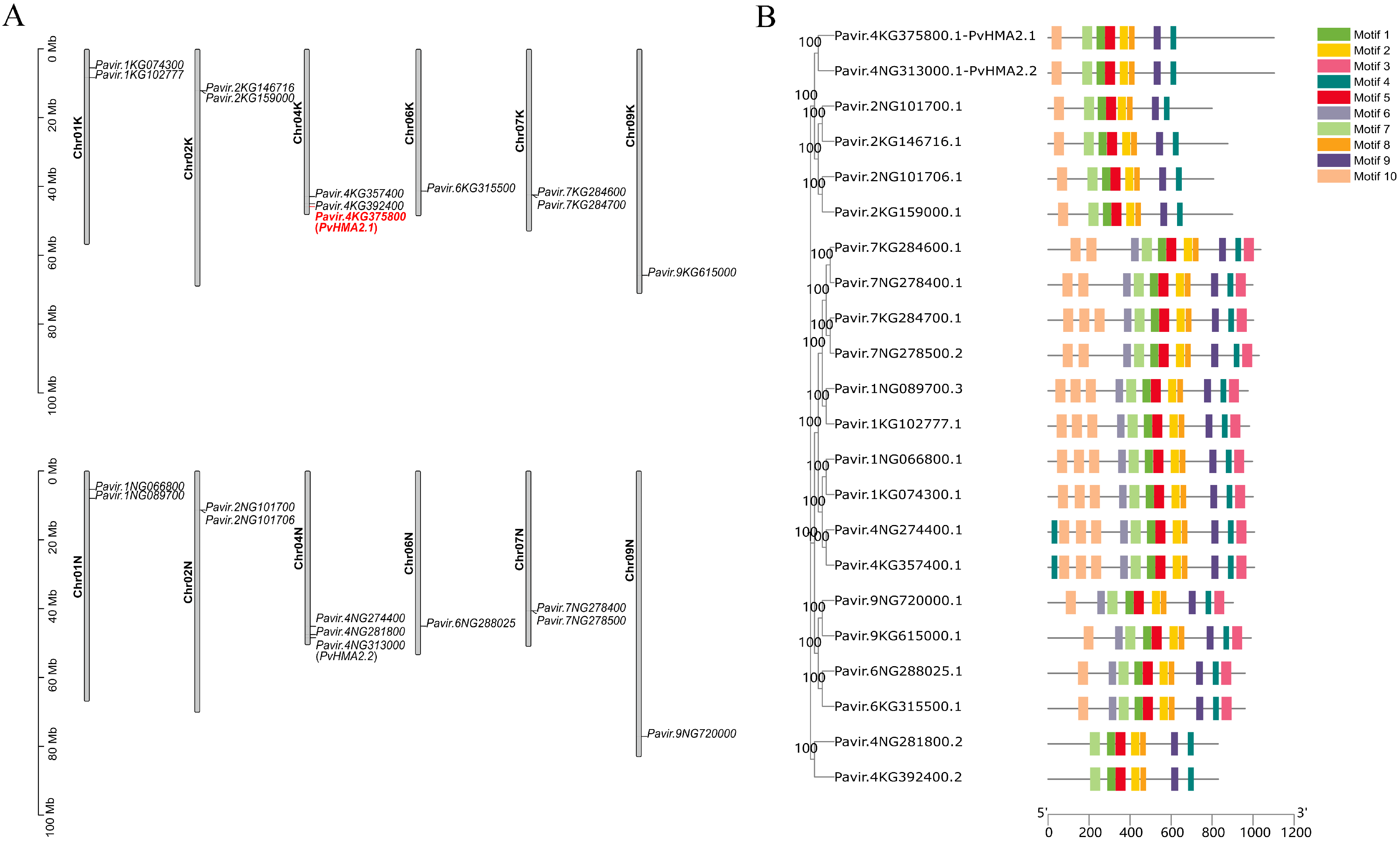
2.2. Chromosomal Location and Protein Structure of HMAs in P. virgatum
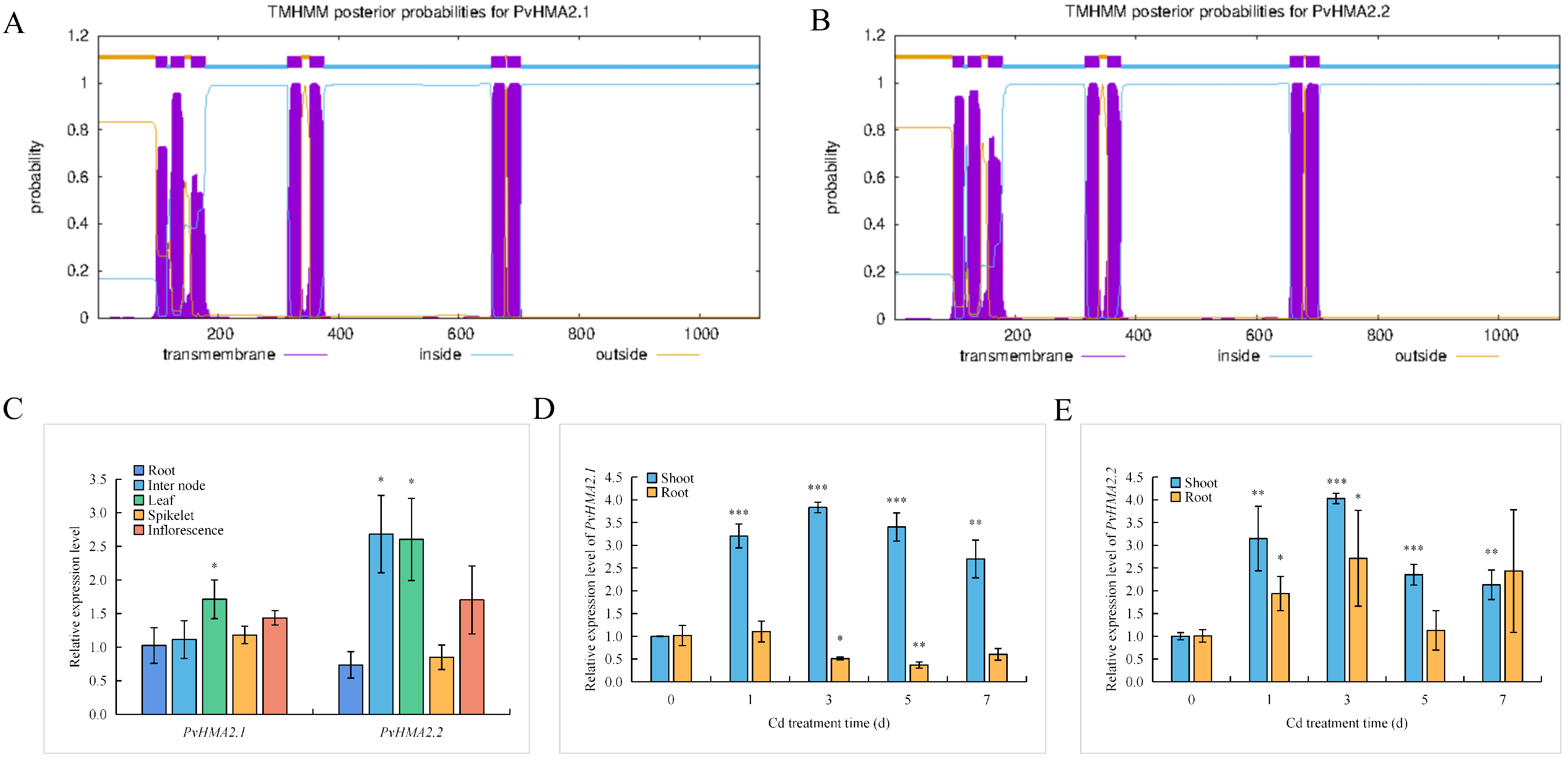
2.3. Protein Structures and Expression Patterns of PvHMA2.1 and PvHMA2.2
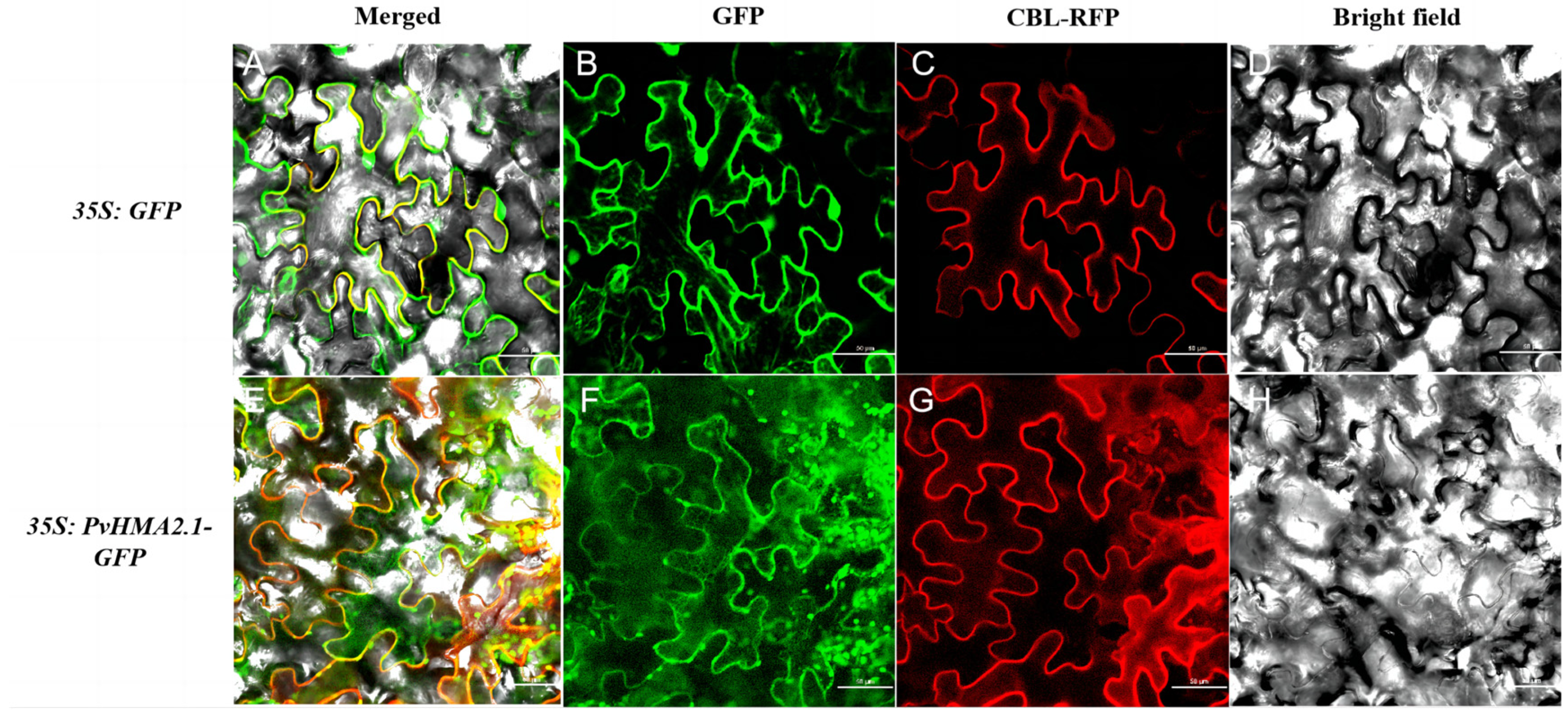
2.4. Subcellular Localization of PvHMA2.1
2.5. Ectopic Expression of PvHMA2.1 Enhanced Cadmium Tolerance in A. thaliana
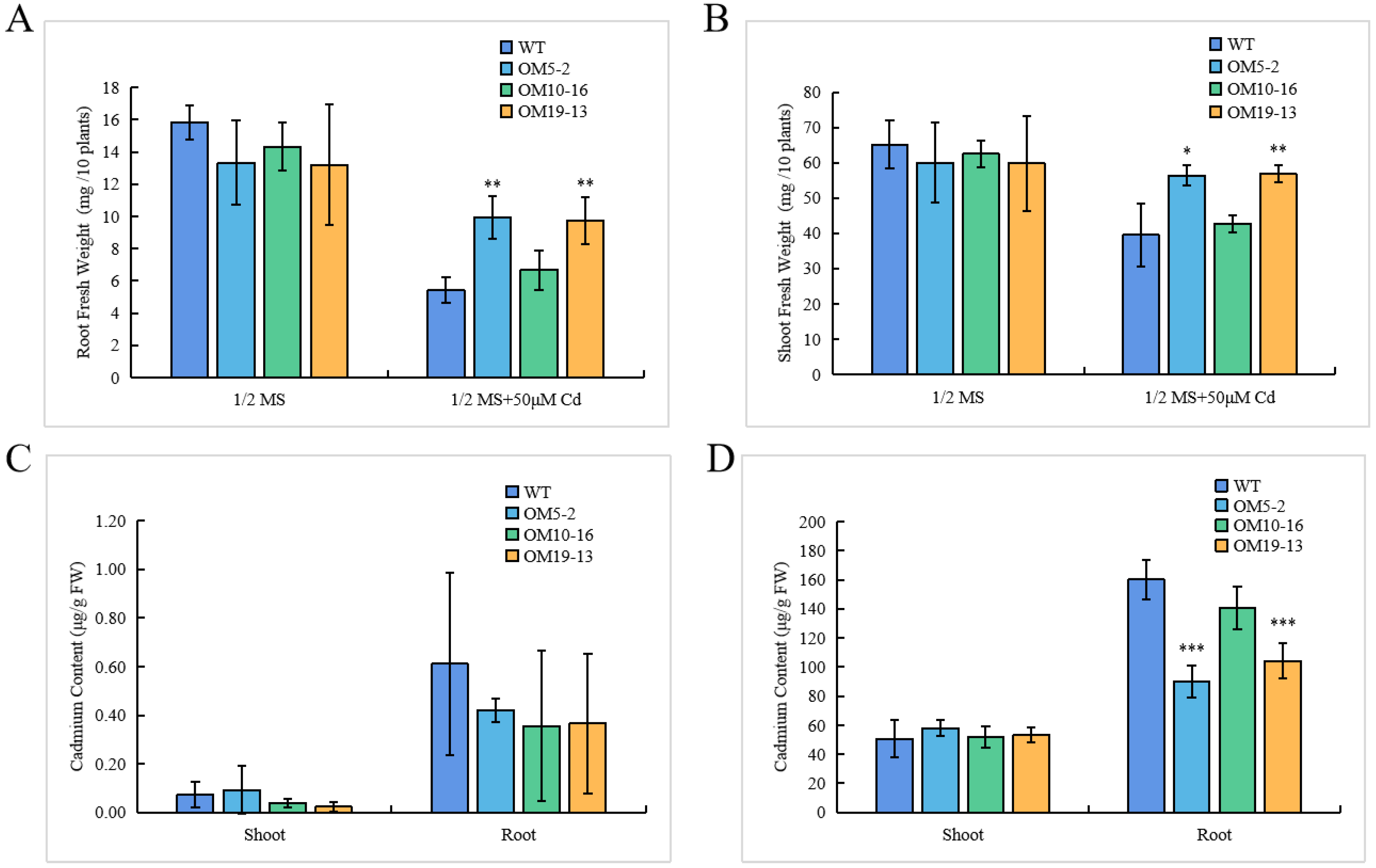
2.6. Cadmium Contents Reduced in PvHMA2.1 Ectopically Expressed Lines in A. thaliana Roots
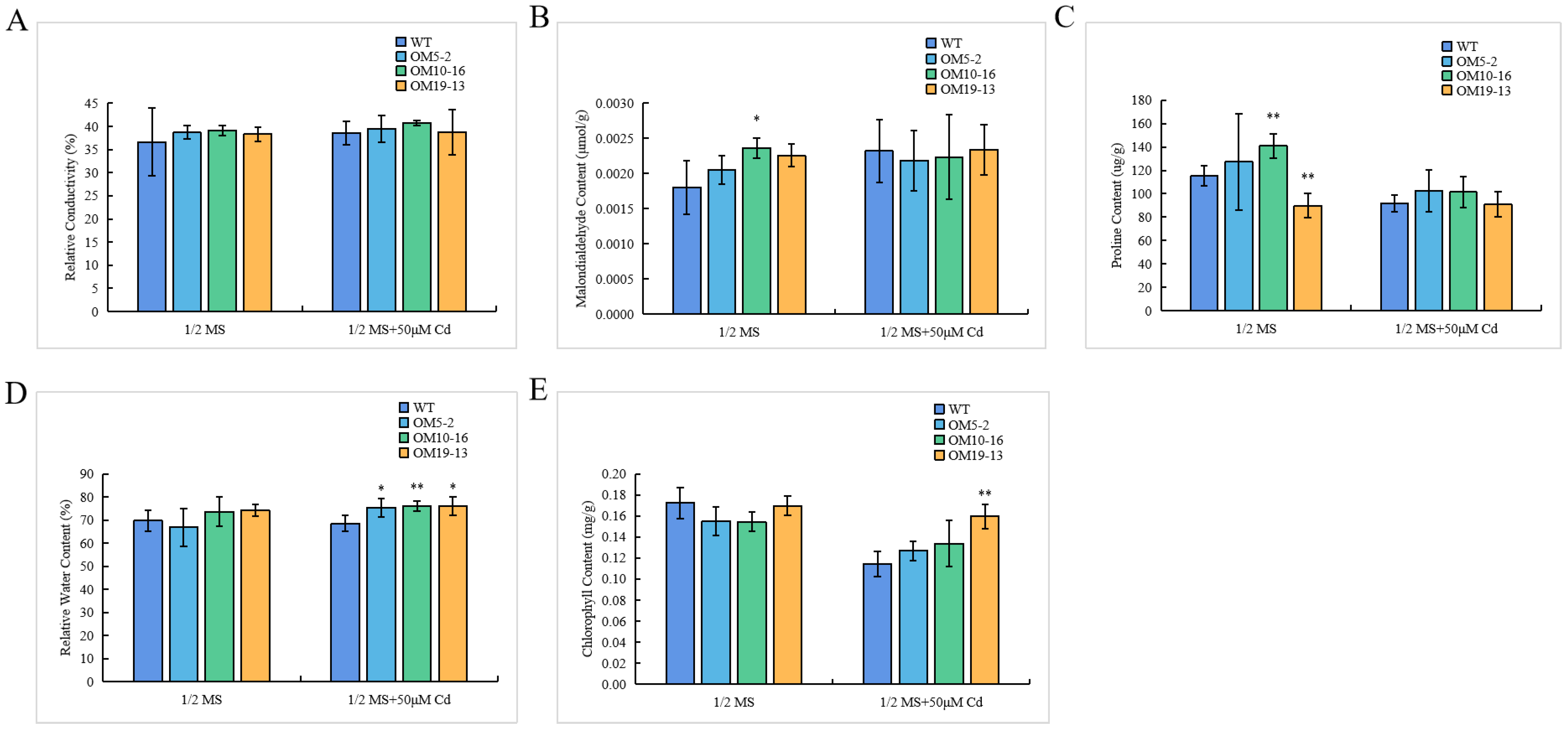
2.7. Ectopic Expression of PvHMA2.1 Enhanced Cadmium Tolerance at the Physiological Level in A. thaliana
3. Discussion
3.1. Analyses of Classification and Protein Structures of P. virgatum HMAs
3.2. Ectopic Expression of PvHMA2.1 Enhances Cadmium Tolerance in A. thaliana
3.3. Expression of PvHMA2.1 Homologous Genes Is Induced by Cadmium Stress
4. Materials and Methods
4.1. Analyses of Gene and Protein Sequences of HMAs
4.2. Materials and Growth Conditions of P. virgatum
4.3. Transcript Analysis by Real-Time Quantitative PCR
4.4. Subcellular Localization of PvHMA2.1
4.5. Expression Vector Construction and Selection for A. thaliana
4.6. Evaluation of Cadmium Tolerance in A. thaliana
4.7. Determination of Cadmium Contents in A. thaliana
4.8. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nawrot, T.; Plusquin, M.; Hogervorst, J.; Roels, H.A.; Celis, H.; Thijs, L.; Vangronsveld, J.; Van Hecke, E.; Staessen, J.A. Environmental exposure to cadmium and risk of cancer: A prospective population-based study. Lancet Oncol. 2006, 7, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Nordberg, G.F. Historical perspectives on cadmium toxicology. Toxicol. Appl. Pharmacol. 2009, 238, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Akesson, A.; Barregard, L.; Bergdahl, I.A.; Nordberg, G.F.; Nordberg, M.; Skerfving, S. Non-renal effects and the risk assessment of environmental cadmium exposure. Environ. Health Perspect. 2014, 122, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.H.; Liu, Y.Y.; Peng, Z.H.; Li, J.C.; Huang, W.P.; Liu, Y.; Wang, X.N.; Xie, S.L.; Sun, L.P.; Han, E.Q.; et al. Ectopic expression of poplar ABC transporter PtoABCG36 confers Cd tolerance in Arabidopsis thaliana. Int. J. Mol. Sci. 2019, 20, 3293. [Google Scholar] [CrossRef]
- Khan, N.; You, F.M.; Datla, R.; Ravichandran, S.; Jia, B.S.; Cloutier, S. Genome-wide identification of ATP binding cassette (ABC) transporter and heavy metal associated (HMA) gene families in flax (Linum usitatissimum L.). BMC Genomics 2020, 21, 722. [Google Scholar] [CrossRef]
- Yu, W.J.; Deng, S.R.; Chen, X.; Cheng, Y.; Li, Z.R.; Wu, J.T.; Zhu, D.Y.; Zhou, J.; Cao, Y.; Fayyaz, P.; et al. PcNRAMP1 enhances cadmium uptake and accumulation in Populus x canescens. Int. J. Mol. Sci. 2022, 23, 7593. [Google Scholar] [CrossRef]
- Tao, J.Y.; Lu, L.L. Advances in genes-encoding transporters for cadmium uptake, translocation, and accumulation in plants. Toxics 2022, 10, 411. [Google Scholar] [CrossRef]
- Arguello, J.M.; Eren, E.; Gonzalez-Guerrero, M. The structure and function of heavy metal transport P1B-ATPases. BioMetals 2007, 20, 233–248. [Google Scholar] [CrossRef]
- Baxter, I.; Tchieu, J.; Sussman, M.R.; Boutry, M.; Palmgren, M.G.; Gribskov, M.; Harper, J.F.; Axelsen, K.B. Genomic comparison of P-type ATPase ion pumps in Arabidopsis and rice. Plant Physiol. 2003, 132, 618–628. [Google Scholar] [CrossRef]
- Zou, W.L.; Li, C.; Zhu, Y.J.; Chen, J.G.; He, H.H.; Ye, G.Y. Rice heavy metal P-type ATPase OsHMA6 is likely a copper efflux protein. Rice Sci. 2020, 27, 143–151. [Google Scholar] [CrossRef]
- Williams, L.E.; Mills, R.F. P1B-ATPases--an ancient family of transition metal pumps with diverse functions in plants. Trends Plant Sci. 2005, 10, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, R.; Bashir, K.; Ishimaru, Y.; Nishizawa, N.K.; Nakanishi, H. The role of heavy-metal ATPases, HMAs, in zinc and cadmium transport in rice. Plant Signal. Behav. 2012, 7, 1605–1607. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.Y.; Choi, H.; Segami, S.; Cho, H.T.; Martinoia, E.; Maeshima, M.; Lee, Y. AtHMA1 contributes to the detoxification of excess Zn(II) in Arabidopsis. Plant J. 2009, 58, 737–753. [Google Scholar] [CrossRef] [PubMed]
- Chao, D.Y.; Silva, A.; Baxter, I.; Huang, Y.S.; Nordborg, M.; Danku, J.; Lahner, B.; Yakubova, E.; Salt, D.E. Genome-wide association studies identify heavy metal ATPase3 as the primary determinant of natural variation in leaf cadmium in Arabidopsis thaliana. PLoS Genet. 2012, 8, 1–14. [Google Scholar] [CrossRef]
- Morel, M.; Crouzet, J.; Gravot, A.; Auroy, P.; Leonhardt, N.; Vavasseur, A.; Richaud, P. AtHMA3, a P1B-ATPase allowing Cd/Zn/Co/Pb vacuolar storage in Arabidopsis. Plant Physiol. 2009, 149, 894–904. [Google Scholar] [CrossRef]
- Verret, F.; Gravot, A.; Auroy, P.; Leonhardt, N.; David, P.; Nussaume, L.; Vavasseur, A.; Richaud, P. Overexpression of AtHMA4 enhances root-to-shoot translocation of zinc and cadmium and plant metal tolerance. FEBS Lett. 2004, 576, 306–312. [Google Scholar] [CrossRef]
- Wong, C.K.E.; Cobbett, C.S. HMA P-type ATPases are the major mechanism for root-to-shoot Cd translocation in Arabidopsis thaliana. New Phytol. 2009, 181, 71–78. [Google Scholar] [CrossRef]
- Mills, R.F.; Valdes, B.; Duke, M.; Peaston, K.A.; Lahner, B.; Salt, D.E.; Williams, L.E. Functional significance of AtHMA4 C-terminal domain in planta. PLoS ONE 2010, 5, e13388. [Google Scholar] [CrossRef]
- Andres-Colas, N.; Sancenon, V.; Rodriguez-Navarro, S.; Mayo, S.; Thiele, D.J.; Ecker, J.R.; Puig, S.; Penarrubia, L. The Arabidopsis heavy metal P-type ATPase HMA5 interacts with metallochaperones and functions in copper detoxification of roots. Plant J. 2006, 45, 225–236. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Kuroda, K.; Kimura, K.; Southron-Francis, J.L.; Furuzawa, A.; Kimura, K.; Iuchi, S.; Kobayashi, M.; Taylor, G.J.; Koyama, H. Amino acid polymorphisms in strictly conserved domains of a P-type ATPase HMA5 are involved in the mechanism of copper tolerance variation in Arabidopsis. Plant Physiol. 2008, 148, 969–980. [Google Scholar] [CrossRef]
- Woeste, K.E.; Kieber, J.J. A strong loss-of-function mutation in RAN1 results in constitutive activation of the ethylene response pathway as well as a rosette-lethal phenotype. Plant Cell 2000, 12, 443–455. [Google Scholar] [CrossRef] [PubMed]
- Catty, P.; Boutigny, S.; Miras, R.; Joyard, J.; Rolland, N.; Seigneurin-Berny, D. Biochemical characterization of AtHMA6/PAA1, a chloroplast envelope Cu(I)-ATPase. J. Biol. Chem. 2011, 286, 36188–36197. [Google Scholar] [CrossRef] [PubMed]
- Satoh-Nagasawa, N.; Mori, M.; Nakazawa, N.; Kawamoto, T.; Nagato, Y.; Sakurai, K.; Takahashi, H.; Watanabe, A.; Akagi, H. Mutations in rice (Oryza sativa) heavy metal ATPase 2 (OsHMA2) restrict the translocation of zinc and cadmium. Plant Cell Physiol. 2012, 53, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Miyadate, H.; Adachi, S.; Hiraizumi, A.; Tezuka, K.; Nakazawa, N.; Kawamoto, T.; Katou, K.; Kodama, I.; Sakurai, K.; Takahashi, H.; et al. OsHMA3, a P1B-type of ATPase affects root-to-shoot cadmium translocation in rice by mediating efflux into vacuoles. New Phytol. 2011, 189, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Olsen, L.I.; Palmgren, M.G. Many rivers to cross: The journey of zinc from soil to seed. Front. Plant Sci. 2014, 5, 30. [Google Scholar] [CrossRef]
- Sasaki, A.; Yamaji, N.; Ma, J.F. Overexpression of OsHMA3 enhances Cd tolerance and expression of Zn transporter genes in rice. J. Exp. Bot. 2014, 65, 6013–6021. [Google Scholar] [CrossRef]
- Huang, X.Y.; Deng, F.L.; Yamaji, N.; Pinson, S.R.M.; Fujii-Kashino, M.; Danku, J.; Douglas, A.; Guerinot, M.L.; Salt, D.E.; Ma, J.F. A heavy metal P-type ATPase OsHMA4 prevents copper accumulation in rice grain. Nat. Commun. 2016, 7, 12138. [Google Scholar] [CrossRef]
- Deng, F.L.; Yamaji, N.; Xia, J.X.; Ma, J.F. A member of the heavy metal P-type ATPase OsHMA5 is involved in xylem loading of copper in rice. Plant Physiol. 2013, 163, 1353–1362. [Google Scholar] [CrossRef]
- Lee, S.; Kim, Y.Y.; Lee, Y.; An, G. Rice P1B-type heavy-metal ATPase, OsHMA9, is a metal efflux protein. Plant Physiol. 2007, 145, 831–842. [Google Scholar] [CrossRef]
- Du, F.; Yang, F.Y.; Casler, M.D.; Zhang, Y.W. The research progress of switchgrass energy grass in America. J. Anhui Agric. Sci. 2010, 38, 20334–20339. [Google Scholar] [CrossRef]
- Guo, Z.P.; Gao, Y.N.; Cao, X.L.; Jiang, W.B.; Liu, X.; Liu, Q.; Chen, Z.; Zhou, W.N.; Cui, J.; Wang, Q.Z. Phytoremediation of Cd and Pb interactive polluted soils by switchgrass (Panicum virgatum L.). Int. J. Phytorem. 2019, 21, 1486–1496. [Google Scholar] [CrossRef]
- Wang, H.Y. Research progress of hyperaccumulator phytoremediation under heavy metal stress. Sci. Technol. Vision 2018, 18, 53–55. [Google Scholar] [CrossRef]
- Begum, N.; Afzal, S.; Zhao, H.H.; Lou, L.Q.; Cai, Q.S. Shoot endophytic plant growth-promoting bacteria reduce cadmium toxicity and enhance switchgrass (Panicum virgatum L.) biomass. Acta Physiol. Plant. 2018, 40, 170. [Google Scholar] [CrossRef]
- Hu, B.Y.; Fang, Z.G.; Lou, L.Q.; Cai, Q.S. Comprehensive evaluation of cadmium tolerance of 14 switchgrass (Panicum virgatum) cultivars in the seedling stage. Acta Pratac. Sin. 2019, 28, 27–36. [Google Scholar] [CrossRef]
- Begum, N.; Hu, Z.Y.; Cai, Q.S.; Lou, L.Q. Influence of PGPB inoculation on HSP70 and HMA3 gene expression in switchgrass under cadmium stress. Plants 2019, 8, 504. [Google Scholar] [CrossRef]
- Takahashi, R.; Ishimaru, Y.; Shimo, H.; Ogo, Y.; Senoura, T.; Nishizawa, N.K.; Nakanishi, H. The OsHMA2 transporter is involved in root-to-shoot translocation of Zn and Cd in rice. Plant, Cell Environ. 2012, 35, 1948–1957. [Google Scholar] [CrossRef]
- Clemens, S.; Aarts, M.G.M.; Thomine, S.; Verbruggen, N. Plant science: The key to preventing slow cadmium poisoning. Trends Plant Sci. 2013, 18, 92–99. [Google Scholar] [CrossRef]
- Park, W.; Ahn, S.J. HMA3 is a key factor for differences in Cd- and Zn-related phenotype between Arabidopsis Ws and Col-0 ecotypes. Plant Biotechnol. Rep. 2017, 11, 209–218. [Google Scholar] [CrossRef]
- Liu, H.; Zhao, H.X.; Wu, L.H.; Liu, A.N.; Zhao, F.J.; Xu, W.Z. Heavy metal ATPase 3 (HMA3) confers cadmium hypertolerance on the cadmium/zinc hyperaccumulator Sedum plumbizincicola. New Phytol. 2017, 215, 687–698. [Google Scholar] [CrossRef]
- Hussain, D.; Haydon, M.J.; Wang, Y.W.; Wong, E.; Sherson, S.M.; Young, J.; Camakaris, J.; Harper, J.F.; Cobbett, C.S. P-type ATPase heavy metal transporters with roles in essential zinc homeostasis in Arabidopsis. Plant Cell 2004, 16, 1327–1339. [Google Scholar] [CrossRef]
- Eren, E.; Arguello, J.M. Arabidopsis HMA2, a divalent heavy metal-transporting P1B-type ATPase, is involved in cytoplasmic Zn2+ homeostasis. Plant Physiol. 2004, 136, 3712–3723. [Google Scholar] [CrossRef] [PubMed]
- Gravot, A.; Lieutaud, A.; Verret, F.; Auroy, P.; Vavasseur, A.; Richaud, P. AtHMA3, a plant P1B-ATPase, functions as a Cd/Pb transporter in yeast. FEBS Lett. 2004, 561, 22–28. [Google Scholar] [CrossRef]
- Mills, R.F.; Krijger, G.C.; Baccarini, P.J.; Hall, J.L.; Williams, L.E. Functional expression of AtHMA4, a P1B-type ATPase of the Zn/Co/Cd/Pb subclass. Plant J. 2003, 35, 164–176. [Google Scholar] [CrossRef] [PubMed]
- Ibuot, A.; Webster, R.E.; Williams, L.E.; Pittman, J.K. Increased metal tolerance and bioaccumulation of zinc and cadmium in Chlamydomonas reinhardtii expressing a AtHMA4 C-terminal domain protein. Biotechnol. Bioeng. 2020, 117, 2996–3005. [Google Scholar] [CrossRef] [PubMed]
- Yamaji, N.; Xia, J.X.; Mitani-Ueno, N.; Yokosho, K.; Ma, J.F. Preferential delivery of zinc to developing tissues in rice is mediated by P-type heavy metal ATPase OsHMA2. Plant Physiol. 2013, 162, 927–939. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.L.; Wang, P.T.; Wang, P.; Yang, M.; Lian, X.M.; Tang, Z.; Huang, C.F.; Salt, D.E.; Zhao, F.J. A loss-of-function allele of OsHMA3 associated with high cadmium accumulation in shoots and grain of Japonica rice cultivars. Plant Cell Environ. 2016, 39, 1941–1954. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.X.; Wang, L.S.; Zhao, F.J.; Wu, L.H.; Liu, A.N.; Xu, W.Z. SpHMA1 is a chloroplast cadmium exporter protecting photochemical reactions in the Cd hyperaccumulator Sedum plumbizincicola. Plant Cell Environ. 2018, 42, 1112–1124. [Google Scholar] [CrossRef]
- Ma, X.D.; Wang, L.; Wang, M.; Peng, H.R. Difference in relative conductivity and ultrastructure of leaf between two wheat cultivars with different thermotolerance under heat acclimation and heat stress. J. China Agric. Univ. 2003, 8, 4–8. [Google Scholar]
- An, M.J.; Wang, H.J.; Fan, H.; Ippolito, J.A.; Meng, C.M.; E, Y.L.; Li, Y.B.; Wang, K.Y.; Wei, C.Z. Effects of modifiers on the growth, photosynthesis, and antioxidant enzymes of cotton under cadmium toxicity. J. Plant Growth Regul. 2019, 38, 1196–1205. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Xing, H.W.; Guo, N.; Han, S.; Zong, M.; Liu, F.; Wang, G.X. The physiological characteristics of ornamental kale for cold resistance. Acta Agric. Boreali-Sin. 2016, 31, 168–174. [Google Scholar] [CrossRef]
- Kishor, P.B.K.; Sreenivasulu, N. Is proline accumulation per se correlated with stress tolerance or is proline homeostasis a more critical issue? Plant, Cell Environ. 2014, 37, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.G.; Baldwin, C.M.; Hu, Q.; Liu, H.B.; Luo, H. Heterologous expression of Arabidopsis H+-pyrophosphatase enhances salt tolerance in transgenic creeping bentgrass (Agrostis stolonifera L.). Plant Cell Environ. 2010, 33, 272–289. [Google Scholar] [CrossRef] [PubMed]
- Ali, B.; Gill, R.A.; Yang, S.; Gill, M.B.; Ali, S.; Rafiq, M.T.; Zhou, W.J. Hydrogen sulfide alleviates cadmium-induced morpho-physiological and ultrastructural changes in Brassica napus. Ecotoxicol. Environ. Saf. 2014, 110, 197–207. [Google Scholar] [CrossRef]
- Lu, C.N.; Zhang, L.X.; Tang, Z.; Huang, X.Y.; Ma, J.F.; Zhao, F.J. Producing cadmium-free Indica rice by overexpressing OsHMA3. Environ. Int. 2019, 126, 619–626. [Google Scholar] [CrossRef]
- Chen, C.J.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.H.; Xia, R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Xie, K.L.; Wang, Y.F.; Bai, X.C.; Ye, Z.; Zhang, C.Q.; Sun, F.L.; Zhang, C.; Xi, Y.J. Overexpression of PvSTK1 gene from Switchgrass (Panicum virgatum L.) affects flowering time and development of floral organ in transgenic Arabidopsis thaliana. Plant Physiol. Biochem. 2022, 178, 93–104. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−△△CT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Huang, Y.H.; Cui, X.; Cen, H.F.; Wang, K.H.; Zhang, Y.W. Transcriptomic analysis reveals vacuolar Na+ (K+)/H+ antiporter gene contributing to growth, development, and defense in switchgrass (Panicum virgatum L.). BMC Plant Biol. 2018, 18, 57. [Google Scholar] [CrossRef]
- Clough, S.J.; Bent, A.F. Floral dip: A simplified method for agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998, 16, 735–743. [Google Scholar] [CrossRef]
- Zhang, X.R.; Henriques, R.; Lin, S.S.; Niu, Q.W.; Chua, N.H. Agrobacterium-mediated transformation of Arabidopsis thaliana using the floral dip method. Nat. Protoc. 2006, 1, 641–646. [Google Scholar] [CrossRef]
- Cen, H.F.; Ye, W.X.; Liu, Y.R.; Li, D.Y.; Wang, K.X.; Zhang, W.J. Overexpression of a chimeric gene, OsDST-SRDX, improved salt tolerance of perennial ryegrass. Sci. Rep. 2016, 6, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.N. Functional analysis of ERF5 gene responding to cadmium stress in Arabidopsis. Master Thesis, Hefei University of Technology, Hefei, China, 2021. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zang, H.; He, J.; Zhang, Q.; Li, X.; Wang, T.; Bi, X.; Zhang, Y. Ectopic Expression of PvHMA2.1 Enhances Cadmium Tolerance in Arabidopsis thaliana. Int. J. Mol. Sci. 2023, 24, 3544. https://doi.org/10.3390/ijms24043544
Zang H, He J, Zhang Q, Li X, Wang T, Bi X, Zhang Y. Ectopic Expression of PvHMA2.1 Enhances Cadmium Tolerance in Arabidopsis thaliana. International Journal of Molecular Sciences. 2023; 24(4):3544. https://doi.org/10.3390/ijms24043544
Chicago/Turabian StyleZang, Hui, Junyi He, Qi Zhang, Xue Li, Tingting Wang, Xiaojing Bi, and Yunwei Zhang. 2023. "Ectopic Expression of PvHMA2.1 Enhances Cadmium Tolerance in Arabidopsis thaliana" International Journal of Molecular Sciences 24, no. 4: 3544. https://doi.org/10.3390/ijms24043544
APA StyleZang, H., He, J., Zhang, Q., Li, X., Wang, T., Bi, X., & Zhang, Y. (2023). Ectopic Expression of PvHMA2.1 Enhances Cadmium Tolerance in Arabidopsis thaliana. International Journal of Molecular Sciences, 24(4), 3544. https://doi.org/10.3390/ijms24043544




