Ejaculatory Abstinence Affects the Sperm Quality in Normozoospermic Men—How Does the Seminal Bacteriome Respond?
Abstract
1. Introduction
2. Results
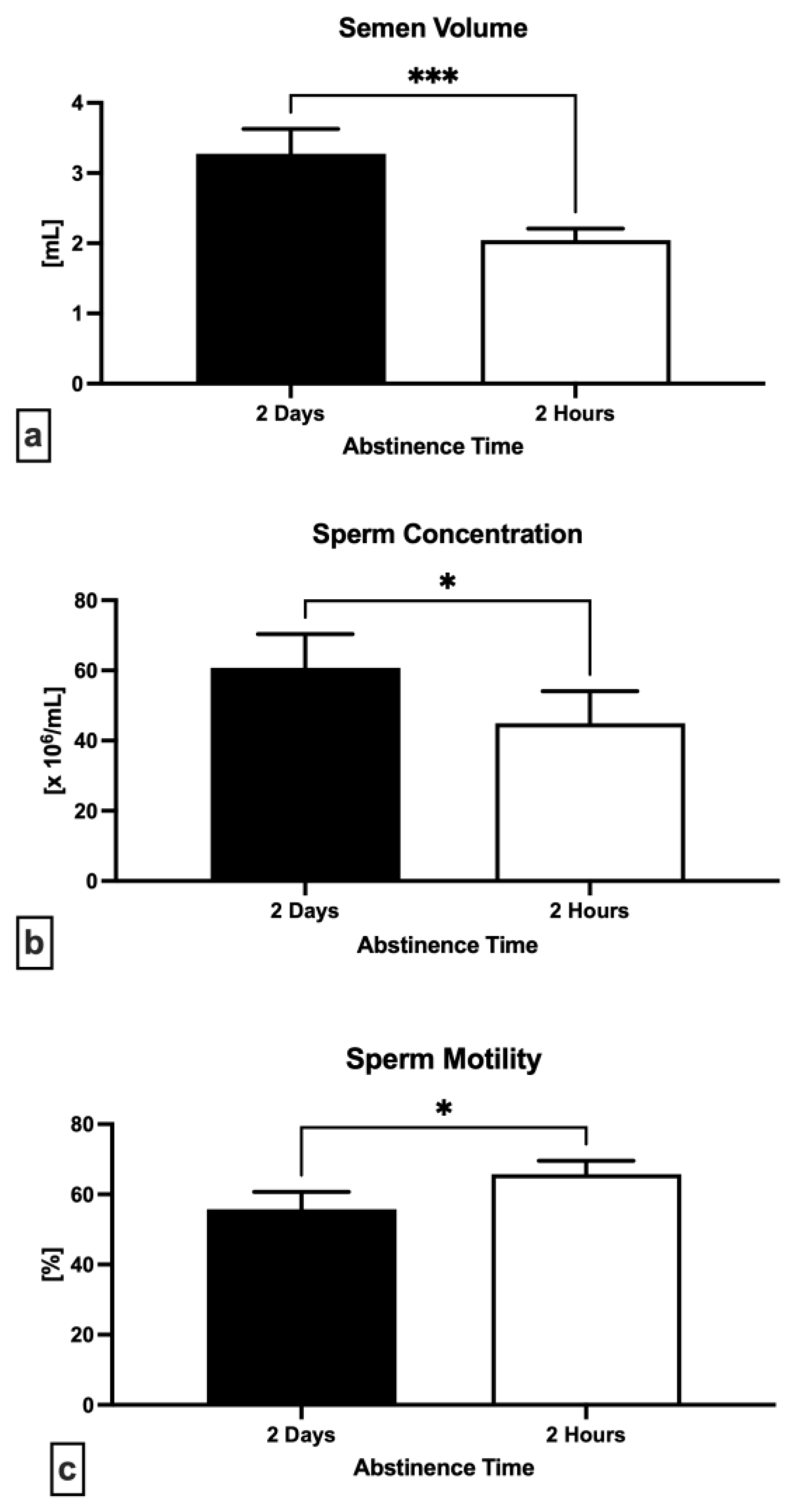
2.1. Semen Quality
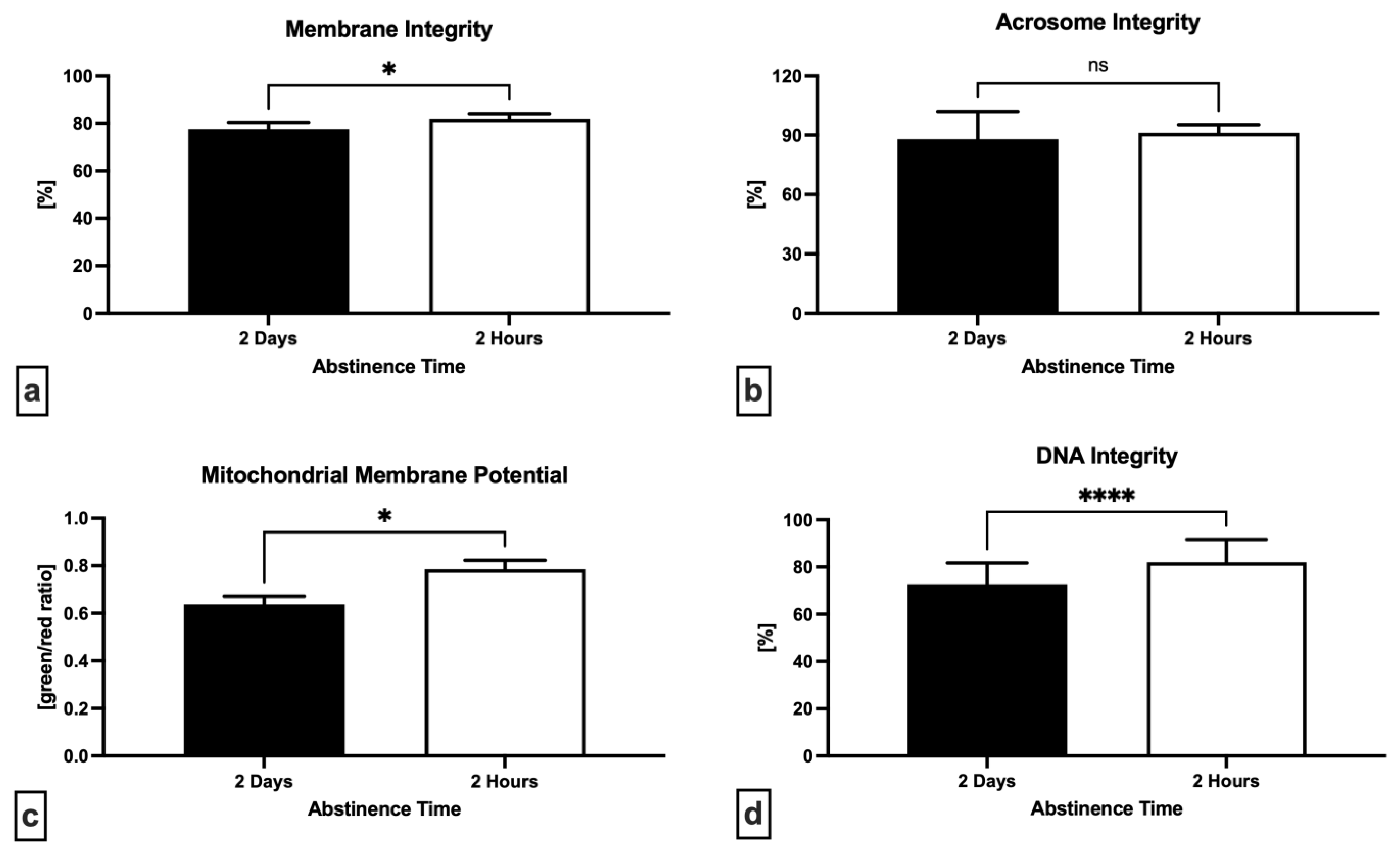
2.2. Oxidative Profile
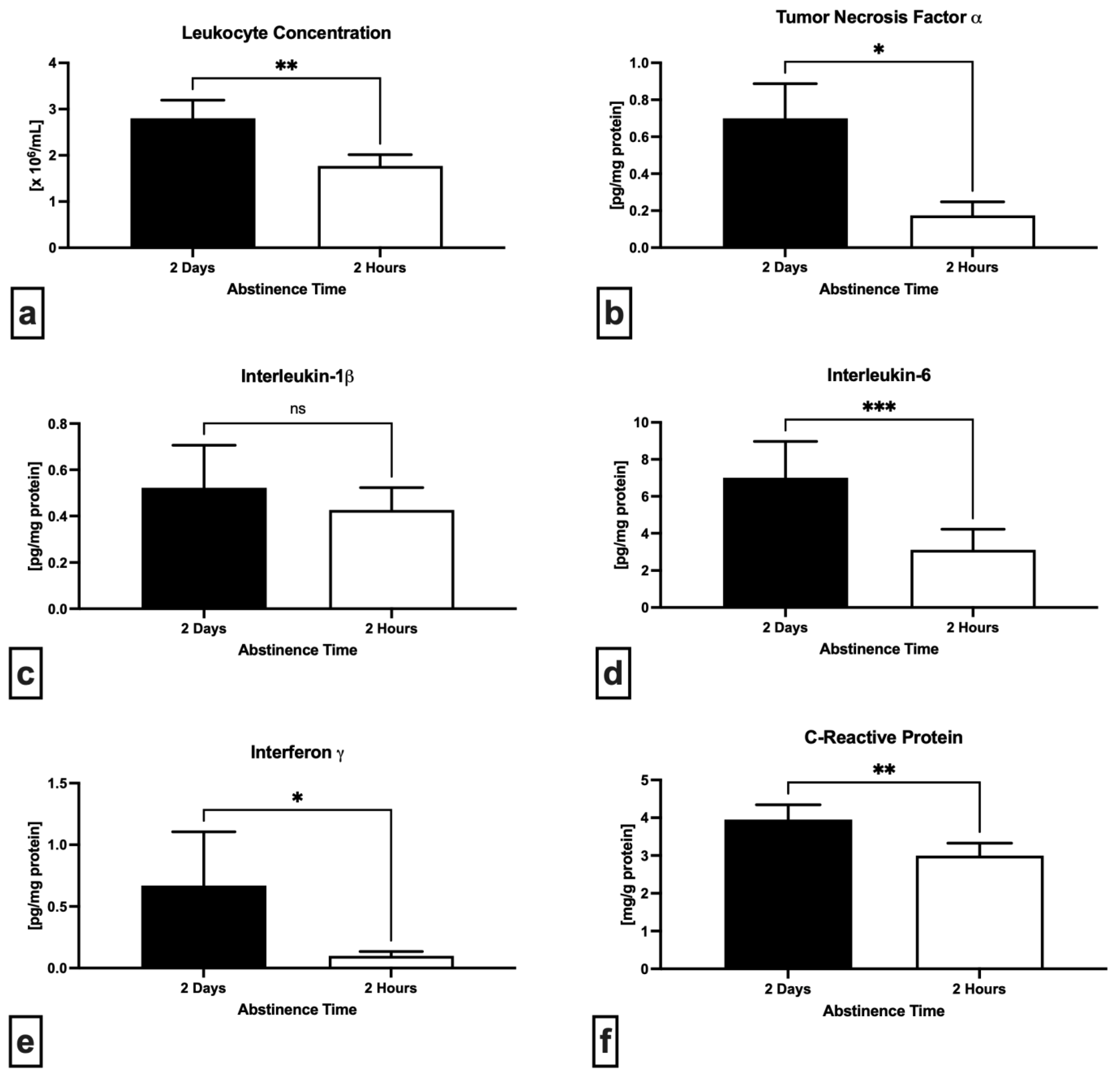
2.3. Immunological Profile
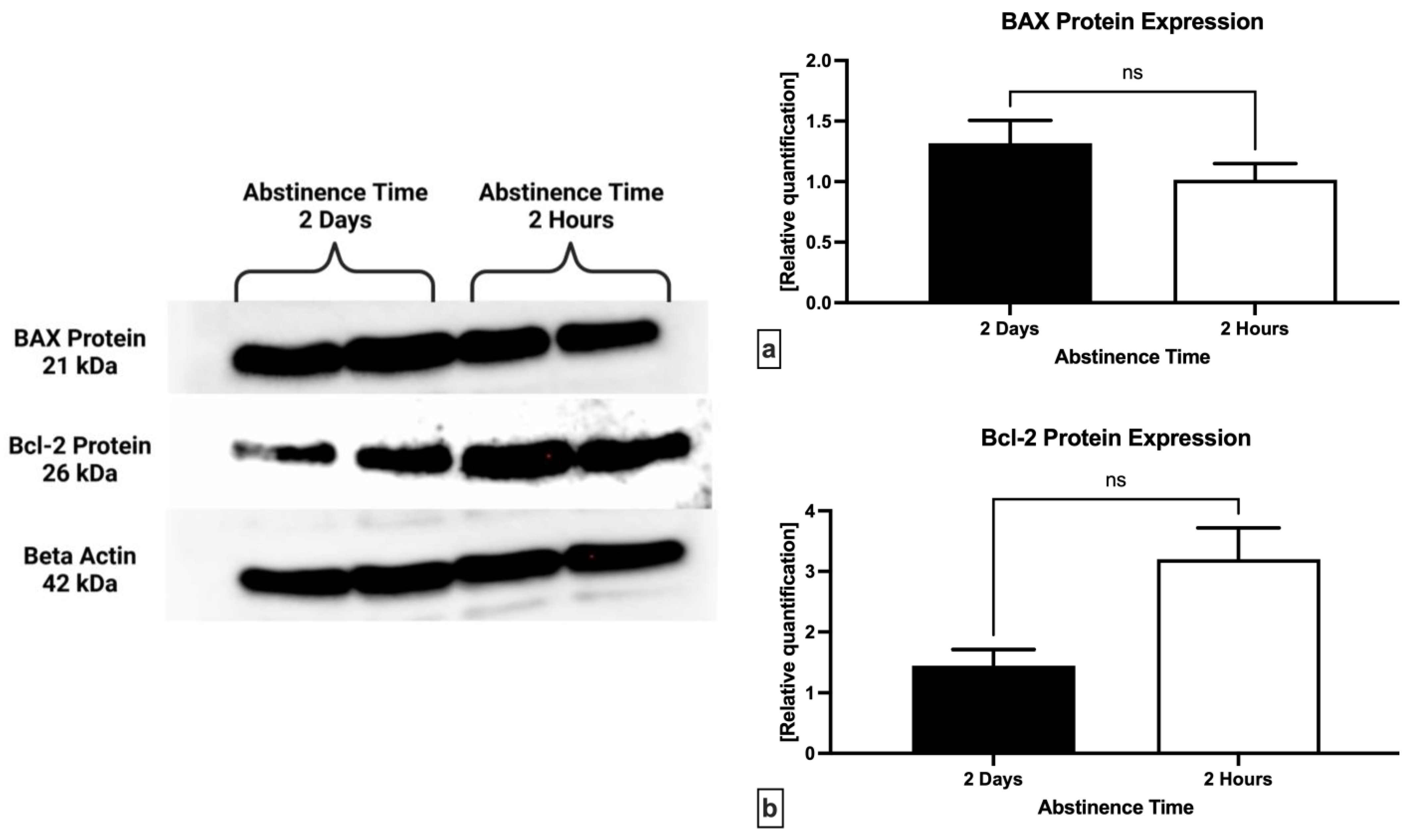
2.4. Western Blot
2.5. Bacteriological Analysis
2.6. Biodiversity Analysis
3. Discussion
4. Materials and Methods
4.1. Collection and Processing of Ejaculates
4.2. Semen Quality Analysis
4.3. Oxidative Profile
4.4. ELISA Assays
4.5. Western Blots
- anti-BAX antibody (BCL2-Associated X Protein) N-Term (#ABIN6990475; Antibodies Online; Dunwoody, GA, USA); Polyclonal/IgG; source: rabbit; 1:1000 in 5% milk in TBS/0.1% Tween-20.
- anti-Bcl-2 antibody (B-Cell CLL/lymphoma 2) N-Term (#ABIN2857047; Antibodies Online; Dunwoody, GA, USA); Polyclonal/IgG; source: rabbit; 1:1000 in 5% milk in TBS/0.1% Tween-20.
- β-actin antibody (AC-15) (#AM4302; Invitrogen, Waltham, MA, USA); Monoclonal/IgG; source: mouse; 1:1000 in 5% milk in TBS/0.1% Tween-20.
4.6. Bacterial Cultures and Identification
4.7. Biodiversity Calculation
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mann, U.; Shiff, B.; Patel, P. Reasons for worldwide decline in male fertility. Curr. Opin. Urol. 2020, 30, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Jafari, H.; Mirzaiinajmabadi, K.; Roudsari, R.L.; Rakhshkhorshid, M. The factors affecting male infertility: A systematic review. Int. J. Reprod. Biomed. 2021, 19, 681–688. [Google Scholar] [CrossRef]
- Baskaran, S.; Finelli, R.; Agarwal, A.; Henkel, R. Diagnostic value of routine semen analysis in clinical andrology. Andrologia 2021, 53, e13614. [Google Scholar] [CrossRef]
- Milachich, T.; Dyulgerova-Nikolova, D. The Sperm: Parameters and Evaluation. In Innovations in Assisted Reproduction Technology, 1st ed.; Sharma, N., Chakrabarti, S., Barak, Y., Ellenbogen, A., Eds.; IntechOpen: London, UK, 2020; pp. 1–20. [Google Scholar] [CrossRef]
- World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen, 6th ed.; WHO Press: Geneva, Switzerland, 2021; 276p. [Google Scholar]
- Cooper, T.G.; Keck, C.; Oberdieck, U.; Nieschlag, E. Effects of multiple ejaculations after extended periods of sexual abstinence on total, motile and normal sperm numbers, as well as accessory gland secretions, from healthy normal and oligozoospermic men. Hum. Reprod. 1993, 8, 1251–1258. [Google Scholar] [CrossRef]
- Pellestor, F.; Girardet, A.; Andreo, B. Effect of long abstinence periods on human sperm quality. Int. J. Fertil. Menopausal. Stud. 1994, 39, 278–282. [Google Scholar]
- Okada, F.K.; Andretta, R.R.; Spaine, D.M. One day is better than four days of ejaculatory abstinence for sperm function. Reprod. Fertil. 2020, 1, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Levitas, E.; Lunenfeld, E.; Weiss, N.; Friger, M.; Har-Vardi, I.; Koifman, A.; Potashnik, G. Relationship between the duration of sexual abstinence and semen quality: Analysis of 9,489 semen samples. Fertil. Steril. 2005, 83, 1680–1686. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Majzoub, A.; Agarwal, A. Oxidative stress and sperm function: A systematic review on evaluation and management. Arab. J. Urol. 2019, 17, 87–97. [Google Scholar] [CrossRef]
- Mayorga-Torres, J.M.; Agarwal, A.; Roychoudhury, S.; Cadavid, A.; Cardona-Maya, W.D. Can a Short Term of Repeated Ejaculations Affect Seminal Parameters? J. Reprod. Infertil. 2016, 17, 177–183. [Google Scholar]
- Gosálvez, J.; González-Martínez, M.; López-Fernández, C.; Fernández, J.L.; Sánchez-Martín, P. Shorter abstinence decreases sperm deoxyribonucleic acid fragmentation in ejaculate. Fertil. Steril. 2011, 96, 1083–1086. [Google Scholar] [CrossRef]
- Agarwal, A.; Gupta, S.; Du Plessis, S.; Sharma, R.; Esteves, S.C.; Cirenza, C.; Eliwa, J.; Al-Najjar, W.; Kumaresan, D.; Haroun, N.; et al. Abstinence Time and Its Impact on Basic and Advanced Semen Parameters. Urology 2016, 94, 102–110. [Google Scholar] [CrossRef]
- De Jonge, C.; LaFromboise, M.; Bosmans, E.; Ombelet, W.; Cox, A.; Nijs, M. Influence of the abstinence period on human sperm quality. Fertil. Steril. 2004, 82, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Tvrdá, E.; Arroyo, F.; Ďuračka, M.; López-Fernández, C.; Gosálvez, J. Dynamic assessment of human sperm DNA damage II: The effect of sperm concentration adjustment during processing. J. Assist. Reprod. Genet. 2019, 36, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Mayorga-Torres, B.J.; Camargo, M.; Agarwal, A.; du Plessis, S.S.; Cadavid, Á.P.; Cardona Maya, W.D. Influence of ejaculation frequency on seminal parameters. Reprod. Biol. Endocrinol. 2015, 13, 47. [Google Scholar] [CrossRef] [PubMed]
- Ayad, B.M.; Van der Horst, G.; du Plessis, S.S. Short abstinence: A potential strategy for the improvement of sperm quality. Middle East Fertil. Soc. J. 2018, 23, 37–43. [Google Scholar] [CrossRef]
- Sánchez-Martín, P.; Sánchez-Martín, F.; González-Martínez, M.; Gosálvez, J. Increased pregnancy after reduced male abstinence. Syst. Biol. Reprod. Med. 2013, 59, 256–260. [Google Scholar] [CrossRef]
- Wang, H.; Xu, A.; Gong, L.; Chen, Z.; Zhang, B.; Li, X. The Microbiome, an Important Factor That Is Easily Overlooked in Male Infertility. Front. Microbiol. 2022, 13, 831272. [Google Scholar] [CrossRef]
- Allen-Vercoe, E. Bringing the gut microbiota into focus through microbial culture: Recent progress and future perspective. Curr. Opin. Microbiol. 2013, 16, 625–629. [Google Scholar] [CrossRef]
- Fraczek, M.; Hryhorowicz, M.; Gill, K.; Zarzycka, M.; Gaczarzewicz, D.; Jedrzejczak, P.; Bilinska, B.; Piasecka, M.; Kurpisz, M. The effect of bacteriospermia and leukocytospermia on conventional and nonconventional semen parameters in healthy young normozoospermic males. J. Reprod. Immunol. 2016, 118, 18–27. [Google Scholar] [CrossRef]
- Tvrdá, E.; Lovíšek, D.; Gálová, E.; Schwarzová, M.; Kováčiková, E.; Kunová, S.; Žiarovská, J.; Kačániová, M. Possible Implications of Bacteriospermia on the Sperm Quality, Oxidative Characteristics, and Seminal Cytokine Network in Normozoospermic Men. Int. J. Mol. Sci. 2022, 23, 8678. [Google Scholar] [CrossRef]
- Perez-Carrasco, V.; Soriano-Lerma, A.; Soriano, M.; Gutiérrez-Fernández, J.; Garcia-Salcedo, J.A. Urinary Microbiome: Yin and Yang of the Urinary Tract. Front. Cell. Infect. Microbiol. 2021, 11, 617002. [Google Scholar] [CrossRef] [PubMed]
- Gottschick, C.; Deng, Z.L.; Vital, M.; Masur, C.; Abels, C.; Pieper, D.H.; Wagner-Döbler, I. The urinary microbiota of men and women and its changes in women during bacterial vaginosis and antibiotic treatment. Microbiome 2017, 5, 99. [Google Scholar] [CrossRef] [PubMed]
- Fouts, D.E.; Pieper, R.; Szpakowski, S.; Pohl, H.; Knoblach, S.; Suh, M.J.; Huang, S.T.; Ljungberg, I.; Sprague, B.M.; Lucas, S.K.; et al. Integrated next-generation sequencing of 16S rDNA and metaproteomics differentiate the healthy urine microbiome from asymptomatic bacteriuria in neuropathic bladder associated with spinal cord injury. J. Transl. Med. 2012, 10, 174. [Google Scholar] [CrossRef]
- Modena, B.D.; Milam, R.; Harrison, F.; Cheeseman, J.A.; Abecassis, M.M.; Friedewald, J.J.; Kirk, A.D.; Salomon, D.R. Changes in Urinary Microbiome Populations Correlate in Kidney Transplants With Interstitial Fibrosis and Tubular Atrophy Documented in Early Surveillance Biopsies. Am. J. Transplant. 2017, 17, 712–723. [Google Scholar] [CrossRef] [PubMed]
- Moustafa, A.; Li, W.; Singh, H.; Moncera, K.J.; Torralba, M.G.; Yu, Y.; Manuel, O.; Biggs, W.; Venter, J.C.; Nelson, K.E.; et al. Microbial metagenome of urinary tract infection. Sci. Rep. 2018, 8, 4333. [Google Scholar] [CrossRef]
- Kogan, M.I.; Naboka, Y.L.; Ibishev, K.S.; Gudima, I.A.; Naber, K.G. Human urine is not sterile—Shift of paradigm. Urol. Int. 2015, 94, 445–452. [Google Scholar] [CrossRef]
- Gonçalves, M.F.M.; Fernandes, Â.R.; Rodrigues, A.G.; Lisboa, C. Microbiome in Male Genital Mucosa (Prepuce, Glans, and Coronal Sulcus): A Systematic Review. Microorganisms 2022, 10, 2312. [Google Scholar] [CrossRef]
- Lundy, S.D.; Sangwan, N.; Parekh, N.V.; Selvam, M.K.P.; Gupta, S.; McCaffrey, P.; Bessoff, K.; Vala, A.; Agarwal, A.; Sabanegh, E.S.; et al. Functional and Taxonomic Dysbiosis of the Gut, Urine, and Semen Microbiomes in Male Infertility. Eur. Urol. 2021, 79, 826–836. [Google Scholar] [CrossRef]
- Alqawasmeh, O.; Fok, E.; Yim, H.; Li, T.; Chung, J.; Chan, D. The microbiome and male infertility: Looking into the past to move forward. Hum. Fertil. 2022. ahead of print. [Google Scholar] [CrossRef]
- Stones, D.H.; Krachler, A.M. Against the tide: The role of bacterial adhesion in host colonization. Biochem. Soc. Trans. 2016, 44, 1571–1580. [Google Scholar] [CrossRef]
- Vander, H.; Gupta, S.; Kaur, S.; Kaur, K.; Prabha, V. Characterization of sperm immobilization factor from Escherichia coli and its receptor to study the underlying mechanism of sperm immobilization. Am. J. Biomed Sci. 2013, 5, 25–33. [Google Scholar] [CrossRef]
- Prabha, V.; Gupta, T.; Kaur, S.; Kaur, N.; Kala, S.; Singh, A. Isolation of a spermatozoal immobilization factor from Staphylococcus aureus filtrates. Can. J. Microbiol. 2009, 55, 874–878. [Google Scholar] [CrossRef] [PubMed]
- Berger, G.K.; Smith-Harrison, L.I.; Sandlow, J.I. Sperm agglutination: Prevalence and contributory factors. Andrologia 2019, 51, e13254. [Google Scholar] [CrossRef]
- Gioannini, T.L.; Weiss, J.P. Regulation of interactions of Gramnegative bacterial endotoxins with mammalian cells. Immunol. Res. 2007, 39, 249–260. [Google Scholar] [CrossRef]
- Fraczek, M.; Szumala-Kakol, A.; Jedrzejczak, P.; Kamieniczna, M.; Kurpisz, M. Bacteria trigger oxygen radical release and sperm lipid peroxidation in in vitro model of semen inflammation. Fertil. Steril. 2007, 88, 1076–1085. [Google Scholar] [CrossRef]
- Fraczek, M.; Szumala-Kakol, A.; Dworacki, G.; Sanocka, D.; Kurpisz, M. In vitro reconstruction of inflammatory reaction in human semen: Effect on sperm DNA fragmentation. J. Reprod. Immunol. 2013, 100, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Loveland, K.L.; Klein, B.; Pueschl, D.; Indumathy, S.; Bergmann, M.; Loveland, B.E.; Hedger, M.P.; Schuppe, H.C. Cytokines in Male Fertility and Reproductive Pathologies: Immunoregulation and Beyond. Front. Endocrinol. 2017, 8, 307. [Google Scholar] [CrossRef]
- Boitrelle, F.; Shah, R.; Saleh, R.; Henkel, R.; Kandil, H.; Chung, E.; Vogiatzi, P.; Zini, A.; Arafa, M.; Agarwal, A. The Sixth Edition of the WHO Manual for Human Semen Analysis: A Critical Review and SWOT Analysis. Life 2021, 11, 1368. [Google Scholar] [CrossRef]
- Valsa, J.; Skandhan, K.P.; Gusani, P.H.; Sahab Khan, P.; Amith, S. Quality of 4-hourly ejaculates--levels of calcium and magnesium. Andrologia 2013, 45, 10–17. [Google Scholar] [CrossRef]
- Küçük, T.; Sozen, E.; Buluc, B. Intrauterine insemination with double ejaculate compared with single ejaculate in male factor infertility: A pilot study. J. Androl. 2008, 29, 404–407. [Google Scholar] [CrossRef]
- Patil, P.S.; Humbarwadi, R.S.; Patil, A.D.; Gune, A.R. Immature germ cells in semen—Correlation with total sperm count and sperm motility. J. Cytol. 2013, 30, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Comar, V.A.; Petersen, C.G.; Mauri, A.L.; Mattila, M.; Vagnini, L.D.; Renzi, A.; Petersen, B.; Nicoletti, A.; Dieamant, F.; Oliveira, J.B.A.; et al. Influence of the abstinence period on human sperm quality: Analysis of 2,458 semen samples. JBRA Assist. Reprod. 2017, 21, 306–312. [Google Scholar] [CrossRef]
- Dahan, M.H.; Mills, G.; Khoudja, R.; Gagnon, A.; Tan, G.; Tan, S.L. Three hour abstinence as a treatment for high sperm DNA fragmentation: A prospective cohort study. J. Assist. Reprod. Genet. 2021, 38, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Agnihotri, S.K.; Agrawal, A.K.; Hakim, B.A.; Vishwakarma, A.L.; Narender, T.; Sachan, R.; Sachdev, M. Mitochondrial membrane potential (MMP) regulates sperm motility. In Vitro Cell. Dev. Biol. Anim. 2016, 52, 953–960. [Google Scholar] [CrossRef] [PubMed]
- Du Plessis, S.S.; Agarwal, A.; Halabi, J.; Tvrda, E. Contemporary evidence on the physiological role of reactive oxygen species in human sperm function. J. Assist. Reprod. Genet. 2015, 32, 509–520. [Google Scholar] [CrossRef]
- Tvrdá, E.; Benko, F.; Ďuračka, M. Oxidative Stress as an Underlying Mechanism of Bacteria-Inflicted Damage to Male Gametes. Oxygen 2022, 2, 36. [Google Scholar] [CrossRef]
- Pahune, P.P.; Choudhari, A.R.; Muley, P.A. The total antioxidant power of semen and its correlation with the fertility potential of human male subjects. J. Clin. Diagn. Res. 2013, 7, 991–995. [Google Scholar] [CrossRef]
- Boe-Hansen, G.B.; Fedder, J.; Ersbøll, A.K.; Christensen, P. The sperm chromatin structure assay as a diagnostic tool in the human fertility clinic. Hum. Reprod. 2006, 21, 1576–1582. [Google Scholar] [CrossRef]
- Chen, G.X.; Li, H.Y.; Lin, Y.H.; Huang, Z.Q.; Huang, P.Y.; Da, L.C.; Shi, H.; Yang, L.; Feng, Y.B.; Zheng, B.H. The effect of age and abstinence time on semen quality: A retrospective study. Asian J. Androl. 2022, 24, 73–77. [Google Scholar] [CrossRef]
- Zandieh, Z.; Vatannejad, A.; Doosti, M.; Zabihzadeh, S.; Haddadi, M.; Bajelan, L.; Rashidi, B.; Amanpour, S. Comparing reactive oxygen species and DNA fragmentation in semen samples of unexplained infertile and healthy fertile men. Ir. J. Med. Sci. 2018, 187, 657–662. [Google Scholar] [CrossRef]
- Eini, F.; Kutenaei, M.A.; Zareei, F.; Dastjerdi, Z.S.; Shirzeyli, M.H.; Salehi, E. Effect of bacterial infection on sperm quality and DNA fragmentation in subfertile men with leukocytospermia. BMC Mol. Cell. Biol. 2021, 22, 42. [Google Scholar] [CrossRef] [PubMed]
- Enwuru, C.A.; Iwalokun, B.; Enwuru, V.N.; Ezechi, O.; Oluwadun, A. The effect of presence of facultative bacteria species on semen and sperm quality of men seeking fertility care. Afr. J. Urol. 2016, 22, 213–222. [Google Scholar] [CrossRef]
- Vilvanathan, S.; Kandasamy, B.; Jayachandran, A.L.; Sathiyanarayanan, S.; Tanjore Singaravelu, V.; Krishnamurthy, V.; Elangovan, V. Bacteriospermia and its impact on basic semen parameters among infertile men. Interdiscip. Perspect. Infect. Dis. 2016, 2016, 2614692. [Google Scholar] [CrossRef]
- Voroshilina, E.S.; Zornikov, D.L.; Ivanov, A.V.; Pochernikov, D.G.; Panacheva, E.A. Microbiota of semen samples with normozoospermia: Analysis of real-time PCR data. Bull. Russ. State Med. Univ. 2021, 5, 54–61. [Google Scholar] [CrossRef]
- Hou, D.; Zhou, X.; Zhong, X.; Settles, M.L.; Herring, J.; Wang, L.; Abdo, Z.; Forney, L.J.; Xu, C. Microbiota of the seminal fluid from healthy and infertile men. Fertil. Steril. 2013, 100, 1261–1269. [Google Scholar] [CrossRef] [PubMed]
- Jedrzejczak, P.; Fraczek, M.; Szumała-Kakol, A.; Taszarek-Hauke, G.; Pawelczyk, L.; Kurpisz, M. Consequences of semen inflammation and lipid peroxidation on fertilization capacity of spermatozoa in vitro conditions. Int. J. Androl. 2005, 28, 275–283. [Google Scholar] [CrossRef]
- Zhang, F.; Dai, J.; Chen, T. Role of Lactobacillus in female infertility via modulating sperm agglutination and immobilization. Front. Cell. Infect. Microbiol. 2021, 10, 620529. [Google Scholar] [CrossRef]
- Wolff, H.; Panhans, A.; Stolz, W.; Meurer, M. Adherence of Escherichia coli to sperm: A mannose mediated phenomenon leading to agglutination of sperm and E. coli. Fertil. Steril. 1993, 60, 154–158. [Google Scholar] [CrossRef]
- Rennemeier, C.; Frambach, T.; Hennicke, F.; Dietl, J.; Staib, P. Microbial quorum-sensing molecules induce acrosome loss and cell death in human spermatozoa. Infect. Immun. 2009, 77, 4990–4997. [Google Scholar] [CrossRef]
- Shang, Y.; Liu, C.; Cui, D.; Han, G.; Yi, S. The effect of chronic bacterial prostatitis on semen quality in adult men: A meta-analysis of case-control studies. Sci. Rep. 2014, 4, 7233. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, K.; Yao, Y.; Li, J.; Deng, S. Bacterial infections affect male fertility: A focus on the oxidative stress-autophagy axis. Front. Cell. Dev. Biol. 2021, 9, 727812. [Google Scholar] [CrossRef] [PubMed]
- Benoff, S.; Cooper, G.W.; Centola, G.M.; Jacob, A.; Hershlag, A.; Hurley, I.R. Metal ions and human sperm mannose receptors. Andrologia 2000, 32, 317–329. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, J.; Srivastava, S.; Singh, M. Pathogenomics of uropathogenic Escherichia coli. Indian J. Med. Microbiol. 2012, 30, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Pant, N.C.; Singh, R.; Gupta, V.; Chauhan, A.; Mavuduru, R.; Prabha, V.; Sharma, P. Contraceptive efficacy of sperm agglutinating factor from Staphylococcus warneri, isolated from the cervix of a woman with inexplicable infertility. Reprod. Biol. Endocrinol. 2019, 17, 85. [Google Scholar] [CrossRef] [PubMed]
- Henderson, B.; Martin, A. Bacterial virulence in the moonlight: Multitasking bacterial moonlighting proteins are virulence determinants in infectious disease. Infect. Immun. 2011, 79, 3476–3491. [Google Scholar] [CrossRef]
- Moretti, E.; Capitani, S.; Figura, N.; Pammolli, A.; Federico, M.G.; Giannerini, V.; Collodel, G. The presence of bacterial species in semen and sperm quality. J. Assisted Reprod. Genet. 2009, 26, 47–56. [Google Scholar] [CrossRef]
- Domes, T.; Lo, K.C.; Grober, E.D.; Mullen, J.B.; Mazzulli, T.; Jarvi, K. The incidence and effect of bacteriospermia and elevated seminal leukocytes on semen parameters. Fertil. Steril. 2012, 97, 1050–1055. [Google Scholar] [CrossRef] [PubMed]
- Berjis, K.; Ghiasi, M.; Sangy, S. Study of seminal infection among an infertile male population in Qom, Iran, and its effect on sperm quality. Iran. J. Microbiol. 2018, 10, 111–116. [Google Scholar]
- Fraczek, M.; Piasecka, M.; Gaczarzewicz, D.; Szumala-Kakol, A.; Kazienko, A.; Lenart, S.; Laszczynska, M.; Kurpisz, M. Membrane stability and mitochondrial activity of human-ejaculated spermatozoa during in vitro experimental infection with Escherichia coli, Staphylococcus haemolyticus and Bacteroides ureolyticus. Andrologia 2012, 44, 315–329. [Google Scholar] [CrossRef]
- Schulz, M.; Sánchez, R.; Soto, L.; Risopatrón, J.; Villegas, J. Effect of Escherichia coli and its soluble factors on mitochondrial membrane potential, phosphatidylserine translocation, viability, and motility of human spermatozoa. Fertil. Steril. 2010, 94, 619–623. [Google Scholar] [CrossRef]
- Sanocka, D.; Fraczek, M.; Jedrzejczak, P.; Szumała-Kakol, A.; Kurpisz, M. Male genital tract infection: An influence of leukocytes and bacteria on semen. J. Reprod. Immunol. 2004, 62, 111–124. [Google Scholar] [CrossRef]
- Rusz, A.; Pilatz, A.; Wagenlehner, F.; Linn, T.; Diemer, T.; Schuppe, H.C.; Lohmeyer, J.; Hossain, H.; Weidner, W. Influence of urogenital infections and inflammation on semen quality and male fertility. World J. Urol. 2012, 30, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Fraczek, M.; Kurpisz, M. Mechanisms of the harmful effects of bacterial semen infection on ejaculated human spermatozoa: Potential inflammatory markers in semen. Folia Histochem. Cytobiol. 2015, 53, 201–217. [Google Scholar] [CrossRef] [PubMed]
- Martínez, P.; Proverbio, F.; Camejo, M.I. Sperm lipid peroxidation and pro-inflammatory cytokines. Asian J. Androl. 2007, 9, 102–107. [Google Scholar] [CrossRef]
- Perdichizzi, A.; Nicoletti, F.; La Vignera, S.; Barone, N.; D’Agata, R.; Vicari, E.; Calogero, A.E. Effects of tumour necrosis factor-alpha on human sperm motility and apoptosis. J. Clin. Immunol. 2007, 27, 152–162. [Google Scholar] [CrossRef]
- Paira, D.A.; Silvera-Ruiz, S.; Tissera, A.; Molina, R.I.; Olmedo, J.J.; Rivero, V.E.; Motrich, R.D. Interferon γ, IL-17, and IL-1β impair sperm motility and viability and induce sperm apoptosis. Cytokine 2022, 152, 155834. [Google Scholar] [CrossRef] [PubMed]
- Dziadecki, W.; Celińska, A.; Fracki, S.; Bablok, L.; Barcz, E. Interleukin 1b and interleukin 18 and their connection with leukocytospermia in human semen. Centr. Eur. J. Immunol. 2010, 35, 157–161. [Google Scholar]
- Koçak, I.; Yenisey, C.; Dündar, M.; Okyay, P.; Serter, M. Relationship between seminal plasma interleukin-6 and tumor necrosis factor alpha levels with semen parameters in fertile and infertile men. Urol. Res. 2002, 30, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Moretti, E.; Collodel, G.; Mazzi, L.; Campagna, M.; Iacoponi, F.; Figura, N. Resistin, interleukin-6, tumor necrosis factor-alpha, and human semen parameters in the presence of leukocytospermia, smoking habit, and varicocele. Fertil. Steril. 2014, 102, 354–360. [Google Scholar] [CrossRef]
- Zeyad, A.; Hamad, M.F.; Hammadeh, M.E. The effects of bacterial infection on human sperm nuclear protamine P1/P2 ratio and DNA integrity. Andrologia 2018, 50, e12841. [Google Scholar] [CrossRef]
- Skandhan, K.P. Hypothesis: Epididymis inhibits sperm motility inside male reproductive tract. Med. Hypotheses 2004, 62, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Tvrdá, E.; Arroyo, F.; Gosálvez, J. Dynamic assessment of human sperm DNA damage I: The effect of seminal plasma-sperm co-incubation after ejaculation. Int. Urol. Nephrol. 2018, 50, 1381–1388. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Sethu, R.; Imlay, J.A. Endogenous superoxide is a key effector of the oxygen sensitivity of a model obligate anaerobe. Proc. Natl. Acad. Sci. USA 2018, 115, E3266–E3275. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.P.; Pramanik, P.; Roy, S. Staphylococcus aureus Infection induced oxidative imbalance in neutrophils: Possible protective role of nanoconjugated vancomycin. ISRN Pharmacol. 2012, 2012, 435214. [Google Scholar] [CrossRef]
- Duracka, M.; Lukac, N.; Kacaniova, M.; Kantor, A.; Hleba, L.; Ondruska, L.; Tvrda, E. Antibiotics Versus Natural Biomolecules: The Case of In Vitro Induced Bacteriospermia by Enterococcus Faecalis in Rabbit Semen. Molecules 2019, 24, 4329. [Google Scholar] [CrossRef]
- Barroso, G.; Morshedi, M.; Oehninger, S. Analysis of DNA fragmentation, plasma membrane translocation of phosphatidylserine and oxidative stress in human spermatozoa. Hum. Reprod. 2000, 15, 1338–1344. [Google Scholar] [CrossRef]
- Collodel, G.; Baccetti, B.; Capitani, S.; Moretti, E. Necrosis in human spermatozoa. I. Ultrastructural features and FISH study in semen from patients with urogenital infections. J. Submicrosc. Cytol. Pathol. 2007, 37, 67–73. [Google Scholar]
- Sharma, R.K.; Seifarth, K.; Agarwal, A. Comparison of single- and two-layer Percoll separation for selection of motile spermatozoa. Int. J. Fertil. Womens Med. 1997, 42, 412–417. [Google Scholar]
- Cui, Z.; Sharma, R.; Agarwal, A. Proteomic analysis of mature and immature ejaculated spermatozoa from fertile men. Asian J. Androl. 2016, 18, 735–746. [Google Scholar] [CrossRef]
- Tvrda, E.; Fik, M.; Kovacik, A.; Duracka, M.; Kacaniova, M. Biochemical and bacteriological characterization of Dachshund semen: A correlation study. Reprod. Domest. Anim. 2022, 57, 60. [Google Scholar] [CrossRef]
- Schuffner, A.; Morshedi, M.; Oehninger, S. Cryopreservation of fractionated, highly motile human spermatozoa: Effect on membrane phosphatidylserine externalization and lipid peroxidation. Hum. Reprod. 2001, 16, 2148–2153. [Google Scholar] [CrossRef] [PubMed]
- Tvrdá, E.; López-Fernández, C.; Sánchez-Martín, P.; Gosálvez, J. Sperm DNA fragmentation in donors and normozoospermic patients attending for a first spermiogram: Static and dynamic assessment. Andrologia 2018, 50, e12986. [Google Scholar] [CrossRef] [PubMed]
- Muller, C.H.; Lee, T.K.; Montaño, M.A. Improved chemiluminescence assay for measuring antioxidant capacity of seminal plasma. Methods Mol. Biol. 2013, 927, 363–376. [Google Scholar] [CrossRef] [PubMed]
- Weber, D.; Davies, M.J.; Grune, T. Determination of Protein Carbonyls in Plasma, Cell Extracts, Tissue Homogenates, Isolated Proteins: Focus on Sample Preparation and Derivatization Conditions. Redox Biol. 2015, 5, 367–380. [Google Scholar] [CrossRef]
- Tvrdá, E.; Kačániová, M.; Baláži, A.; Vašíček, J.; Vozaf, J.; Jurčík, R.; Ďuračka, M.; Žiarovská, J.; Kováč, J.; Chrenek, P. The Impact of Bacteriocenoses on Sperm Vitality, Immunological and Oxidative Characteristics of Ram Ejaculates: Does the Breed Play a Role? Animals 2022, 12, 54. [Google Scholar] [CrossRef]
- Tvrdá, E.; Lovíšek, D.; Kyzek, S.; Kováčik, D.; Gálová, E. The Effect of Non-Thermal Plasma on the Structural and Functional Characteristics of Human Spermatozoa. Int. J. Mol. Sci. 2021, 22, 4979. [Google Scholar] [CrossRef]
- Benko, F.; Fialková, V.; Žiarovská, J.; Ďuračka, M.; Lukáč, N.; Tvrdá, E. In Vitro versus Cryo-Induced Capacitation of Bovine Spermatozoa, Part 2: Changes in the Expression Patterns of Selected Transmembrane Channels and Protein Kinase A. Int. J. Mol. Sci. 2022, 23, 14646. [Google Scholar] [CrossRef]

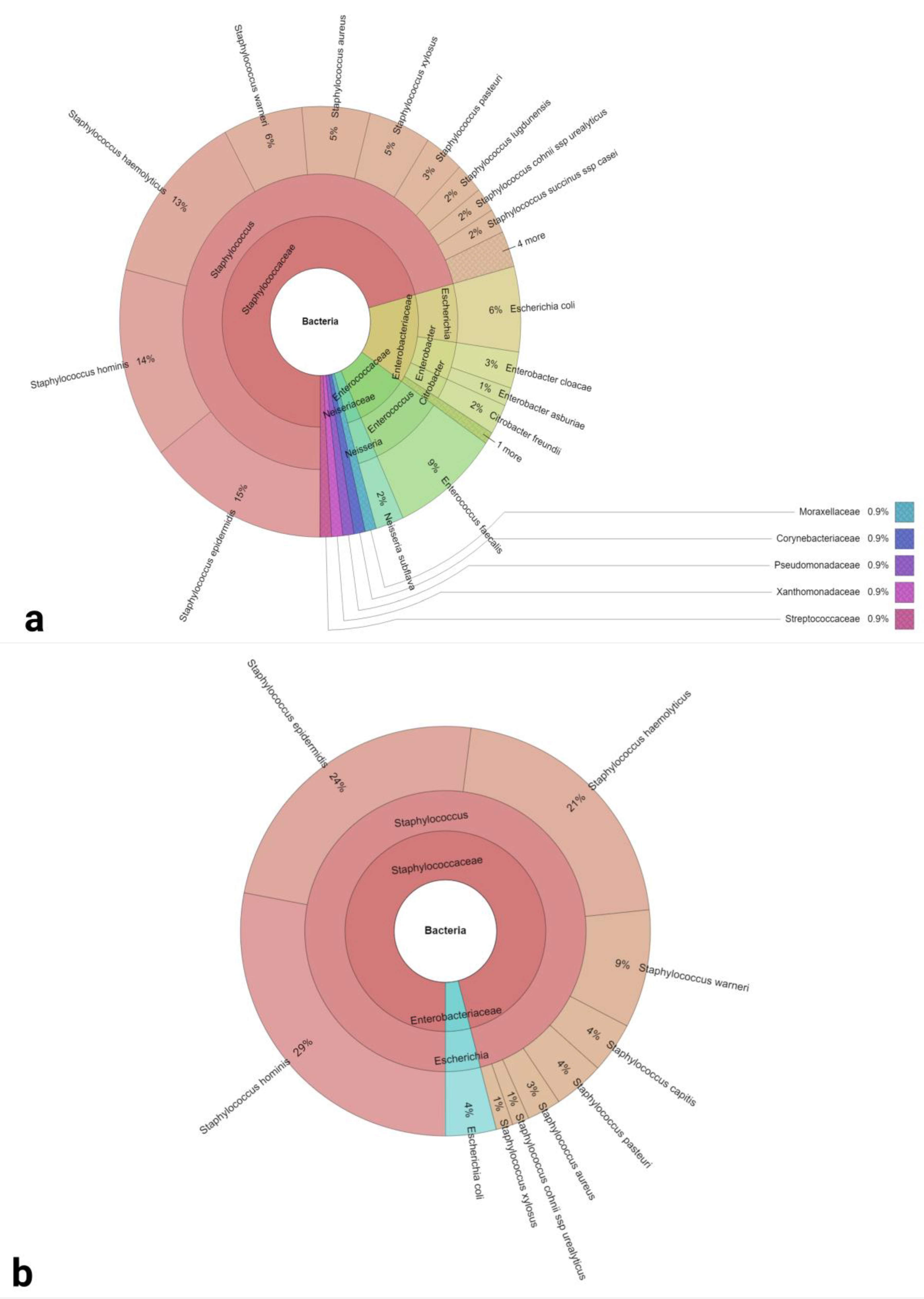
| Groups | 2 Days of Abstinence | 2 h of Abstinence |
|---|---|---|
| Bacterial Load (log10 CFU/mL) | 3.27 ± 0.34 | 1.59 ± 0.35 **** |
| Identified Bacterial Species and Sample Positivity | Staphylococcus epidermidis (62.75%) | Staphylococcus epidermidis (35.29%) |
| Staphylococcus hominis (60.78%) | Staphylococcus hominis (41.18%) | |
| Staphylococcus haemolyticus (56.86%) | Staphylococcus haemolyticus (29.41%) | |
| Staphylococcus warneri (27.45%) | Staphylococcus warneri (13.73%) | |
| Escherichia coli (25.49%) | Escherichia coli (5.88%) | |
| Staphylococcus xylosus (21.56%) | Staphylococcus xylosus (1.96%) | |
| Staphylococcus cohnii (15.69%) | Staphylococcus cohnii (3.92%) | |
| Staphylococcus pasteuri (13.73%) | Staphylococcus pasteuri (5.88%) | |
| Staphylococcus capitis (13.73%) | Staphylococcus capitis (5.88%) | |
| Staphylococcus aureus (11.76%) | Staphylococcus aureus (3.92%) | |
| Enterococcus faecalis (37.25%) | N/A | |
| Enterobacter cloacae (11.76%) | N/A | |
| Citrobacter freundii (9.80%) | N/A | |
| Neisseria subflava (9.80%) | N/A | |
| Staphylococcus lugdunensis (9.80%) | N/A | |
| Staphylococcus succinus (7.84%) | N/A | |
| Enterobacter asburiae (5.88%) | N/A | |
| Acinetobacter lwoffii (3.92%) | N/A | |
| Corynebacterium glucuronolyticum (3.92%) | N/A | |
| Pseudomonas aeruginosa (3.92%) | N/A | |
| Rahnella aquatilis (3.92%) | N/A | |
| Staphylococcus chromogenes (1.96%) | N/A | |
| Staphylococcus schleiferi (3.92%) | N/A | |
| Staphylococcus simiae (3.92%) | N/A | |
| Stenotrophomonas maltophilia (3.92%) | N/A | |
| Streptococcus agalactiae (3.92%) | N/A |
| Species | 2 Days of Abstinence | 2 h of Abstinence |
|---|---|---|
| Staphylococcus epidermidis | 32 | 18 |
| Staphylococcus hominis | 31 | 21 |
| Staphylococcus haemolyticus | 29 | 16 |
| Enterococcus faecalis | 19 | - |
| Escherichia coli | 14 | 3 |
| Staphylococcus warneri | 14 | 7 |
| Staphylococcus xylosus | 11 | 1 |
| Staphylococcus pasteuri | 7 | 3 |
| Staphylococcus aureus | 6 | 2 |
| Enterobacter cloacae | 6 | - |
| Staphylococcus capitis | 6 | 3 |
| Staphylococcus lugdunensis | 5 | - |
| Neisseria subflava | 5 | - |
| Citrobacter freundii | 5 | - |
| Staphylococcus cohnii ssp. Urealyticus | 4 | 1 |
| Staphylococcus succinus ssp. casei | 4 | - |
| Enterobacter asburiae | 3 | - |
| Corynebacterium glucuronolyticum | 2 | - |
| Pseudomonas aeruginosa | 2 | - |
| Streptococcus agalactiae | 2 | - |
| Staphylococcus simiae | 2 | - |
| Stenotrophomonas maltophilia | 2 | - |
| Staphylococcus schleiferi | 2 | - |
| Rahnella aquatilis | 2 | - |
| Acinetobacter lwoffii | 2 | - |
| Staphylococcus chromogenes | 1 | - |
| Staphylococcus capitis ssp. capitis | 1 | - |
| N/A | - | 4 |
| Groups | 2 Days of Abstinence | 2 h of Abstinence |
|---|---|---|
| Richness (R) | 27 | 11 |
| Berger Parker Dominance Index | 0.16 | 0.27 |
| Shannon α-diversity | 2.54 | 1.96 |
| Simpson dominance | 0.1 | 0.18 |
| Source | Sum of Squares (SS) | Degrees of Freedom (ν) | Mean Square (MS) | F Statistic | p-Value |
|---|---|---|---|---|---|
| Treatment | 7.69 | 1 | 7.69 | 80.7043 | 1.1102 × 10−16 |
| Error | 252.8235 | 2751 | 0.0919 | ||
| Total | 260.2405 | 2752 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tvrdá, E.; Ďuračka, M.; Benko, F.; Kováčik, A.; Lovíšek, D.; Gálová, E.; Žiarovská, J.; Schwarzová, M.; Kačániová, M. Ejaculatory Abstinence Affects the Sperm Quality in Normozoospermic Men—How Does the Seminal Bacteriome Respond? Int. J. Mol. Sci. 2023, 24, 3503. https://doi.org/10.3390/ijms24043503
Tvrdá E, Ďuračka M, Benko F, Kováčik A, Lovíšek D, Gálová E, Žiarovská J, Schwarzová M, Kačániová M. Ejaculatory Abstinence Affects the Sperm Quality in Normozoospermic Men—How Does the Seminal Bacteriome Respond? International Journal of Molecular Sciences. 2023; 24(4):3503. https://doi.org/10.3390/ijms24043503
Chicago/Turabian StyleTvrdá, Eva, Michal Ďuračka, Filip Benko, Anton Kováčik, Daniel Lovíšek, Eliška Gálová, Jana Žiarovská, Marianna Schwarzová, and Miroslava Kačániová. 2023. "Ejaculatory Abstinence Affects the Sperm Quality in Normozoospermic Men—How Does the Seminal Bacteriome Respond?" International Journal of Molecular Sciences 24, no. 4: 3503. https://doi.org/10.3390/ijms24043503
APA StyleTvrdá, E., Ďuračka, M., Benko, F., Kováčik, A., Lovíšek, D., Gálová, E., Žiarovská, J., Schwarzová, M., & Kačániová, M. (2023). Ejaculatory Abstinence Affects the Sperm Quality in Normozoospermic Men—How Does the Seminal Bacteriome Respond? International Journal of Molecular Sciences, 24(4), 3503. https://doi.org/10.3390/ijms24043503











