Long-Term Ingestion of Sicilian Black Bee Chestnut Honey and/or D-Limonene Counteracts Brain Damage Induced by High Fat-Diet in Obese Mice
Abstract
1. Introduction
2. Results
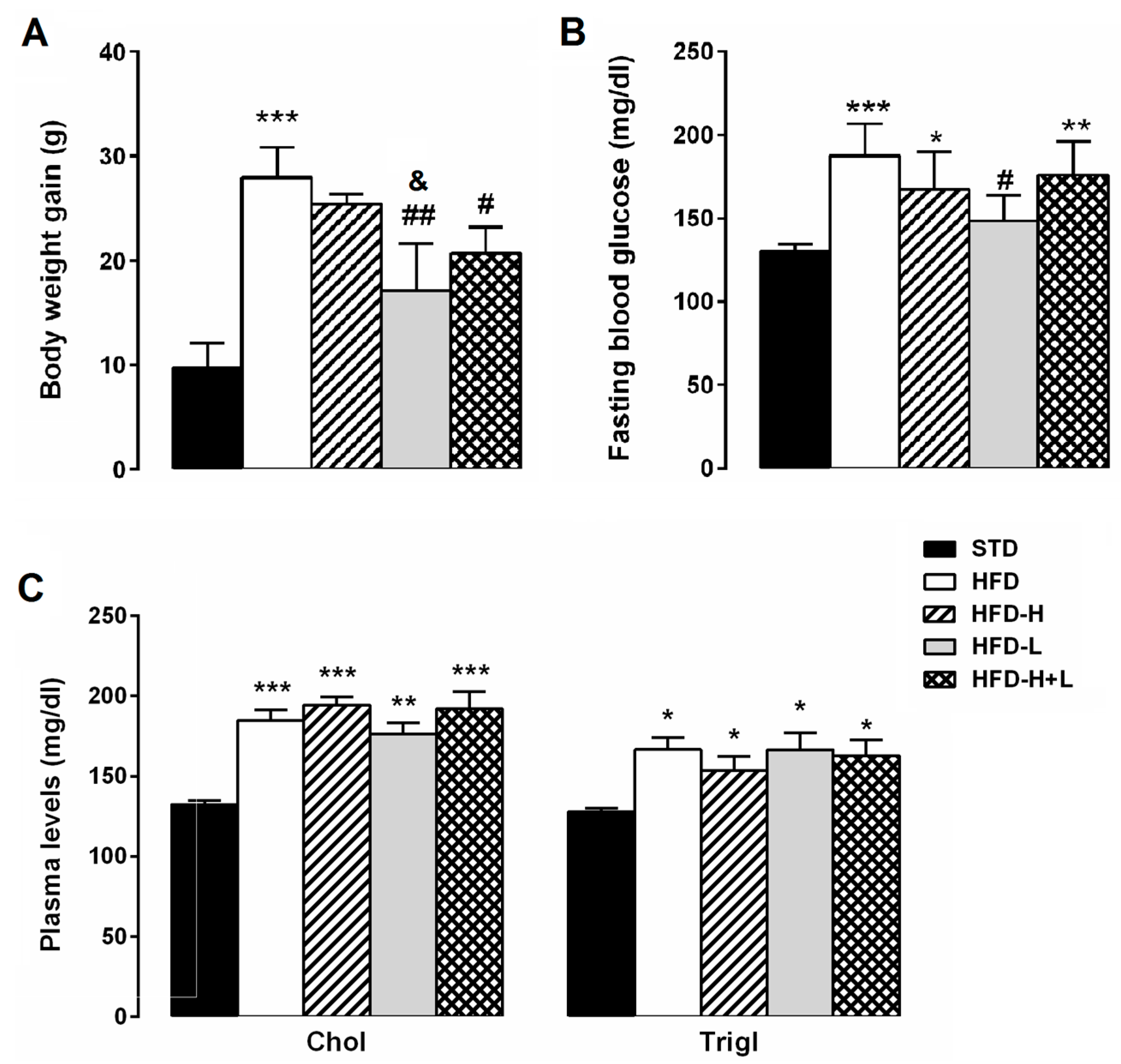
2.1. Body Weight, Glycaemia and Serum Lipids
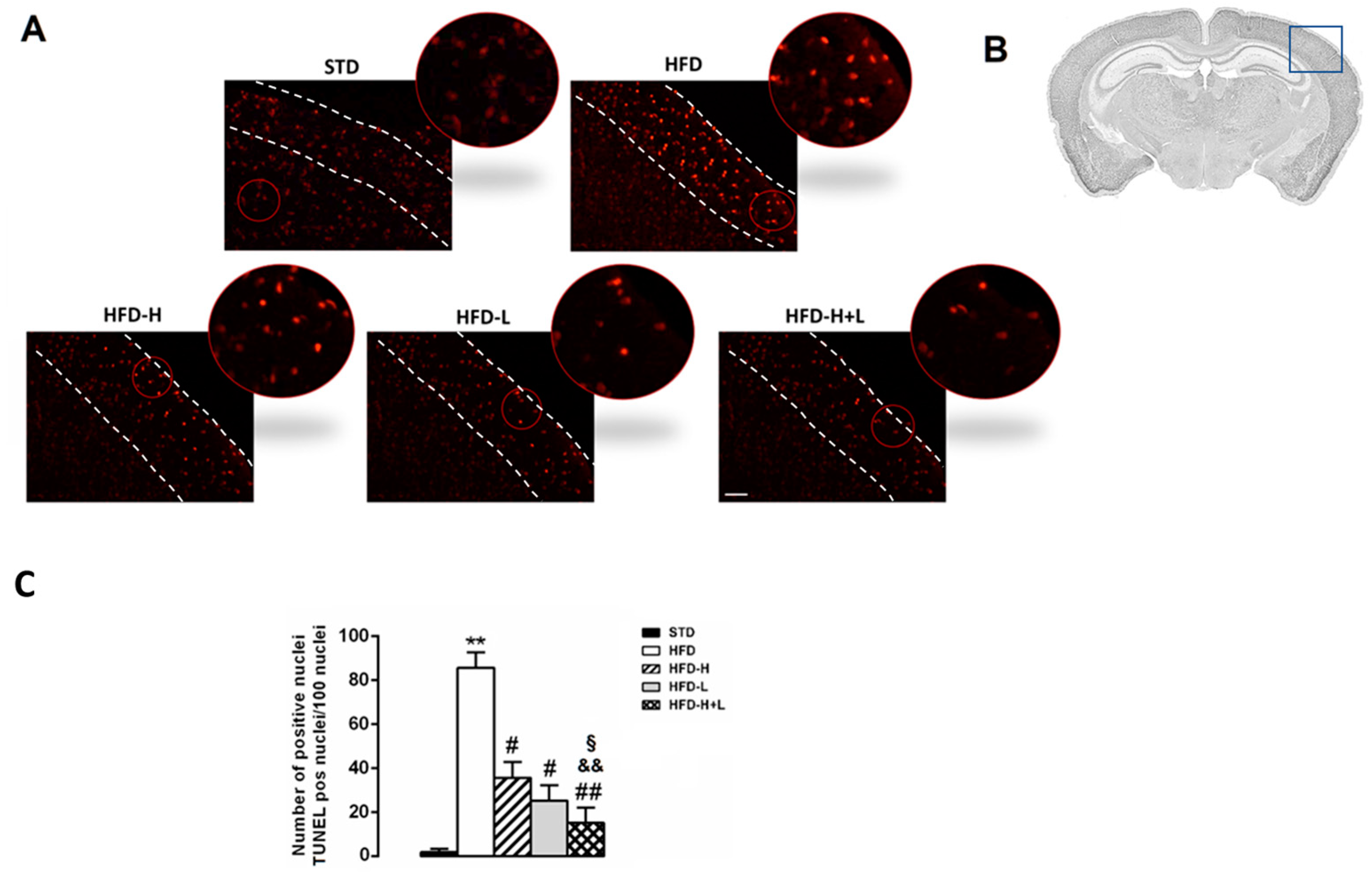
2.2. Neurodegeneration: TUNEL Assay
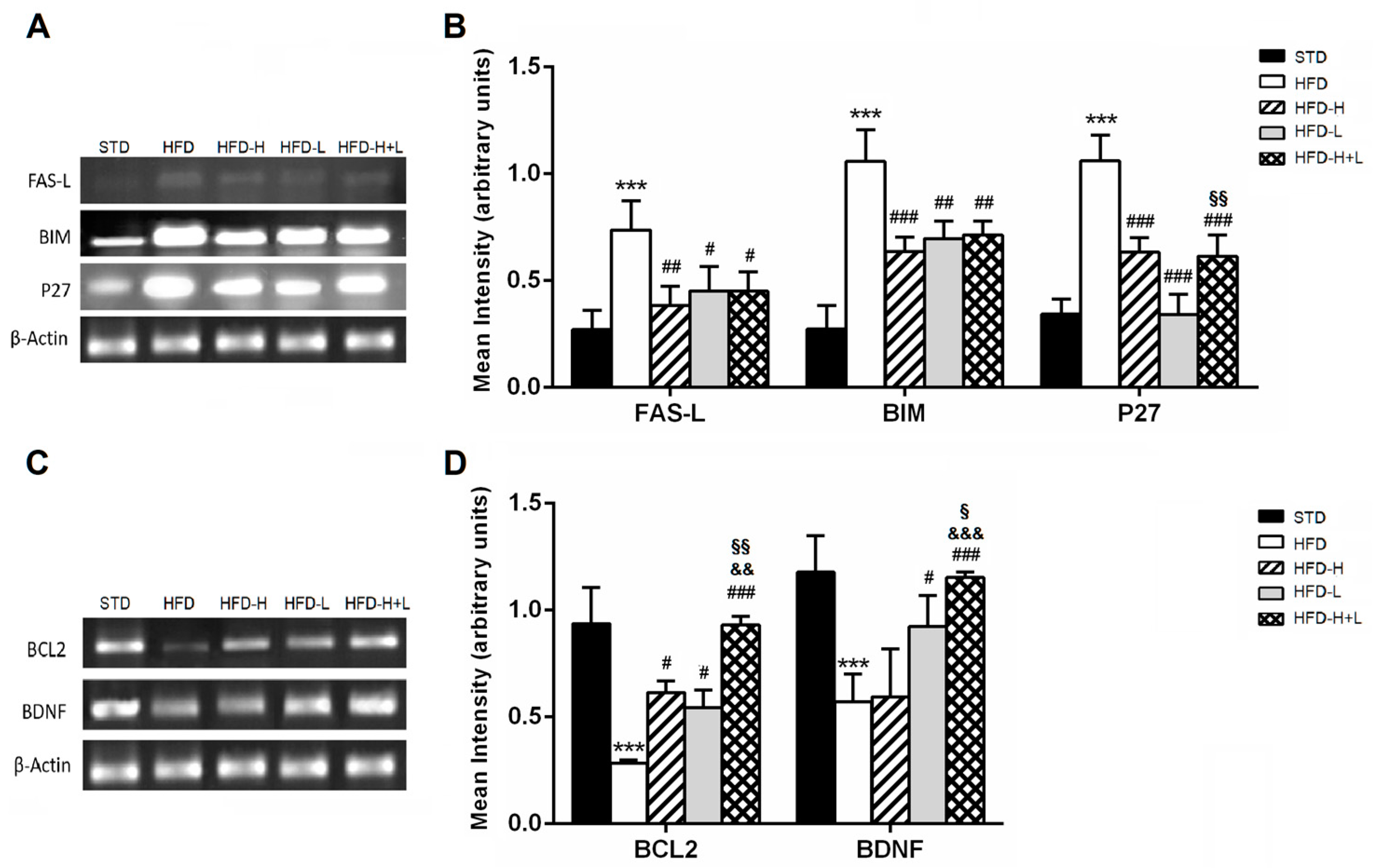
2.3. Pro-Apoptosis and Anti-Apoptosis Genes Expression
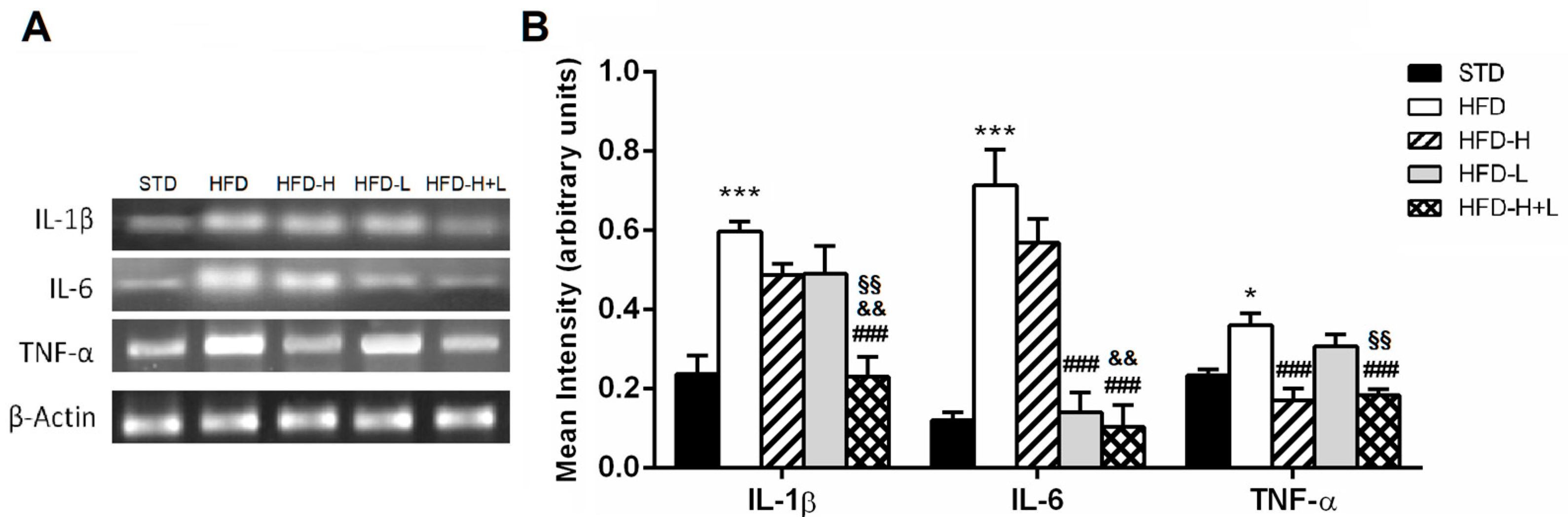
2.4. Brain Pro-Inflammatory Gene and Protein Expression
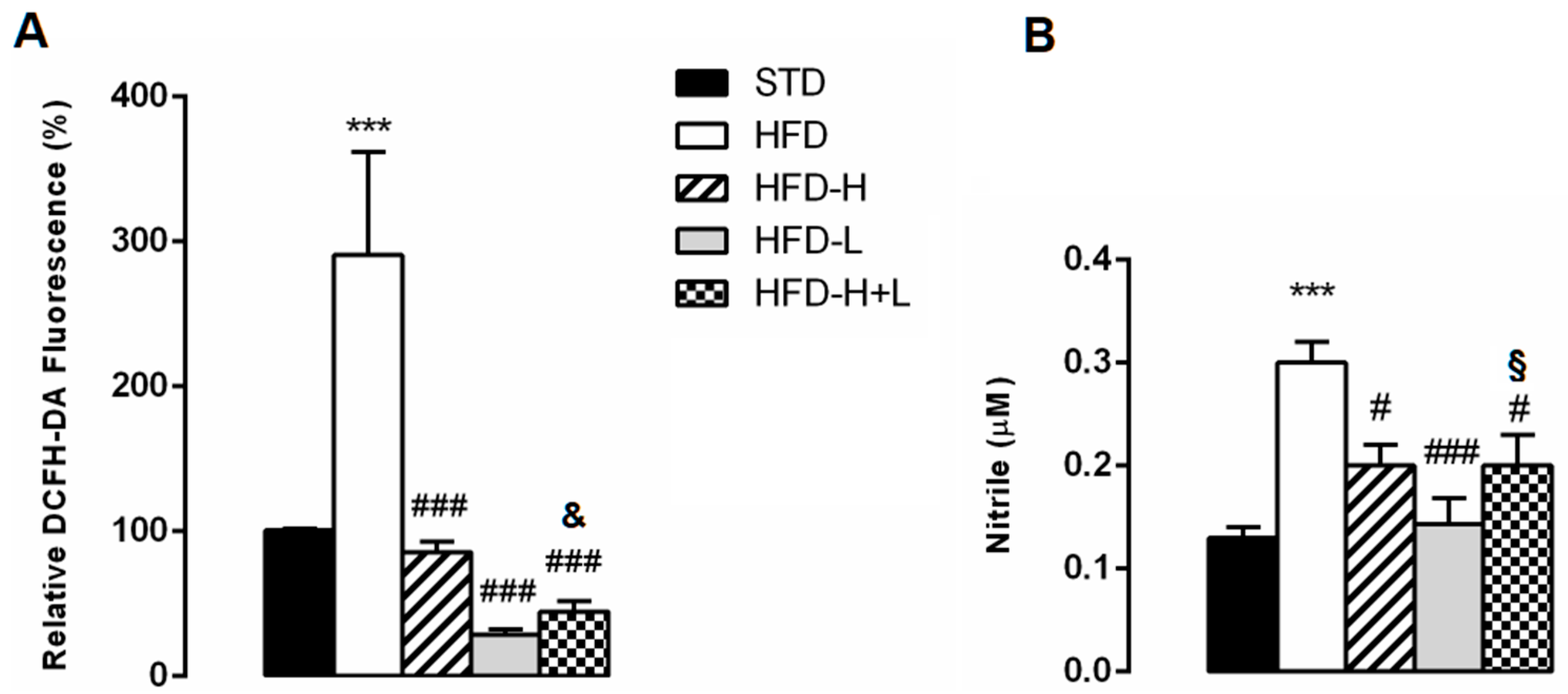
2.5. Brain Oxidative Stress
2.6. Expression of Genes Involved in AD
3. Discussion
4. Materials and Methods
4.1. Animals and Diets
4.2. Biochemical Analyses
4.3. Apoptosis Investigation
4.4. Reactive Oxygen Species Analysis
4.5. Determination of Nitric Oxide (NO) Levels
4.6. Molecular Analyses
4.7. RT2 Profiler PCR Array
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Livingston, G.; Huntley, J.; Sommerlad, A.; Ames, D.; Ballard, C.; Banerjee, S.; Brayne, C.; Burns, A.; Cohen-Mansfield, J.; Cooper, C.; et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 2020, 396, 413–446. [Google Scholar] [CrossRef] [PubMed]
- Reitz, C.; Brayne, C.; Mayeux, R. Epidemiology of Alzheimer disease. Nat. Rev. Neurol. 2011, 7, 137–152. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative stress: A key modulator in neurodegenerative diseases. Molecules 2019, 24, 1583. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, J.; Nutma, E.; van der Valk, P.; Amor, S. Inflammation in CNS neurodegenerative diseases. Immunology 2018, 154, 204–219. [Google Scholar] [CrossRef]
- Terzo, S.; Amato, A.; Mulè, F. From obesity to Alzheimer’s disease through insulin resistance. J. Diabetes Complicat. 2021, 35, 108026. [Google Scholar] [CrossRef]
- Frazier, H.; Ghoweri, A.; Anderson, K.; Lin, R.; Porter, N.M.; Thibault, O. Broadening the definition of brain insulin resistance in aging and Alzheimer’s disease. Exp. Neurol. 2019, 313, 79–87. [Google Scholar] [CrossRef]
- De la Monte, S. Type 3 diabetes is sporadic Alzheimer’s disease: Mini-review. Eur. Neuropsychopharmacol. 2014, 24, 1954–1960. [Google Scholar] [CrossRef]
- Farr, S.; Yamada, K.; Butterfield, D.; Abdul, H.M.; Xu, L.; Miller, N.E.; Banks, W.A.; Morley, J.E. Obesity and hypertriglyceridemia produce cognitive impairment. Endocrinology 2008, 149, 2628–2636. [Google Scholar] [CrossRef]
- Valladolid-Acebes, I.; Stucchi, P.; Cano, V.; Fernández-Alfonso, M.; Merino, B.; Gil-Ortega, M.; Fole, A.; Morales, L.; Ruiz-Gayo, M.; Del Olmo, N. High-fat diets impair spatial learning in the radial-arm maze in mice. Neurobiol. Learn. Mem. 2011, 95, 80–85. [Google Scholar] [CrossRef]
- Nuzzo, D.; Picone, P.; Baldassano, S.; Caruana, L.; Messina, E.; Marino Gammazza, A.; Cappello, F.; Mulè, F.; Di Carlo, M. Insulin resistance as common molecular denominator linking obesity to Alzheimer’s disease. Curr. Alzheimer Res. 2015, 12, 723–735. [Google Scholar] [CrossRef]
- Stranahan, A.; Norman, E.; Lee, K.; Cutler, R.G.; Telljohann, R.S.; Egan, J.M.; Mattson, M.P. Diet-induced insulin resistance impairs hippocampal synaptic plasticity and cognition in middle-aged rats. Hippocampus 2008, 18, 1085–1088. [Google Scholar] [CrossRef]
- Siino, V.; Amato, A.; Di Salvo, F.; Caldara, G.F.; Filogamo, M.; James, P.; Vasto, S. Impact of diet-induced obesity on the mouse brain phosphoproteome. J. Nutr. Biochem. 2018, 58, 102–109. [Google Scholar] [CrossRef]
- Siino, V.; Jensen, P.; James, P.; Vasto, S.; Amato, A.; Mulè, F.; Accardi, G.; Larsen, M.R. Obesogenic Diets Cause Alterations on Proteins and Theirs Post-Translational Modifications in Mouse Brains. Nutr. Metab. Insights 2021, 14, 11786388211012405. [Google Scholar] [CrossRef]
- Salman, M.; Al-Obaidi, Z.; Kitchen, P.; Loreto, A.; Bill, R.M.; Wade-Martins, R. Advances in Applying Computer-Aided Drug Design for Neurodegenerative Diseases. Int. J. Mol. Sci. 2021, 22, 4688. [Google Scholar] [CrossRef]
- Aldewachi, H.; Al-Zidan, R.; Conner, M.; Salman, M. High-Throughput Screening Platforms in the Discovery of Novel Drugs for Neurodegenerative Diseases. Bioengineering 2021, 8, 30. [Google Scholar] [CrossRef]
- Carvalho, J.; Fernandes, C.; Daleprane, J.; Alves, M.S.; Stien, D.; Dhammika Nanayakkara, N.P. Role of Natural Antioxidants from Functional Foods in Neurodegenerative and Metabolic Disorders. Oxid. Med. Cell Longev. 2018, 18, 459753. [Google Scholar] [CrossRef]
- Nuzzo, D.; Amato, A.; Picone, P.; Terzo, S.; Galizzi, G.; Bonina, F.P.; Mulè, F.; Di Carlo, M. A natural dietary supplement with a combination of nutrients prevents neurodegeneration induced by a high fat diet in mice. Nutrients 2018, 10, 1130. [Google Scholar] [CrossRef]
- Nuzzo, D.; Galizzi, G.; Amato, A.; Terzo, S.; Picone, P.; Cristaldi, L.; Mulè, F.; Di Carlo, M. Regular intake of pistachio mitigates the deleterious effects of a high fat-diet in the brain of obese mice. Antioxidants 2020, 9, 317. [Google Scholar] [CrossRef]
- Storz, M. Is there a lack of support for whole-food, plant-based diets in the medical community? Perm. J. 2018, 23, 18-068. [Google Scholar] [CrossRef]
- González-Castejón, M.; Rodriguez-Casado, A. Dietary phytochemicals and their potential effects on obesity: A review. Pharm. Res. 2011, 64, 438–455. [Google Scholar] [CrossRef]
- Terzo, S.; Mulè, F.; Amato, A. Honey and obesity-related dysfunctions: A summary on health benefits. J. Nutr. Biochem. 2020, 82, 108401. [Google Scholar] [CrossRef] [PubMed]
- Lo Dico, G.A.; Ulrici, A.; Pulvirenti, A.; Camilleri, G.; Macaluso, A. Multivariate statistical analysis of the polyphenols content for the discrimination of honey produced in Sicily (Southern Italy). J. Food Compos. Anal. 2019, 82, 103225. [Google Scholar] [CrossRef]
- Terzo, S.; Calvi, P.; Nuzzo, D.; Picone, P.; Galizzi, G.; Caruana, L.; Di Carlo, M.; Lentini, L.; Puleio, R.; Mulè, F. Preventive impact of long-term ingestion of chestnut honey on glucose disorders and neurodegeneration in obese mice. Nutrients 2022, 14, 756. [Google Scholar] [CrossRef] [PubMed]
- Eddin, L.; Jha, N.; Meeran, M.; Kesari, K.K.; Beiram, R.; Ojha, S. Neuroprotective Potential of Limonene and Limonene Containing Natural Products. Molecules 2021, 26, 4535. [Google Scholar] [CrossRef]
- Vieira, A.; Beserra, F.; Souza, M.; Totti, B.M.; Rozza, A.L. Limonene: Aroma of innovation in health and disease. Chem. Biol. Interact. 2018, 283, 97–106. [Google Scholar] [CrossRef]
- Jesudoss, V.A.S.; Jayachitra, J.; Shenbagam, M.; Nalini, N. Dietary d-limonene alleviates insulin resistance and oxidative stress-induced liver injury in high-fat diet and L-NAME-treated rats. Eur. J. Nutr. 2012, 51, 57–68. [Google Scholar] [CrossRef]
- Jing, L.; Zhang, Y.; Fan, S.; Gu, M.; Guan, Y.; Lu, X.; Huang, C.; Zhou, Z. Preventive and ameliorating effects of citrus D-limonene on dyslipidemia and hyperglycemia in mice with high-fat diet-induced obesity. Eur. J. Pharm. 2013, 715, 46–55. [Google Scholar] [CrossRef]
- Szwajgier, D.; Baranowska-Wójcik, E. Terpenes and phenylpropanoids as acetyl- and butyrylcholinesterase inhibitors: A comparative study. Curr. Alzheimer Res. 2019, 16, 963–973. [Google Scholar] [CrossRef]
- Shin, M.; Liu, Q.; Choi, B.; Shin, C.; Lee, B.; Yuan, C.; Song, Y.J.; Yun, H.S.; Lee, I.S.; Koo, B.S. Neuroprotective Effects of Limonene (+) against Aβ42-Induced Neurotoxicity in a Drosophila Model of Alzheimer’s Disease. Biol. Pharm. Bull. 2020, 43, 409–417. [Google Scholar] [CrossRef]
- Joglekar, M.; Bavkar, L.; Sistla, S.; Arvindekar, A. Effective inhibition of protein glycation by combinatorial usage of limonene and aminoguanidine through differential and synergistic mechanisms. Int. J. Biol. Macromol. 2017, 99, 563–569. [Google Scholar] [CrossRef]
- Ortega, R.; Requejo, A.; Andres, P.; López-Sobaler, A.M.; Quintas, M.E.; Redondo, M.R.; Navia, B.; Rivas, T. Dietary intake and cognitive function in a group of elderly people. Am. J. Clin. Nutr. 1997, 66, 803–809. [Google Scholar] [CrossRef]
- Freeman, L.R.; Haley-Zitlin, V.; Rosenberger, D.S.; Granholm, A.C. Damaging effects of a high- fat diet to the brain and cognition: A review of proposed mechanisms. Nutr. Neurosci. 2014, 17, 241–251. [Google Scholar] [CrossRef]
- Yeo, E.; Wong, K.; See, M.; See, M.L.; Wong, K.Y.; Yap, J.K.Y.; Gan, S.Y. Piper sarmentosum Roxb. confers neuroprotection on beta-amyloid (Aβ)-induced microglia-mediated neuroinflammation and attenuates tau hyperphosphorylation in SH-SY5Y cells. J. Ethnopharmacol. 2018, 217, 187–194. [Google Scholar] [CrossRef]
- Collins, S.; Martin, T.L.; Surwit, R.S.; Robidoux, J. Genetic vulnerability to diet-induced obesity in the C57BL/6J mouse: Physiological and molecular characteristics. Physiol. Behav. 2004, 81, 243–248. [Google Scholar] [CrossRef]
- Amato, A.; Caldara, G.; Nuzzo, D.; Baldassano, S.; Picone, P.; Rizzo, M.; Mulè, F.; Di Carlo, M. NAFLD and Atherosclerosis Are Prevented by a Natural Dietary Supplement Containing Curcumin, Silymarin, Guggul, Chlorogenic Acid and Inulin in Mice Fed a High-Fat Diet. Nutrients 2017, 9, 492. [Google Scholar] [CrossRef]
- Baldassano, S.; Rappa, F.; Amato, A.; Cappello, F.; Mulè, F. GLP-2 as Beneficial Factor in the Glucose Homeostasis in Mice Fed a High Fat Diet. J. Cell. Physiol. 2015, 230, 3029–3036. [Google Scholar] [CrossRef]
- Glass, C.; Saijo, K.; Winner, B.; Marchetto, M.C.; Gage, F.H. Mechanisms underlying inflammation in neurodegeneration. Cell 2010, 140, 918–934. [Google Scholar] [CrossRef]
- Petrov, D.; Pedrós, I.; Artiach, G.; Sureda, F.X.; Barroso, E.; Pallàs, M.; Casadesús, G.; Beas-Zarate, C.; Carro, E.; Ferrer, I. High-fat diet-induced deregulation of hippocampal insulin signaling and mitochondrial homeostasis deficiences contribute to Alzheimer disease pathology in rodents. Biochim. Biophys. Acta 2015, 1852, 1687–1699. [Google Scholar] [CrossRef]
- Busquets, O.; Ettcheto, M.; Pallàs, M.; Beas-Zarate, C.; Verdaguer, E.; Auladell, C.; Folch, J.; Camins, A. Long-term exposition to a high fat diet favors the appearance of β-amyloid depositions in the brain of C57BL/6J mice. A potential model of sporadic Alzheimer’s disease. Mech. Ageing Dev. 2017, 162, 38–45. [Google Scholar] [CrossRef]
- Ghavami, S.; Shojaei, S.; Yeganeh, B.; Ande, S.R.; Jangamreddy, J.R.; Mehrpour, M.; Christoffersson, J.; Chaabane, W.; Moghadam, A.R.; Kashani, H.H.; et al. Autophagy and apoptosis dysfunction in neurodegenerative disorders. Prog. Neurobiol. 2014, 112, 24–49. [Google Scholar] [CrossRef]
- Planells-Ferrer, L.; Urresti, J.; Coccia, E.; Galenkamp, K.M.; Calleja-Yagüe, I.; López-Soriano, J.; Carriba, P.; Barneda-Zahonero, B.; Segura, M.F.; Comella, J.X. Fas apoptosis inhibitory molecules: More than death-receptor antagonists in the nervous system. J. Neurochem. 2016, 139, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, S.; Sharma, P. Role and regulation of p27 in neuronal apoptosis. J. Neurochem. 2017, 140, 576–588. [Google Scholar] [CrossRef] [PubMed]
- Park, H.R.; Park, M.; Choi, J.; Park, K.Y.; Chung, H.Y.; Lee, J. A high-fat diet impairs neurogenesis: Involvement of lipid peroxidation and brain-derived neurotrophic factor. Neurosci. Lett. 2010, 482, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.; Ying, Z.; Gomez-Pinilla, F. The interplay between oxidative stress and brain-derived neurotrophic factor modulates the outcome of a saturated fat diet on synaptic plasticity and cognition. Eur. J. Neurosci. 2004, 19, 1699–1707. [Google Scholar] [CrossRef] [PubMed]
- Kempuraj, D.; Thangavel, R.; Natteru, P.; Selvakumar, G.P.; Saeed, D.; Zahoor, H.; Zaheer, S.; Iyer, S.S.; Zaheer, A. Neuroinflammation induces neurodegeneration. J. Neurol. Neurosurg. Spine 2016, 1, 1003. [Google Scholar]
- Duffy, C.; Hofmeister, J.; Nixon, J.; Butterick, T. High fat diet increases cognitive decline and neuroinflammation in a model of orexin loss. Neurobiol. Learn. Mem. 2019, 157, 41–47. [Google Scholar] [CrossRef]
- Spencer, S.; D’Angelo, H.; Soch, A.; Watkins, L.R.; Maier, S.F.; Barrientos, R.M. High-fat diet and aging interact to produce neuroinflammation and impair hippocampal and amygdala-dependent memory. Neurobiol. Aging 2017, 58, 88–101. [Google Scholar] [CrossRef]
- D’Alessio, P.; Ostan, R.; Bisson, J.; Schulzke, J.D.; Ursini, M.V.; Béné, M.C. Oral administration of d-limonene controls inflammation in rat colitis and displays anti-inflammatory properties as diet supplementation in humans. Life Sci. 2013, 92, 1151–1156. [Google Scholar] [CrossRef]
- Roberto, D.; Micucci, P.; Sebastian, T.; Graciela, F.; Anesini, C. Antioxidant activity of limonene on normal murine lymphocytes: Relation to H2O2 modulation and cell proliferation. Basic Clin. Pharm. Toxicol. 2010, 106, 38–44. [Google Scholar] [CrossRef]
- Nuzzo, D.; Baldassano, S.; Amato, A.; Picone, P.; Galizzi, G.; Caldara, G.F.; Di Carlo, M.; Mulè, F. Glucagon-like peptide-2 reduces the obesity-associated inflammation in the brain. Neurobiol. Dis. 2019, 121, 296–304. [Google Scholar] [CrossRef]
- Nuzzo, D.; Presti, G.; Picone, P.; Galizzi, G.; Gulotta, E.; Giuliano, S.; Mannino, C.; Gambino, V.; Scoglio, S.; Di Carlo, M.; et al. Effects of the aphanizomenon flos-aquae extract (Klamin®) on a neurodegeneration cellular model. Oxid. Med. Cell Longev. 2018, 17, 9089016. [Google Scholar] [CrossRef]
- Shimazu, R.; Anada, M.; Miyaguchi, A.; Nomi, Y.; Matsumoto, H. Evaluation of Blood-Brain Barrier Permeability of Polyphenols, Anthocyanins, and Their Metabolites. Agric. Food Chem. 2021, 69, 11676–11686. [Google Scholar] [CrossRef]
- Khan, H.; Ullah, H.; Aschner, M.; Cheang, W.; Akkol, E. Neuroprotective Effects of Quercetin in Alzheimer’s Disease. Biomolecules 2019, 10, 59. [Google Scholar] [CrossRef]
- Siddique, Y.R. Neurodegenerative Diseases and Flavonoids: Special Reference to Kaempferol. CNS Neurol. Disord. Drug Targets 2021, 20, 327–342. [Google Scholar] [CrossRef]
- Beg, T.; Jyoti, S.; Naz, F.; Rahul Ali, F.; Ali, S.K.; Reyad, A.M.; Siddique, Y.H. Protective Effect of Kaempferol on the Transgenic Drosophila Model of Alzheimer’s Disease. CNS Neurol. Disord. Drug Targets 2018, 17, 421–429. [Google Scholar] [CrossRef]
- Vinklarova, L.; Schmidt, M.; Benek, O.; Kuca, K.; Gunn-Moore, F.; Musilek, K. Friend or enemy? Review of 17β-HSD10 and its role in human health or disease. J. Neurochem. 2020, 155, 231–249. [Google Scholar] [CrossRef]
- Nikolac Perkovic, M.; Pivac, N. Genetic Markers of Alzheimer’s Disease. Adv. Exp. Med. Biol. 2019, 1192, 27–52. [Google Scholar] [CrossRef]
- Serrano-Pozo, A.; Das, S.; Hyman, B.T. APOE and Alzheimer’s disease: Advances in genetics, pathophysiology, and therapeutic approaches. Lancet Neurol. 2021, 20, 68–80. [Google Scholar] [CrossRef]
- Liu, S.L.; Wang, C.; Jiang, T.; Tan, L.; Xing, A.; Yu, J.T. The Role of Cdk5 in Alzheimer’s Disease. Mol. Neurobiol. 2016, 53, 4328–4342. [Google Scholar] [CrossRef]
- Uddin, M.S.; Kabir, M.T.; Begum, M.M.; Islam, M.S.; Behl, T.; Ashraf, G.M. Exploring the Role of CLU in the Pathogenesis of Alzheimer’s Disease. Neurotox. Res. 2021, 39, 2108–2119. [Google Scholar] [CrossRef]
- Nasuti, C.; Gabbianelli, R.; Falcioni, G.; Cantalamessa, F. Antioxidative and gastroprotective activities of anti-inflammatory formulations derived from chestnut honey in rats. Nutr. Res. 2006, 26, 130–137. [Google Scholar] [CrossRef]
- Terzo, S.; Attanzio, A.; Calvi, P.; Mulè, F.; Tesoriere, L.; Allegra, M.; Amato, A. Indicaxanthin from Opuntia ficus-indica Fruit Ameliorates Glucose Dysmetabolism and Counteracts Insulin Resistance in High-Fat, Diet-Fed Mice. Antioxidants 2021, 11, 80. [Google Scholar] [CrossRef]

| Gene Name | Protein | HFD/Lean | HFD-H/HFD | HFD-L/HFD | HFD-H + L/HFD |
|---|---|---|---|---|---|
| Ache | Acetylcholinesterase | 2.10 | −30.84 | −12.01 | −16.31 |
| Apba3 | Amyloid beta (A4) precursor protein-binding, family A, member 3 | 44.94 | −59.17 | −30.02 | −29.88 |
| Apbb2 | Amyloid beta (A4) precursor protein-binding, family B, member 2 | 2.60 | −18.46 | −10.71 | −17.39 |
| Aplp1 | Amyloid beta (A4) precursor-like protein 1 | 5.17 | −8.79 | −8.53 | −5.00 |
| Aplp2 | Amyloid beta (A4) precursor-like protein 2 | 15.97 | −45.55 | −15.49 | −17.72 |
| Apoe | Apolipoprotein E | 5.16 | −26.20 | −9.36 | −9.77 |
| App | Amyloid beta (A4) precursor protein | 6.05 | −15.85 | −9.94 | −10.12 |
| Bdnf | Brain derived neurotrophic factor | −17.99 | −5.49 | −3.44 | −2.67 |
| Cdk5 | Cyclin-dependent kinase 5 | 2.92 | −36.93 | −17.68 | −13.99 |
| Clu | Clusterin | 26.50 | −4.04 | −3.76 | −2.19 |
| Ctsl | Cathepsin L | 5.08 | −13.34 | −4.65 | −4.90 |
| Gsk3a | Glycogen synthase kinase 3 α | 7.20 | −22.22 | −14.11 | −15.35 |
| Hsd17b10 | Hydroxy steroid deydrogenase10 | 5.87 | −15.93 | −9.38 | −11.82 |
| Il1a | Interleukin 1 α | 4.31 | −90.55 | −49.60 | −98.59 |
| Mapt | Microtubule-associated protein tau | 44.58 | −23.78 | −9.87 | −8.28 |
| Mpo | Myeloperoxidase | 14.87 | −33.10 | −14.25 | −28.87 |
| Prkca | Protein kinase C, alpha | 6.17 | −16.16 | −15.98 | −10.33 |
| Prkce | Protein kinase C, epsilon | 5.89 | −16.58 | −12.56 | −16.61 |
| Psen1 | Presenilin 1 | 4.68 | −225.85 | −152.13 | −193.57 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Terzo, S.; Calvi, P.; Nuzzo, D.; Picone, P.; Allegra, M.; Mulè, F.; Amato, A. Long-Term Ingestion of Sicilian Black Bee Chestnut Honey and/or D-Limonene Counteracts Brain Damage Induced by High Fat-Diet in Obese Mice. Int. J. Mol. Sci. 2023, 24, 3467. https://doi.org/10.3390/ijms24043467
Terzo S, Calvi P, Nuzzo D, Picone P, Allegra M, Mulè F, Amato A. Long-Term Ingestion of Sicilian Black Bee Chestnut Honey and/or D-Limonene Counteracts Brain Damage Induced by High Fat-Diet in Obese Mice. International Journal of Molecular Sciences. 2023; 24(4):3467. https://doi.org/10.3390/ijms24043467
Chicago/Turabian StyleTerzo, Simona, Pasquale Calvi, Domenico Nuzzo, Pasquale Picone, Mario Allegra, Flavia Mulè, and Antonella Amato. 2023. "Long-Term Ingestion of Sicilian Black Bee Chestnut Honey and/or D-Limonene Counteracts Brain Damage Induced by High Fat-Diet in Obese Mice" International Journal of Molecular Sciences 24, no. 4: 3467. https://doi.org/10.3390/ijms24043467
APA StyleTerzo, S., Calvi, P., Nuzzo, D., Picone, P., Allegra, M., Mulè, F., & Amato, A. (2023). Long-Term Ingestion of Sicilian Black Bee Chestnut Honey and/or D-Limonene Counteracts Brain Damage Induced by High Fat-Diet in Obese Mice. International Journal of Molecular Sciences, 24(4), 3467. https://doi.org/10.3390/ijms24043467











