Odorant-Binding and Chemosensory Proteins in Anthonomus eugenii (Coleoptera: Curculionidae) and Their Tissue Expression
Abstract
1. Introduction
2. Results
2.1. Library Assembly
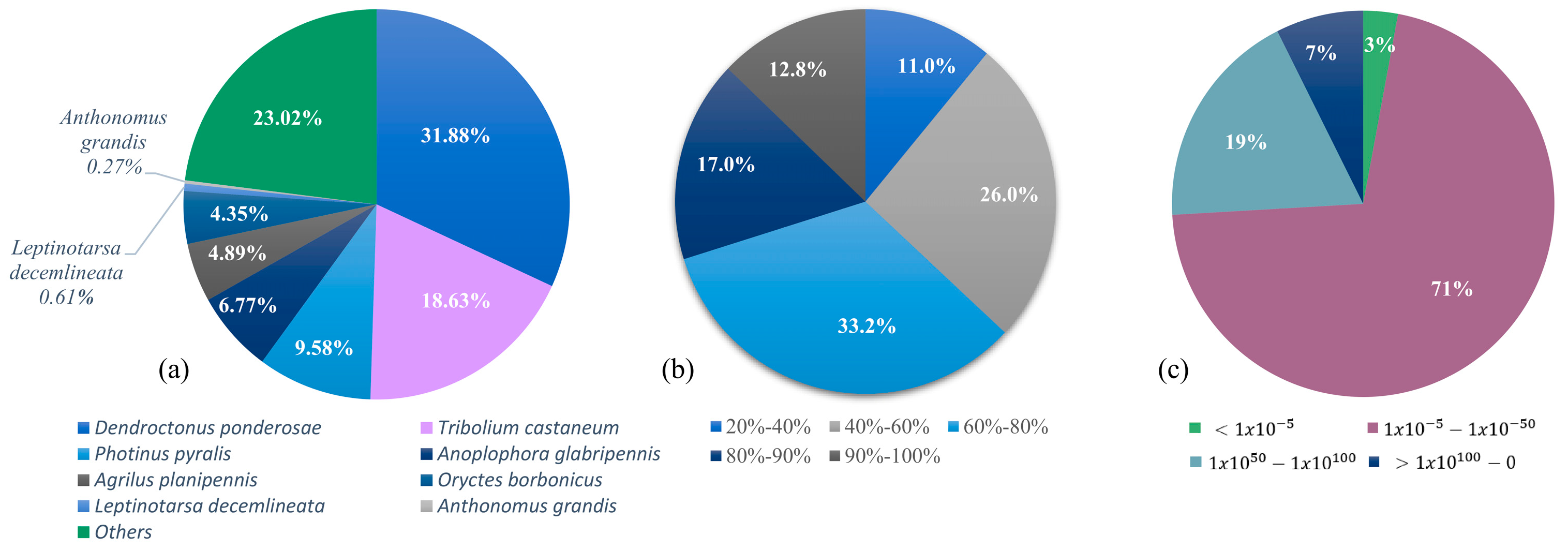
2.2. GO Terms, Homology Annotation and Chemosensory Sequences Extraction
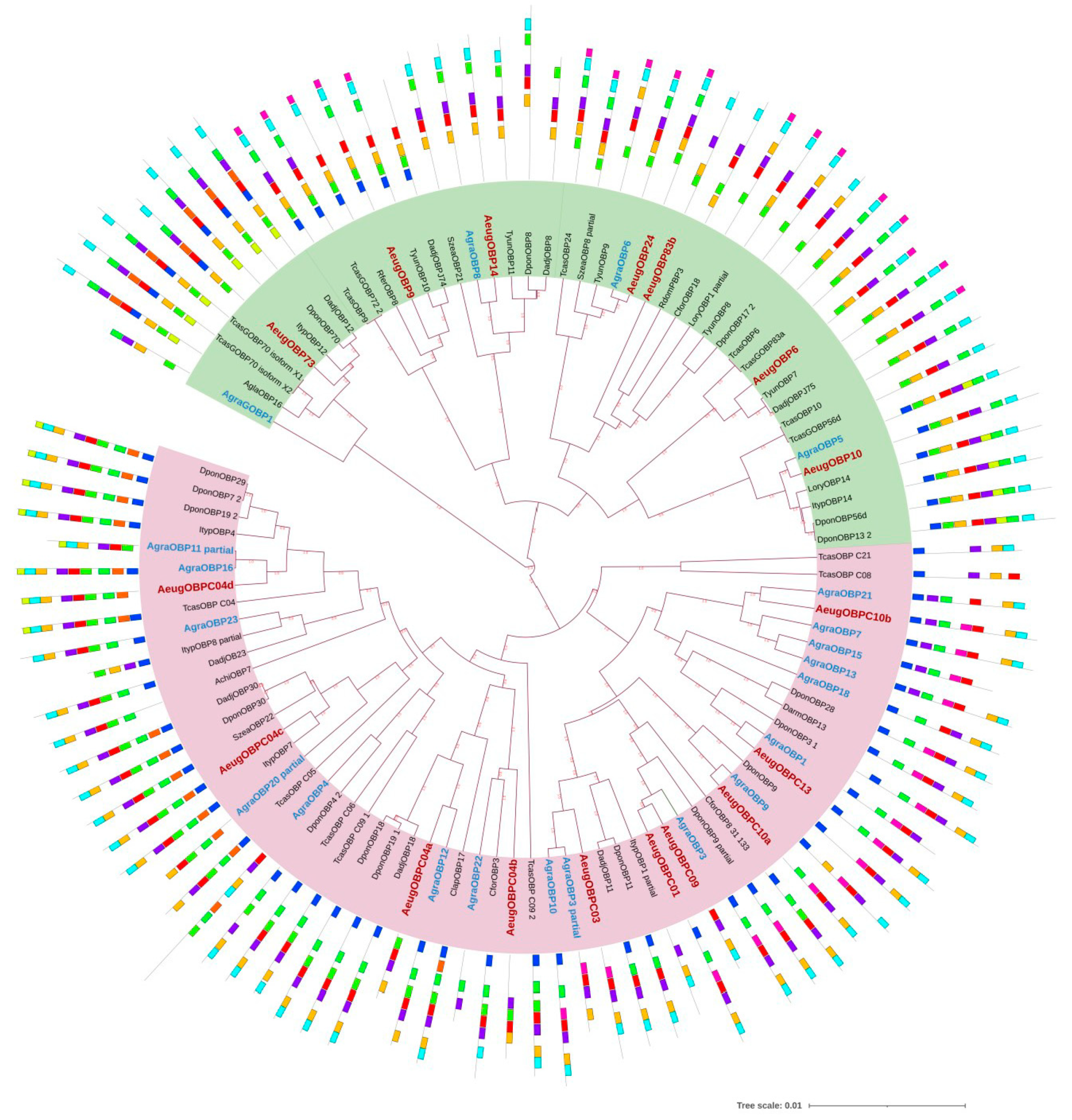
2.3. Odorant-Binding Proteins
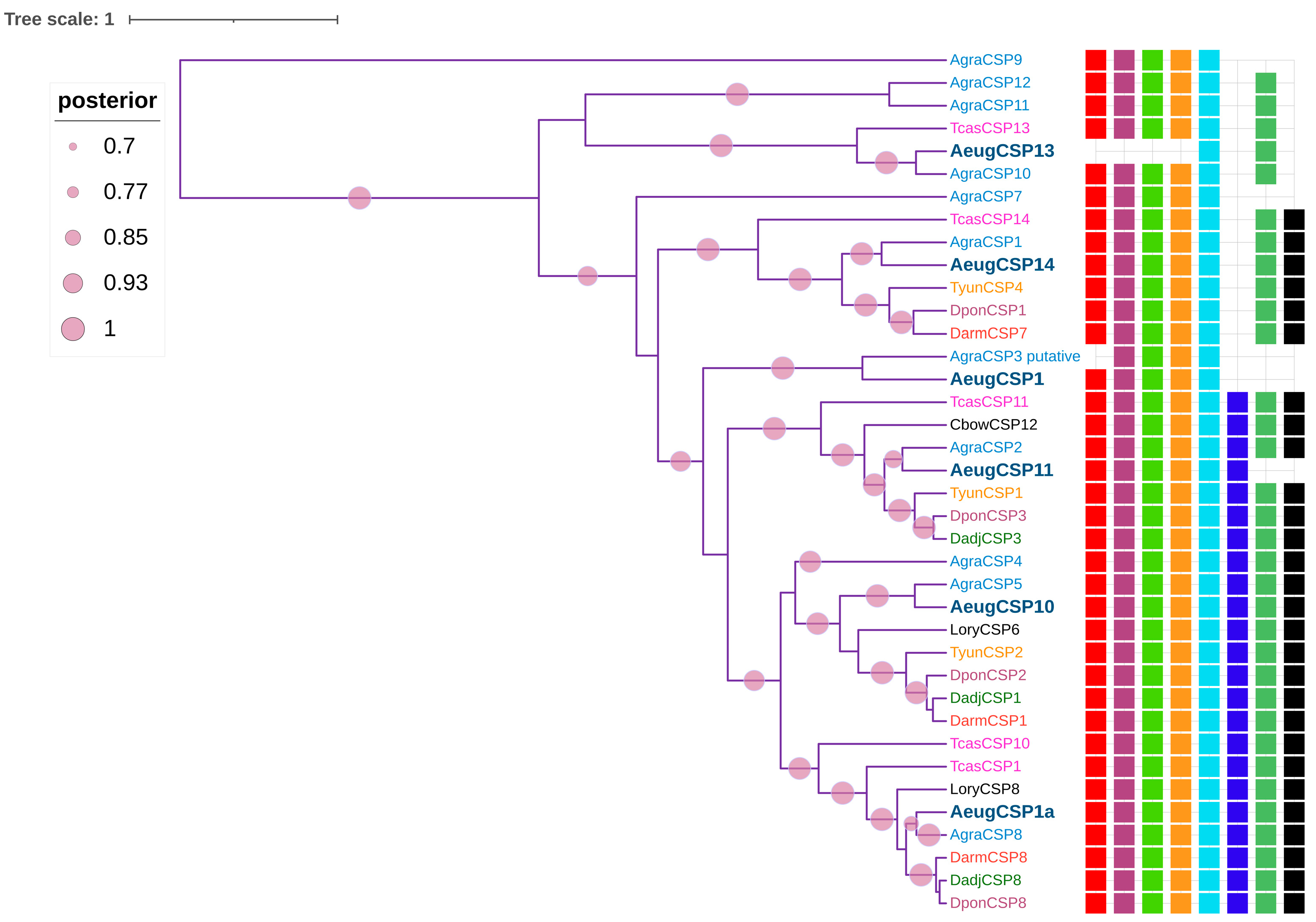
2.4. Chemosensory Proteins
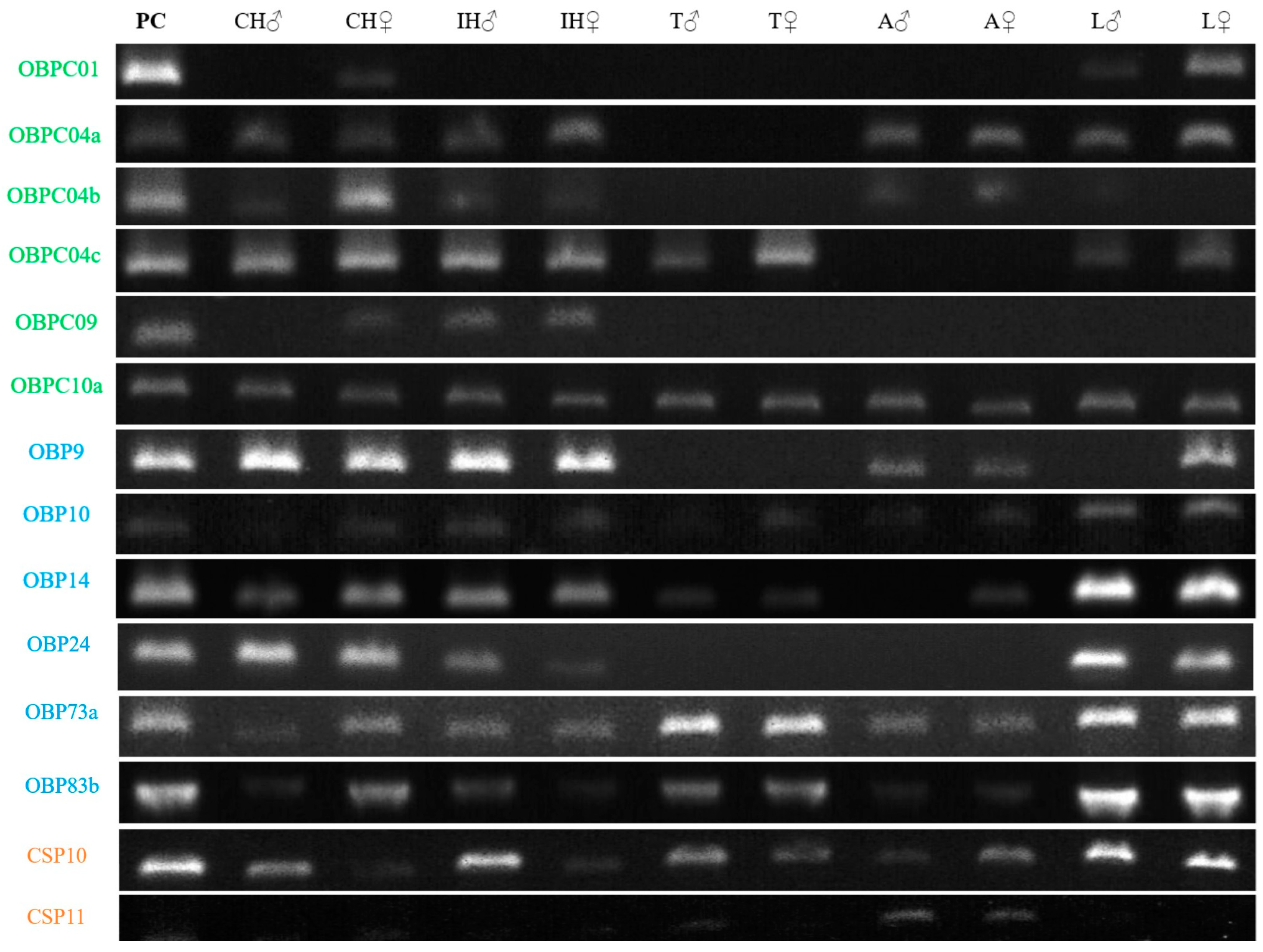
2.5. OBP and CSP Expression Profile
3. Discussion
4. Materials and Methods
4.1. Insect Collection, cDNA Library Construction, Sequencing, and De Novo Assembly
4.2. Gene Ontology (GO Terms) Annotation
4.3. Classification of OBPs, CSPs, and Functional Annotation
4.4. Motif Discovery and Phylogenetic Analysis
4.5. Gene Amplification of OBP, CSP, and Expression Pattern per Tissue and Sex
4.6. Sequence Information
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goff, C.; Wilson, W. The pepper weevil. Fla. Agric. Exp. Stn. Bull. 1937, 3–11. Available online: https://edis.ifas.ufl.edu/publication/IN555 (accessed on 1 March 2020).
- Torres-Ruíz, A.; Rodríguez-Leyva, E. Guía Para el Manejo Integrado de Plagas del Pimiento Bajo Invernadero, con Énfasis en el Picudo del Chile 2012. Available online: https://www.researchgate.net/publication/277004440_Guia_para_el_manejo_integrado_de_plagas_del_pimiento_bajo_invernadero_con_enfasis_en_el_picudo_del_chile (accessed on 1 March 2020).
- Labbé, R.M.; Gagnier, D.; Rizzato, R.; Tracey, A.; McCreary, C. Assessing New Tools for Management of the Pepper Weevil (Coleoptera: Curculionidae) in Greenhouse and Field Pepper Crops. J. Econ. Entomol. 2020, 113, 1903–1912. [Google Scholar] [CrossRef] [PubMed]
- Capinera, J.L. Pepper Weevil, Anthonomus eugenii Cano (Insecta: Coleoptera: Curculionidae). Univ. Fla. Georg. A Smathers Libr. Edis 1969, 2004, 1–6. [Google Scholar] [CrossRef]
- Fernández, D.C.; VanLaerhoven, S.L.; Labbé, R. Host utilization by the pepper weevil (Anthonomus eugenii): Suitability, preference, and offspring performance. Pest Manag. Sci. 2021, 77, 4719–4729. [Google Scholar] [CrossRef]
- Labbé, R.M.; Hilker, R.; Gagnier, D.; McCreary, C.; Gibson, G.A.P.; Fernández-Triana, J.; Mason, P.G.; Gariepy, T.D. Natural enemies of Anthonomus eugenii (Coleoptera: Curculionidae) in Canada. Can. Entomol. 2018, 150, 404–411. [Google Scholar] [CrossRef]
- Eller, F.; Palmquist, D. Factors Affecting Pheromone Production by the Pepper Weevil, Anthonomus Eugenii Cano Collect. Efficiency. Insects 2014, 5, 909–920. [Google Scholar] [CrossRef]
- Velázquez-González, J.C. Compuestos Volátiles que Median la Interacción Entre Anthonomus Eugenii Cano y Capsicum Annuum L. Ph.D. Thesis, Phytopatology-Entomology and Acaroloy Colegio de Postgraduados, Montecillo Texcoco, México, 2012. [Google Scholar]
- Muñiz-Merino, M.; Cibrián-Tovar, J.; Hidalgo-Moreno, C.; Bautista-Martínez, N.; Vaquera-Huerta, H.; Aldama-Aguilera, C. Compuestos volátiles atraen al picudo (Anthonomus eugenii Cano) del chile (Capsicum spp.) y presentan sinergia con su feromona de agregación. Agrociencia 2014, 8, 819–832. [Google Scholar]
- Bautista-San Juan, A.; Cibrián-Tovar, J.; López-Romero, R.M.; Bautista-Martínez, N.; Gómez-Domínguez, N.S. Adult Attraction of Anthonomus eugenii (Cano) Coleoptera: Curculionidae to Volatile Synthetic Compound Mixtures. Southwest. Entomol. 2019, 44, 743–754. [Google Scholar] [CrossRef]
- Bautista-Hernández, C.F.; Cibrián-Tovar, J.; Velázquez-González, J.C.; Rodríguez-Guzmán, M. del P. Evaluación en campo de atrayentes para la captura de Anthonomus eugenii Cano (Coleoptera: Curculionidae). Rev. Chil. Entomol. 2020, 46, 211–219. [Google Scholar] [CrossRef]
- Zeng, B.; Ye, Y.; Ma, J.; Song, J. Juvenile hormone upregulates sugar baby for vitellogenesis and egg development in the migratory locust Locusta migratoria. Arch. Insect Biochem. Physiol. 2021, 106, 1–12. [Google Scholar] [CrossRef]
- Venthur, H.; Zhou, J.J. Odorant receptors and odorant-binding proteins as insect pest control targets: A comparative analysis. Front. Physiol. 2018, 9, 1163. [Google Scholar] [CrossRef]
- Pelosi, P.; Iovinella, I.; Zhu, J.; Wang, G.; Dani, F.R. Beyond chemoreception: Diverse tasks of soluble olfactory proteins in insects. Biol. Rev. 2018, 93, 184–200. [Google Scholar] [CrossRef] [PubMed]
- Leal, W.S. Odorant reception in insects: Roles of receptors, binding proteins, and degrading enzymes. Annu. Rev. Entomol. 2013, 58, 373–391. [Google Scholar] [CrossRef] [PubMed]
- Tegoni, M.; Campanacci, V.; Cambillau, C. Structural aspects of sexual attraction and chemical communication in insects. Trends Biochem. Sci. 2004, 29, 257–264. [Google Scholar] [CrossRef]
- Ozaki, M. Odorant-Binding Proteins in Taste System: Putative Roles in Taste Sensation and Behavior. In Olfactory Concepts of Insect Control-Alternative to insecticides; Springer: Cham, Switzerland, 2019; pp. 187–204. [Google Scholar] [CrossRef]
- Fan, J.; Francis, F.; Liu, Y.; Chen, J.L.; Cheng, D.F. An overview of odorant-binding protein functions in insect peripheral olfactory reception. Genet. Mol. Res. 2011, 10, 3056–3069. [Google Scholar] [CrossRef]
- Wei, H.S.; Duan, H.X.; Li, K.; Zhang, S.; Wei, Z.J.; Yin, J. The mechanism underlying OBP heterodimer formation and the recognition of odors in Holotrichia oblita Faldermann. Int. J. Biol. Macromol. 2020, 152, 957–968. [Google Scholar] [CrossRef]
- Pelosi, P.; Zhou, J.J.; Ban, L.P.; Calvello, M. Soluble proteins in insect chemical communication. Cell. Mol. Life Sci. 2006, 63, 1658–1676. [Google Scholar] [CrossRef]
- Lartigue, A.; Campanacci, V.; Roussel, A.; Larsson, A.M.; Jones, T.A.; Tegoni, M.; Cambillau, C. X-ray Structure and Ligand Binding Study of a Moth Chemosensory Protein. J. Biol. Chem. 2002, 277, 32094–32098. [Google Scholar] [CrossRef]
- Oppenheim, S.J.; Baker, R.H.; Simon, S.; Desalle, R. We can’t all be supermodels: The value of comparative transcriptomics to the study of non-model insects. Insect Mol. Biol. 2015, 24, 139–154. [Google Scholar] [CrossRef]
- Blum, M.; Chang, H.-Y.; Chuguransky, S.; Grego, T.; Kandasaamy, S.; Mitchell, A.; Nuka, G.; Paysan-Lafosse, T.; Qureshi, M.; Raj, S.; et al. The InterPro protein families and domains database: 20 years on. Nucleic Acids Res. 2021, 49, D344–D354. [Google Scholar] [CrossRef]
- Keeling, C.I.; Yuen, M.M.; Liao, N.Y.; Docking, T.R.; Chan, S.K.; Taylor, G.A.; Palmquist, D.L.; Jackman, S.D.; Nguyen, A.; Li, M.; et al. Draft genome of the mountain pine beetle, Dendroctonus ponderosae Hopkins, a major forest pest. Genome Biol. 2013, 14, R27. [Google Scholar] [CrossRef]
- Hu, P.; Wang, J.; Cui, M.; Tao, J.; Luo, Y. Antennal transcriptome analysis of the Asian longhorned beetle Anoplophora Glabripennis. Sci. Rep. 2016, 6, 26652. [Google Scholar] [CrossRef] [PubMed]
- Torres-Huerta, B.; Segura-León, O.L.; Aragón-Magadan, M.A.; González-Hernández, H. Identification and motif analyses of candidate nonreceptor olfactory genes of Dendroctonus adjunctus Blandford (Coleoptera: Curculionidae) from the head transcriptome. Sci. Rep. 2020, 10, 20695. [Google Scholar] [CrossRef] [PubMed]
- Venthur, H.; Mutis, A.; Zhou, J.J.; Quiroz, A. Ligand binding and homology modelling of insect odorant-binding proteins. Physiol. Entomol. 2014, 39, 183–198. [Google Scholar] [CrossRef]
- El-Gebali, S.; Mistry, J.; Bateman, A.; Eddy, S.R.; Luciani, A.; Potter, S.C.; Qureshi, M.; Richardson, L.J.; Salazar, G.A.; Smart, A.; et al. The Pfam protein families database in 2019. Nucleic Acids Res. 2019, 47, D427–D432. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.L.; He, P.; Zhang, L.; Fang, S.Q.; Dong, S.L.; Zhang, Y.J.; Li, F. Large-scale identification of odorant-binding proteins and chemosensory proteins from expressed sequence tags in insects. BMC Genom. 2009, 10, 632. [Google Scholar] [CrossRef]
- Dippel, S.; Oberhofer, G.; Kahnt, J.; Gerischer, L.; Opitz, L.; Schachtner, J.; Stanke, M.; Schütz, S.; Wimmer, E.A.; Angeli, S. Tissue-specific transcriptomics, chromosomal localization, and phylogeny of chemosensory and odorant binding proteins from the red flour beetle Tribolium castaneum reveal subgroup specificities for olfaction or more general functions. BMC Genom. 2014, 15, 1141. [Google Scholar] [CrossRef]
- Paula, D.P.; Togawa, R.C.; Costa, M.M.d.C.; Grynberg, P.; Martins, N.F.; Andow, D.A. Systemic and sex-biased regulation of OBP expression under semiochemical stimuli. Sci. Rep. 2018, 8, 6035. [Google Scholar] [CrossRef]
- Heringa, J. Protein domains. In Encyclopedia of Genetics, Genomics, Proteomics, and Bioinformatics; John Wiley & Sons: Hoboken, NJ, USA, 2005. [Google Scholar] [CrossRef]
- Zhou, J.J. Odorant-binding proteins in insects. Vitam. Horm. 2010, 83, 241–272. [Google Scholar] [CrossRef]
- Khan, H. De Novo Annotation of Non-Model Organisms Using Whole-Genome and Transcriptome Shotgun Sequencing. Master’s Thesis, The University of British Columbia, Vancouver, BC, Canada, 2016. [Google Scholar] [CrossRef]
- Vieira, F.; Rozas, J. Comparative Genomics of the Odorant-Binding and Chemosensory Protein Gene Families across the Arthropoda: Origin and Evolutionary History of the Chemosensory System. Genome Biol. Evol. 2011, 3, 476–490. [Google Scholar] [CrossRef]
- Rihani, K.; Ferveur, J.F.; Briand, L. The 40-Year Mystery of Insect Odorant-Binding Proteins. Biomolecules 2021, 4, 509. [Google Scholar] [CrossRef] [PubMed]
- Hong, B.; Chang, Q.; Zhai, Y.; Ren, B.; Zhang, F. Functional Characterization of Odorant Binding Protein PyasOBP2 From the Jujube Bud Weevil, Pachyrhinus yasumatsui (Coleoptera: Curculionidae). Front Physiol. 2022, 13, 900752. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.Q.; Zhang, S.; Luo, J.Y.; Wang, S.B.; Wang, C.Y.; Lv, L.M.; Dong, S.L.; Cui, J.J. Identification and expression pattern of candidate olfactory genes in Chrysoperla sinica by antennal transcriptome analysis. Comp. Biochem. Physiol. Part D Genom. Proteom. 2015, 15, 28–38. [Google Scholar] [CrossRef]
- Hekmat-Scafe, D.S.; Scafe, C.R.; McKinney, A.J.; Tanouye, M.A. Genome-wide analysis of the odorant-binding protein gene family in Drosophila melanogaster. Genome Res. 2002, 12, 1357–1369. [Google Scholar] [CrossRef]
- Zhou, J.J.; Vieira, F.G.; He, X.L.; Smadja, C.; Liu, R.; Rozas, J.; Field, L.M. Genome annotation and comparative analyses of the odorant-binding proteins and chemosensory proteins in the pea aphid Acyrthosiphon pisum. Insect Mol. Biol. 2010, 19, 113–122. [Google Scholar] [CrossRef]
- Behrens, S.; Peuß, R.; Milutinović, B.; Eggert, H.; Esser, D.; Rosenstiel, P.; Schulenburg, H.; Bornberg-Bauer, E.; Kurtz, J. Infection routes matter in population-specific responses of the red flour beetle to the entomopathogen Bacillus thuringiensis. BMC Genom. 2014, 15, 445. [Google Scholar] [CrossRef]
- Andersson, M.N.; Grosse-Wilde, E.; Keeling, C.I.; Bengtsson, J.M.; Yuen, M.M.S.; Li, M.; Hillbur, Y.; Bohlmann, J.; Hansson, B.S.; Schlytetr, F.; et al. Antennal transcriptome analysis of the chemosensory gene families in the tree killing bark beetles, Ips typographus and Dendroctonus ponderosae(Coleoptera: Curculionidae: Scolytinae). BMC Genom. 2013, 14, 198. [Google Scholar] [CrossRef]
- Zhang, F.; Merchant, A.; Zhao, Z.; Zhang, Y.; Zhang, J.; Zhang, Q.; Wang, Q.; Zhou, X.; Li, X. Characterization of MaltOBP1, a Minus-C Odorant-Binding Protein, From the Japanese Pine Sawyer Beetle, Monochamus alternatus Hope (Coleoptera: Cerambycidae). Front Physiol. 2020, 11, 212. [Google Scholar] [CrossRef] [PubMed]
- Dickens, J.C.; Prestwich, G.D.; Sun, W.C. Behavioral and neurosensory responses of the boll weevil Anthonomus grandis Boh. (Coleoptera: Curculionidae), to fluorinated analogs of aldehyde components of its pheromone. J. Chem. Ecol. 1991, 17, 1007–1020. [Google Scholar] [CrossRef]
- Li, F.; Dewer, Y.; Li, D.; Qu, C.; Luo, C. Functional and evolutionary characterization of chemosensory protein CSP2 in the whitefly, Bemisia Tabaci. Pest Manag. Sci. 2021, 77, 378–388. [Google Scholar] [CrossRef]
- Scheuermann, E.A.; Smith, D.P. Odor-Specific Deactivation Defects in a Drosophila Odorant-Binding Protein Mutant. Genetics 2019, 213, 897–909. [Google Scholar] [CrossRef] [PubMed]
- Picimbon, J.F.; Dietrich, K.; Angeli, S.; Scaloni, A.; Krieger, J.; Breer, H.; Pelosi, P. Purification and molecular cloning of chemosensory proteins fromBombyx mori. Arch. Insect Biochem. Physiol. 2000, 44, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Dani, F.R.; Michelucci, E.; Francese, S.; Mastrobuoni, G.; Cappellozza, S.; La Marca, G.; Niccolini, A.; Felicioli, A.; Moneti, G.; Pelosi, P. Odorant-binding proteins and chemosensory proteins in pheromone detection and release in the silkmoth Bombyx mori. Chem Senses. 2011, 36, 335–344. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Li, Z.Q.; Zhang, Y.F.; Chen, L.; Wang, J.; Xu, L.; Zhang, Y.N.; He, M. Identification of odorant-binding and chemosensory protein genes and the ligand affinity of two of the encoded proteins suggest a complex olfactory perception system in Periplaneta americana. Insect Mol. Biol. 2017, 6, 687–701. [Google Scholar] [CrossRef]
- Khuhro, S.A.; Yan, Q.; Liao, H.; Zhu, G.H.; Sun, J.; Dong, S.L. Expression Profile and Functional Characterization Suggesting the Involvement of Three Chemosensory Proteins in Perception of Host Plant Volatiles in Chilo suppressalis (Lepidoptera: Pyralidae). J. Insect Sci. 2018, 18, 5. [Google Scholar] [CrossRef]
- Li, Z.; Dai, L.; Chu, H.; Fu, D.; Sun, Y.; Chen, H. Identification, Expression Patterns, and Functional Characterization of Chemosensory Proteins in Dendroctonus armandi (Coleoptera: Curculionidae: Scolytinae). Front. Physiol. 2018, 9, 291. [Google Scholar] [CrossRef]
- Qiao, H.L.; Deng, P.Y.; Li, D.D.; Chen, M.; Jiao, Z.J.; Liu, Z.C.; Zhang, Y.Z.; Kan, Y.C. Expression analysis and binding experiments of chemosensory proteins indicate multiple roles in Bombyx mori. J. Insect Physiol. 2013, 59, 667–675. [Google Scholar] [CrossRef]
- Liu, G.; Ma, H.; Xie, H.; Xuan, N.; Guo, X.; Fan, Z.; Rajashekar, B.; Arnaud, P.; Offmann, B.; Picimbon, J.F. Biotype Characterization, Developmental Profiling, Insecticide Response and Binding Property of Bemisia tabaci Chemosensory Proteins: Role of CSP in Insect Defense. PLoS ONE 2016, 11, e0154706. [Google Scholar] [CrossRef]
- Xiong, W.; Gao, S.; Lu, Y.; Wei, L.; Mao, J.; Xie, J.; Cao, Q.; Liu, J.; Bi, J.; Song, X.; et al. Latrophilin participates in insecticide susceptibility through positively regulating CSP10 and partially compensated by OBPC01 in Tribolium castaneum. Pestic. Biochem. Physiol. 2019, 159, 107–117. [Google Scholar] [CrossRef]
- Ingham, V.A.; Anthousi, A.; Douris, V.; Harding, N.J.; Lycett, G.; Morris, M.; Vontas, J.; Ranson, H. A sensory appendage protein protects malaria vectors from pyrethroids. Nature 2019, 577, 376–380. [Google Scholar] [CrossRef]
- Chen, X.; Lei, Y.; Li, H.; Xu, L.; Yang, H.; Wang, J.; Jiang, H. CRISPR/Cas9 mutagenesis abolishes odorant-binding protein BdorOBP56f-2 and impairs the perception of methyl eugenol in Bactrocera dorsalis (Hendel). Insect Biochem. Mol. Biol. 2021, 139, 103656. [Google Scholar] [CrossRef]
- Martinazzo, J.; Ballen, C.S.; Steffens, J.; Steffens, C. Sensing of pheromones from Euschistus heros (F.) stink bugs by nanosensors. Sens. Actuators Rep. 2022, 4, 100071. [Google Scholar] [CrossRef]
- Liu, G.; Xuan, N.; Rajashekar, B.; Arnaud, P.; Offmann, B.; Picimbon, J.F. Comprehensive History of CSP Genes: Evolution, Phylogenetic Distribution and Functions. Genes 2020, 11, 413. [Google Scholar] [CrossRef] [PubMed]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Haas, B.J.; Papanicolaou, A.; Yassour, M.; Grabherr, M.; Blood, P.D.; Bowden, J.; Couger, M.B.; Eccles, D.; Li, B.; Lieber, M.; et al. De novo transcript sequence reconstruction from RNA-seq using the Trinity platform for reference generation and analysis. Nat. Protoc. 2013, 8, 1494–1512. [Google Scholar] [CrossRef]
- Mikheenko, A.; Prjibelski, A.; Saveliev, V.; Antipov, D.; Gurevich, A. Versatile genome assembly evaluation with QUAST-LG. Bioinformatics 2018, 34, i142–i150. [Google Scholar] [CrossRef]
- Staton, E. Sestaton/Hmmer2Go: Hmmer2Go; Version 0.17.8; Recovered in May 18th 2020; Zenodo: Genève, Switzerland, 2018. [Google Scholar] [CrossRef]
- Ye, J.; Zhang, Y.; Cui, H.; Liu, J.; Wu, Y.; Cheng, Y.; Xu, H.; Huang, X.; Li, S.; Zhou, A.; et al. WEGO 2.0: A web tool for analyzing and plotting GO annotations, 2018 update. Nucleic Acids Res. 2018, 46, W71–W75. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, D.L.; Barrett, T.; Benson, D.A.; Bryant, S.H.; Canese, K.; Chetvernin, V.; Church, D.M.; DiCuccio, M.; Edgar, R.; Federhen, S.; et al. Database resources of the National Center for Biotechnology Information. Nucleic Acids Res. 2007, 35, D5–D12. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef]
- Zhao, B.; Katuwawala, A.; Oldfield, C.J.; Dunker, A.K.; Faraggi, E.; Gsponer, J.; Kloczkowski, A.; Malhis, N.; Mirdita, M.; Obradovic, Z.; et al. DescribePROT: Database of amino acid-level protein structure and function predictions. Nucleic Acids Res. 2021, 49, D298–D308. [Google Scholar] [CrossRef]
- Mitchell, A.; Chang, H.Y.; Daugherty, L.; Fraser, M.; Hunter, S.; López, R.; McAnulla, C.; McMenamin, C.; Nuka, G.; Pesseat, S.; et al. The InterPro protein families’ database: The classification resource after 15 years. Nucleic Acids Res. 2015, 43, D213–D221. [Google Scholar] [CrossRef] [PubMed]
- Bailey, T.L.; Johnson, J.; Grant, C.E.; Noble, W.S. The MEME Suite. Nucleic Acids Res. 2015, 43, W39–W49. [Google Scholar] [CrossRef]
- Darriba, D.; Posada, D.; Kozlov, A.M.; Stamatakis, A.; Morel, B.; Flouri, T. ModelTest-NG: A New and Scalable Tool for the Selection of DNA and Protein Evolutionary Models. Mol. Biol. Evol. 2020, 37, 291–294. [Google Scholar] [CrossRef] [PubMed]
- Bouckaert, R.; Heled, J.; Kühnert, D.; Vaughan, T.; Wu, C.H.; Xie, D.; Suchard, M.A.; Rambaut, A.; Drummond, A.J. BEAST 2: A Software Platform for Bayesian Evolutionary Analysis. PLOS Comput. Biol. 2014, 10, e1003537. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive Tree Of Life (iTOL) v5: An online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021, 49, W293–W296. [Google Scholar] [CrossRef] [PubMed]
- Untergasser, A.; Cutcutache, I.; Koressaar, T.; Ye, J.; Faircloth, B.C.; Remm, M.; Rozen, S.G. Primer3—New capabilities and interfaces. Nucleic Acids Res. 2012, 40, e115. [Google Scholar] [CrossRef]

| OBP | PBP-GOBP Superfamily | PhBP Family | ||
|---|---|---|---|---|
| CDD (cl11600)/PF01395 | Smart (smart00708) | |||
| Range | E-Value | Range | E-Value | |
| AeugOBPC01 | 21–128 | 2.84 × 10−9 | 37–129 | 1.28 × 10−5 |
| AeugOBPC03 | 34–98 | 5.24 × 10−4 | 30–84 | 7.50 × 10−3 |
| AeugOBPC04a | 1–59 | 1.65 × 10−5 | − | − |
| AeugOBPC04b | 20–72 | 4.38 × 10−4 | − | − |
| AeugOBPC04c | 18–127 | 1.61 × 10−14 | 32–127 | 2.64 × 10−8 |
| AeugOBPC04d | 15–122 | 1.70 × 10−22 | 29–122 | 7.99 × 10−12 |
| AeugOBP6 | 1–108 | 3.18 × 10−15 | 17–108 | 1.98 × 10−10 |
| AeugOBP9 | 25–137 | 2.12 × 10−12 | 40–137 | 8.01 × 10−7 |
| AeugOBPC09 | 1–63 | 2.49 × 10−6 | 1–63 | 1.51 × 10−5 |
| AeugOBPC10a | 10–98 | 5.11 × 10−5 | − | − |
| AeugOBPC10b | 79–173 | 1.19 × 10−10 | 79–173 | 8 × 10−10 |
| AeugOBP10 | 21–128 | 5.05 × 10−24 | 34–127 | 4.62 × 10−14 |
| AeugOBP13 | 25–134 | 1.84 × 10−8 | 70–133 | 1.90 × 10−3 |
| AeugOBP14 | 20–134 | 1.91 × 10−14 | 35–135 | 8.27 × 10−7 |
| AeugOBP24 | 23–127 | 1.71 × 10−7 | 59–130 | 1.52 × 10−8 |
| AeugOBP73a | 35–144 | 2.77 × 10−5 | 30–145 | 5.08 × 10−3 |
| AeugOBP83b | 16–125 | 7.50 × 10−11 | 31–125 | 2.92 × 10−6 |
| CSP | Insect Pheromone-Binding Family, A10/OS-D | |||
|---|---|---|---|---|
| CDD (cl04042) | Pfam (PF03392) | |||
| Range | E-Value | Range | E-Value | |
| AeugCSP1 | 34–89 | 1.32 × 10−32 | 34–89 | 1.32 × 10−32 |
| AeugCSP1a | - | - | 34–127 | 1.08 × 10−47 |
| AeugCSP10 | 20–110 | 2.73 × 10−52 | 20–110 | 2.73 × 10−52 |
| AeugCSP11 | 30–107 | 5.18 × 10−44 | 30–107 | 5.18 × 10−44 |
| AeugCSP13 | 10–61 | 3.19 × 10−18 | - | - |
| AeugCSP14 | 39–130 | 1.44 × 10−48 | 39–130 | 1.44 × 10−48 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lechuga-Paredes, P.; Segura-León, O.L.; Cibrián-Tovar, J.; Torres-Huerta, B.; Velázquez-González, J.C.; Cruz-Jaramillo, J.L. Odorant-Binding and Chemosensory Proteins in Anthonomus eugenii (Coleoptera: Curculionidae) and Their Tissue Expression. Int. J. Mol. Sci. 2023, 24, 3406. https://doi.org/10.3390/ijms24043406
Lechuga-Paredes P, Segura-León OL, Cibrián-Tovar J, Torres-Huerta B, Velázquez-González JC, Cruz-Jaramillo JL. Odorant-Binding and Chemosensory Proteins in Anthonomus eugenii (Coleoptera: Curculionidae) and Their Tissue Expression. International Journal of Molecular Sciences. 2023; 24(4):3406. https://doi.org/10.3390/ijms24043406
Chicago/Turabian StyleLechuga-Paredes, Pablo, Obdulia Lourdes Segura-León, Juan Cibrián-Tovar, Brenda Torres-Huerta, Julio César Velázquez-González, and José Luis Cruz-Jaramillo. 2023. "Odorant-Binding and Chemosensory Proteins in Anthonomus eugenii (Coleoptera: Curculionidae) and Their Tissue Expression" International Journal of Molecular Sciences 24, no. 4: 3406. https://doi.org/10.3390/ijms24043406
APA StyleLechuga-Paredes, P., Segura-León, O. L., Cibrián-Tovar, J., Torres-Huerta, B., Velázquez-González, J. C., & Cruz-Jaramillo, J. L. (2023). Odorant-Binding and Chemosensory Proteins in Anthonomus eugenii (Coleoptera: Curculionidae) and Their Tissue Expression. International Journal of Molecular Sciences, 24(4), 3406. https://doi.org/10.3390/ijms24043406





