Enhanced UV-B Radiation Induced the Proanthocyanidins Accumulation in Red Rice Grain of Traditional Rice Cultivars and Increased Antioxidant Capacity in Aging Mice
Abstract
1. Introduction
2. Results
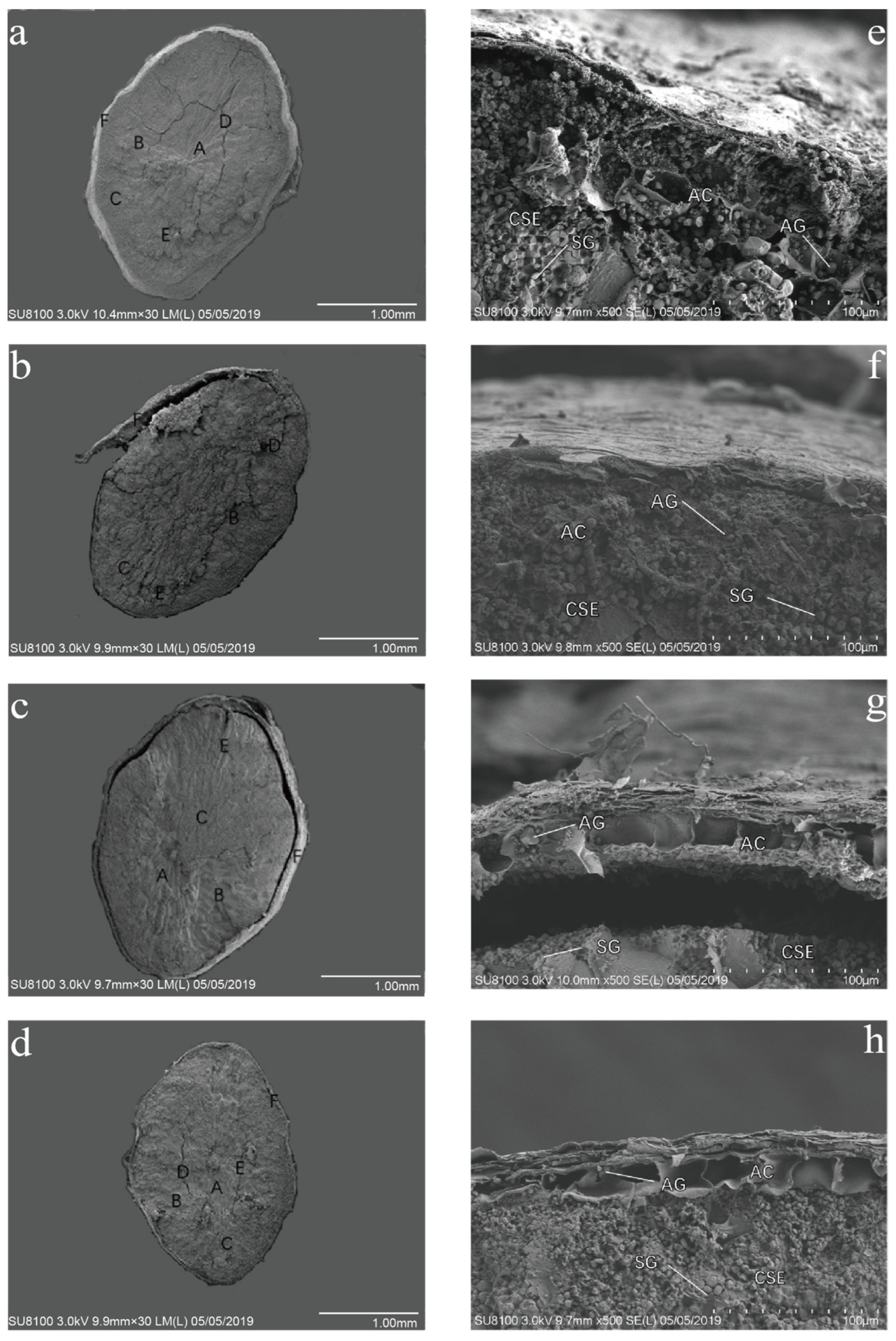
2.1. Effect of Enhanced UV-B Radiation on Grain Morphology of Red Rice
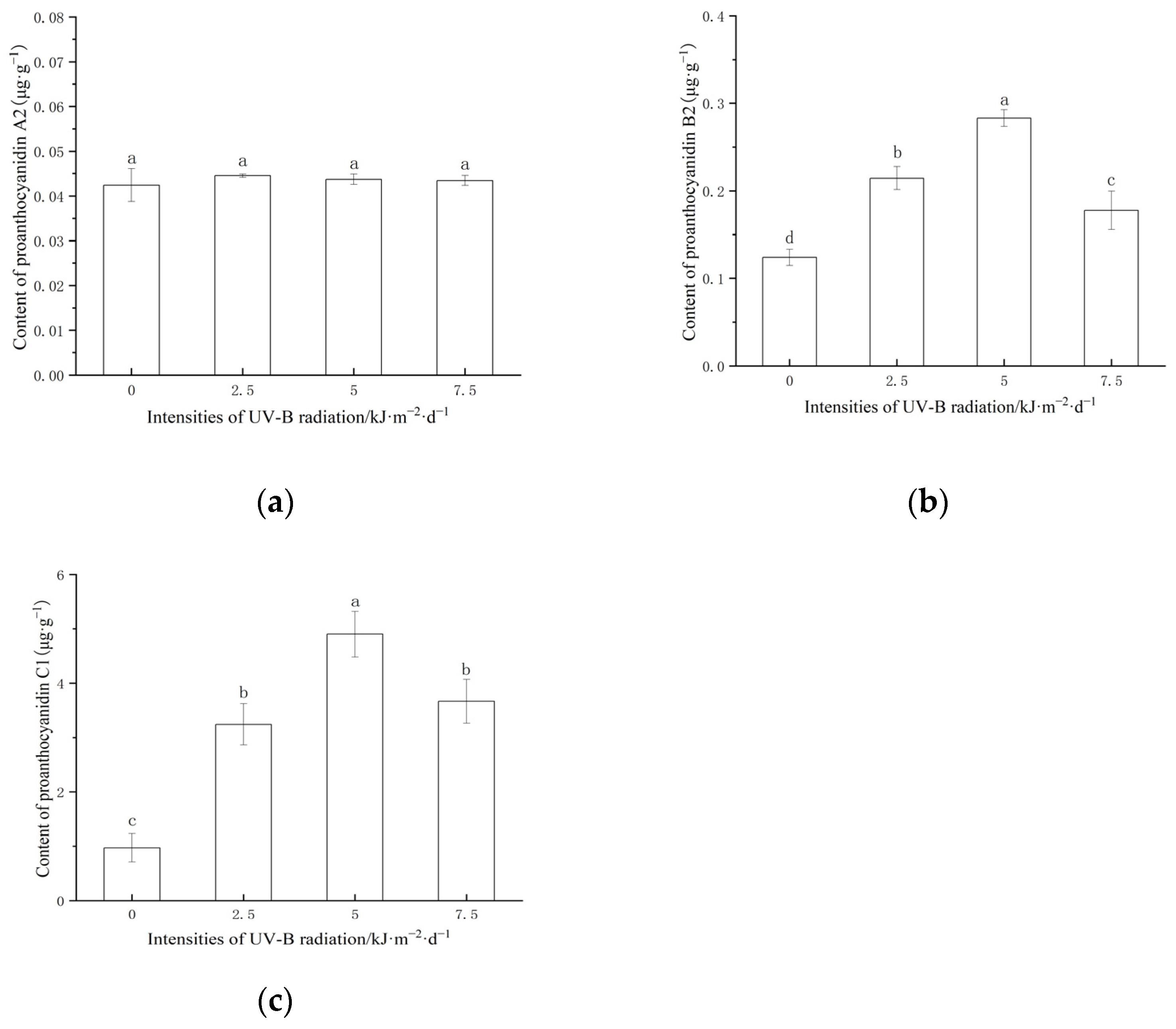
2.2. Effect of Enhanced UV-B Radiation on Proanthocyanidin with Different Polymerization Degrees Content in Red Rice
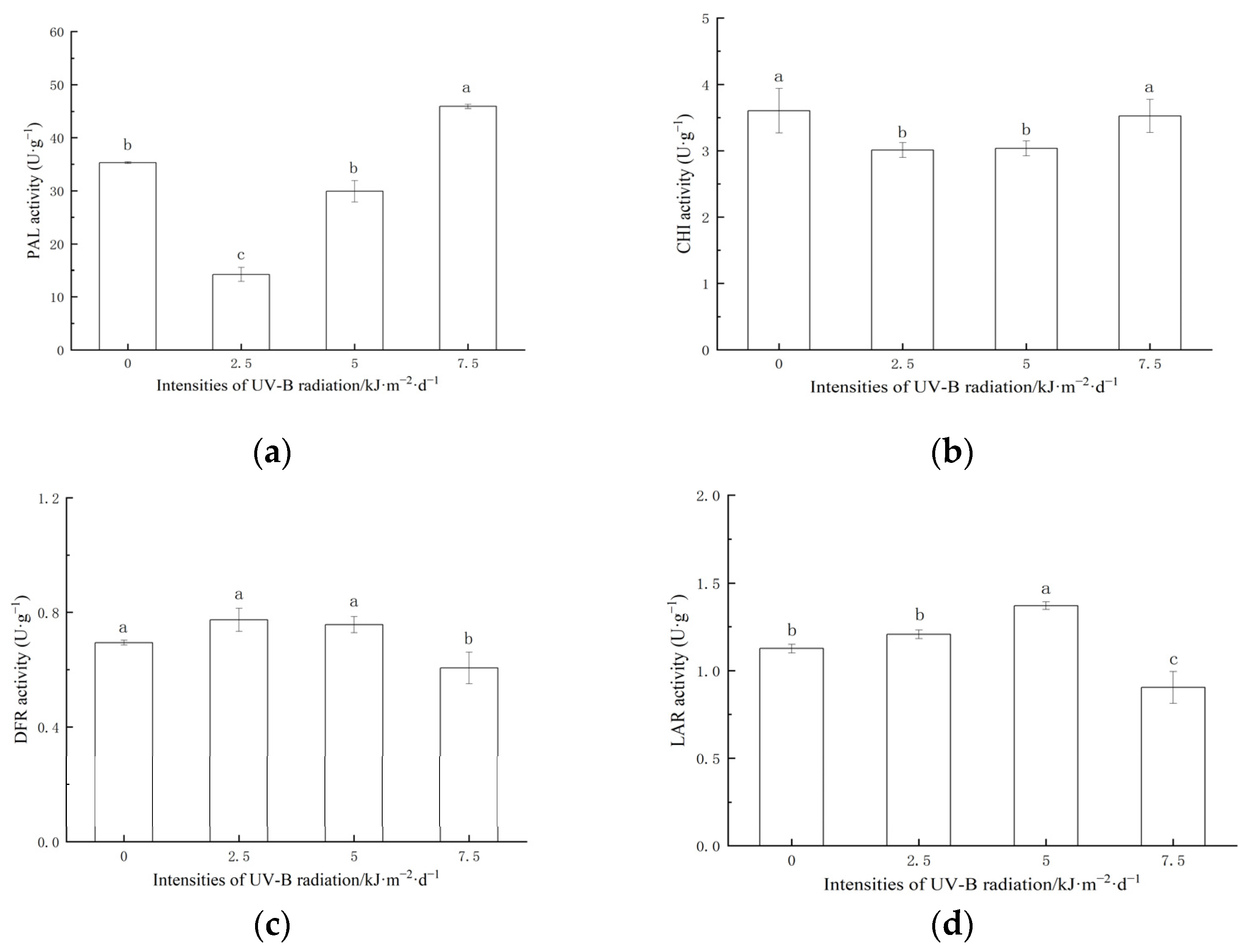
2.3. Effect of Enhanced UV-B Radiation on the Activities of Key Enzymes for Proanthocyanidins Synthesis in Red Rice
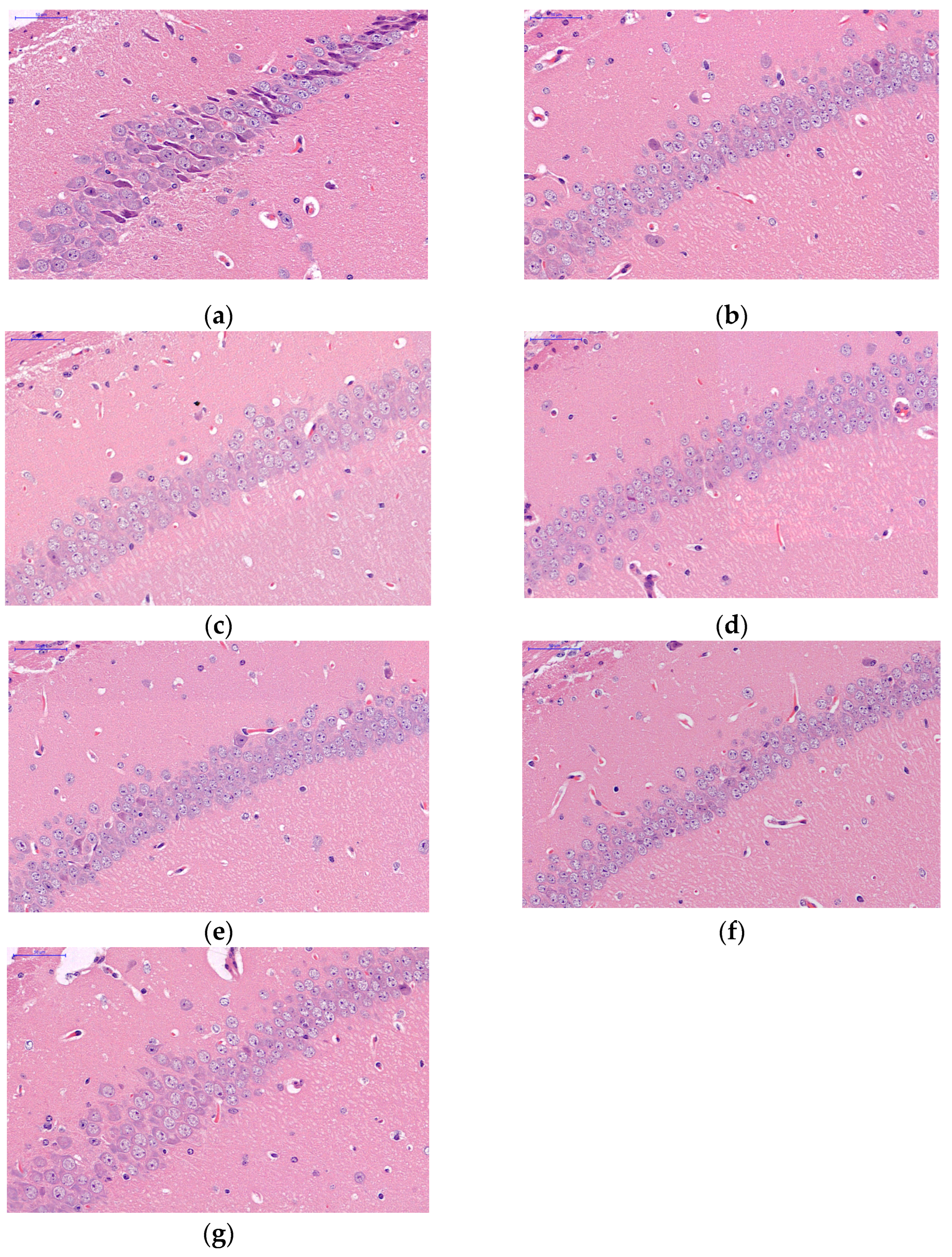
2.4. Morphological Changes in Hippocampus in Mice Brain
2.5. Effect of UV-B Radiation on Antioxidant Capacity of Red Rice
2.6. Comprehensive Quantitative Evaluation of Red Rice Antioxidant Capacity
3. Discussion
3.1. UV-B Radiation and Red Rice Structure
3.2. UV-B Radiation and Proanthocyanidin Synthesis in Red Rice
3.3. UV-B Radiation and Antioxidant Capacity of Red Rice
4. Materials and Methods
4.1. Summary of Test Site
4.2. Field Test Design
4.3. Observation on Morphological Structure of Rice Grain
4.4. Determination of Proanthocyanidin with Different Polymerization Degrees Content
4.5. Determination of Proanthocyanidins Synthase Activity
4.6. Animals Experimental Protocol
4.7. Brain Tissue Section
4.8. Determination of Antioxidant Index
4.9. Comprehensive Quantitative Evaluation of Antioxidant Capacity
4.10. Data Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bais, A.F.; Bernhard, G.; McKenzie, R.L.; Aucamp, P.J.; Young, P.J.; Ilyas, M.; Jockel, P.; Deushi, M. Ozone-climate interactions and effects on solar ultraviolet radiation. Photochem. Photobiol. Sci. 2019, 18, 602–640. [Google Scholar] [CrossRef] [PubMed]
- Bernhard, G.H.; Neale, R.E.; Barnes, P.W.; Neale, P.J.; Zepp, R.G.; Wilson, S.R.; Andrady, A.L.; Bais, A.F.; McKenzie, R.L.; Aucamp, P.J.; et al. Environmental effects of stratospheric ozone depletion, UV radiation and interactions with climate change: UNEP Environmental Effects Assessment Panel, update 2019. Photochem. Photobiol. Sci. 2020, 19, 542–584. [Google Scholar] [CrossRef] [PubMed]
- Parihar, P.; Singh, S.; Singh, R.; Singh, V.P.; Prasad, S.M. Changing scenario in plant UV-B research:UV-B from a generic stressor to a specific regulator. J. Photochem. Photobiol. B-Biol. 2015, 153, 334–343. [Google Scholar] [CrossRef]
- Julkunen-Tiitto, R.; Nenadis, N.; Neugart, S.; Robson, M.; Agati, G.; Vepsalainen, J.; Zipoli, G.; Nybakken, L.; Winkler, B.; Jansen, M.A.K. Assessing the response of plant flavonoids to UV radiation: An overview of appropriate techniques. Phytochem. Rev. 2015, 14, 273–297. [Google Scholar] [CrossRef]
- Valenta, K.; Dimac-Stohl, K.; Baines, F.; Smith, T.; Piotrowski, G.; Hill, N.; Kuppler, J.; Nevo, O. Ultraviolet radiation changes plant color. BMC Plant Biol. 2020, 20, 253. [Google Scholar] [CrossRef] [PubMed]
- Mahmad, N.; Taha, R.M. Effects of pH, UV-B radiation and NaCl on anthocyanin stability from vivid blue petals of Clitoria ternatea L., a potential natural colourant from legume crop. Pigment. Resin Technol. 2018, 47, 507–510. [Google Scholar] [CrossRef]
- Rizi, M.R.; Azizi, A.; Sayyari, M.; Mirzaie-Asl, A.; Conti, L. Increased phenylpropanoids production in UV-B irradiated Salvia verticillata as a consequence of altered genes expression in young leaves. Plant Physiol. Biochem. 2021, 167, 174–184. [Google Scholar] [CrossRef]
- Hong, L.; Qian, Q.; Tang, D.; Wang, K.; Li, M.; Cheng, Z. A mutation in the rice chalcone isomerase gene causes the golden hull and internode 1 phenotype. Planta 2012, 236, 141–151. [Google Scholar] [CrossRef]
- Mouradov, A.; Spangenberg, G. Flavonoids: A metabolic network mediating plants adaptation to their real estate. Front. Plant Sci. 2014, 5, 620. [Google Scholar] [CrossRef]
- Ferrer, J.L.; Austin, M.B.; Stewart, C., Jr.; Noel, J.P. Structure and function of enzymes involved in the biosynthesis of phenylpropanoids. Plant Physiol. Biochem. 2008, 46, 356–370. [Google Scholar] [CrossRef]
- Jaakola, L.; Maatta-Riihinen, K.; Karenlampi, S.; Hohtola, A. Activation of flavonoid biosynthesis by solar radiation in bilberry (Vaccinium myrtillus L) leaves. Planta 2004, 218, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Scattino, C.; Castagna, A.; Neugart, S.; Chan, H.M.; Schreiner, M.; Crisosto, C.H.; Tonutti, P.; Ranieri, A. Post-harvest UV-B irradiation induces changes of phenol contents and corresponding biosynthetic gene expression in peaches and nectarines. Food Chem. 2014, 163, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Cos, P.; Bruyne, T.D.; Hermans, N.; Apers, S.; Berghe, D.V.; Vlietinck, A.J. Proanthocyanidins in health care: Current and new trends. Curr. Med. Chem. 2004, 11, 1345–1359. [Google Scholar] [CrossRef]
- de La Iglesia, R.; Milagro, F.I.; Campión, J.; Boqué, N.; Martínez, J.A. Healthy properties of proanthocyanidins. Biofactors 2010, 36, 159–168. [Google Scholar] [CrossRef]
- Yousuf, B.; Gul, K.; Wani, A.A.; Singh, P. Health Benefits of Anthocyanins and Their Encapsulation for Potential Use in Food Systems: A Review. Crit. Rev. Food Sci. Nutr. 2016, 56, 2223–2230. [Google Scholar] [CrossRef]
- Oki, T.; Masuda, M.; Kobayashi, M.; Nishiba, Y.; Furuta, S.; Suda, I.; Sato, T. Polymeric procyanidins as radical-scavenging components in red-hulled rice. J. Agric. Food Chem. 2002, 50, 7524–7529. [Google Scholar] [CrossRef]
- Quan, G.; Liu, Z. Research status of colored rice. Seed 2017, 36, 51–53. [Google Scholar]
- Li, F.B.; Lu, G.D.; Zhou, X.Y.; Ni, H.X.; Xu, C.C.; Yue, C.; Yang, X.M.; Feng, J.F.; Fang, F.P. Elevation and land use types have significant impacts on spatial variability of soil organic matter content in Hani terraced field of Yuanyang County, China. Rice Sci. 2015, 22, 27–34. [Google Scholar]
- Min, B.; Gu, L.W.; McClung, A.M.; Bergman, C.J.; Chen, M.H. Free and bound total phenolic concentrations, antioxidant capacities, and profiles of proanthocyanidins and anthocyanins in whole grain rice (Oryza sativa L.) of different bran colours. Food Chem. 2012, 133, 715–722. [Google Scholar] [CrossRef]
- Pereira-Caro, G.; Cros, G.; Yokota, T.; Crozier, A. Phytochemical profiles of black, red, brown, and white rice from the Camargue region of France. J. Agric. Food Chem. 2013, 61, 7976–7986. [Google Scholar] [CrossRef]
- Xie, D.-Y.; Dixon, R.A. Proanthocyanidin biosynthesis—Still more questions than answers? Phytochemistry 2005, 66, 2127–2144. [Google Scholar] [CrossRef] [PubMed]
- Nile, S.H.; Park, S.W. Edible berries: Bioactive components and their effect on human health. Nutrition 2014, 30, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Sieniawska, E. Activities of Tannins—From in Vitro Studies to Clinical Trials. Nat. Prod. Commun. 2015, 10, 1934578X1501001. [Google Scholar] [CrossRef]
- Jin, K. Modern Biological Theories of Aging. Aging Dis. 2010, 1, 72–74. [Google Scholar] [PubMed]
- Niu, L.; Han, D. Chemical Analysis of Antioxidant Capacity; De Gruyter: Berlin, Germany, 2020; ISBN 9783110573763. [Google Scholar]
- Alkadhi, K.A. Cellular and Molecular Differences between Area CA1 and the Dentate Gyrus of the Hippocampus. Mol. Neurobiol. 2019, 56, 6566–6580. [Google Scholar] [CrossRef]
- López-González, I.; Tebé Cordomí, C.; Ferrer, I. Regional Gene Expression of Inflammation and Oxidative Stress Responses Does Not Predict Neurodegeneration in Aging. J. Neuropathol. Exp. Neurol. 2017, 76, 135–150. [Google Scholar] [CrossRef]
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 408, 239–247. [Google Scholar] [CrossRef]
- Zhang, Y.; Lin, H.; Liu, C.; Huang, J.; Liu, Z. A review for physiological activities of EGCG and the role in improving fertility in humans/mammals. Biomed. Pharmacother. 2020, 127, 110186. [Google Scholar] [CrossRef]
- Ahangarpour, A.; Najimi, S.A.; Farbood, Y. Effects of Vitex agnus-castus fruit on sex hormones and antioxidant indices in a d-galactose-induced aging female mouse model. J. Chin. Med. Assoc. 2016, 79, 589–596. [Google Scholar] [CrossRef]
- Xia, E.-Q.; Deng, G.-F.; Guo, Y.-J.; Li, H.-B. Biological activities of polyphenols from grapes. Int. J. Mol. Sci. 2010, 11, 622–646. [Google Scholar] [CrossRef]
- Gulcin, İ. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef] [PubMed]
- Gunaratne, A.; Wu, K.; Li, D.Q.; Bentota, A.; Corke, H.; Cai, Y.Z. Antioxidant activity and nutritional quality of traditional red-grained rice varieties containing proanthocyanidins. Food Chem. 2013, 138, 1153–1161. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Zhang, H.Y.; Xu, P.Z.; Li, Y.; Wang, X.D.; Wu, X.J. Genetic analysis and histological study of red seed in rice. Acta Genet. Sin. 2006, 33, 559–564. [Google Scholar] [CrossRef]
- Furukawa, T.; Maekawa, M.; Oki, T.; Suda, I.; Iida, S.; Shimada, H.; Takamure, I.; Kadowaki, K.I. The Rc and Rd genes are involved in proanthocyanidin synthesis in rice pericarp. Plant J. 2007, 49, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Zuo, J.; Li, J. Molecular genetic dissection of quantitative trait loci regulating rice grain size. Annu. Rev. Genet. 2014, 48, 99–118. [Google Scholar] [CrossRef]
- Morazzoni, P.; Vanzani, P.; Santinello, S.; Gucciardi, A.; Zennaro, L.; Miotto, G.; Ursini, F. Grape seeds proanthocyanidins: Advanced technological preparation and analytical characterization. Antioxidants 2021, 10, 418. [Google Scholar] [CrossRef]
- Meyer, P.; van de Poel, B.; De Coninck, B. UV-B light and its application potential to reduce disease and pest incidence in crops. Hortic. Res. 2021, 8, 194. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.K.; Zeng, D.E.; Wei, H.P.; Xu, Y.; GU, Y.J.; Wang, Z. Structure observation of rice endosperm tissues. Chin. J. Rice Sci. 2017, 31, 91–98. [Google Scholar]
- Zheng, Y.; Wang, Z. Differentiation mechanism and function of the cereal aleurone cells and hormone effects on them. Plant Cell Rep. 2014, 33, 1779–1787. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, Z. Protein accumulation in aleurone cells, sub-aleurone cells and the center starch endosperm of cereals. Plant Cell Rep. 2014, 33, 1607–1615. [Google Scholar] [CrossRef]
- Xu, J.J.; Zhang, X.F.; Xue, H.W. Rice aleurone layer specific OsNF-YB1 regulates grain filling and endosperm development by interacting with an ERF transcription factor. J. Exp. Bot. 2016, 67, 6399–6411. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Chen, J.; Chen, S.; Ye, X.; Liu, D. Inhibition effect of three common proanthocyanidins from grape seeds, peanut skins and pine barks on maize starch retrogradation. Carbohydr. Polym. 2021, 252, 117172. [Google Scholar] [CrossRef] [PubMed]
- Feng, N.; She, S.; Hu, H.; Tang, S.; Tan, J.; Wu, Q.; Xiao, J. Effects of Oligomeric Procyanidins From Lotus Seedpod on the Retrogradation Properties of Rice Starch. Front. Nutr. 2021, 8, 751627. [Google Scholar] [CrossRef]
- Ma, M.; Wang, P.; Yang, R.; Gu, Z. Effects of UV-B radiation on the isoflavone accumulation and physiological-biochemical changes of soybean during germination: Physiological-biochemical change of germinated soybean induced by UV-B. Food Chem. 2018, 250, 259–267. [Google Scholar] [CrossRef]
- Jiao, J.; Gai, Q.Y.; Wang, W.; Luo, M.; Gu, C.B.; Fu, Y.J.; Ma, W. Ultraviolet radiation-elicited enhancement of isoflavonoid accumulation, biosynthetic gene expression, and antioxidant activity in astragalus membranaceus hairy root cultures. J. Agric. Food Chem. 2015, 63, 8216–8224. [Google Scholar] [CrossRef] [PubMed]
- Veiga, T.; Solis-Escalante, D.; Romagnoli, G.; ten Pierick, A.; Hanemaaijer, M.; Deshmukh, A.T.; Wahl, A.; Pronk, J.T.; Daran, J.M. Resolving phenylalanine metabolism sheds light on natural synthesis of penicillin G in Penicillium chrysogenum. Eukaryot Cell 2012, 11, 238–249. [Google Scholar] [CrossRef]
- Palacio, L.; Cantero, J.J.; Cusido, R.M.; Goleniowski, M.E. Phenolic compound production in relation to differentiation in cell and tissue cultures of Larrea divaricata (Cav.). Plant Sci. 2012, 193–194, 1–7. [Google Scholar] [CrossRef]
- Lister, C.E.; Lancaster, J.E.; Walker, J.R.L. Developmental changes in enzymes of flavonoid biosynthesis in the skins of red and green apple cultivars. J. Sci. Food Agric. 1996, 71, 313–320. [Google Scholar] [CrossRef]
- Jorgensen, K.; Rasmussen, A.V.; Morant, M.; Nielsen, A.H.; Bjarnholt, N.; Zagrobelny, M.; Bak, S.; Moller, B.L. Metabolon formation and metabolic channeling in the biosynthesis of plant natural products. Curr. Opin. Plant Biol. 2005, 8, 280–291. [Google Scholar] [CrossRef]
- Pang, Y.Z.; Peel, G.J.; Wright, E.; Wang, Z.Y.; Dixon, R.A. Early steps in proanthocyanidin biosynthesis in the model legume Medicago truncatula. Plant Physiol. 2007, 145, 601–615. [Google Scholar] [CrossRef]
- Kitamura, S.; Shikazono, N.; Tanaka, A. TRANSPARENT TESTA 19 is involved in the accumulation of both anthocyanins and proanthocyanidins in Arabidopsis. Plant J. 2004, 37, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Hideg, E.; Jansen, M.A.; Strid, A. UV-B exposure, ROS, and stress: Inseparable companions or loosely linked associates? Trends Plant Sci. 2013, 18, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.-Q.; Yin, G.; Huang, J.-R.; Xi, S.-J.; Qian, F.; Lee, R.-X.; Peng, X.-C.; Tang, F.-R. Ionizing Radiation-Induced Brain Cell Aging and the Potential Underlying Molecular Mechanisms. Cells 2021, 10, 3570. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Zhang, Y.; Zhang, L.; Li, X.; Dong, R.; Song, C.; Cheng, L.; Shi, M.; Zhao, H. Combination of tea polyphenols and proanthocyanidins prevents menopause-related memory decline in rats via increased hippocampal synaptic plasticity by inhibiting p38 MAPK and TNF-α pathway. Nutr. Neurosci. 2022, 25, 1909–1927. [Google Scholar] [CrossRef] [PubMed]
- Banji, D.; Banji, O.J.F.; Dasaroju, S.; Kranthi, K.C.H. Curcumin and piperine abrogate lipid and protein oxidation induced by D-galactose in rat brain. Brain Res. 2013, 1515, 1–11. [Google Scholar] [CrossRef] [PubMed]
- McCleery, J.; Abraham, R.P.; Denton, D.A.; Rutjes, A.W.; Chong, L.-Y.; Al-Assaf, A.S.; Griffith, D.J.; Rafeeq, S.; Yaman, H.; Malik, M.A.; et al. Vitamin and mineral supplementation for preventing dementia or delaying cognitive decline in people with mild cognitive impairment. Cochrane Database Syst. Rev. 2018, 11, CD011905. [Google Scholar] [CrossRef]
- Mukherjee, A.; Haldar, C. Melatonin membrane receptor (MT1R) expression and nitro-oxidative stress in testis of golden hamster, Mesocricetus auratus: An age-dependent study. Exp. Gerontol. 2015, 69, 211–220. [Google Scholar] [CrossRef]
- Peng, C.; Wang, X.; Chen, J.; Jiao, R.; Wang, L.; Li, Y.M.; Zuo, Y.; Liu, Y.; Lei, L.; Ma, K.Y.; et al. Biology of ageing and role of dietary antioxidants. Biomed. Res. Int. 2014, 2014, 831841. [Google Scholar] [CrossRef]
- Jin, S.; Yin, Y. In vivo antioxidant activity of total flavonoids from indocalamus leaves in aging mice caused by D-galactose. Food Chem. Toxicol. 2012, 50, 3814–3818. [Google Scholar] [CrossRef]
- Engelsma, G. On the mechanism of the changes in phenylalanine ammonia-lyase activity induced by ultraviolet and blue light in gherkin hypocotyls. Plant Physiol. 1974, 54, 702–705. [Google Scholar] [CrossRef]
- Rehman, S.U.; Shah, S.A.; Ali, T.; Chung, J.I.; Kim, M.O. Anthocyanins reversed D-galactose-induced oxidative stress and neuroinflammation mediated cognitive impairment in adult rats. Mol. Neurobiol. 2017, 54, 255–271. [Google Scholar] [CrossRef] [PubMed]
| Treatment (kJ·m−2·d−1) | 1000-Seeds Dry Weight (g) | 1000-Seeds Fresh Weight (g) | Moisture Content (%) | Grain Length/mm | Grain Width/mm | Empty Grain Rate (%) |
|---|---|---|---|---|---|---|
| 0 | 22.05 ± 0.51 a | 27.75 ± 1.05 a | 25.85 ± 3.64 ab | 8.05 ± 0.55 a | 3.54 ± 0.35 a | 17.01 ± 2.24 a |
| 2.5 | 21.35 ± 0.56 ab | 28.50 ± 0.30 a | 33.53 ± 2.43 a | 7.21 ± 0.46 b | 3.47 ± 0.30 ab | 10.42 ± 1.43 b |
| 5.0 | 20.81 ± 0.72 b | 27.50 ± 1.00 a | 32.34 ± 7.43 ab | 7.21 ± 0.41 b | 3.32 ± 0.39 ab | 14.53 ± 1.47 ab |
| 7.5 | 20.50 ± 0.36 b | 25.25 ± 0.55 b | 23.22 ± 4.84 b | 7.19 ± 0.68 b | 3.27 ± 2.80 b | 10.69 ± 1.95 b |
| Treatment | Gender | CAT(U·g−1) | SOD(U·g−1) | MDA(μmol·g−1) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serum | Liver | Brain | Heart | Serum | Liver | Brain | Heart | Serum | Liver | Brain | Heart | ||
| CK | Male | 25.65 ± 3.86 ef | 17.05 ± 4.52 cd | 82.38 ± 4.81 abc | 45.73 ± 5.76 cd | 272.77 ± 3.95 b | 217.51 ± 3.39 d | 180.46 ± 6.41 e | 279.93 ± 12.49 de | 5.64 ± 1.31 cde | 12.23 ± 1.55 e | 14.64 ± 0.78 ef | 25.87 ± 1.82 b |
| Female | 28.84 ± 3.86 de | 19.92 ± 1.46 c | 83.01 ± 1.46 abc | 42.86 ± 8.67 cd | 278.76 ± 11.77 b | 227.67 ± 4.80 d | 186.28 ± 5.55 e | 314.56 ± 21.17 c | 7.22 ± 1.57 bc | 14.92 ± 1.23 cde | 12.7 ± 0.80 f | 16.93 ± 3.26 efg | |
| R | Male | 37.44 ± 2.41 bc | 31.71 ± 2.92 a | 90.98 ± 6.78 a | 94.48 ± 7.96 a | 299.78 ± 1.94 a | 259.95 ± 8.30 c | 254.25 ± 11.89 b | 376.55 ± 11.07 b | 4.99 ± 0.12 def | 15.31 ± 3.20 cde | 18.26 ± 1.24 d | 13.63 ± 2.41 g |
| Female | 35.85 ± 2.87 bc | 25.33 ± 0.96 b | 85.56 ± 8.60 ab | 91.30 ± 1.66 a | 316.28 ± 11.65 a | 264.90 ± 7.30 bc | 268.57 ± 2.20 b | 256.47 ± 5.43 ef | 4.82 ± 0.16 defg | 14.61 ± 1.15 cde | 21.57 ± 1.43 c | 17.87 ± 1.56 def | |
| T | Male | 18.97 ± 3.07 g | 12.91 ± 2.53 efg | 65.17 ± 3.36 de | 31.71 ± 5.76 e | 179.81 ± 3.87 g | 124.63 ± 9.59 e | 101.72 ± 4.36 f | 148.53 ± 16.19 h | 13.51 ± 1.11 a | 27.47 ± 9.96 a | 30.53 ± 1.20 a | 28.54 ± 0.95 a |
| Female | 21.83 ± 4.72 fg | 10.04 ± 0.96 gh | 53.06 ± 7.65 e | 19.28 ± 4.91 f | 186.66 ± 5.52 g | 123.98 ± 3.86 e | 101.75 ± 6.99 f | 153.24 ± 9.97 h | 14.53 ± 0.62 a | 23.99 ± 2.50 ab | 31.12 ± 3.09 a | 29.97 ± 1.68 a | |
| TR | Male | 49.87 ± 5.44 a | 14.50 ± 1.46 def | 92.89 ± 8.35 a | 45.09 ± 6.14 cd | 260.81 ± 5.01 bcd | 256.90 ± 9.78 c | 215.14 ± 7.12 cd | 273.49 ± 7.55 de | 7.60 ± 2.14 b | 13.22 ± 1.30 de | 25.68 ± 2.73 b | 24.18 ± 0.86 bc |
| Female | 46.68 ± 2.41 a | 14.18 ± 1.46 def | 90.98 ± 9.67 a | 47.64 ± 2.76 cd | 253.35 ± 7.47 cde | 254.41 ± 4.48 c | 195.11 ± 8.71 de | 272.99 ± 10.78 de | 6.08 ± 1.17 bcd | 13.36 ± 1.36 de | 25.67 ± 1.04 b | 14.92 ± 3.11 gh | |
| TRL | Male | 37.12 ± 1.99 bc | 10.68 ± 0.55 fgh | 54.65 ± 2.41 e | 51.46 ± 6.71 c | 270.90 ± 9.97 bc | 257.41 ± 5.06 c | 180.73 ± 4.90 e | 290.60 ± 10.03 d | 3.00 ± 1.07 g | 20.08 ± 1.39 bc | 21.47 ± 0.95 c | 22.59 ± 1.42 bc |
| Female | 39.67 ± 2.53 b | 13.22 ± 1.46 efg | 55.93 ± 4.17 e | 44.45 ± 4.17 cd | 308.46 ± 2.57 a | 321.61 ± 12.78 a | 188.41 ± 2.24 e | 284.69 ± 12.66 d | 3.95 ± 1.04 efg | 18.14 ± 2.74 bcde | 21.76 ± 0.55 c | 17.46 ± 0.70 efg | |
| TRM | Male | 36.81 ± 2.53 bc | 12.27 ± 3.07 efg | 74.09 ± 8.33 bcd | 79.82 ± 5.74 b | 251.89 ± 8.91 de | 266.37 ± 12.74 bc | 228.24 ± 12.89 c | 372.26 ± 16.75 b | 3.56 ± 0.48 fg | 16.49 ± 1.89 cde | 23.97 ± 0.77 bc | 21.67 ± 2.26 cd |
| Female | 33.30 ± 2.41 cd | 15.14 ± 1.46 de | 82.38 ± 4.91 abc | 76.64 ± 7.73 b | 236.03 ± 6.95 e | 276.78 ± 18.86 b | 331.37 ± 36.62 a | 432.74 ± 25.96 a | 3.88 ± 0.23 efg | 19.61 ± 1.84 bcd | 17.10 ± 1.79 de | 20.68 ± 2.65 cde | |
| TRH | Male | 25.02 ± 4.31 ef | 7.17 ± 0.96 h | 64.21 ± 3.86 de | 38.40 ± 7.67 de | 237.66 ± 2.21 e | 220.67 ± 4.98 d | 183.97 ± 7.50 e | 233.30 ± 14.02 f | 3.01 ± 0.24 g | 23.74 ± 4.31 ab | 14.75 ± 0.19 ef | 22.03 ± 1.89 bc |
| Female | 26.93 ± 1.99 ef | 11.95 ± 0.96 efg | 71.86 ± 10.53 cd | 38.72 ± 2.87 de | 211.01 ± 28.23 f | 233.14 ± 5.00 d | 192.20 ± 4.13 e | 201.86 ± 9.36 g | 3.74 ± 0.66 efg | 17.97 ± 1.79 bcde | 13.51 ± 0.33 f | 13.59 ± 3.85 g | |
| Treatment | CAT | SOD | MDA | CEV | Rank |
|---|---|---|---|---|---|
| CK | 0.45 | 0.52 | 0.77 | 0.58 | 4 |
| R | 0.83 | 0.73 | 0.78 | 0.78 | 1 |
| T | 0.11 | 0.01 | 0.05 | 0.06 | 7 |
| TR | 0.64 | 0.53 | 0.63 | 0.60 | 3 |
| TRL | 0.32 | 0.62 | 0.66 | 0.53 | 5 |
| TRM | 0.55 | 0.72 | 0.67 | 0.65 | 2 |
| TRH | 0.24 | 0.37 | 0.77 | 0.46 | 6 |
| Time (min) | Flow Rate (mL·min−1) | Acetonitrile Solvent Gradient (%) | Curve |
|---|---|---|---|
| Initial | 0.30 | 10 | Initial |
| 2.00 | 0.30 | 10 | 6 |
| 6.00 | 0.30 | 90 | 6 |
| 7.00 | 0.30 | 90 | 6 |
| 7.10 | 0.30 | 10 | 6 |
| 8.00 | 0.30 | 10 | 6 |
| Compounds | Q1 Mass (Da) | Q3 Mass (Da) | Retention Time (min) | Declustering Potential (V) | Collision Energy (V) | Cell Exit Potential (V) |
|---|---|---|---|---|---|---|
| Proanthocyanidin A2 | 575.1 | 285 | 4.03 | −190 | −38 | −9 |
| Proanthocyanidin A2 | 575.1 | 448.9 | 4.03 | −190 | −30 | −14 |
| Proanthocyanidin A2 | 575.1 | 539.1 | 4.03 | −199 | −35 | −11 |
| Proanthocyanidin B2 | 577 | 289 | 3.30 | −171 | −36 | −8 |
| Proanthocyanidin B2 | 577 | 407.1 | 3.30 | −172 | −33 | −8 |
| Proanthocyanidin B2 | 577 | 425.1 | 3.30 | −173 | −20 | −13 |
| Proanthocyanidin C1 | 865.1 | 287 | 3.67 | −48 | −43 | −8 |
| Proanthocyanidin C1 | 865.1 | 425 | 3.67 | −25 | −39 | −13 |
| Proanthocyanidin C1 | 865.1 | 695 | 3.67 | −13 | −35 | −14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Sheng, J.; Li, Z.; He, Y.; Zu, Y.; Li, Y. Enhanced UV-B Radiation Induced the Proanthocyanidins Accumulation in Red Rice Grain of Traditional Rice Cultivars and Increased Antioxidant Capacity in Aging Mice. Int. J. Mol. Sci. 2023, 24, 3397. https://doi.org/10.3390/ijms24043397
Li X, Sheng J, Li Z, He Y, Zu Y, Li Y. Enhanced UV-B Radiation Induced the Proanthocyanidins Accumulation in Red Rice Grain of Traditional Rice Cultivars and Increased Antioxidant Capacity in Aging Mice. International Journal of Molecular Sciences. 2023; 24(4):3397. https://doi.org/10.3390/ijms24043397
Chicago/Turabian StyleLi, Xiang, Jianjun Sheng, Zuran Li, Yongmei He, Yanqun Zu, and Yuan Li. 2023. "Enhanced UV-B Radiation Induced the Proanthocyanidins Accumulation in Red Rice Grain of Traditional Rice Cultivars and Increased Antioxidant Capacity in Aging Mice" International Journal of Molecular Sciences 24, no. 4: 3397. https://doi.org/10.3390/ijms24043397
APA StyleLi, X., Sheng, J., Li, Z., He, Y., Zu, Y., & Li, Y. (2023). Enhanced UV-B Radiation Induced the Proanthocyanidins Accumulation in Red Rice Grain of Traditional Rice Cultivars and Increased Antioxidant Capacity in Aging Mice. International Journal of Molecular Sciences, 24(4), 3397. https://doi.org/10.3390/ijms24043397






