The Role of Reactive Oxygen Species in Plant Response to Radiation
Abstract
1. Introduction
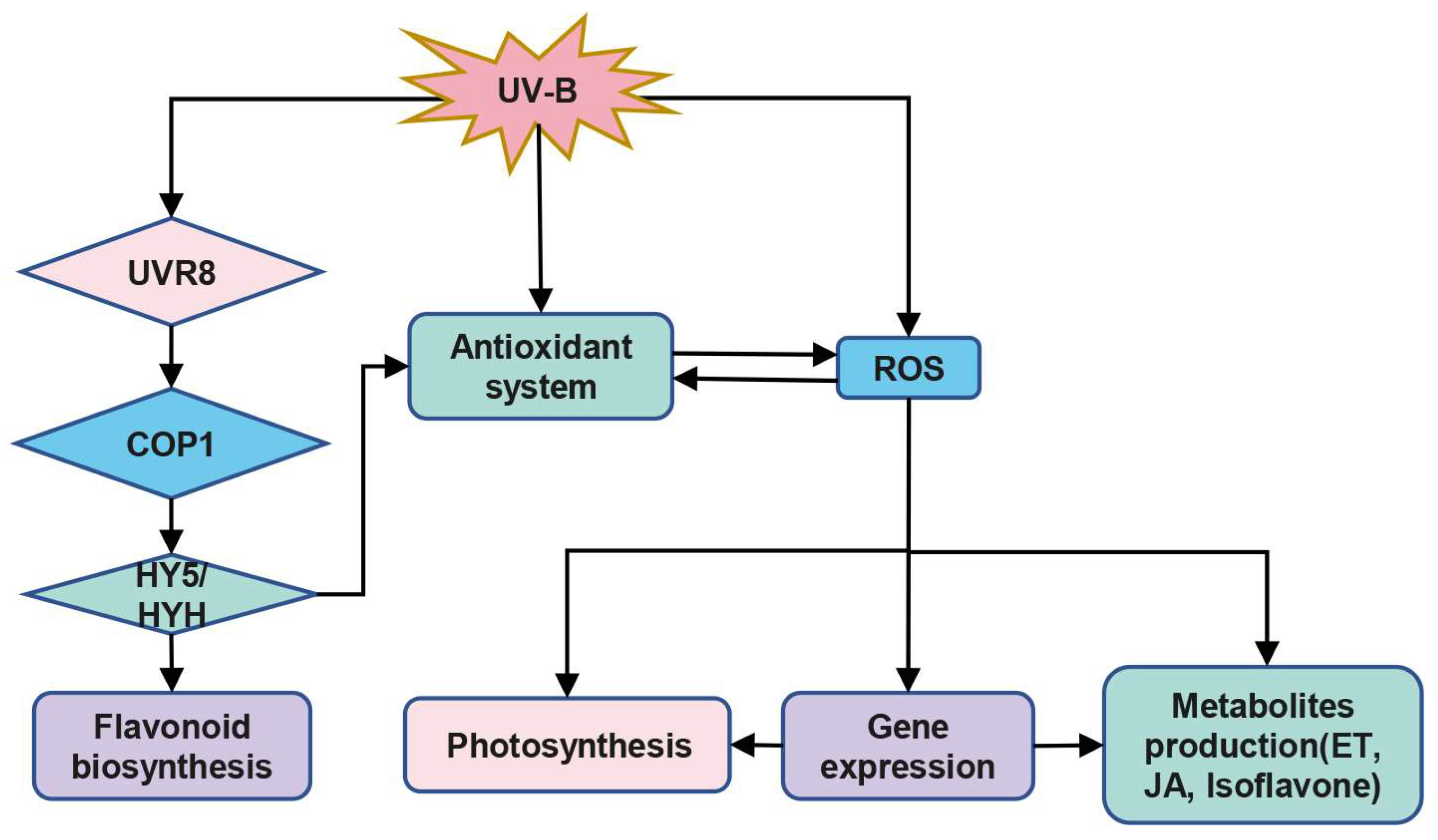
2. The Role of ROS in Plant Responses to UV Radiation
2.1. UV Radiation May Induce ROS Production and Activates Plant Antioxidant Systems
2.2. UV May Affect Metabolites Production via ROS
2.3. UV May Affect Photosynthesis via ROS
2.4. ROS May Be Involved in the Regulation of Gene Expression under UV Radiation
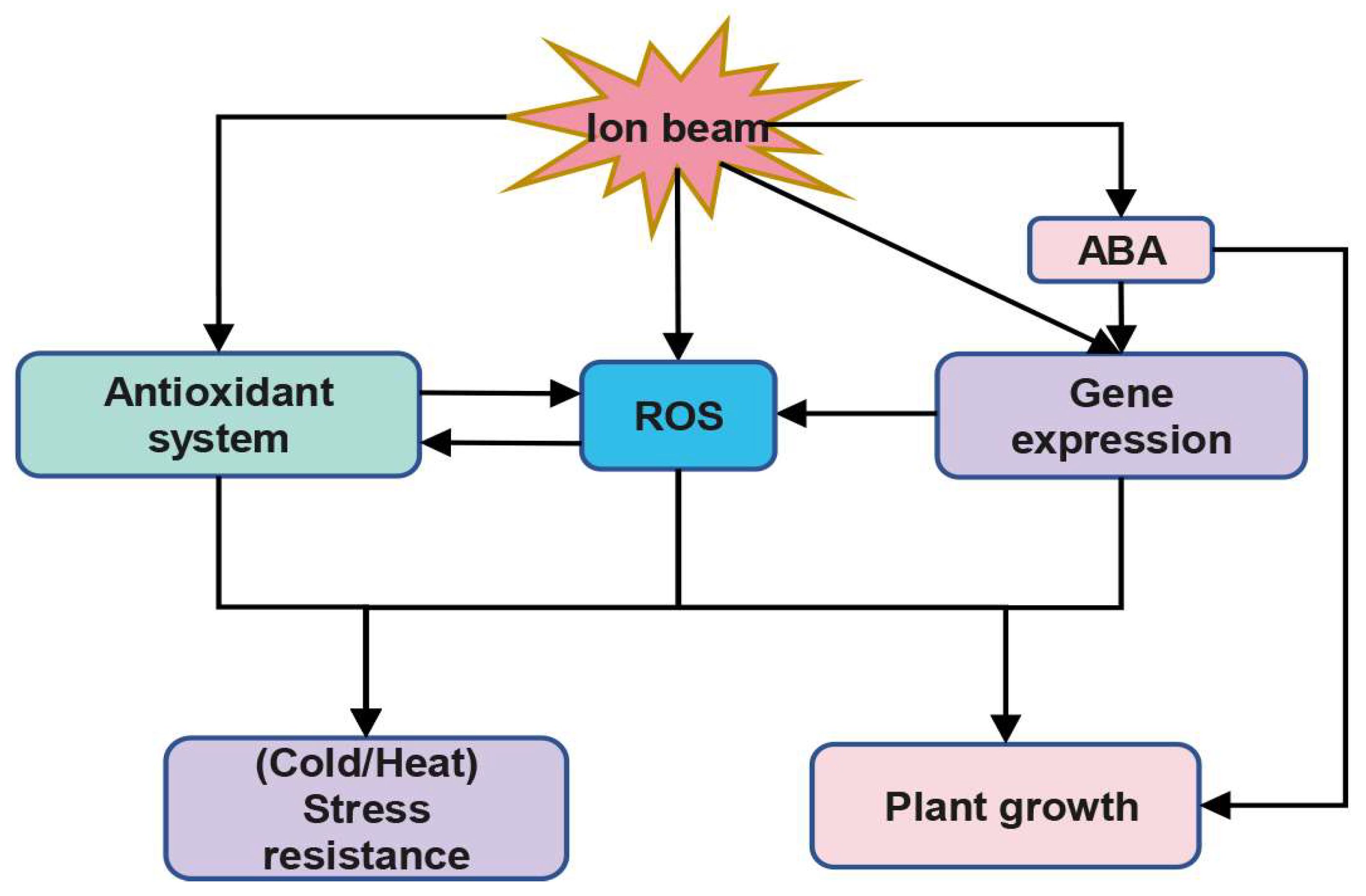
3. The Function of ROS in Plant Response to Ion Beam
3.1. Ion Beam May Enhance Plant Stress Resistance by Modulating ROS Levels
3.2. Ion Beam May Affect Plant Growth via ROS
4. The Function of ROS in Plant Response to Plasma
4.1. Plasma May Induce Seed Germination via ROS
4.2. Plasma May Promote Seedling Growth via ROS
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Turner, J.E. Interaction of ionizing radiation with matte. Health Phys. 2004, 86, 228–252. [Google Scholar] [CrossRef]
- Fedorowski, A.; Steciwko, A. Biological effects of non-ionizing electromagnetic radiation. Med. Pr. 1998, 49, 93–105. [Google Scholar]
- Kataria, S.; Jajoo, A.; Guruprasad, K.N. Impact of increasing ultraviolet-B (UV-B) radiation on photosynthetic processes. J. Photochem. Photobiol. B Biol. 2014, 137, 55–66. [Google Scholar] [CrossRef]
- Xiong, F.S.; Day, T.A. Effect of solar ultraviolet-B radiation during springtime ozone depletion on photosynthesis and biomass production of Antarctic vascular plants. Plant Physiol. 2001, 125, 738–751. [Google Scholar]
- Vanhoudt, N.; Horemans, N.; Wannijn, J.; Nauts, R.; Van Hees, M.; Vandenhove, H. Primary stress responses in Arabidopsis thaliana exposed to gamma radiation. J. Environ. Radioact. 2014, 129, 1–6. [Google Scholar] [CrossRef]
- Kurdziel, M.; Filek, M.; Łabanowska, M. The impact of short-term UV irradiation on grains of sensitive and tolerant cereal genotypes studied by EPR. J. Sci. Food Agric. 2018, 98, 2607–2616. [Google Scholar] [CrossRef]
- Zhang, L.; Qi, W.; Xu, H.; Wang, L.; Jiao, Z. Effects of low-energy N+-beam implantation on root growth in Arabidopsis seedlings. Ecotoxicol. Environ. Saf. 2016, 124, 111–119. [Google Scholar]
- Priatama, R.A.; Pervitasari, A.N.; Park, S.; Park, S.J.; Lee, Y.K. Current advancements in the molecular mechanism of plasma treatment for seed germination and plant growth. Int. J. Mol. Sci. 2022, 23, 4609. [Google Scholar] [CrossRef]
- Esnault, M.A.; Legue, F.; Chenal, C. Ionizing radiation: Advances in plant response. Environ. Exp. Bot. 2010, 68, 231–237. [Google Scholar]
- Kovalchuk, I.; Molinier, J.; Yao, Y.; Arkhipov, A.; Kovalchuk, O. Transcriptome analysis reveals fundamental differences in plant response to acute and chronic exposure to ionizing radiation. Mutat. Res./Fundam. Mol. Mech. Mutagen. 2007, 624, 101–113. [Google Scholar]
- Gudkov, S.V.; Grinberg, M.A.; Sukhov, V.; Vodeneev, V. Effect of ionizing radiation on physiological and molecular processes in plants. J. Environ. Radioact. 2019, 202, 8–24. [Google Scholar] [PubMed]
- Hollósy, F. Effects of ultraviolet radiation on plant cells. Micron 2002, 33, 179–197. [Google Scholar] [PubMed]
- Mishra, V.; Srivastava, G.; Prasad, S.M. Antioxidant response of bitter gourd (Momordica charantia L.) seedlings to interactive effect of dimethoate and UV-B irradiation. Sci. Hortic. 2009, 120, 373–378. [Google Scholar]
- Qi, W.; Zhang, L.; Feng, W.; Xu, H.; Wang, L.; Jiao, Z. ROS and ABA signaling are involved in the growth stimulation induced by low-dose gamma irradiation in Arabidopsis seedling. Appl. Biochem. Biotechnol. 2015, 175, 1490–1506. [Google Scholar]
- Wang, L.; Ma, R.; Yin, Y.; Jiao, Z. Antioxidant response of Arabidopsis thaliana seedlings to oxidative stress induced by carbon ion beams irradiation. J. Environ. Radioact. 2018, 195, 1–8. [Google Scholar] [CrossRef]
- Waskow, A.; Howling, A.; Furno, I. Mechanisms of plasma-seed treatments as a potential Seed Processing Technology. Front. Phys. 2021, 9, 17345. [Google Scholar]
- Fridovich, I. Oxygen free radicals and tissue damage: Chairman’s introduction. In Ciba Foundation Symposium 65—Oxygen Free Radicals and Tissue Damage; Ciba Foundation: Glendale, CA, USA, 1978; pp. 1–4. [Google Scholar]
- Huang, H.; Ullah, F.; Zhou, D.-X.; Yi, M.; Zhao, Y. Mechanisms of ROS regulation of plant development and stress responses. Front. Plant Sci. 2019, 10, 800. [Google Scholar]
- Tripathy, B.C.; Oelmuller, R. Reactive oxygen species generation and signaling in plants. Plant Signal. Behav. 2012, 7, 1621–1633. [Google Scholar] [CrossRef]
- Piccini, C.; Cai, G.M.; Dias, M.C. UV-B radiation affects photosynthesis-related processes of two Italian Olea europaea (L.) varieties differently. Plants 2020, 9, 1712. [Google Scholar] [CrossRef]
- Piccini, C.; Cai, G.; Dias, M.C.; Araújo, M.; Parri, S.; Romi, M.; Faleri, C.; Cantini, C. Olive varieties under UV-B stress show distinct responses in terms of antioxidant machinery and isoform/activity of RubisCO. Int. J. Mol. Sci. 2021, 22, 11214. [Google Scholar]
- Nisa, M.-U.; Huang, Y.; Benhamed, M.; Raynaud, C. The plant DNA damage response: Signaling pathways leading to growth inhibition and putative role in response to stress conditions. Front. Plant Sci. 2019, 10, 653. [Google Scholar] [CrossRef] [PubMed]
- Ludovici, G.M.; de Souza, S.O.; Chierici, A. Adaptation to ionizing radiation of higher plants: From environmental radioactivity to chernobyl disaster. J. Environ. Radioact. 2020, 222, 106375. [Google Scholar] [CrossRef] [PubMed]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar]
- Prasad, S.M.; Dwivedi, R.; Zeeshan, M. Growth, photosynthetic electron transport, and antioxidant responses of young soybean seedlings to simultaneous exposure of nickel and UV-B stress. Photosynthetica 2005, 43, 177–185. [Google Scholar] [CrossRef]
- Gechev, T.S.; Van Breusegem, F.; Stone, J.M.; Denev, I.; Laloi, C. Reactive oxygen species as signals that modulate plant stress responses and programmed cell death. Bioessays 2006, 28, 1091–1101. [Google Scholar] [PubMed]
- Ozgur, R.; Uzilday, B.; Yalcinkaya, T.; Akyol, T.Y.; Yildirim, H.; Turkan, I. Differential responses of the scavenging systems for reactive oxygen species (ROS) and reactive carbonyl species (RCS) to UV-B irradiation in Arabidopsis thaliana and its high altitude perennial relative Arabis alpina. Photochem. Photobiol. Sci. 2021, 20, 889–901. [Google Scholar] [CrossRef]
- Puač, N.; Živković, S.; Selaković, N.; Milutinović, M.; Boljević, J.; Malović, G.; Petrović, Z.L. Long and short term effects of plasma treatment on meristematic plant cells. Appl. Phys. Lett. 2014, 104, 214106. [Google Scholar] [CrossRef]
- Ma, M.; Xu, W.; Wang, P.; Gu, Z.; Zhang, H.; Yang, R. UV-B-triggered H2O2 production mediates isoflavones synthesis in germinated soybean. Food Chem. X 2022, 14, 100331. [Google Scholar]
- Cui, D.; Yin, Y.; Li, H.; Hu, X.; Zhuang, J.; Ma, R.; Jiao, Z. Comparative transcriptome analysis of atmospheric pressure cold plasma enhanced early seedling growth in Arabidopsis thaliana. Plasma Sci. Technol. 2021, 23, 085502. [Google Scholar] [CrossRef]
- Surjadinata, B.B.; Jacobo-Velazquez, D.A.; Cisneros-Zevallos, L. Physiological role of reactive oxygen species, ethylene, and jasmonic acid on UV light induced phenolic biosynthesis in wounded carrot tissue. Postharvest Biol. Technol. 2021, 172, 111388. [Google Scholar]
- Wu, Q.; Su, N.; Zhang, X.; Liu, Y.; Cui, J.; Liang, Y. Hydrogen peroxide, nitric oxide and UV RESISTANCE LOCUS8 interact to mediate UV-B-induced anthocyanin biosynthesis in radish sprouts. Sci. Rep. 2016, 6, 29164. [Google Scholar] [CrossRef] [PubMed]
- Swarna, K.; Bhanumathi, G.; Murthy, S.D.S. Studies on the UV-B radiation induced oxidative damage in thylakoid photofunctions and analysis of the role of antioxidant enzymes in maize primary leaves. Bioscan 2012, 7, 609610. [Google Scholar]
- Elstner, E.F.; Heupel, A. Inhibition of nitrite formation from hydroxylammonium chloride: A simple assay for superoxide dismutase. Anal. Biochem. 1976, 70, 616–620. [Google Scholar]
- Machado, F.; Dias, M.C.; de Pinho, P.G.; Araújo, A.M.; Pinto, D.; Silva, A.; Correia, C.; Moutinho-Pereira, J.; Santos, C. Photosynthetic performance and volatile organic compounds profile in Eucalyptus globulus after UVB radiation. Environ. Exp. Bot. 2017, 140, 141–149. [Google Scholar] [CrossRef]
- Dias, M.C.; Pinto, D.C.G.A.; Correia, C.; Moutinho-Pereira, J.; Oliveira, H.; Freitas, H.; Silva, A.M.S.; Santos, C. UV-B radiation modulates physiology and lipophilic metabolite profile in Olea europaea. J. Plant Physiol. 2018, 222, 39–50. [Google Scholar]
- Jordan, B.R.; He, J.; Chow, W.S.; Anderson, J.M. Changes in mRNA levels and polypeptide subunits of ribulose-1,5-bisphosphate carboxylase in response to supplemental UV-B radiation. Plant Cell Environ. 1992, 15, 91–98. [Google Scholar]
- Caldwell, C.R. Ultraviolet-induced photodegradation of cucumber (Cucumis sativus L.) microsomal and soluble protein tryptophanyl residues in vitro. Plant Physiol. 1993, 101, 947–953. [Google Scholar] [PubMed]
- Desimone, M.; Wagner, E.; Johanningmeier, U. Degradation of active oxygen modified ribulose-1,5-bisphosphate carboxylase/oxygenase by chloroplastic proteases requires ATP hydrolysis. Planta 1998, 205, 459–466. [Google Scholar] [CrossRef]
- Mackerness, S.A.H.; John, C.F.; Jordan, B.; Thomas, B. Early signaling components in ultraviolet-B responses: Distinct roles for different reactive oxygen species and nitric oxide. FEBS Lett. 2001, 489, 237–242. [Google Scholar]
- Wang, L.; Ma, R.; Yin, Y.; Jiao, Z. Physio-Biochemical and transcriptional responses to heat stress in seedlings following carbon ion beam irradiation of Arabidopsis seeds. J. Plant Growth Regul. 2021, 41, 3243–3254. [Google Scholar] [CrossRef]
- Wang, L.; Ma, R.N.; Yin, Y.; Jiao, Z. Role of carbon ion beams irradiation in mitigating cold stress in Arabidopsis thaliana. Ecotoxicol. Environ. Saf. 2018, 162, 341–347. [Google Scholar] [PubMed]
- Zhang, L.W.; Zhang, H.; Zhang, X.F.; Zhang, J.L. Assessment of biological changes in wheat seedlings induced by 12C6+-ion irradiation. Nucl. Sci. Tech. 2008, 19, 138–141. [Google Scholar]
- Yang, G.; Mei, T.; Yuan, H.; Zhang, W.; Chen, L.; Xue, J.; Wu, L.; Wang, Y. Bystander/abscopal effects induced in intact Arabidopsis seeds by low-energy heavy-ion radiation. Radiat. Res. 2008, 170, 372–380. [Google Scholar] [PubMed]
- Chen, H.; Li, F.H.; Yuan, H.; Xiao, X.; Yang, G.; Wu, L. Abscopal signals mediated bio-effects in low-energy ion irradiated Medicago truncatula seeds. J. Radiat. Res. 2010, 51, 651–656. [Google Scholar]
- Sivachandiran, L.; Khacef, A. Enhanced seed germination and plant growth by atmospheric pressure cold air plasma: Combined effect of seed and water treatment. RSC Adv. 2017, 7, 1822–1832. [Google Scholar] [CrossRef]
- Bafoil, M.; Jemmat, A.; Martinez, Y.; Merbahi, N.; Eichwald, O.; Dunand, C.; Yousfi, M. Effects of low temperature plasmas and plasma activated waters on Arabidopsis thaliana germination and growth. PLoS ONE 2018, 13, e0195512. [Google Scholar]
- Waskow, A.; Howling, A.; Furno, I. Advantages and limitations of surface analysis techniques on plasma-treated Arabidopsis thaliana seeds. Front. Mater. 2021, 8, 123. [Google Scholar]
- Cui, D.J.; Yin, Y.; Wang, X.J.; Wang, Z.W.; Ding, H.B.; Ma, R.N.; Jiao, Z. Research on the physio-biochemical mechanism of non-thermal plasma regulated seed germination and early seedling development in Arabidopsis. Front. Plant. Sci. 2019, 10, 1322. [Google Scholar] [CrossRef] [PubMed]
- Whitaker, C.; Beckett, R.P.; Minibayeva, F.V.; Kranner, I. Alleviation of dormancy by reactive oxygen species in Bidens pilosa L. seeds. S. Afr. J. Bot. 2010, 76, 601–605. [Google Scholar]
- Mujahid, Z.; Tounekti, T.; Khemira, H. Cold plasma treatment to release dormancy and improve growth in grape buds: A promising alternative to natural chilling and rest breaking chemicals. Sci. Rep. 2020, 10, 2667. [Google Scholar] [CrossRef]
- Adhikari, B.; Adhikari, M.; Ghimire, B.; Adhikari, B.C.; Choi, E.H. Cold plasma seed priming modulates growth, redox homeostasis and stress response by inducing reactive species in tomato (Solanum lycopersicum). Free Radic. Biol. Med. 2020, 156, 57–69. [Google Scholar] [PubMed]
- Degutytė-Fomins, L.; Pauzaite, G.; Zukiene, R.; Mildaziene, V.; Koga, K.; Shiratani, M. Relationship between cold plasma treatment-induced changes in radish seed germination and phytohormone balance. Jpn. J. Appl. Phys. 2020, 59, SH1001. [Google Scholar]
- Paul, N.D.; Gwynn-Jones, D. Ecological roles of solar UV radiation: Towards an integrated approach. Trends Ecol. Evol. 2003, 18, 48–55. [Google Scholar]
- Caldwell, M.M.; Bornman, J.F.; Ballaré, C.L.; Flint, S.D.; Kulandaivelu, G. Terrestrial ecosystems, increased solar ultraviolet radiation, and interactions with other climate change factors. Photochem. Photobiol. Sci. 2007, 6, 252–266. [Google Scholar] [PubMed]
- Newsham, K.K.; Robinson, S.A. Responses of plants in polar regions to UVB exposure: A meta-analysis. Glob. Change Biol. 2009, 15, 2574–2589. [Google Scholar] [CrossRef]
- Ballaré, C.L.; Caldwell, M.M.; Flint, S.D.; Robinson, S.A.; Bornman, J.F. Effects of solar ultraviolet radiation on terrestrial ecosystems. Patterns, mechanisms, and interactions with climate change. Photochem. Photobiol. Sci. 2011, 10, 226–241. [Google Scholar] [CrossRef]
- Jansen, M.A.K.; Gaba, V.; Greenberg, B.M. Higher plants and UV-B radiation: Balancing damage, repair and acclimation. Trends Plant Sci. 1998, 3, 131–135. [Google Scholar]
- Frohnmeyer, H.; Staiger, D. Ultraviolet-B radiation-mediated responses in plants. Balancing damage and protection. Plant Physiol. 2003, 133, 1420–1428. [Google Scholar]
- Panagopoulos, I.; Bornman, J.F.; Björn, L.O. Effects of ultraviolet radiation and visible light on growth, fluorescence induction, ultraweak luminescence and peroxidase activity in sugar beet plants. J. Photochem. Photobiol. B Biol. 1990, 8, 73–87. [Google Scholar]
- Foyer, C.H.; Lelandais, M.; Kunert, K.J. Photooxidative stress in plants. Physiol. Plant 1994, 92, 696–717. [Google Scholar] [CrossRef]
- Smirnoff, N. Plant resistance to environmental stress. Curr. Opin. Biotechnol. 1998, 9, 214–219. [Google Scholar]
- Mahdavian, K.; Ghorbanli, M.; Kalantari, K.M. The effects of ultraviolet radiation on the contents of chlorophyll, flavonoid, anthocyanin and proline in Capsicum annuum L. Turk. J. Bot. 2008, 32, 25–33. [Google Scholar]
- Jansen, M.A.K.; Hectors, K.; O’Brien, N.M.; Guisez, Y.; Potters, G. Plant stress and human health; do human consumers benefit from UV-B acclimated crops? Plant Sci. 2008, 175, 449458. [Google Scholar] [CrossRef]
- Fedina, I.; Hidema, J.; Velitchkova, M.; Georgieva, K.; Nedeva, D. UV-B induced stress responses in three rice cultivars. Biol. Plant. 2010, 54, 571–574. [Google Scholar]
- Hideg, E.; Nagy, T.; Oberschall, A.; Dudits, D.; Vass, I. Detoxification function of aldose/aldehyde reductase during drought and UV-B (280–320 nm) stresses. Plant Cell Environ. 2003, 26, 513–522. [Google Scholar]
- Lidon, F.C.; Ramalho, J.C. Impact of UV-B irradiation on photosynthetic performance and chloroplast membrane components in Oryza sativa. J. Photochem. Photobiol. B Biol. 2011, 104, 457–466. [Google Scholar]
- Hideg, É.; Mano, J.; Ohno, C.; Asada, K. Increased levels of monodehydroascorbate radical in UV-B irradiated broad bean leaves. Plant Cell Physiol. 1997, 38, 684–690. [Google Scholar] [CrossRef]
- Dai, Q.; Yan, B.; Huang, S. Response of oxidative stress defence systems in rice (Oryza sativa) leaves with supplemental UV-B radiation. Physiol. Plant 1997, 101, 301–308. [Google Scholar] [CrossRef]
- Kalbin, G.; Ohlsson, A.B.; Berglund, T.; Rydström, J.; Strid, A. Ultraviolet-B-radiation induced changes in nicotinamide and glutathione metabolism and gene expression in plants. Eur. J. Biochem. 1997, 249, 465–472. [Google Scholar] [CrossRef]
- Barta, C.; Kálai, T.; Hideg, K.; Vass, I.; Hideg, É. Differences in the ROS-generating efficacy of various ultraviolet wavelengths in detached spinach leaves. Funct. Plant Biol. 2004, 31, 23–28. [Google Scholar]
- Hideg, E.; Vass, I. UV-B induced free radical production in plant leaves and isolated thylakoids. Plant Sci. 1996, 115, 251–260. [Google Scholar]
- Renger, G.; Völker, M.; Eckert, H.J.; Fromme, R.; Hohm-Veit, S.; Gräber, P. On the mechanism of photosystem II deterioration by UV-B irradiation. Photochem. Photobiol. 1989, 49, 97–105. [Google Scholar]
- Vass, I.; Sass, L.; Spetea, C.; Bakou, A.; Ghanotakis, D.F.; Petrouleas, V. UV-B-induced inhibition of photosystem II electron transport studied by EPR and chlorophyll fluorescence. Impairment of donor and acceptor side components. Biochemistry 1996, 35, 8964–8973. [Google Scholar] [CrossRef]
- Czégény, G.; Wu, M.; Dér, A.; Eriksson, L.A.; Strid, Å.; Hideg, É. Hydrogen peroxide contributes to the ultraviolet-B (280–315 nm) induced oxidative stress of plant leaves through multiple pathways. FEBS Lett. 2014, 588, 2255–2261. [Google Scholar] [CrossRef]
- Surjadinata, B.B.; Jacobo-Velázquez, D.A.; Cisneros-Zevallos, L. UVA, UVB and UVC light enhances the biosynthesis of phenolic antioxidants in fresh-cut carrot through a synergistic effect with wounding. Molecules 2017, 22, 668. [Google Scholar] [CrossRef]
- Ortega-Hernández, E.; Nair, V.; Serrano-Sandoval, S.N.; Welti-Chanes, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Wounding and UVB light synergistically induce the postharvest biosynthesis of indicaxanthin and betanin in red prickly pears. Postharvest Biol. Technol. 2020, 167, 111247. [Google Scholar] [CrossRef]
- Rao, M.V.; Paliyath, G.; Ormrod, D.P. Ultraviolet-B and ozone induced biochemical changes in antioxidant enzymes of Arabidopsis thaliana. Plant Physiol. 1996, 110, 125–136. [Google Scholar]
- Kubo, A.; Aono, M.; Nakajima, N.; Saji, H.; Tanaka, K.; Kondo, N. Differential responses in activity of antioxidant enzymes to different environmental stresses in Arabidopsis thaliana. J. Plant Res. 1999, 112, 279–290. [Google Scholar] [CrossRef]
- Carvalho, F.E.L.; Silveira, J.A.G. H2O2-retrograde signaling as a pivotal mechanism to understand priming and cross stress tolerance in plants. In Priming-Mediated Stress and Cross-Stress Tolerance in Crop Plants, 1st ed.; Hossain, M.A., Liu, F., Burritt, D.J., Fujita, M., Huang, B., Eds.; Academic Press: Pittsburgh, PA, USA, 2020; pp. 57–78. [Google Scholar]
- Xue, S.; Zang, Y.; Chen, J.; Shang, S.; Gao, L.; Tang, X. Ultraviolet-B radiation stress triggers reactive oxygen species and regulates the antioxidant defense and photosynthesis systems of intertidal red algae Neoporphyra haitanensis. Front. Mar. Sci. 2022, 9, 1043462. [Google Scholar]
- Örvar, B.L.; Ellis, B.E. Transgenic tobacco plants expressing antisense RNA for cytosolic ascorbate peroxidase show increased susceptibility to ozone injury. Plant J. 1997, 11, 1297–1305. [Google Scholar] [CrossRef]
- Brosché, M.; Strid, Å. Molecular events following perception of ultraviolet-B radiation by plants. Physiol. Plant. 2003, 117, 1–10. [Google Scholar] [CrossRef]
- Fedina, I.; Nedeva, D.; Georgieva, K.; Velitchkova, M. Methyl jasmonate counteract UV-B stress in barley seedlings. J. Agron. Crop Sci. 2009, 195, 204–212. [Google Scholar]
- Tossi, V.; Amenta, M.; Lamattina, L.; Cassia, R. Nitric oxide enhances plant ultraviolet-B protection up-regulating gene expression of the phenylpropanoid biosynthetic pathway. Plant Cell Environ. 2011, 34, 909–921. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.H.; Al-Whaibi, M.H.; Basalah, M.O. Role of nitric oxide in tolerance of plants to abiotic stress. Protoplasma 2011, 248, 447–455. [Google Scholar]
- Bandurska, H.; Niedziela, J.; Chadzinikolau, T. Separate and combined responses to water deficit and UV-B radiation. Plant Sci. 2013, 213, 98–105. [Google Scholar]
- Kasote, D.M.; Katyare, S.S.; Hegde, M.V.; Bae, H. Significance of antioxidant potential of plants and its relevance to therapeutic applications. Int. J. Biol. Sci. 2015, 11, 982. [Google Scholar] [CrossRef]
- Jacobo-Velázquez, D.A.; González-Agüero, M.; Cisneros-Zevallos, L. Cross-talk between signaling pathways: The link between plant secondary metabolite production and wounding stress response. Sci. Rep. 2015, 5, 8608. [Google Scholar]
- Czarnocka, W.; Karpinski, S. Friend or foe? Reactive oxygen species production, scavenging and signaling in plant response to environmental stresses. Free Radic. Biol. Med. 2018, 122, 4–20. [Google Scholar]
- Delledonne, M.; Xia, Y.; Dixon, R.A.; Lamb, C. Nitric oxide functions as a signal in plant disease resistance. Nature 1998, 394, 585–588. [Google Scholar]
- Jain, K.; Kataria, S.; Guruprasad, K.N. Changes in antioxidant defenses of cucumber cotyledons in response to UV-B and to the free radical generating compound AAPH. Plant Sci. 2003, 165, 551–557. [Google Scholar] [CrossRef]
- Vass, I.; Szilard, A.; Sicora, C. Adverse effects of UV-B light on the structure and function of the photosynthetic apparatus. In Handbook of Photosynthesis, 2nd ed.; Pessarakli, M., Ed.; CRC Press: Boca Raton, FL, USA, 2005; pp. 827–843. [Google Scholar]
- Iwanzik, W.; Tevini, M.; Dohnt, G.; Voss, M. Action of UV-B radiation on photo-synthetic primary reaction in spinach chloroplasts. Plant Physiol. 1983, 58, 401–407. [Google Scholar] [CrossRef]
- Chow, W.S.; Strid, A.; Anderson, J.M. Short-term treatment of pea plants with supplementary ultraviolet-B radiation: Recovery time-courses of some photosynthetic functions and components. Res. Photosynth. 1992, 4, 361–364. [Google Scholar]
- Doughty, J.C.; Hope, A.B. Effects of ultraviolet radiation on the plasma membranes of Chara corallina. J. Plant Physiol. 1976, 3, 693–699. [Google Scholar]
- Murphy, T.M.; Wilson, C. UV-stimulated K+ efflux from cells: Counterion and inhibitor studies. Plant Physiol. 1982, 70, 709–713. [Google Scholar] [CrossRef]
- Tyystjärvi, E. Photoinhibition of photosystem II and photodamage of the oxygen evolving manganese cluster. Coord. Chem. Rev. 2008, 252, 361–376. [Google Scholar]
- Hakala, M.; Tuominen, I.; Keranen, M.; Tyystjärvi, T.; Tyystjärvi, E. Evidence for the role of the oxygen-evolving manganese complex in photoinhibition of photosystem II. Biochim. Biophys. Acta 2005, 1706, 68–80. [Google Scholar]
- Takahashi, S.; Milward, S.E.; Yamori, W.; Evans, J.R.; Hillier, W.; Badger, M.R. The solar action spectrum of photosystem II damage. Plant Physiol. 2010, 153, 988–993. [Google Scholar] [CrossRef]
- Lidon, F.J.C.; Reboredo, F.H.; Leitão, A.E.; Silva, M.M.; Duarte, M.; Ramalho, J. Impact of UV-B radiation on photosynthesis—An overview. Emir. J. Food Agric. 2012, 24, 546–556. [Google Scholar] [CrossRef]
- Szilard, A.; Sass, L.; Vass, I. Photoinactivation of photosystem II at low light intensity, mathematical model. Acta Biol. Szeged. 2002, 46, 167–169. [Google Scholar]
- Ishida, H.; Makino, A.; Mae, T. Fragmentation of the large subunit of ribulose1,5-bisphosphate carboxylase by reactive oxygen species occurs near Gly329. J. Biol. Chem. 1999, 274, 5222–5226. [Google Scholar]
- Lang-Mladek, C.; Xie, L.S.; Nigam, N.; Chumak, N.; Binkert, M.; Neubert, S.; Hauser, M. UV-B signaling pathways and fluence rate dependent transcriptional regulation of ARIADNE12. Physiol. Plant. 2012, 145, 527–539. [Google Scholar] [CrossRef]
- Nawkar, G.M.; Maibam, P.; Park, J.H.; Sahi, V.P.; Lee, S.Y.; Kang, C.H. UV-induced cell death in plants. Int. J. Mol. Sci. 2013, 14, 1608–1628. [Google Scholar] [CrossRef]
- González-Besteiro, M.A.; Bartels, S.; Albert, A.; Ulm, R. Arabidopsis MAP kinase phosphatase 1 and its target MAP kinases 3 and 6 antagonistically determine UV-B stress tolerance, independent of the UVR8 photoreceptor pathway. Plant J. 2011, 68, 727–737. [Google Scholar]
- Chen, Z.; Dong, Y.; Huang, X. Plant responses to UV-B radiation: Signaling, acclimation and stress tolerance. Stress Biol. 2022, 2, 51. [Google Scholar]
- Geras’kin, S.A. Ecological effects of exposure to enhanced levels of ionizing radiation. J. Environ. Radioact. 2016, 162, 347–357. [Google Scholar]
- Walle, J.V.D.; Horemans, N.; Saenen, E.; Hees, M.V.; Wannijn, J.; Nauts, R.; Gompel, A.V.; Vangronsveld, J.; Vandenhove, H.; Cuypers, A. Arabidopsis plants exposed to gamma radiation in two successive generations show a different oxidative stress response. J. Environ. Radioact. 2016, 165, 270–279. [Google Scholar] [CrossRef]
- Gu, S.B.; Li, S.C.; Feng, H.Y.; Wu, Y.; Yu, Z.L. A novel approach to microbial breeding—Low-energy ion implantation. Appl. Microbiol. Biotechnol. 2008, 78, 201–209. [Google Scholar]
- Stoilov, L.; Georgieva, M.; Manova, V.; Liu, L.X.; Gecheff, K. Karyotype reconstruction modulates the sensitivity of barley genome to radiation-induced DNA and chromosomal damage. Mutagenesis 2013, 28, 153–160. [Google Scholar] [CrossRef]
- Rakwal, R.; Kimura, S.; Shibato, J.; Nojima, K.; Kim, Y.K.; Nahm, B.H.; Jwa, N.S.; Endo, S.; Tanaka, K.; Iwahashi, H. Growth retardation and death of rice plants irradiated with carbon ion beams is preceded by very early dose- and time-dependent gene expression changes. Mol. Cells 2008, 25, 272–278. [Google Scholar]
- Ya, H.; Chen, Q.; Wang, W.; Cheng, Y. Gene expression characteristics of growth-inhibited rice seedlings induced by low-energy N(þ)-beam implantation. Genet. Mol. Res. 2014, 13, 6259–6271. [Google Scholar] [CrossRef]
- Yang, G.; Wu, L.J.; Chen, L.Y.; Pei, B.; Wang, Y.G.; Zhan, F.R.; Wu, Y.J.; Yu, Z.L. Targeted irradiation of shoot apical meristem of Arabidopsis embryos induces long-distance bystander/abscopal effects. Radiat. Res 2007, 167, 298–305. [Google Scholar] [CrossRef]
- Liu, Q.; Kasuga, M.; Sakuma, Y.; Abe, H.; Miura, S.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Two transcription factors, DREB1 and DREB2, with an EREBP/AP2 DNA binding domain separate two cellular signal transduction pathways in drought and low-temperature-responsive gene expression, respectively, in Arabidopsis. Plant Cell 1998, 10, 1391–1406. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.Z.; Jin, Y.N.; Ding, X.H.; Wang, W.J.; Zhai, S.S.; Bai, L.P.; Guo, Z.F. Gene regulation and signal transduction in the ICE-CBF-COR signaling pathway during cold stress in plants. Biochemistry (Moscow) 2017, 82, 1103–1117. [Google Scholar]
- Chinnusamy, V.; Zhu, J.H.; Zhu, J.K. Cold stress regulation of gene expression in plants. Trends Plant Sci. 2007, 12, 444–451. [Google Scholar] [PubMed]
- Lee, H.G.; Seo, P.J. The MYB96-HHP module integrates cold and abscisic acid signaling to activate the CBF-COR pathway in Arabidopsis. Plant J. 2015, 82, 962–977. [Google Scholar] [CrossRef]
- Lohmann, C.; Eggers-Schumacher, G.; Wunderlich, M.; Schöffl, F. Two different heat shock transcription factors regulate immediate early expression of stress genes in Arabidopsis. Mol. Genet. Genom. 2004, 271, 11–21. [Google Scholar] [CrossRef]
- Kavi Kishor, P.B.; Sangam, S.; Amrutha, R.N.; Laxmi, P.S.; Sreenivasulu, N. Regulation of proline biosynthesis, degradation, uptake and transport in higher plants: Its implications in plant growth and abiotic stress tolerance. Curr. Sci. 2005, 88, 424–438. [Google Scholar]
- Ábrahám, E.; Rigó, G.; Székely, G.; Nagy, R.; Koncz, C.; Szabados, L. Light-dependent induction of proline biosynthesis by abscisic acid and salt stress is inhibited by brassinosteroid in Arabidopsis. Plant Mol. Biol. 2003, 51, 363–372. [Google Scholar] [CrossRef]
- Ling, A.P.; Ung, Y.C.; Hussein, S.; Harun, A.R.; Tanaka, A.; Yoshihiro, H. Morphological and biochemical responses of Oryza sativa L. (cultivar MR219) to ion beam irradiation. J. Zhejiang Univ.—Sci. B 2013, 14, 1132–1143. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.F.; Wang, Z.Z.; Zhou, L.B.; Qu, Y.; Lu, D.; Yu, L.X.; Du, Y.; Jin, W.J.; Li, W.J. Relationship between plant growth and cytological effect in root apical meristem after exposure of wheat dry seeds to carbon ion beams. Nucl. Instrum. Methods B 2013, 305, 9–15. [Google Scholar] [CrossRef]
- Sjahril, R.; Riadi, M.; Rafiuddin; Sato, T.; Toriyama, K.; Abe, T.; Trisnawaty, A.R. Effect of heavy ion beam irradiation on germination of local Toraja rice seed (M1-M2) mutant generation. IOP Conf. Ser. Earth Environ. Sci. 2018, 157, 012046. [Google Scholar] [CrossRef]
- Vilaithong, T.; Yu, L.D.; Alisi, C.; Phanchaisri, B.; Apavatjrut, P.; Anuntalabhochai, S. A study of low-energy ion beam effects on outer plant cell structure for exogenous macromolecule transferring. Surf. Coat. Technol. 2000, 128, 133–138. [Google Scholar]
- Apavatjrut, P.; Alisi, C.; Phanchaisri, B.; Yu, L.D.; Vilaithong, T. Induction of exogenous molecule transfer into plant cells by ion beam bombardment. ScienceAsia 2003, 29, 99–107. [Google Scholar] [CrossRef]
- Li, Y.Q.; Gu, J.Y.; Irshad, A.; Zhao, L.; Guo, H.; Xiong, H.; Xie, Y.; Zhao, S.; Ding, Y.; Zhou, L.; et al. Physiological and differential proteomic analysis at seedling stage by induction of heavy-ion beam radiation in wheat seeds. Front. Genet. 2022, 13, 942806. [Google Scholar] [CrossRef] [PubMed]
- Fifika, A.W.; Ikmal, A.M.; Faiz, A.; Nor’Aishah, H.; Rahim, H.A.; Sobri, H.; Noraziyah, A.S. Carbon-ion beam radiosensitivity study and biological responses of high-yielding rice line, MR219-PL-5. Sains Malays. 2021, 50, 3481–3491. [Google Scholar]
- Sagi, M.; Fluhr, R. Production of reactive oxygen species by plant NADPH oxidases. Plant Physiol. 2006, 141, 336–340. [Google Scholar]
- Kwak, J.M.; Mori, I.C.; Pei, Z.M.; Leonhardt, N.; Torres, M.A.; Dangl, J.L.; Bloom, R.E.; Bodde, S.; Jones, J.D.; Schroeder, J.I. NADPH oxidase AtrbohD and AtrbohF genes function in ROS-dependent ABA signaling in Arabidopsis. EMBO J. 2003, 22, 2623–2633. [Google Scholar] [PubMed]
- Chen, Q.F.; Ya, H.Y.; Feng, Y.R.; Jiao, Z. Expression of key genes involved in ABA biosynthesis in rice implanted by ion beam. Appl. Biochem. Biotechnol. 2014, 173, 239–247. [Google Scholar] [CrossRef]
- Starič, P.; Vogel-Mikuš, K.; Mozetič, M.; Junkar, I. Effects of nonthermal plasma on morphology, genetics and physiology of seeds: A Review. Plants 2020, 9, 1736. [Google Scholar] [CrossRef]
- Jiang, J.F.; Lu, Y.F.; Li, J.G.; Li, L.; He, X.; Shao, H.L.; Dong, Y.H. Effect of seed treatment by cold plasma on the resistance of tomato to Ralstonia solanacearum (Bacterial Wilt). PLoS ONE 2014, 9, e97753. [Google Scholar]
- Šerá, B.; Gajdova, I.; Gavril, B.; Hnatiuc, E.; Šerý, M.; Špatenka, P. Hemp (Cannabis sativa L.) seeds after plasma treatment. In Proceedings of the 13th International Conference on Optimization of Electridcal and Electronic Equipment, Brasov, Romania, 24–26 May 2012. [Google Scholar]
- Šerá, B.; Gajdova, I.; Sery, M.; Gajdová, I.; Šerý, M.; Špatenka, P. New physicochemical treatment method of poppy seeds for agriculture and food industries. Plasma Sci. Technol. 2013, 15, 935. [Google Scholar] [CrossRef]
- Li, L.; Li, J.G.; Shen, M.C.; Zhang, C.L.; Dong, Y.H. Cold plasma treatment enhances oilseed rape seed germination under drought stress. Sci. Rep. 2015, 5, 13033. [Google Scholar]
- Selcuk, M.; Oksuz, L.; Basaran, P. Decontamination of grains and legumes infected with Aspergillus spp. and Penicillum spp. by cold plasma treatment. Bioresour. Technol. 2008, 99, 5104–5109. [Google Scholar] [CrossRef]
- Dong, X.Y.; Yang, Y.L. A novel approach to enhance blueberry quality during storage using cold plasma at atmospheric air pressure. Food Bioprocess Technol. 2019, 12, 1409–1421. [Google Scholar] [CrossRef]
- Thirumdas, R.; Sarangapani, C.; Annapure, U.S. Cold plasma: A novel non-thermal technology for food processing. Food Biophys. 2015, 10, 1–11. [Google Scholar] [CrossRef]
- Bradu, C.; Kutasi, K.; Magureanu, M.; Puač, N.; Živković, S. Reactive titrogen species in plasma-activated water: Generation, chemistry and application in agriculture. J. Phys. Appl. Phys. 2020, 53, 223001. [Google Scholar]
- Arjunan, K.P.; Sharma, V.K.; Ptasinska, S. Effects of atmospheric pressure plasmas on isolated and cellular DNA—A review. Int. J. Mol. Sci. 2015, 16, 2971–3016. [Google Scholar] [CrossRef]
- Singh, R.; Singh, S.; Parihar, P.; Mishra, R.K.; Prasad, S.M. Reactive oxygen species (ROS): Beneficial companions of plants’ developmental processes. Front. Plant Sci. 2016, 7, 1299. [Google Scholar]
- Billah, M.; Sajib, S.A.; Roy, N.C.; Rashid, M.M.; Reza, M.A.; Hasan, M.M.; Talukder, M.R. Effects of DBD air plasma treatment on the enhancement of black gram (Vigna mungo L.) seed germination and growth. Arch. Biochem. Biophys. 2020, 681, 108253. [Google Scholar]
- Liu, Y.; Ye, N.H.; Liu, R.; Chen, M.X.; Zhang, J.H. H2O2 mediates the regulation of ABA catabolism and GA biosynthesis in Arabidopsis seed dormancy and germination. J. Exp. Bot. 2010, 61, 2979–2990. [Google Scholar] [CrossRef]
- Barba-Espín, G.; Diaz-Vivancos, P.; Job, D.; Belghazi, M.; Job, C.; Hernandez, J.A. Understanding the role of H2O2 during pea seed germination: A combined proteomic and hormone profiling approach. Plant Cell Environ. 2011, 34, 1907–1919. [Google Scholar] [CrossRef] [PubMed]
- Kurek, K.; Plitta-Michalak, B.; Ratajczak, E. Reactive oxygen species as potential drivers of the seed aging process. Plants 2019, 8, 174. [Google Scholar] [CrossRef] [PubMed]
- Pandiselvam, R.; Mayookha, V.P.; Kothakota, A.; Sharmila, L.; Ramesh, S.V.; Bharathi, C.P.; Gomathy, K.; Srikanth, V. Impact of ozone treatment on seed germination—A systematic review. Ozone Sci. Eng. 2019, 42, 331–346. [Google Scholar] [CrossRef]
- Iranbakhsh, A.; Oraghi Ardebili, Z.; Molaei, H.; Ardebili, N.O.; Amini, M. Cold plasma up-regulated expressions of WRKY1 transcription factor and genes involved in biosynthesis of cannabinoids in hemp (Cannabis sativa L.). Plasma Chem. Plasma Process. 2020, 40, 527–537. [Google Scholar] [CrossRef]
- Pérez-Pizá, M.C.; Prevosto, L.; Grijalba, P.E.; Zilli, C.G.; Cejas, E.; Mancinelli, B.; Balestrasse, K.B. Improvement of growth and yield of soybean plants through the application of non-thermal plasmas to seeds with different health status. Heliyon 2019, 5, e01495. [Google Scholar]
- Ghasempour, M.; Iranbakhsh, A.; Ebadi, M.; Ardebili, Z.O. Seed priming with cold plasma improved seedling performance, secondary metabolism, and expression of deacetylvindoline O-acetyltransferase gene in Catharanthus roseus. Contrib. Plasma Phys. 2020, 60, e201900159. [Google Scholar]
- Puligundla, P.; Kim, J.W.; Mok, C. Effect of corona discharge plasma jet treatment on decontamination and sprouting of rapeseed (Brassica napus L.) seeds. Food Control 2017, 71, 376–382. [Google Scholar]
- Cui, D.J.; Yin, Y.; Sun, H.; Wang, X.J.; Zhuang, J.; Wang, L.; Ma, R.N.; Jiao, Z. Regulation of cellular redox homeostasis in Arabidopsis thaliana seedling by atmospheric pressure cold plasma-generated reactive oxygen/nitrogen species. Ecotoxicol. Environ. Saf. 2022, 240, 113703. [Google Scholar]
- Marzol, E.; Borassi, C.; Juarez, S.P.D.; Mangano, S.; Estevez, J.M. RSL4 takes control: Multiple signals, one transcription factor. Trends Plant Sci. 2017, 22, 553–555. [Google Scholar]

| Radiation | Plants | ROS | Major Advances | Reference |
|---|---|---|---|---|
| Low-energy N+ beam | Arabidopsis thaliana | ROS | Interfere with cellular activity, leading to reduced meristematic cell viability and inhibited meristematic cell division | [7] |
| Carbon ion beam | Arabidopsis thaliana | ROS | Promote seedling growth | [15] |
| Atmospheric pressure cold plasma (APCP) | Daucus carota | ROS | Have a long-term positive effect on growth | [28] |
| UV-B | Glycine max | H2O2 | Mediate isoflavones synthesis | [29] |
| Atmospheric pressure cold plasma (APCP) | Arabidopsis thaliana | ROS | Regulate the expression of GSH and phytohormone genes | [30] |
| UV | Daucus carota | ROS | Activate ethylene (ET) and jasmonic acid (JA) biosynthesis | [31] |
| UV-B | Raphanus sativus | H2O2 | Mediate anthocyanin biosynthesis | [32] |
| UV-B | Zea mays | ROS | Reduce PS II photochemical efficiency | [33] |
| UV-B | Vicia faba | ·OH | May be one of the mechanisms of UV-B-induced damage | [34] |
| UV-B | Eucalyptus globulus and Olea europea | ROS | ROS was associated with a decrease in pigmentation | [35,36] |
| UV-B | Pisum sativum, Cucumis sativus and Hordeum vulgare | ROS | Degrade Rubisco via proteolytic degradation of large subunits (LSU) | [37,38,39] |
| UV-B | Arabidopsis thaliana | O2•− | Induce the expression of PDF1.2 | [40] |
| UV-B | Arabidopsis thaliana | H2O2 | Increase the expression of PR-1 but inhibits Lhcb | [40] |
| Carbon ion beam | Arabidopsis thaliana | ROS | Increase heat tolerance | [41] |
| Carbon ion beam | Arabidopsis thaliana | ROS | Increase cold tolerance | [42] |
| 12C6+-ion beam | Triticum aestivum | H2O2, O2•− | Improve disease resistance | [43] |
| Ar-ion beam | Arabidopsis thaliana | ROS | Inhibit seeding growth | [44] |
| Ar+ ion beam | Medicago truncatula | ROS | Suppress seed germination and seedling establishment, as well as decrease primary root and primary stem length | [45] |
| Cold air plasma | Solanum lycopersicum and Capsicum annum | H2O2 | Improve seed germination | [46] |
| Plasma-activated water (PAW) | Arabidopsis thaliana | H2O2 | A positive effect on germination | [47] |
| Plasma | Arabidopsis thaliana | O3 | Modify the coat of seeds | [48] |
| Plasma | Arabidopsis and Bidens pilosa | O2•−, ·OH | Break seed dormancy and thus increasing seed germination | [49,50] |
| Plasma | Arabidopsis and Bidens pilosa | H2O2 | Inhibit seed germination | [49,50] |
| Plasma | Bidens pilosa | ROS | Regulate the expression of gibberellin-related genes | [50] |
| Cold plasma (CP) | Vitis vinifera | ·OH | Cause cell wall loosening, which in turn promotes seed germination | [51] |
| Cold plasma (CP) | Solanum lycopersicum and Raphanus sativus | ROS | Affect seed germination, plant growth and development, and stress resistance | [52,53] |
| Antioxidants | Reactions Catalyzed |
|---|---|
| Catalase (CAT) | 2H2O2 → 2H2O + O2 |
| Peroxidase (POD) | 2H2O2 → 2H2O + O2 |
| Ascorbate peroxidase (APX) | H2O2 + AsA → 2H2O + MDHA |
| Glutathione reductase (GR) | GSSG + NADPH + H+ → GSH + NADP+ |
| Superoxide dismutase (SOD) | 2O2 •− + 2H+→ O2 + H2O2 |
| Ascorbic acid (ASA) | Scavenges O2 •–, H2O2, ·OH, and 1O2 |
| Glutathione (GSH) | Scavenges H2O2, ·OH, and 1O2 |
| Flavonoids | Scavenges O2 •–, H2O2, and 1O2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, Y.; Duan, Y.; Chi, Q.; Wang, R.; Yin, Y.; Cui, D.; Li, S.; Wang, A.; Ma, R.; Li, B.; et al. The Role of Reactive Oxygen Species in Plant Response to Radiation. Int. J. Mol. Sci. 2023, 24, 3346. https://doi.org/10.3390/ijms24043346
Tan Y, Duan Y, Chi Q, Wang R, Yin Y, Cui D, Li S, Wang A, Ma R, Li B, et al. The Role of Reactive Oxygen Species in Plant Response to Radiation. International Journal of Molecular Sciences. 2023; 24(4):3346. https://doi.org/10.3390/ijms24043346
Chicago/Turabian StyleTan, Yuantao, Yaoke Duan, Qing Chi, Rong Wang, Yue Yin, Dongjie Cui, Shuang Li, Aiying Wang, Ruonan Ma, Bing Li, and et al. 2023. "The Role of Reactive Oxygen Species in Plant Response to Radiation" International Journal of Molecular Sciences 24, no. 4: 3346. https://doi.org/10.3390/ijms24043346
APA StyleTan, Y., Duan, Y., Chi, Q., Wang, R., Yin, Y., Cui, D., Li, S., Wang, A., Ma, R., Li, B., Jiao, Z., & Sun, H. (2023). The Role of Reactive Oxygen Species in Plant Response to Radiation. International Journal of Molecular Sciences, 24(4), 3346. https://doi.org/10.3390/ijms24043346







