Sustained Release of Antifungal and Antibacterial Agents from Novel Hybrid Degradable Nanofibers for the Treatment of Polymicrobial Osteomyelitis
Abstract
1. Introduction
2. Results
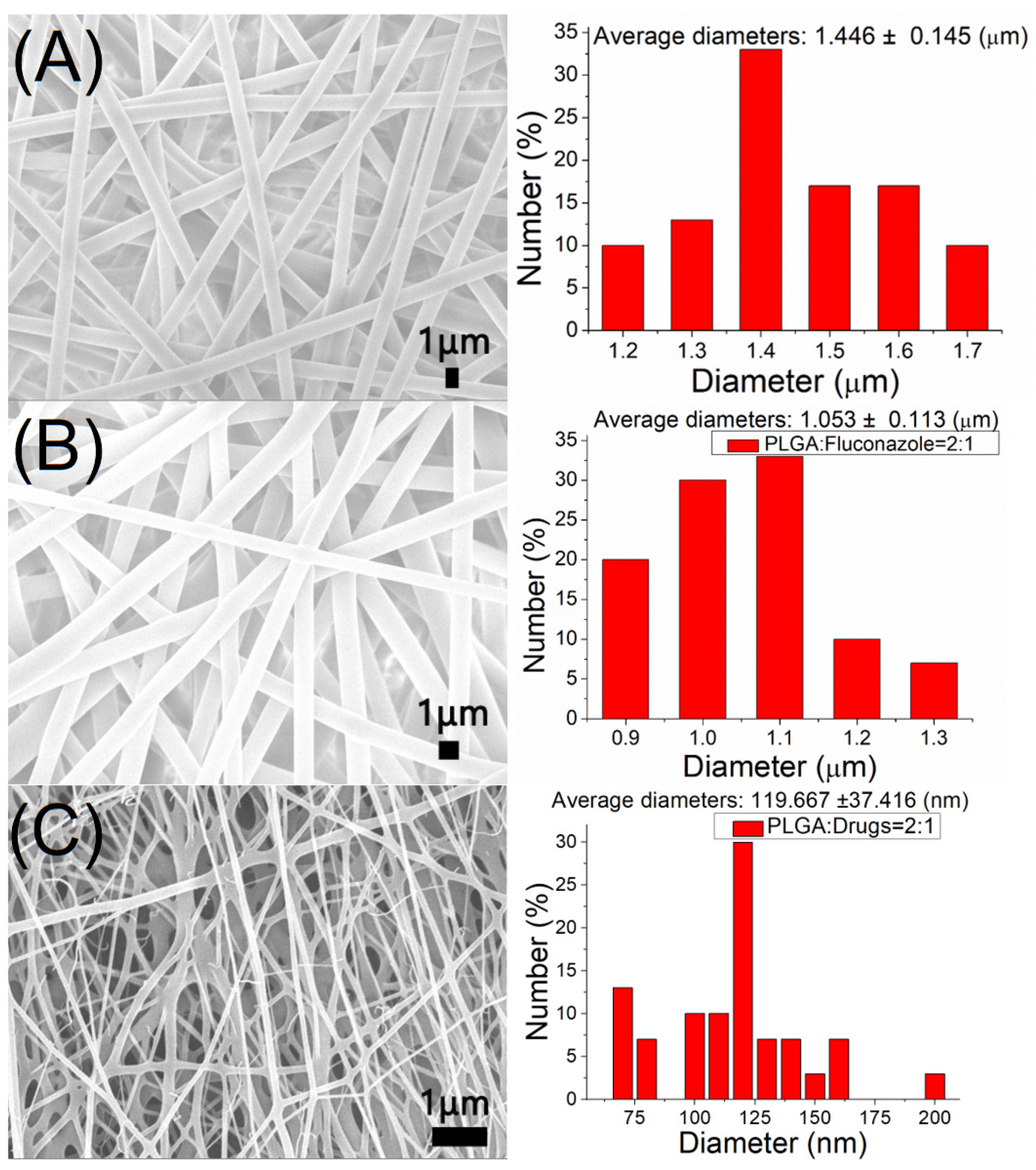
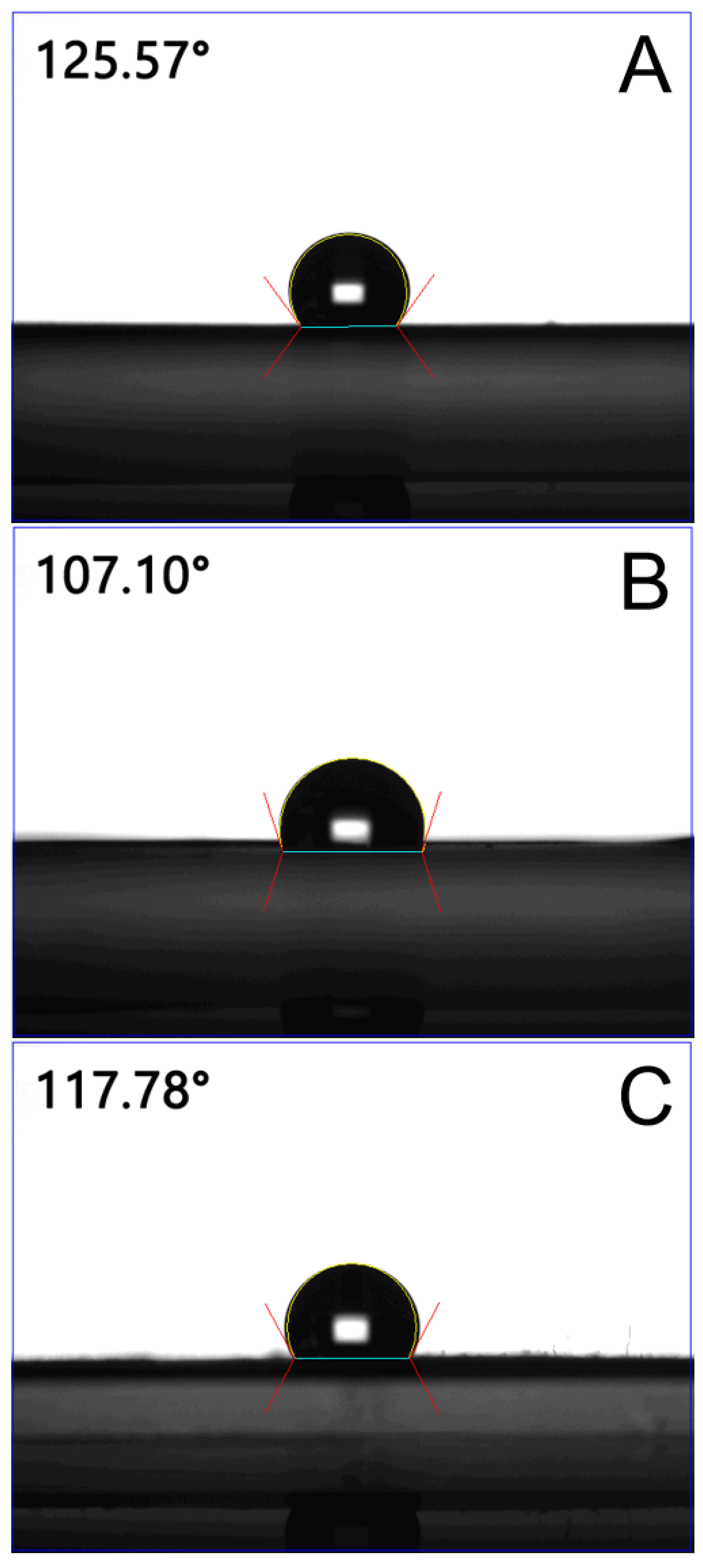
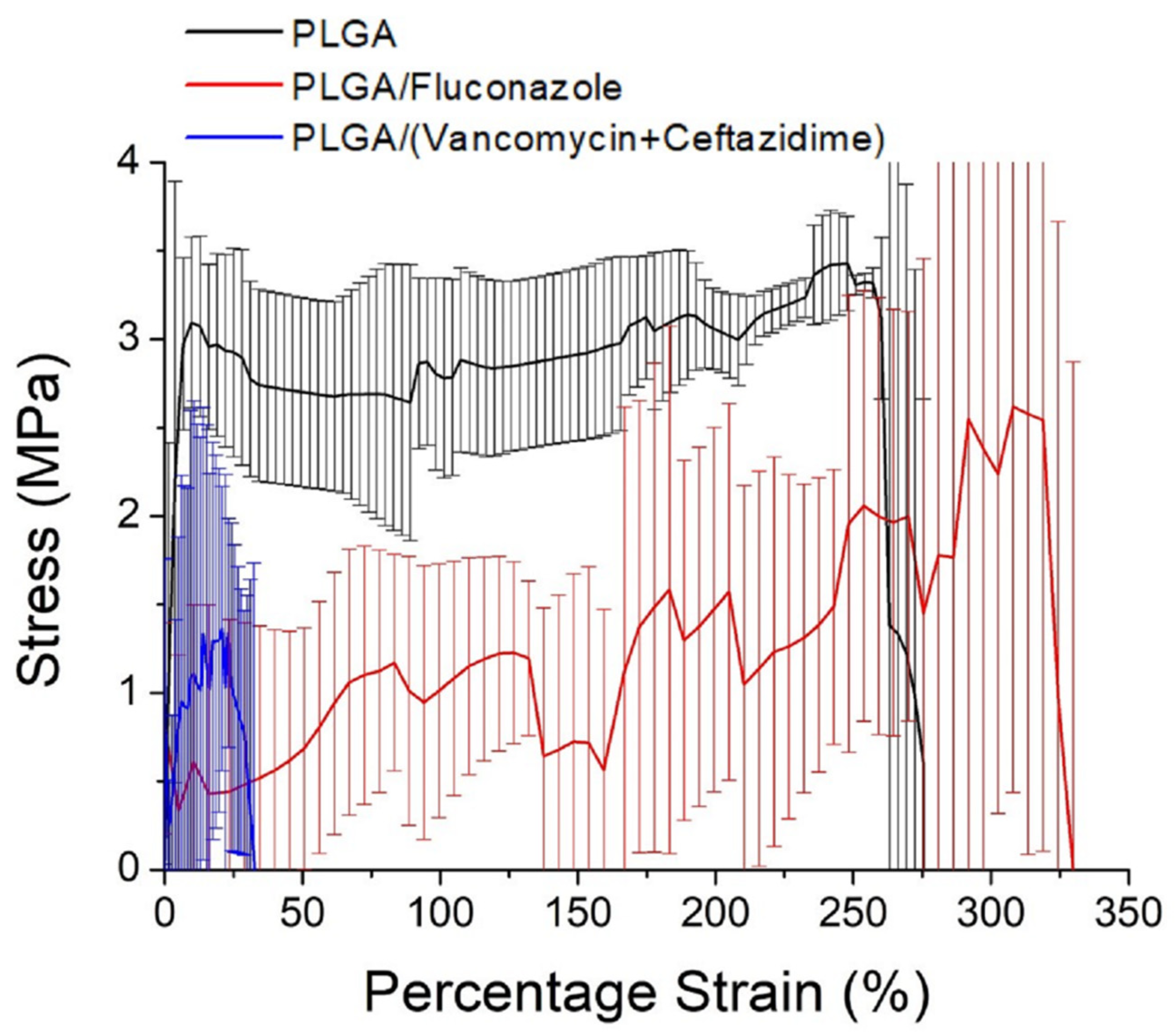
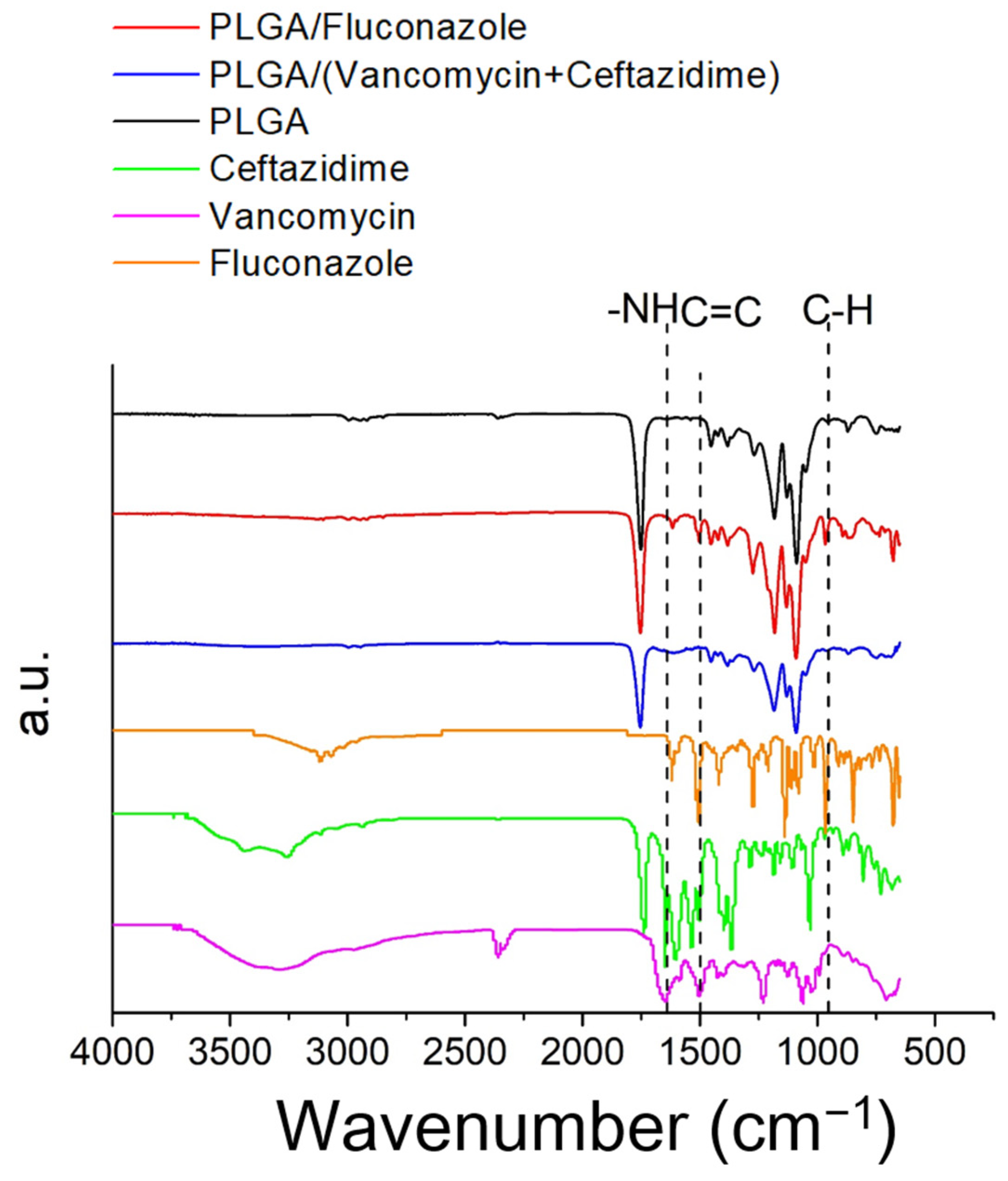
2.1. Characterization of Electrospun Nanofibers
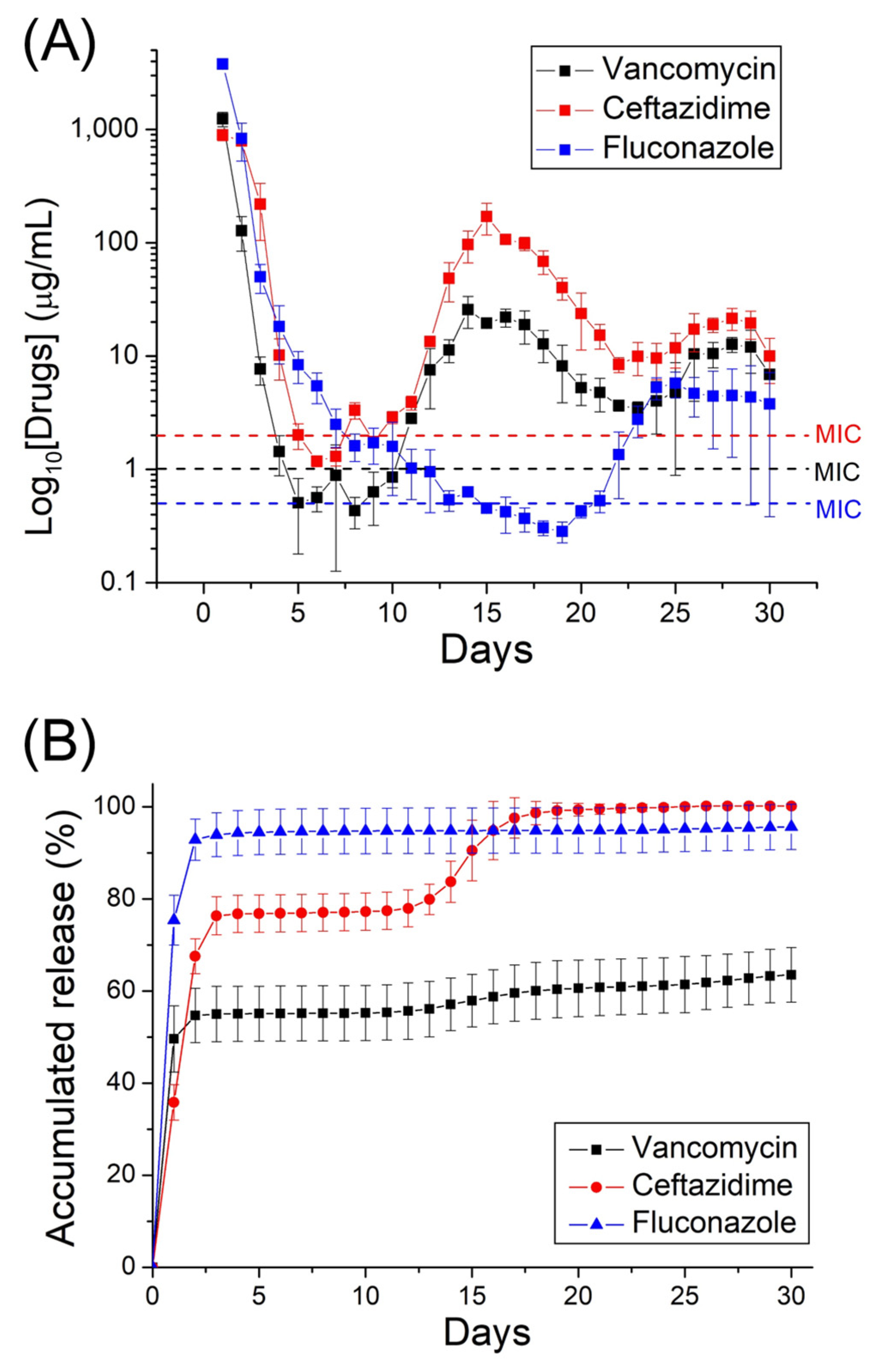
2.2. In Vitro and In Vivo Release Patterns of Antimicrobial Agents
2.3. Histological Analysis
3. Discussion
4. Material and Methods
4.1. Manufacturing of Hybrid Drug-Loaded Nanofibers
4.2. Assessment of Electrospun Nanofibers
4.3. Entrapment Efficiency
4.4. In Vitro Pharmaceutical Discharge
4.5. In Vivo Investigations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pincher, B.; Fenton, C.; Jeyapalan, R.; Barlow, G.; Sharma, H.K. A systematic review of the single-stage treatment of chronic osteomyelitis. J. Orthop. Surg. Res. 2019, 14, 393. [Google Scholar] [CrossRef] [PubMed]
- Gamaletsou, M.N.; Walsh, T.J.; Sipsas, N.V. Epidemiology of fungal osteomyelitis. Curr. Fungal. Infect. Rep. 2014, 8, 262–270. [Google Scholar] [CrossRef]
- Desai, J.V.; Mitchell, A.P.; Andes, D.R. Fungal biofilms, drug resistance, and recurrent infection. Cold Spring Harb. Perspect. Med. 2014, 4, a019729. [Google Scholar] [CrossRef]
- Sanz-Rodriguez, C.; Hernandez-Surmann, F.; Bueno, A.G.; Goizueta, C.; Noguerado, A. Candida and bacterial mandibular osteomyelitis in an AIDS patient. Eur. J. Clin. Microbiol. Infect. Dis. 1998, 17, 531–532. [Google Scholar] [CrossRef]
- Lerch, K.; Kalteis, T.; Schubert, T.; Lehn, N.; Grifka, J. Prosthetic joint infections with osteomyelitis due to Candida albicans. Mycoses 2003, 46, 462–466. [Google Scholar] [CrossRef] [PubMed]
- Scheunemann, G.; Fortes, B.N.; Lincopan, N.; Ishida, K. Caspofungin inhibits mixed biofilms of Candida albicans and methicillin-resistant Staphylococcus aureus and displays effectiveness in coinfected Galleria mellonella larvae. Microbiol. Spectr. 2021, 9, e0074421. [Google Scholar] [CrossRef] [PubMed]
- Kong, E.F.; Tsui, C.; Kucharíková, S.; Andes, D.; Van Dijck, P.; Jabra-Rizk, M.A. Commensal protection of Staphylococcus aureus against antimicrobials by Candida albicans biofilm matrix. mBio 2016, 7, e01365-16. [Google Scholar] [CrossRef]
- Eichelberger, K.R.; Cassat, J.E. Metabolic adaptations during Staphylococcus aureus and Candida albicans co-Infection. Front. Immunol. 2021, 12, 797550. [Google Scholar] [CrossRef]
- Høiby, N.; Bjarnsholt, T.; Givskov, M.; Molin, S.; Ciofu, O. Antibiotic resistance of bacterial biofilms. Int. J. Antimicrob. Agents 2010, 35, 322–332. [Google Scholar] [CrossRef]
- Kuhn, D.M.; George, T.; Chandra, J.; Mukherjee, P.K.; Ghannoum, M.A. Antifungal susceptibility of Candida biofilms: Unique efficacy of amphotericin B lipid formulations and echinocandins. Antimicrob. Agents Chemother. 2002, 46, 1773–1780. [Google Scholar] [CrossRef]
- Barakat, A.; Schilling, W.H.K.; Sharma, S.; Guryel, E.; Freeman, R. Chronic osteomyelitis: A review on current concepts and trends in treatment. Orthop. Trauma 2019, 33, 181–187. [Google Scholar] [CrossRef]
- Hsu, Y.H.; Chen, H.Y.; Chen, J.C.; Yu, Y.H.; Chou, Y.C.; Ueng, S.W.N.; Liu, S.J. Resorbable beads provide extended release of antifungal medication: In vitro and in vivo analyses. Pharmaceutics 2019, 11, 550. [Google Scholar] [CrossRef] [PubMed]
- Mader, J.T.; Calhoun, J.; Cobos, J. In vitro evaluation of antibiotic diffusion from antibiotic-impregnated biodegradable beads and polymethylmethacrylate beads. Antimicrob. Agents Chemother. 1997, 41, 415–418. [Google Scholar] [CrossRef] [PubMed]
- Kontogiannopoulos, K.N.; Assimopoulou, A.N.; Tsivintzelis, I.; Panayiotou, C.; Papageorgiou, V.P. Electrospun fiber mats containing shikonin and derivatives with potential biomedical applications. Int. J. Pharm. 2011, 409, 216–228. [Google Scholar] [CrossRef]
- Hsu, Y.H.; Hu, C.C.; Hsieh, P.H.; Shih, H.N.; Ueng, S.W.N.; Chang, Y. Vancomycin and ceftazidime in bone cement as a potentially effective treatment for knee periprosthetic joint infection. J. Bone Joint Surg. Am. 2017, 99, 223–231. [Google Scholar] [CrossRef]
- Wu, M.H.; Hsu, K.Y. Candidal arthritis in revision knee arthroplasty successfully treated with sequential parenteral-oral fluconazole and amphotericin B-loaded cement spacer. Knee Surg. Sports Traumatol. Arthrosc. 2011, 19, 273–276. [Google Scholar] [CrossRef]
- Sealy, P.I.; Nguyen, C.; Tucci, M.; Benghuzzi, H.; Cleary, J.D. Delivery of antifungal agents using bioactive and non-bioactive bone cements. Ann. Pharmacother. 2009, 43, 1606–1615. [Google Scholar] [CrossRef]
- Goss, B.; Lutton, C.; Weinrauch, P.; Jabur, M.; Gillett, G.; Crawford, R. Elution and mechanical properties of antifungal bone cement. J. Arthroplast. 2007, 22, 902–908. [Google Scholar] [CrossRef]
- Liu, S.J.; Ueng, S.W.N.; Lin, S.S.; Chan, E.C. In vivo release of vancomycin from biodegradable beads. J. Biomed. Mater. Res. 2002, 63, 807–813. [Google Scholar] [CrossRef]
- Hsu, Y.H.; Chen, D.W.; Li, M.J.; Yu, Y.H.; Chou, Y.C.; Liu, S.J. Sustained delivery of analgesic and antimicrobial agents to the knee joint by direct injections of electrosprayed multipharmaceutical-loaded nano/microparticles. Polymers 2018, 10, 890. [Google Scholar] [CrossRef]
- Islam, M.S.; Ang, B.C.; Andriyana, A.; Afifi, A.M. A review on fabrication of nanofibers via electrospinning and their applications. SN Appl. Sci. 2019, 1, 1248. [Google Scholar] [CrossRef]
- Fadil, F.; Affandi, N.D.N.; Misnon, M.I.; Bonnia, N.N.; Harun, A.M.; Alam, M.K. Review on electrospun nanofiber-applied products. Polymers 2021, 13, 2087. [Google Scholar] [CrossRef] [PubMed]
- Torres-Martinez, E.J.; Bravo, J.M.C.; Medina, A.S.; Gonzalez, G.L.P.; Gomez, L.J.V. A summary of electrospun nanofibers as drug delivery system: Drugs loaded and biopolymers used as matrices. Curr. Drug Deliv. 2018, 15, 1360–1374. [Google Scholar] [CrossRef] [PubMed]
- Hrib, J.; Sirc, J.; Hobzova, R.; Hampejsova, Z.; Bosakova, Z.; Munzarova, M.; Michalek, J. Nanofibers for drug delivery—Incorporation and release of model molecules, influence of molecular weight and polymer structure. Beilstein J. Nanotechnol. 2015, 6, 1939–1945. [Google Scholar] [CrossRef]
- Cleeton, C.; Keirouz, A.; Chen, X.; Radacsi, N. Electrospun nanofibers for drug delivery and biosensing. ACS Biomater. Sci. Eng. 2019, 5, 4183–4205. [Google Scholar] [CrossRef]
- Madhukiran, D.R.; Jha, A.; Kumar, M.; Ajmal, G.; Bonde, G.V.; Mishra, B. Electrospun nanofiber-based drug delivery platform: Advances in diabetic foot ulcer management. Expert Opin. Drug Deliv. 2021, 18, 25–42. [Google Scholar] [CrossRef] [PubMed]
- Ghitman, J.; Biru, E.I.; Stan, R.; Iovu, H. Review of hybrid PLGA nanoparticles: Future of smart drug delivery and theranostics medicine. Mater. Des. 2020, 193, 108805. [Google Scholar] [CrossRef]
- Chiu, H.I.; Samad, N.A.; Fang, L.; Lim, V. Cytotoxicity of targeted PLGA nanoparticles: A systematic review. RSC Adv. 2021, 11, 9433–9449. [Google Scholar] [CrossRef] [PubMed]
- Hagbani, T.A.; Yadav, H.; Moin, A.; Lila, A.S.A.; Mehmood, K.; Alshammari, F.; Khan, S.; Khafagy, E.S.; Hussain, T.; Rizvi, S.M.D.; et al. Enhancement of vancomycin potential against pathogenic bacterial strains via gold nano-formulations: A nano-antibiotic approach. Materials 2022, 15, 1108. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, Y.; Yan, Z.; Liao, S. Assay of ceftazidime and cefepime based on fluorescence quenching of carbon quantum dots. Luminescence 2015, 30, 1133–1138. [Google Scholar] [CrossRef]
- Atay, O.; Selck, F. Quantitative determination of fluconazole by infrared spectrophotometry. Anal. Lett. 1996, 29, 2163–2176. [Google Scholar] [CrossRef]
- Zhou, J.; Fang, T.; Wen, J.; Shao, Z.; Dong, J. Silk coating on poly(ε-caprolactone) microspheres for the delayed release of vancomycin. J. Microencapsul. 2011, 28, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Safdari, M.; Shakiba, E.; Kiaie, S.H.; Fattahi, A. Preparation and characterization of ceftazidime loaded electrospun silk fibroin/gelatin mat for wound dressing. Fiber Polym. 2016, 17, 744–750. [Google Scholar] [CrossRef]
- Alkhamis, K.A.; Obaidat, A.A.; Nuseirat, A.F. Solid-state characterization of fluconazole. Pharm. Dev. Technol. 2002, 7, 491–503. [Google Scholar] [CrossRef] [PubMed]
- Shirtliff, M.E.; Peters, B.M.; Jabra-Rizk, M.A. Cross-kingdom interactions: Candida albicans and bacteria. FEMS Microbiol Lett 2009, 299, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Jorge, L.S.; Chueire, A.G.; Rossit, A.R.B. Osteomyelitis: A current challenge. Braz. J. Infect. Dis. 2010, 14, 310–315. [Google Scholar] [CrossRef]
- Hatzenbuehler, J.; Pulling, T.J. Diagnosis and management of osteomyelitis. Am. Fam. Physician 2011, 84, 1027–1033. [Google Scholar] [PubMed]
- Blyth, C.C.; Gomes, L.; Sorrell, T.C.; da Cruz, M.; Sud, A.; Chen, S.C. Skull-base osteomyelitis: Fungal vs. bacterial infection. Clin. Microbiol. Infect. 2011, 17, 306–311. [Google Scholar] [CrossRef]
- Stewart, R.L.; Cox, J.T.; Volgas, D.; Stannard, J.; Duffy, L.; Waites, K.B.; Chu, T.M. The use of a biodegradable, load-bearing scaffold as a carrier for antibiotics in an infected open fracture model. J. Orthop. Trauma 2010, 24, 587–591. [Google Scholar] [CrossRef]
- Khan, A.A.; Alanazi, A.M.; Jabeen, M.; Khan, S.; Malik, A. Additive potential of combination therapy against cryptococcosis employing a novel amphotericin B and fluconazole loaded dual delivery system. Eur. J. Pharm. Sci. 2018, 119, 171–178. [Google Scholar] [CrossRef]
- Hsu, Y.H.; Chen, D.W.C.; Tai, C.D.; Chou, Y.C.; Liu, S.J.; Ueng, S.W.N.; Chan, E.C. Biodegradable drug-eluting nanofiber-enveloped implants for sustained release of high bactericidal concentrations of vancomycin and ceftazidime: In vitro and in vivo studies. Int. J. Nanomed. 2014, 9, 4347–4355. [Google Scholar] [CrossRef]
- Chen, Y.P.; Liu, H.Y.; Liu, Y.W.; Lee, T.Y.; Liu, S.J. Determination of electrospinning parameters’ strength in Poly(D,L)-lactide-co-glycolide micro/nanofibers diameter tailoring. J. Nanomater. 2019, 2019, 2626085. [Google Scholar] [CrossRef]
- Liu, S.J.; Kau, Y.C.; Chou, C.Y.; Chen, J.K.; Wu, R.C.; Yeh, W.L. Electrospun PLGA/collagen nanofibrous membrane as early-stage wound dressing. J. Membr. Sci. 2010, 355, 53–59. [Google Scholar] [CrossRef]
- Hsu, Y.H.; Lin, C.T.; Yu, Y.H.; Chou, Y.C.; Liu, S.J.; Chan, E.C. Dual delivery of active antibactericidal agents and bone morphogenetic protein at sustainable high concentrations using biodegradable sheath-core-structured drug-eluting nanofibers. Int. J. Nanomed. 2016, 11, 3927–3937. [Google Scholar] [CrossRef]
- Luraghi, A.; Peri, F.; Moroni, L. Electrospinning for drug delivery applications: A review. J. Control. Release 2021, 334, 463–484. [Google Scholar] [CrossRef]
- Martínez-Pérez, C.A. Electrospinning: A promising technique for drug delivery systems. Rev. Adv. Mater. Sci. 2020, 59, 441–454. [Google Scholar] [CrossRef]
- Varma, M.V.; Kaushal, A.M.; Garg, A.; Garg, S. Factors Affecting mechanism and kinetics of drug release from matrix-based oral controlled drug delivery systems. Am. J. Drug Deliv. 2004, 2, 43–57. [Google Scholar] [CrossRef]
- Liu, K.S.; Kao, C.W.; Tseng, Y.Y.; Chen, S.K.; Lin, Y.T.; Lu, C.J.; Liu, S.J. Assessment of antimicrobial agents, analgesics, and epidermal growth factors-embedded anti-adhesive poly (lactic-co-glycolic acid) nanofibrous membranes: In vitro and in vivo studies. Int. J. Nanomed. 2021, 16, 4471–4480. [Google Scholar] [CrossRef]
- Eccleston, D.S.; Horrigan, M.C.; Ellis, S.G. Rationale for local drug delivery. Semin. Interv. Cardiol. 1996, 1, 8–16. [Google Scholar]
- McLaren, A.C. Alternative materials to acrylic bone cement for delivery of depot antibiotics in orthopaedic infections. Clin. Orthop. Relat. Res. 2004, 427, 101–106. [Google Scholar] [CrossRef]
- Roy, M.E.; Peppers, M.P.; Whiteside, L.A.; LaZear, R.M. Vancomycin concentration in synovial fluid: Direct injection into the knee vs. intravenous infusion. J. Arthroplast. 2014, 29, 564–568. [Google Scholar] [CrossRef] [PubMed]
- Haidar, R.; Der Boghossian, A.; Atiyeh, B. Duration of post-surgical antibiotics in chronic osteomyelitis: Empiric or evidence-based? Int. J. Infect. Dis. 2010, 14, e752–e758. [Google Scholar] [CrossRef] [PubMed]
- Lou Aie Banerjee, P.; Drusano, G.L.; Shayegani, M.; Miller, M.H. Interaction between fluconazole and amphotericin B in mice with systemic infection due to fluconazole-susceptible or -resistant strains of Candida albicans. Antimicrob. Agents Chemother. 1999, 43, 2841–2847. [Google Scholar] [CrossRef]
- Ye, P.; Wei, S.; Luo, C.; Wang, Q.; Li, A.; Wei, F. Long-term effect against Methicillin-resistant Staphylococcus aureus of emodin released from coaxial electrospinning nanofiber membranes with a biphasic profile. Biomolecules 2020, 10, 362. [Google Scholar] [CrossRef] [PubMed]



| Drug | Membrane Weight (mg) | Theoretical Drug Weight (μg) | Determined Drug Weight (μg) | Entrapment Efficiency (%) |
|---|---|---|---|---|
| Vancomycin | 17.3 | 4325 | 2694.5 | 62.3 |
| Ceftazidime | 17.3 | 4325 | 4290.4 | 99.2 |
| Fluconazole | 15.8 | 7900 | 7805.2 | 98.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, Y.-H.; Yu, Y.-H.; Chou, Y.-C.; Lu, C.-J.; Lin, Y.-T.; Ueng, S.W.-N.; Liu, S.-J. Sustained Release of Antifungal and Antibacterial Agents from Novel Hybrid Degradable Nanofibers for the Treatment of Polymicrobial Osteomyelitis. Int. J. Mol. Sci. 2023, 24, 3254. https://doi.org/10.3390/ijms24043254
Hsu Y-H, Yu Y-H, Chou Y-C, Lu C-J, Lin Y-T, Ueng SW-N, Liu S-J. Sustained Release of Antifungal and Antibacterial Agents from Novel Hybrid Degradable Nanofibers for the Treatment of Polymicrobial Osteomyelitis. International Journal of Molecular Sciences. 2023; 24(4):3254. https://doi.org/10.3390/ijms24043254
Chicago/Turabian StyleHsu, Yung-Heng, Yi-Hsun Yu, Ying-Chao Chou, Chia-Jung Lu, Yu-Ting Lin, Steve Wen-Neng Ueng, and Shih-Jung Liu. 2023. "Sustained Release of Antifungal and Antibacterial Agents from Novel Hybrid Degradable Nanofibers for the Treatment of Polymicrobial Osteomyelitis" International Journal of Molecular Sciences 24, no. 4: 3254. https://doi.org/10.3390/ijms24043254
APA StyleHsu, Y.-H., Yu, Y.-H., Chou, Y.-C., Lu, C.-J., Lin, Y.-T., Ueng, S. W.-N., & Liu, S.-J. (2023). Sustained Release of Antifungal and Antibacterial Agents from Novel Hybrid Degradable Nanofibers for the Treatment of Polymicrobial Osteomyelitis. International Journal of Molecular Sciences, 24(4), 3254. https://doi.org/10.3390/ijms24043254






