APE1/Ref-1 Inhibits Adipogenic Transcription Factors during Adipocyte Differentiation in 3T3-L1 Cells
Abstract
1. Introduction
2. Results
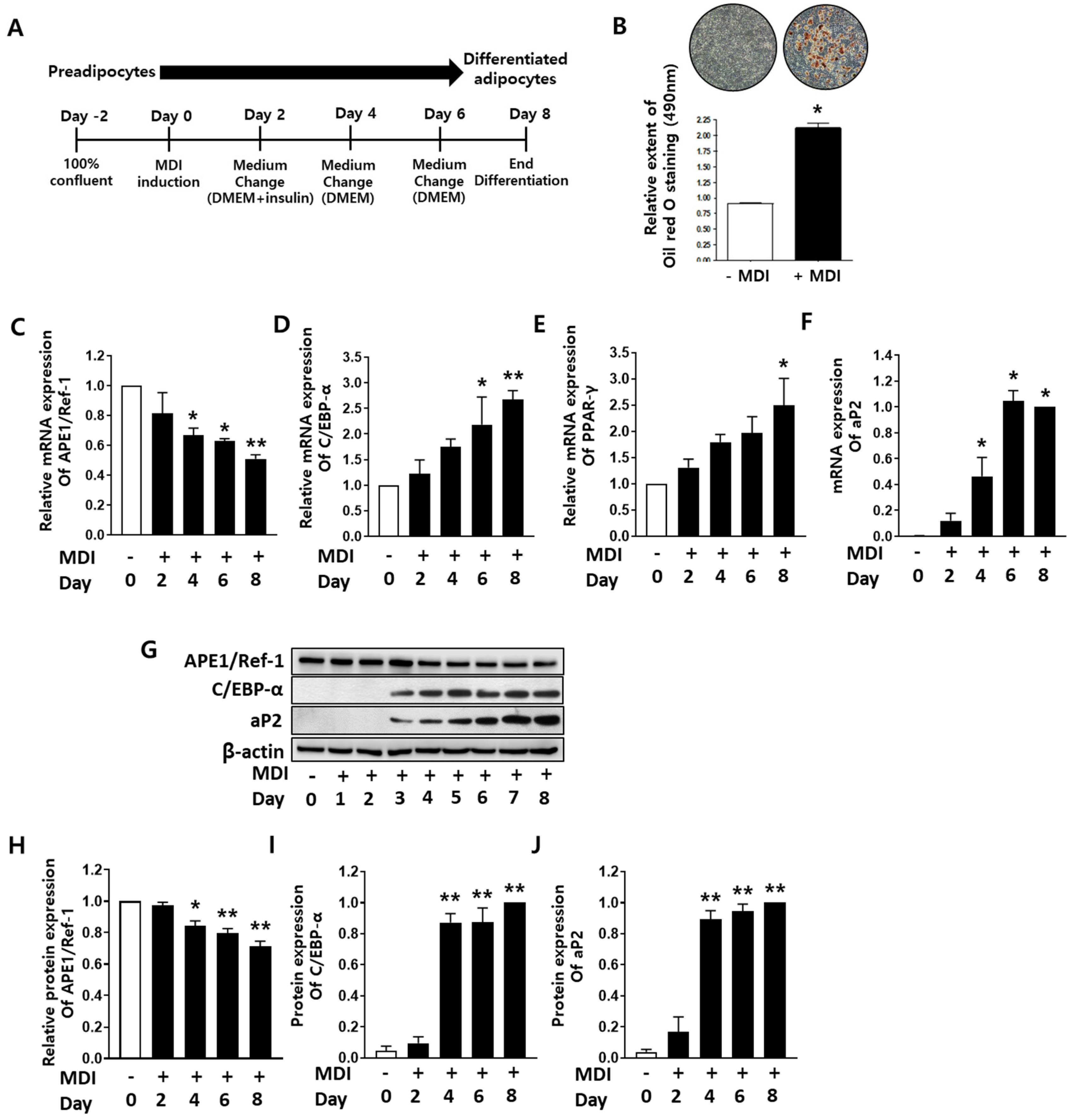
2.1. APE1/Ref-1 Expression Decreased during Adipocyte Differentiation
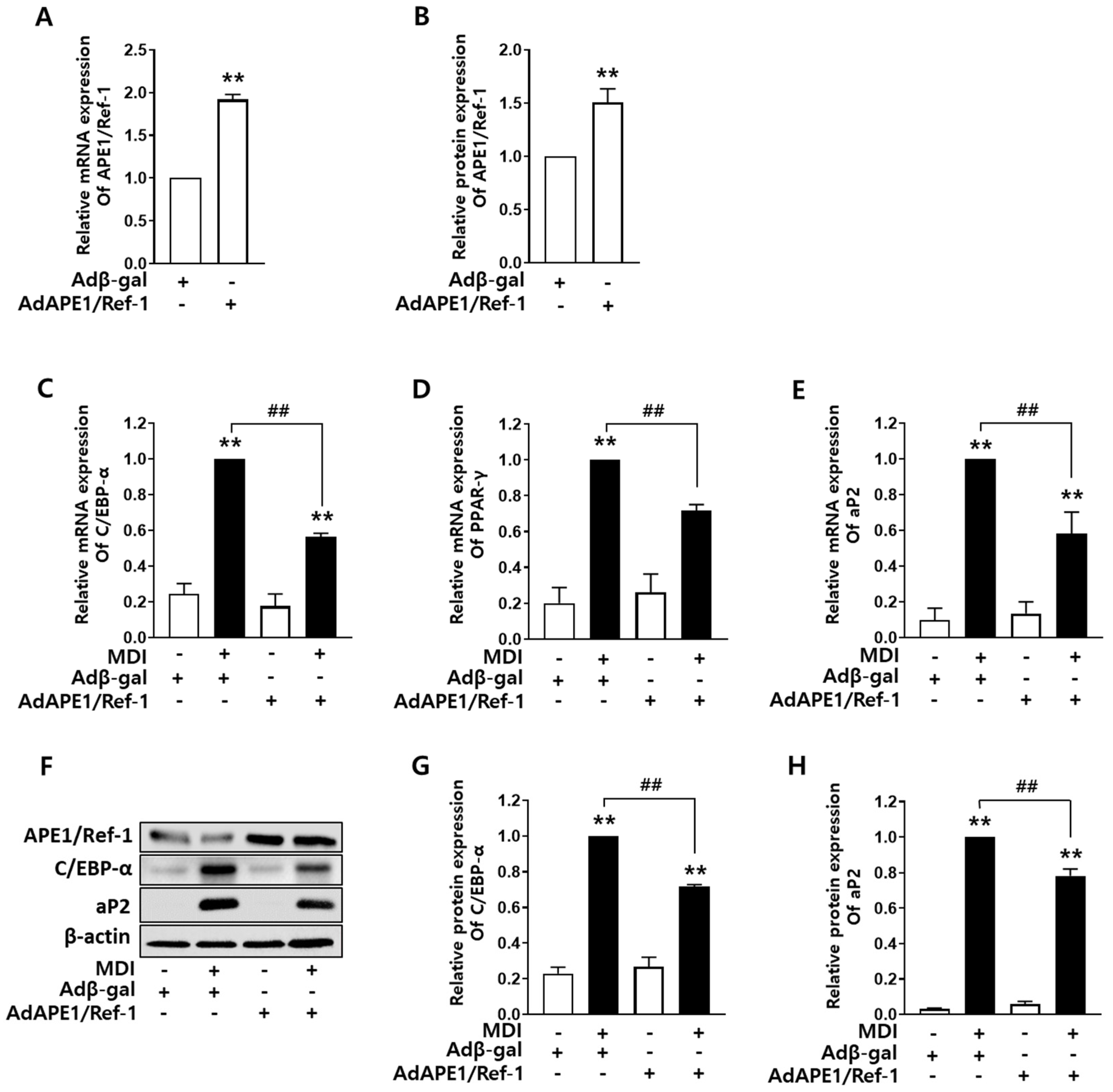
2.2. APE1/Ref-1 Overexpression Decreased Adipocyte Differentiation
2.3. Silencing APE1/Ref-1 Promotes Adipocyte Differentiation
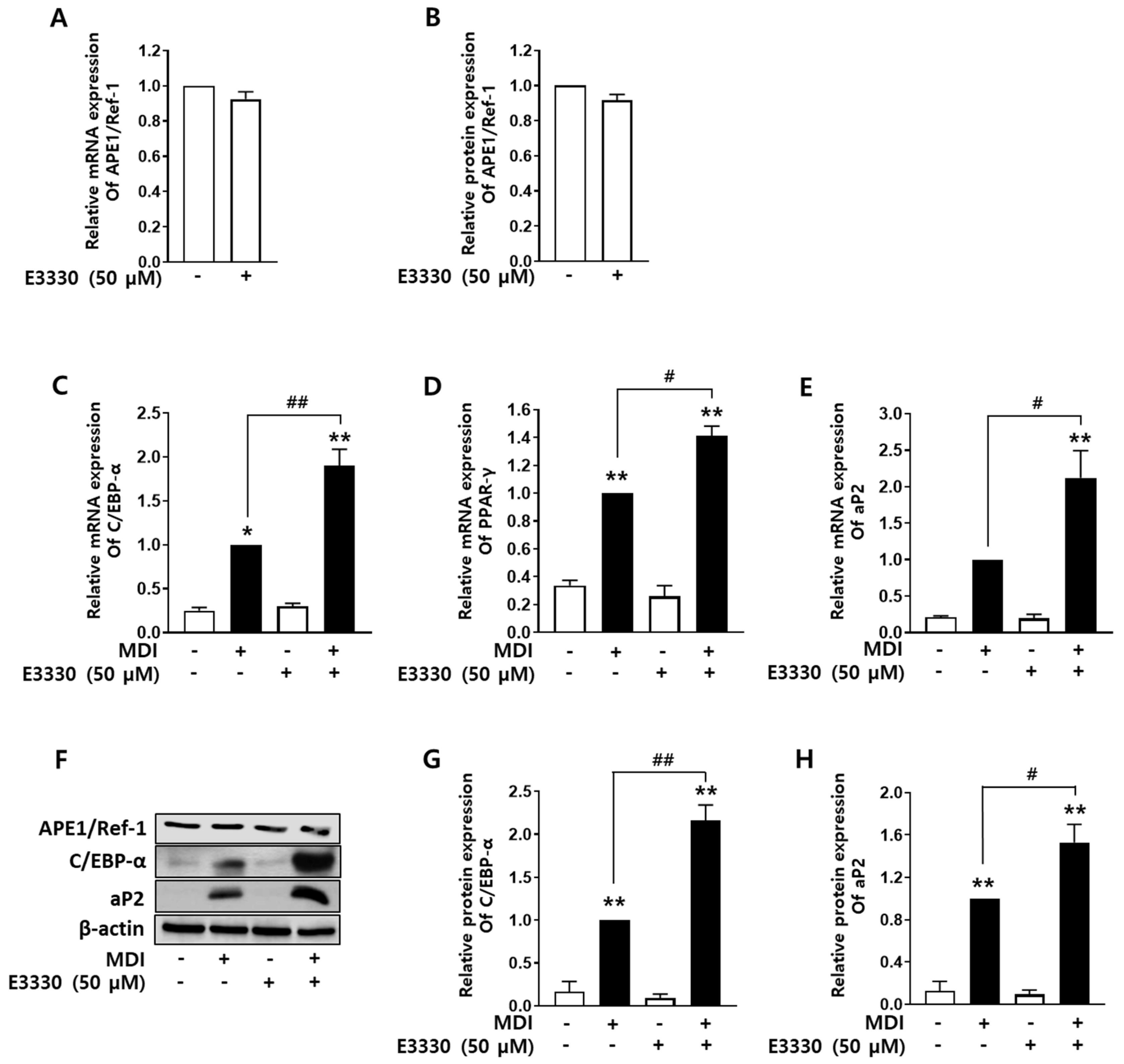
2.4. Redox Inhibition of APE1/Ref-1 Augments Adipocyte Differentiation
3. Discussion
4. Materials and Methods
4.1. Cells and Reagents
4.2. Culture and Adipocyte Differentiation of 3T3-L1 Cells
4.3. Oil Red O Staining
4.4. Transfection of Small-Interfering RNA
4.5. Transfection of Adenoviruses
4.6. Western Blot Analysis
4.7. Quantitative Real-Time Reverse Transcription-Polymerase Chain Reaction (qRT-PCR)
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sarjeant, K.; Stephens, J.M. Adipogenesis. Cold Spring Harb. Perspect. Biol. 2012, 4, a008417. [Google Scholar] [CrossRef]
- Gregoire, F.M.; Smas, C.M.; Sul, H.S. Understanding adipocyte differentiation. Physiol. Rev. 1998, 78, 783–809. [Google Scholar] [CrossRef]
- Hsu, C.-L.; Yen, G.-C. Effects of capsaicin on induction of apoptosis and inhibition of adipogenesis in 3T3-L1 cells. J. Agric. Food Chem. 2007, 55, 1730–1736. [Google Scholar] [CrossRef]
- Song, Y.; Park, H.J.; Kang, S.N.; Jang, S.-H.; Lee, S.-J.; Ko, Y.-G.; Kim, G.-S.; Cho, J.-H. Blueberry peel extracts inhibit adipogenesis in 3T3-L1 cells and reduce high-fat diet-induced obesity. PLoS ONE 2013, 8, e69925. [Google Scholar] [CrossRef]
- Ozaki, M.; Suzuki, S.; Irani, K. Redox factor-1/APE suppresses oxidative stress by inhibiting activity of the rac1 GTPase. FASEB J. 2002, 16, 889–890. [Google Scholar] [CrossRef]
- Lee, K.M.; Lee, E.O.; Lee, Y.R.; Joo, H.K.; Park, M.S.; Kim, C.-S.; Choi, S.; Jeong, J.-O.; Jeon, B.H. APE1/Ref-1 inhibits phosphate-induced calcification and osteoblastic phenotype changes in vascular smooth muscle cells. Int. J. Mol. Sci. 2017, 18, 2053. [Google Scholar] [CrossRef]
- Chen, T.; Liu, Z.; Sun, W.; Li, J.; Liang, Y.; Yang, X.; Xu, Y.; Yu, M.; Tian, W.; Chen, G. Inhibition of Ape1 redox activity promotes odonto/osteogenic differentiation of dental papilla cells. Sci. Rep. 2015, 5, 1–13. [Google Scholar] [CrossRef]
- Lee, Y.R.; Joo, H.K.; Jeon, B.H. The biological role of apurinic/apyrimidinic endonuclease1/redox factor-1 as a therapeutic target for vascular inflammation and as a serologic biomarker. Biomedicines 2020, 8, 57. [Google Scholar] [CrossRef]
- Oliveira, T.T.; Coutinho, L.G.; de Oliveira, L.O.A.; de Souza Timoteo, A.R.; Farias, G.C.; Agnez-Lima, L.F. APE1/Ref-1 Role in Inflammation and Immune Response. Front. Immunol. 2022, 13, 793096. [Google Scholar] [CrossRef]
- Unnikrishnan, A.; Raffoul, J.J.; Patel, H.V.; Prychitko, T.M.; Anyangwe, N.; Meira, L.B.; Friedberg, E.C.; Cabelof, D.C.; Heydari, A.R. Oxidative stress alters base excision repair pathway and increases apoptotic response in apurinic/apyrimidinic endonuclease 1/redox factor-1 haploinsufficient mice. Free Radic. Biol. Med. 2009, 46, 1488–1499. [Google Scholar] [CrossRef]
- Choi, S.; Joo, H.K.; Jeon, B.H. Dynamic regulation of APE1/Ref-1 as a therapeutic target protein. Chonnam Med. J. 2016, 52, 75–80. [Google Scholar] [CrossRef]
- Ahima, R.S.; Flier, J.S. Adipose tissue as an endocrine organ. Trends Endocrinol. Metab. 2000, 11, 327–332. [Google Scholar] [CrossRef]
- Spiegelman, B.M.; Flier, J.S. Adipogenesis and obesity: Rounding out the big picture. Cell 1996, 87, 377–389. [Google Scholar] [CrossRef]
- Farmer, S.R. Transcriptional control of adipocyte formation. Cell Metab. 2006, 4, 263–273. [Google Scholar] [CrossRef]
- Ntambi, J.M.; Young-Cheul, K. Adipocyte differentiation and gene expression. J. Nutr. 2000, 130, 3122S–3126S. [Google Scholar] [CrossRef]
- Rahman, N.; Jeon, M.; Kim, Y.S. Delphinidin, a major anthocyanin, inhibits 3T3-L1 pre-adipocyte differentiation through activation of Wnt/β-catenin signaling. Biofactors 2016, 42, 49–59. [Google Scholar]
- Zou, G.-M.; Luo, M.-H.; Reed, A.; Kelley, M.R.; Yoder, M.C. Ape1 regulates hematopoietic differentiation of embryonic stem cells through its redox functional domain. Blood 2007, 109, 1917–1922. [Google Scholar] [CrossRef]
- Jeon, B.H.; Irani, K. APE1/Ref-1: Versatility in progress. Antioxid. Redox Signal. 2009, 11, 571–574. [Google Scholar] [CrossRef]
- Campos, J.T.A.d.M.; Oliveira, M.S.d.; Soares, L.P.; Medeiros, K.A.d.; Campos, L.R.d.S.; Lima, J.G. DNA repair-related genes and adipogenesis: Lessons from congenital lipodystrophies. Genet. Mol. Biol. 2022, 45, e20220086. [Google Scholar] [CrossRef]
- Tell, G.; Quadrifoglio, F.; Tiribelli, C.; Kelley, M.R. The many functions of APE1/Ref-1: Not only a DNA repair enzyme. Antioxid. Redox Signal. 2009, 11, 601–619. [Google Scholar] [CrossRef]
- Fishel, M.L.; Kelley, M.R. The DNA base excision repair protein Ape1/Ref-1 as a therapeutic and chemopreventive target. Mol. Asp. Med. 2007, 28, 375–395. [Google Scholar] [CrossRef]
- Moreno-Navarrete, J.M.; Fernández-Real, J.M. Adipocyte differentiation. In Adipose Tissue Biology; Springer: New York, NY, USA, 2017; pp. 69–90. [Google Scholar]
- Fève, B. Adipogenesis: Cellular and molecular aspects. Best Pract. Res. Clin. Endocrinol. Metab. 2005, 19, 483–499. [Google Scholar] [CrossRef]
- Domenis, R.; Bergamin, N.; Gianfranceschi, G.; Vascotto, C.; Romanello, M.; Rigo, S.; Vagnarelli, G.; Faggiani, M.; Parodi, P.; Kelley, M.R. The redox function of APE1 is involved in the differentiation process of stem cells toward a neuronal cell fate. PLoS ONE 2014, 9, e89232. [Google Scholar] [CrossRef]
- Ali, A.T.; Hochfeld, W.E.; Myburgh, R.; Pepper, M.S. Adipocyte and adipogenesis. Eur. J. Cell Biol. 2013, 92, 229–236. [Google Scholar] [CrossRef]
- Villiers, D.d.; Potgieter, M.; Ambele, M.A.; Adam, L.; Durandt, C.; Pepper, M.S. The role of reactive oxygen species in adipogenic differentiation. Stem Cells Biol. Eng. 2017, 1083, 125–144. [Google Scholar]
- Zhou, X.; Gao, H.; Guo, Y.; Chen, Y.; Ruan, X.Z. Knocking down Stard3 decreases adipogenesis with decreased mitochondrial ROS in 3T3-L1 cells. Biochem. Biophys. Res. Commun. 2018, 504, 387–392. [Google Scholar] [CrossRef]
- Sekiya, M.; Hiraishi, A.; Touyama, M.; Sakamoto, K. Oxidative stress induced lipid accumulation via SREBP1c activation in HepG2 cells. Biochem. Biophys. Res. Commun. 2008, 375, 602–607. [Google Scholar] [CrossRef]
- Lee, H.M.; Jeon, B.H.; Won, K.-J.; Lee, C.-K.; Park, T.-K.; Choi, W.S.; Bae, Y.M.; Kim, H.S.; Lee, S.K.; Park, S.H. Gene Transfer of Redox Factor-1 Inhibits Neointimal Formation: Involvement of Platelet-Derived Growth Factor-β Receptor Signaling via the Inhibition of the Reactive Oxygen Species–Mediated Syk Pathway. Circ. Res. 2009, 104, 219–227. [Google Scholar] [CrossRef]
- Joo, H.K.; Lee, Y.R.; Park, M.S.; Choi, S.; Park, K.; Lee, S.K.; Kim, C.-S.; Park, J.B.; Jeon, B.H. Mitochondrial APE1/Ref-1 suppressed protein kinase C-induced mitochondrial dysfunction in mouse endothelial cells. Mitochondrion 2014, 17, 42–49. [Google Scholar] [CrossRef]
- Gurusamy, N.; Mukherjee, S.; Lekli, I.; Bearzi, C.; Bardelli, S.; Das, D.K. Inhibition of ref-1 stimulates the production of reactive oxygen species and induces differentiation in adult cardiac stem cells. Antioxid. Redox Signal. 2009, 11, 589–599. [Google Scholar] [CrossRef]
- Imhoff, B.R.; Hansen, J.M. Extracellular redox environments regulate adipocyte differentiation. Differentiation 2010, 80, 31–39. [Google Scholar] [CrossRef]
- Orlicky, D.J.; Schaack, J. Adenovirus transduction of 3T3-L1 cells. J. Lipid Res. 2001, 42, 460–466. [Google Scholar] [CrossRef]
- Ross, S.A.; Song, X.; Burney, M.W.; Kasai, Y.; Orlicky, D.J. Efficient adenovirus transduction of 3T3-L1 adipocytes stably expressing coxsackie-adenovirus receptor. Biochem. Biophys. Res. Commun. 2003, 302, 354–358. [Google Scholar] [CrossRef]
- Park, M.S.; Kim, C.-S.; Joo, H.K.; Lee, Y.R.; Kang, G.; Kim, S.J.; Choi, S.; Lee, S.D.; Park, J.B.; Jeon, B.H. Cytoplasmic localization and redox cysteine residue of APE1/Ref-1 are associated with its anti-inflammatory activity in cultured endothelial cells. Mol. Cells 2013, 36, 439–445. [Google Scholar] [CrossRef]
- Zou, G.-M.; Maitra, A. Small-molecule inhibitor of the AP endonuclease 1/REF-1 E3330 inhibits pancreatic cancer cell growth and migration. Mol. Cancer Ther. 2008, 7, 2012–2021. [Google Scholar] [CrossRef] [PubMed]
- Mijit, M.; Wireman, R.; Armstrong, L.; Gampala, S.; Hassan, Z.; Schneeweis, C.; Schneider, G.; Zhang, C.; Fishel, M.L.; Kelley, M.R. RelA Is an Essential Target for Enhancing Cellular Responses to the DNA Repair/Ref-1 Redox Signaling Protein and Restoring Perturbated Cellular Redox Homeostasis in Mouse PDAC Cells. Front. Oncol. 2022, 12, 826617. [Google Scholar] [CrossRef]
- Zebisch, K.; Voigt, V.; Wabitsch, M.; Brandsch, M. Protocol for effective differentiation of 3T3-L1 cells to adipocytes. Anal. Biochem. 2012, 425, 88–90. [Google Scholar] [CrossRef]
- Kim, S.; Lee, Y.-R.; Lee, E.-O.; Jin, H.; Choi, Y.-H.; Joo, H.-K.; Jeon, B.-H. Capsanthin Inhibits Atherosclerotic Plaque Formation and Vascular Inflammation in ApoE−/−Mice. Biomedicines 2022, 10, 1780. [Google Scholar] [CrossRef]
- Kim, S.J.; Kim, T.; Choi, H.N.; Cho, E.J.; Park, J.B.; Jeon, B.H.; Do Lee, S. TonEBP suppresses adipocyte differentiation via modulation of early signaling in 3T3-L1 cells. Korean J. Physiol. Pharmacol. Off. J. Korean Physiol. Soc. Korean Soc. Pharmacol. 2016, 20, 649. [Google Scholar] [CrossRef]
- Kraus, N.A.; Ehebauer, F.; Zapp, B.; Rudolphi, B.; Kraus, B.J.; Kraus, D. Quantitative assessment of adipocyte differentiation in cell culture. Adipocyte 2016, 5, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Joo, H.K.; Lee, Y.R.; Lee, E.-O.; Park, M.S.; Choi, S.; Kim, C.-S.; Park, J.-B.; Jeon, B.H. The extracellular role of Ref-1 as anti-inflammatory function in lipopolysaccharide-induced septic mice. Free Radic. Biol. Med. 2019, 139, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-R.; Joo, H.-K.; Lee, E.-O.; Kim, S.; Jin, H.; Choi, Y.-H.; Kim, C.-S.; Jeon, B.-H. 17 β-Estradiol Increases APE1/Ref-1 Secretion in Vascular Endothelial Cells and Ovariectomized Mice: Involvement of Calcium-Dependent Exosome Pathway. Biomedicines 2021, 9, 1040. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, E.-O.; Joo, H.-K.; Lee, Y.-R.; Kim, S.; Lee, K.-H.; Lee, S.-D.; Jeon, B.-H. APE1/Ref-1 Inhibits Adipogenic Transcription Factors during Adipocyte Differentiation in 3T3-L1 Cells. Int. J. Mol. Sci. 2023, 24, 3251. https://doi.org/10.3390/ijms24043251
Lee E-O, Joo H-K, Lee Y-R, Kim S, Lee K-H, Lee S-D, Jeon B-H. APE1/Ref-1 Inhibits Adipogenic Transcription Factors during Adipocyte Differentiation in 3T3-L1 Cells. International Journal of Molecular Sciences. 2023; 24(4):3251. https://doi.org/10.3390/ijms24043251
Chicago/Turabian StyleLee, Eun-Ok, Hee-Kyoung Joo, Yu-Ran Lee, Sungmin Kim, Kwon-Ho Lee, Sang-Do Lee, and Byeong-Hwa Jeon. 2023. "APE1/Ref-1 Inhibits Adipogenic Transcription Factors during Adipocyte Differentiation in 3T3-L1 Cells" International Journal of Molecular Sciences 24, no. 4: 3251. https://doi.org/10.3390/ijms24043251
APA StyleLee, E.-O., Joo, H.-K., Lee, Y.-R., Kim, S., Lee, K.-H., Lee, S.-D., & Jeon, B.-H. (2023). APE1/Ref-1 Inhibits Adipogenic Transcription Factors during Adipocyte Differentiation in 3T3-L1 Cells. International Journal of Molecular Sciences, 24(4), 3251. https://doi.org/10.3390/ijms24043251







