Fluro-Protein C-Phycocyanin Docked Silver Nanocomposite Accelerates Cell Migration through NFĸB Signaling Pathway
Abstract
1. Introduction
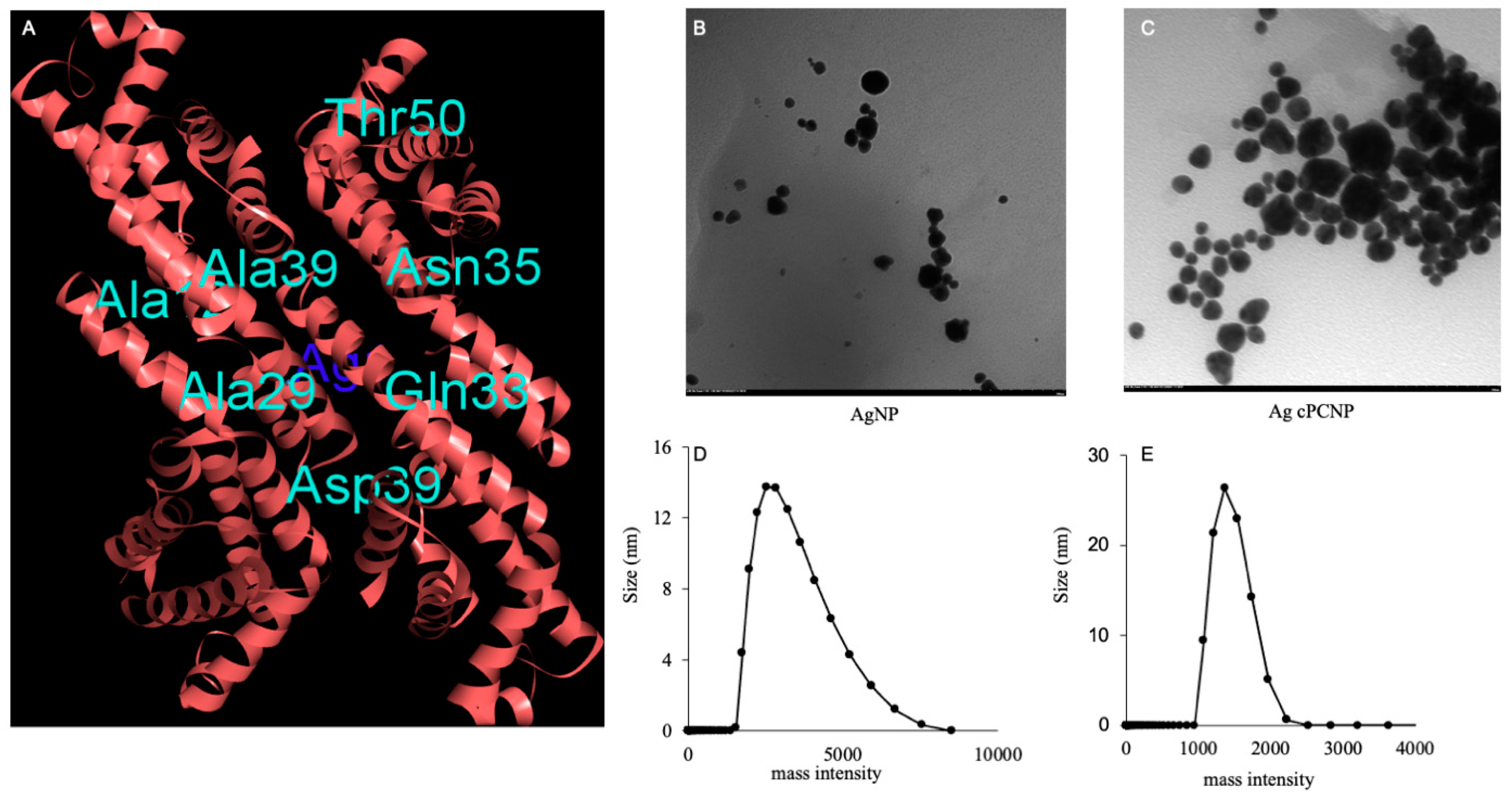
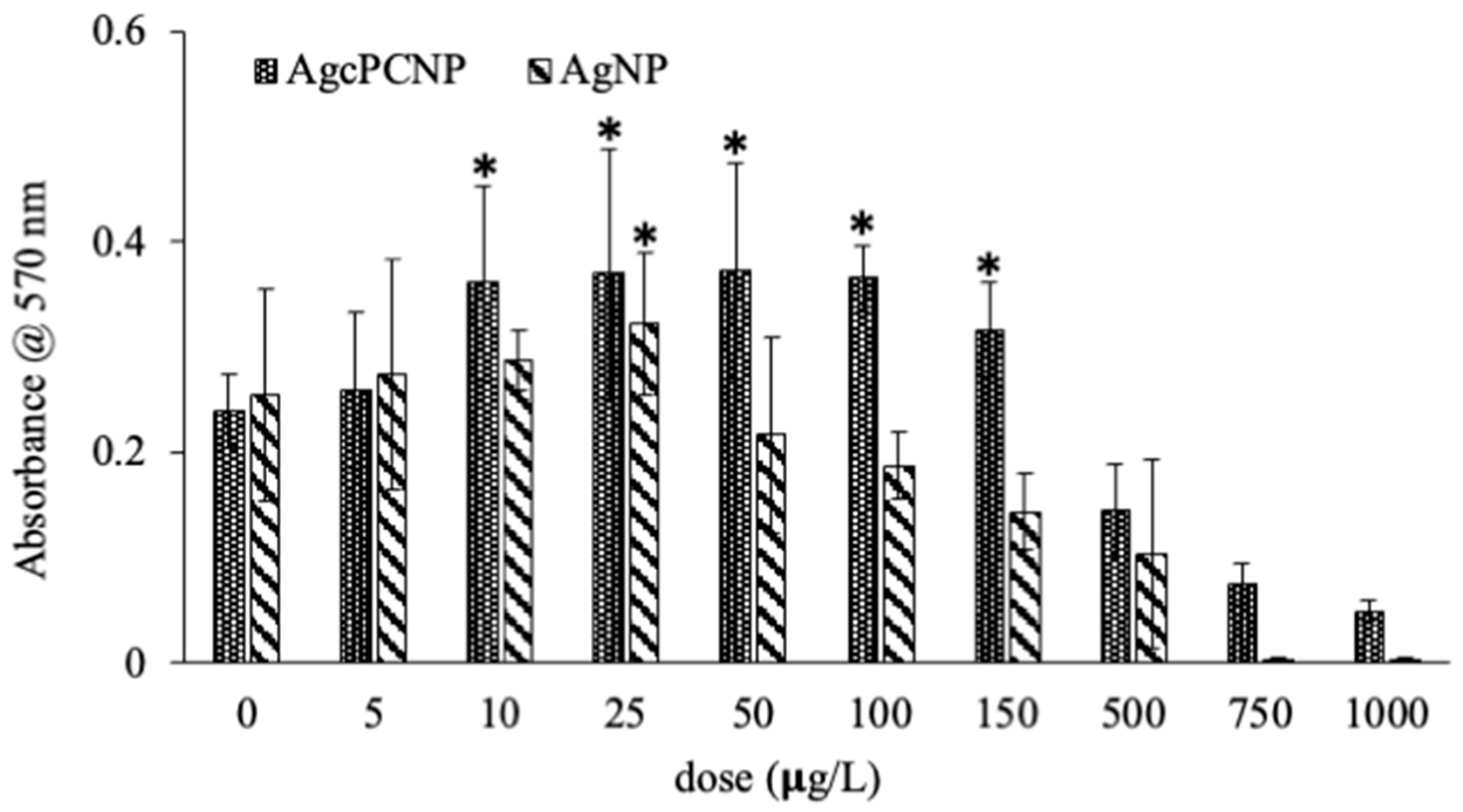
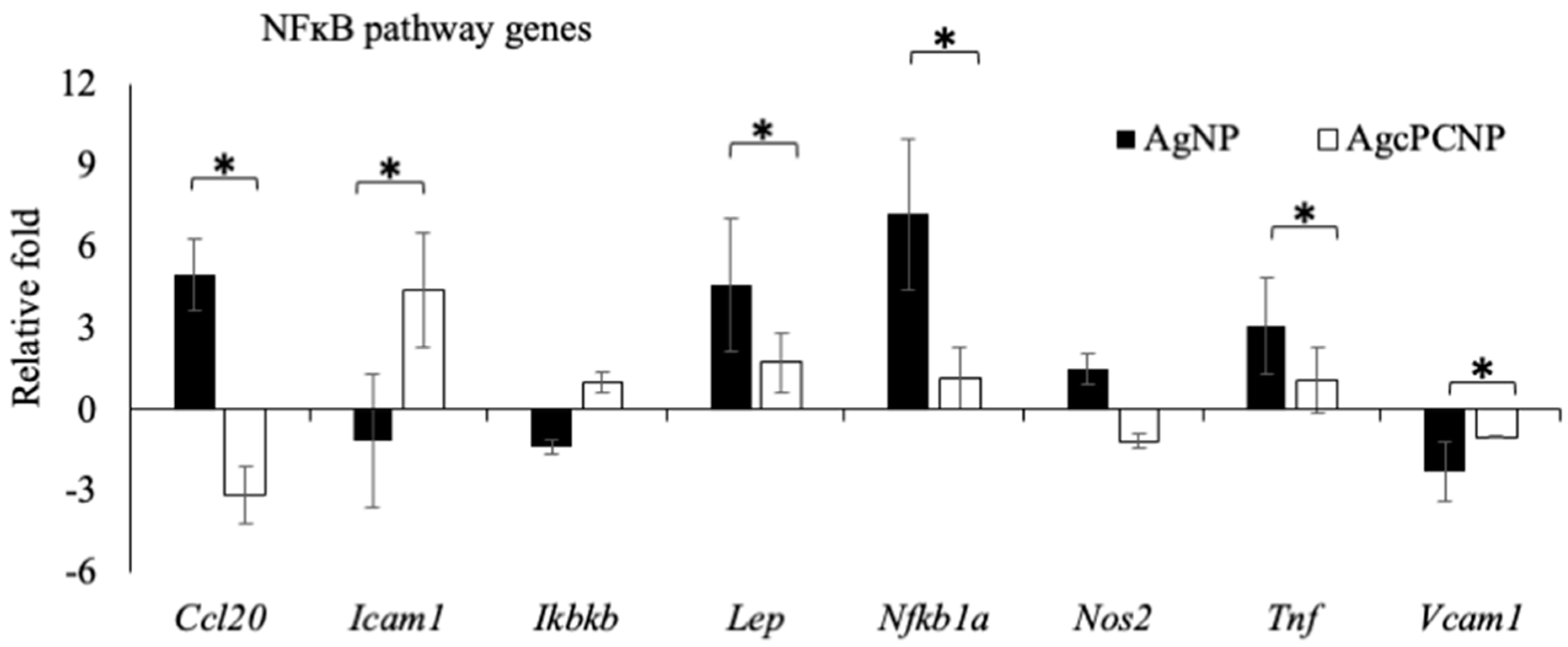
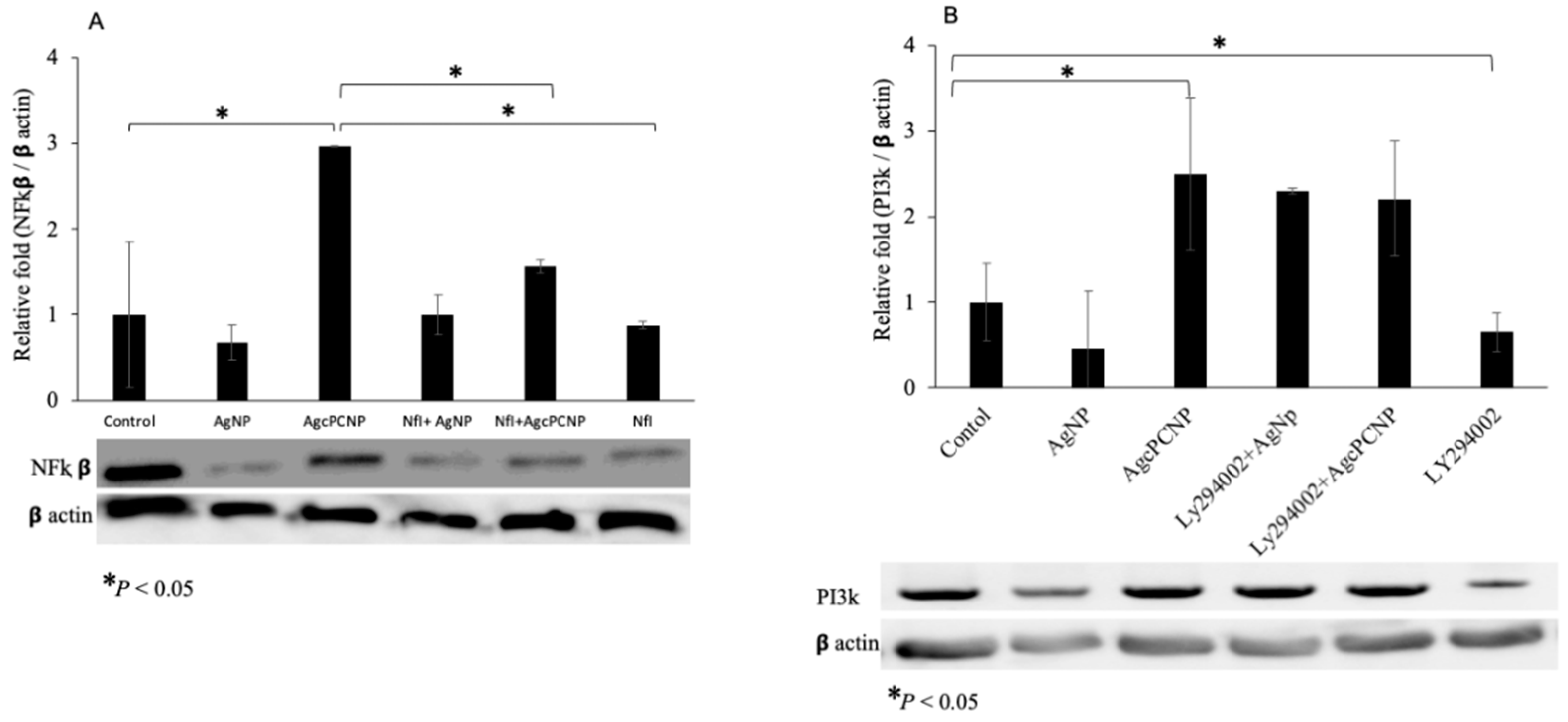
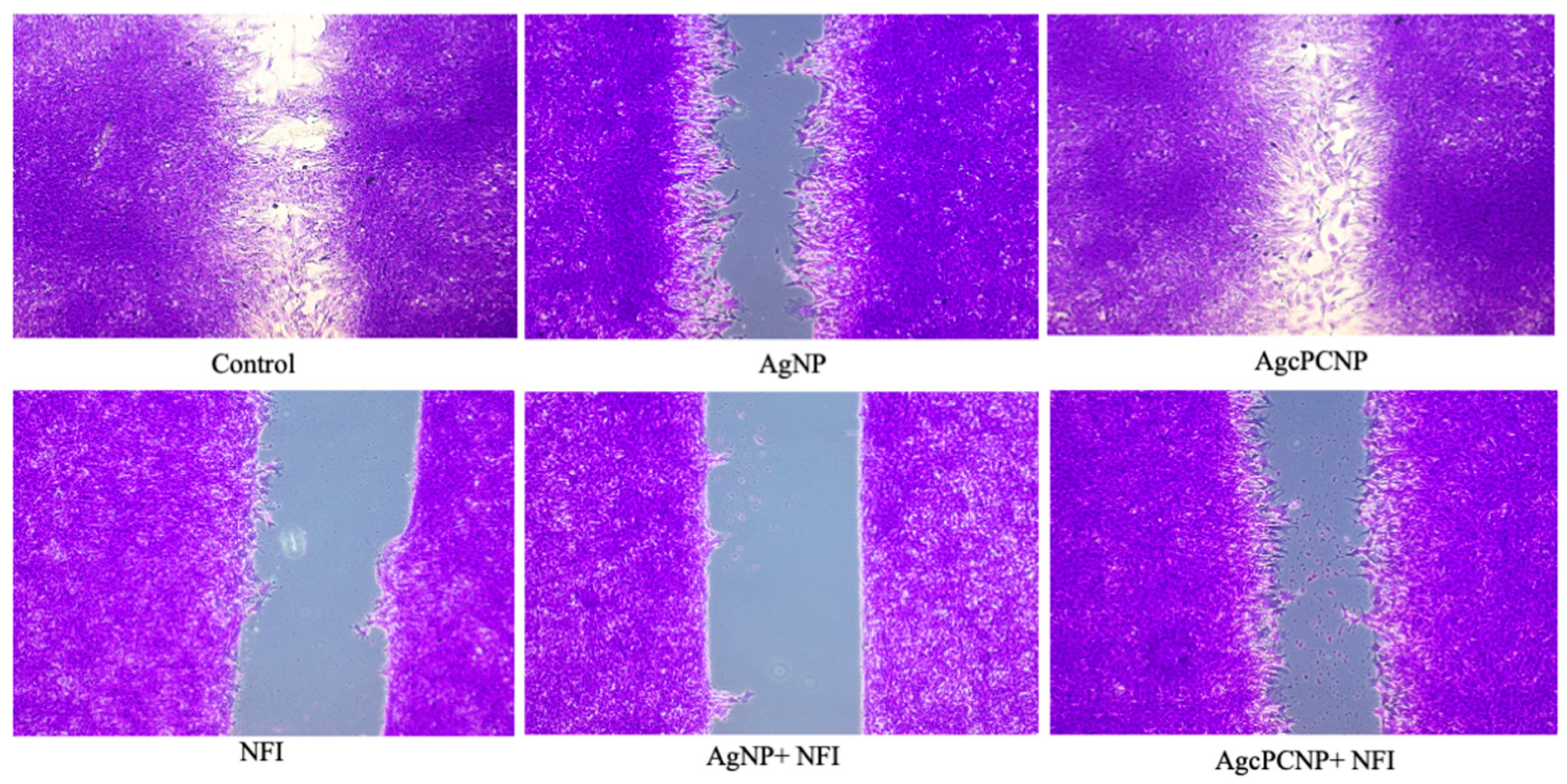
2. Results and Discussion
3. Materials and Method
3.1. Chemicals and Materials
3.2. Cell Culture and Experimental Design
3.3. Bio-Informatic and Molecular Docking Analysis
3.4. Characterization of AgcPCNP (TEM, DLS, ICPS)
3.5. End Point Toxicity Assay
3.6. Radical Scavenging Assay
3.7. c-Phycocyanin Entrapment Assay
3.8. Nanoparticle Ageing and Stability Assay
3.9. Colloidal Stability Assay
3.10. RT2 Profiler Signal Transduction Pathway Finder PCR Array
3.11. Immunoblot Assay
3.12. In Vitro Wound Healing Assay
3.13. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AgNP | Silver nanoparticle |
| AgcPCNPs | C-phycocyanin conjugated silver nanoparticles |
| FRAC | Ferric Reducing Anti-oxidant power |
| ABTS | 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) |
| HSBS | Hank’s Balanced Salt Solution |
| FBS | Fetal Bovine Serum |
| PBS | Phosphate buffered solution |
| ICPS | Integrated Coupled Plasma Emission Spectroscopy |
| Nfi | NFĸB inhibitor |
| LY294002 | PI3K inhibitor |
| cPC | c-Phycocyanin |
References
- Husen, A.; Siddiqi, K.S. Phytosynthesis of nanoparticles: Concept, controversy and application. Nanoscale Res. Lett. 2014, 9, 229. [Google Scholar] [CrossRef] [PubMed]
- Gherasim, O.; Puiu, R.A.; Bîrcă, A.C.; Burdușel, A.C.; Grumezescu, A.M. An Updated Review on Silver Nanoparticles in Biomedicine. Nanomaterials 2020, 10, 2318. [Google Scholar] [CrossRef]
- Arafa, M.G.; El-Kased, R.F.; Elmazar, M.M. Thermoresponsive gels containing gold nanoparticles as smart antibacterial and wound healing agents. Sci. Rep. 2018, 8, 13674. [Google Scholar] [CrossRef]
- Sharma, S.; Madhyastha, H.; Swetha, K.L.; Maravajjala, K.S.; Singh, A.; Madhyastha, R.; Roy, A. Development of an in-situ forming, self-healing scaffold for dermal wound healing: In-vitro and in-vivo studies. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 128, 112263. [Google Scholar] [CrossRef] [PubMed]
- Fatrekar, A.P.; Morajkar, R.; Krishnan, S.; Dusane, A.; Madhyastha, H.; Vernekar, A.A. Delineating the Role of Tailored Gold Nanostructures at the Biointerface. ACS Appl. Bio Mater. 2021, 4, 8172–8191. [Google Scholar] [CrossRef]
- Daeschlein, G. Antimicrobial and antiseptic strategies in wound management. Int. Wound J. 2013, 10 (Suppl. 1), 9–14. [Google Scholar] [CrossRef]
- Madhyastha, H.; Madhyastha, R.; Thakur, A.; Kentaro, S.; Dev, A.; Singh, S.; Maruyama, M. c-Phycocyanin primed silver nano conjugates: Studies on red blood cell stress resilience mechanism. Colloids Surf. B Biointerfaces 2020, 194, 111211. [Google Scholar] [CrossRef]
- Umapathi, A.; Nagaraju, N.P.; Madhyastha, H.; Jain, D.; Srinivas, S.P.; Rotello, V.M.; Daima, H.K. Highly efficient and selective antimicrobial isonicotinylhydrazide-coated polyoxometalate-functionalized silver nanoparticles. Colloids Surf. B Biointerfaces 2019, 184, 110522. [Google Scholar] [CrossRef] [PubMed]
- Dhapte, V.; Kadam, S.; Moghe, A.; Pokharkar, V. Probing the wound healing potential of biogenic silver nanoparticles. J. Wound Care 2014, 23, 431–432. [Google Scholar] [CrossRef]
- Gupta, A.; Briffa, S.M.; Swingler, S.; Gibson, H.; Kannappan, V.; Adamus, G.; Radecka, I. Synthesis of Silver Nanoparticles Using Curcumin-Cyclodextrins Loaded into Bacterial Cellulose-Based Hydrogels for Wound Dressing Applications. Biomacromolecules 2020, 21, 1802–1811. [Google Scholar] [CrossRef]
- Ugru, M.M.; Sheshadri, S.; Jain, D.; Madhyastha, H.; Madhyastha, R.; Maruyama, M.; Daima, H.K. Insight into the composition and surface corona reliant biological behavior pf quercetin engineered nanoparticles. Colloids Surf. A 2018, 548, 1–9. [Google Scholar] [CrossRef]
- Harishkumar, M.; Radha, M.; Yuchi, N.; Kumar, D.H.; Paduvarahalli, N.; Masugi, M. An opinion on nano-medicine and toxicology-cellular crosstalk: Considerations and Caveats. Mater. Today Proc. 2019, 10, 100–105. [Google Scholar]
- Madhyastha, H.; Madhyastha, R.; Nakajima, Y.; Omura, S.; Maruyama, M. Regulation of growth factors-associated cell migration by C-phycocyanin scaffold in dermal wound healing. Clin. Exp. Pharm. Physiol. 2012, 39, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Süntar, I.; Çetinkaya, S.; Panieri, E.; Saha, S.; Buttari, B.; Profumo, E.; Saso, L. Regulatory Role of Nrf2 Signaling Pathway in Wound Healing Process. Molecules 2021, 26, 2424. [Google Scholar] [CrossRef]
- Houschyar, K.S.; Tapking, C.; Puladi, B.; Popp, D.; Duscher, D.; Rein, S.; Reumuth, G.; Grieb, G.; Branski, L.K.; Siemers, F.; et al. Wnt signaling in cutaneous wound healing. Handchir. Mikrochir. Plast Chir. 2020, 52, 151–158. [Google Scholar] [PubMed]
- Kunkemoeller, B.; Kyriakides, T.R. Redox Signaling in Diabetic Wound Healing Regulates Extracellular Matrix Deposition. Antioxid. Redox Signal. 2017, 27, 823–838. [Google Scholar] [CrossRef]
- Chen, G.; He, L.; Zhang, P.; Zhang, J.; Mei, X.; Wang, D.; Zhang, Y.; Ren, X.; Chen, Z. Encapsulation of green tea polyphenol nanospheres in PVA/alginate hydrogel for promoting wound healing of diabetic rats by regulating PI3K/AKT pathway. Mater. Sci. Eng. C 2020, 110, 110686. [Google Scholar] [CrossRef]
- Pils, V.; Terlecki-Zaniewicz, L.; Schosserer, M.; Grillari, J.; Lämmermann, I. The role of lipid-based signalling in wound healing and senescence. Mech. Ageing Dev. 2021, 198, 111527. [Google Scholar] [CrossRef]
- Hori, K.; Ding, J.; Marcoux, Y.; Iwashina, T.; Sakurai, H.; Tredget, E.E. Impaired cutaneous wound healing in transforming growth factor-β inducible early gene1 knockout mice. Wound Repair Regen. 2012, 20, 166–177. [Google Scholar] [CrossRef] [PubMed]
- Natoli, G.; Ghisletti, S.; Barozzi, I. The genomic landscapes of inflammation. Genes Dev. 2011, 25, 101–106. [Google Scholar] [CrossRef]
- Gour, A.; Jain, N.K. Advances in green synthesis of nanoparticles. Artif. Cells Nanomed. Biotechnol. 2019, 47, 844–851. [Google Scholar] [CrossRef] [PubMed]
- Bannu, S.M.; Lomada, D.; Gulla, S.; Chandrasekhar, T.; Reddanna, P.; Reddy, M.C. Potential Therapeutic Applications of C-Phycocyanin. Curr. Drug Metab. 2019, 20, 967–976. [Google Scholar] [CrossRef] [PubMed]
- Castangia, I.; Manca, M.L.; Catalán-Latorre, A.; Maccioni, A.M.; Fadda, A.M.; Manconi, M. Phycocyanin-encapsulating hyalurosomes as carrier for skin delivery and protection from oxidative stress damage. J. Mater. Sci. Mater. Med. 2016, 27, 75. [Google Scholar] [CrossRef]
- Wiley, B.J.; Im, S.H.; Li, Z.Y.; McLellan, J.; Siekkinen, A.; Xia, Y. Maneuvering the surface plasmon resonance of silver nanostructures through shape-controlled synthesis. J. Phys. Chem. B 2006, 110, 15666–15675. [Google Scholar] [CrossRef] [PubMed]
- Birinci, Y.; Niazi, J.H.; Aktay-Çetin, O.; Basaga, H. Quercetin in the form of a nano-antioxidant (QTiO). Enzym. Microb. Technol. 2020, 138, 109559. [Google Scholar] [CrossRef]
- Stevens, J.L.; Feelisch, M.; Martin, D.S. Perioperative Oxidative Stress: The Unseen Enemy. Anesth. Analg. 2019, 129, 1749–1760. [Google Scholar] [CrossRef]
- Lee, H.P.; Gaharwar, A.K. Light-Responsive Inorganic Biomaterials for Biomedical Applications. Adv. Sci. (Weinh) 2020, 7, 2000863. [Google Scholar] [CrossRef] [PubMed]
- Madhyastha, H.; Halder, S.; Madhyastha, R.; Mohanapriya, A.; Sudhakaran, R.; Sajitha, L.S.; Nakajima, Y. Surface refined Au Quercetin nanoconjugate stimulates dermal cell migration: Possible implication in wound healing. RSC Adv. 2020, 10, 37683–37694. [Google Scholar]
- Sahoo, P.R.; Madhyastha, H.; Madhyastha, R.; Maruyama, M.; Nakajima, Y. Recent Progress in Nanotheranostic Medicine; Springer: Cham, Switzerland, 2020. [Google Scholar]
- Larios, J.; Mercier, V.; Roux, A.; Gruenberg, J. ALIX- and ESCRT-III-dependent sorting of tetraspanins to exosomes. J. Cell Biol. 2020, 219. [Google Scholar] [CrossRef] [PubMed]
- Mathé, E.; Busby, B.; Piontkivska, H.; Developers, T. Matchmaking in Bioinformatics. F1000 Res. 2018, 7, 171. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Schubert, J.; Chanana, M. Coating Matters: Review on Colloidal Stability of Nanoparticles with Biocompatible Coatings in Biological Media, Living Cells and Organisms. Curr. Med. Chem. 2018, 25, 4553–4586. [Google Scholar] [CrossRef] [PubMed]



| Pathway | Marker Gene |
|---|---|
| Mitogenic pathway | Egr1, Fos, Jun, Nab2 |
| WNT pathway | Birc5, Cend1, Cdh1, Fgf4, Jun, Lef1, Myc, Pparg, Tcf7, Vegfa, Wisp1 |
| Hedgehog pathway | Bmp2, Bmp4, En1, Foxa2, Hhip, Ptch1 |
| TFGβ pathway | Cdkn 1a, Cdkn 1b, Cdkn 2a, Cdkn 2b |
| JNK pathway | Bcl2, Bcl 211 |
| P53 pathway | Bax, Cdkn1a, Ei24, Fas, Gadd 45a, Igfbp3, Mdm2 |
| Stress signal pathway | Atf2, Fos, Hsf1, Hspb1, Myc, Trp53 |
| NFAT pathway | Cd5, Fas1 |
| CREB pathway | Cyp19a1, Egr1, Fos |
| JAK/STAT pathway | Cxcl9, Li4ra, Irf1, Mmp10, Nos2 |
| Estrogen regulation pathway | Bcl2, Brca1, Grb1, Igfbp4, Nrip1 |
| Androgen regulation pathway | Cdk2, cdkn 1a |
| Ca2+-Protein Kinase C pathway | Csf2, Fos, II2ra, Jun, Myc, Odc1, Tfrc |
| Phospholipase C pathway | Bcl2, Egr1, Fos, Icam1, Jun, Nos2, Ptgs2, Vcam1 |
| Insulin regulation pathway | Cebpb, Fasn, Gys1, Hk2, Lep |
| LDL pathway | Ccl2, Csf2, Sele, Vcam1 |
| Retinoic acid regulation pathway | En1, Hoxa1, Rbpn1 |
| PI3K pathway | Bcl2, Ccnd1, Fnf1, Jun, Mmp7, Myc |
| NFĸB pathway | Ccl20, Icam1, Ikbĸβ, Lep, NFĸb1a, Nos2, Tnf, Vcam1 |
| Gene Name | AgNP | AgcPCNP | cPC |
|---|---|---|---|
| Mitogenic pathway genes | |||
| Egr1 | 9.4657 | 6.3892 | 7.3787 |
| Fos | 4.5002 | 1.3673 | 3.6734 |
| Jun | 1.7291 | 2.0034 | 1.7854 |
| Nab2 | 1.3665 | −2.2763 | −1.3673 |
| Wnt signaling pathway genes | |||
| Birc5 | −2.20783 | −1.43904 | 0.00934 |
| Cend1 | −1.7532 | −2.2605 | −2.0094 |
| Cdh1 | −4.3169 | −6.75623 | −6.0934 |
| Fgf4 | −4.7632 | 5.5281 | −39095 |
| Jun | 1.7236 | 2.0465 | 1.9734 |
| Lef1 | 1.5911 | 1.7092 | 1.5763 |
| Myc | −1.3864 | 1.3887 | 1.3872 |
| Pparg | 4.6873 | 3.8956 | 2.8945 |
| Tcf7 | 2.0345 | −7453 | 1.9835 |
| Vegfa | 1.38874 | −1.5463 | −1.8934 |
| Wisp1 | 1.7843 | 1.3288 | 1.4352 |
| Hedgehog signaling pathway genes | |||
| Bmp2 | 2.9032 | −19834 | 1.9645 |
| Bmp4 | −1.3762 | 1.8932 | −1.4763 |
| En1 | −1.7834 | −1.4782 | −1.9034 |
| Foxa2 | −16.354 | −14.3784 | −12.9834 |
| Hhip | 1.6723 | 1.2328 | 1.2673 |
| Ptch1 | 1.3723 | −1.2673 | 1.9453 |
| TGFβ pathway genes | |||
| Cdkn 1a | −1.2664 | −1.3784 | 0.9945 |
| Cdkn 1b | −9.3623 | −8.4872 | −7.3725 |
| Cdkn 2a | −1.3765 | −1.4783 | −1.3744 |
| Cdkn 2b | −1.3784 | −1.3674 | 1.3364 |
| JNK pathway genes | |||
| Bcl2 | −1.7634 | −1.9983 | −1.2783 |
| Bcl211 | 2.4563 | 1.8964 | 2.3321 |
| p53 pathway genes | |||
| Bax | −1.3564 | −1.8873 | −1.2234 |
| Cdkn 1a | −1.0734 | −1.8435 | −1.0034 |
| Ei24 | −1.6873 | −1.7763 | −1.5342 |
| Fas | −3.8734 | −1.2763 | −2.8934 |
| Gadd45a | 1.6723 | 1.6624 | 1.3784 |
| Igfbp3 | 1.6724 | 1.7724 | 1.0935 |
| Mdm2 | 2.9835 | 2.0934 | 1.2753 |
| Gene | AgNP | AgcPCNP | cPC |
|---|---|---|---|
| Stress marker genes | |||
| Atf 2 | 10.6723 | 9.3452 | 10.2674 |
| Fos | 4.2754 | 1.8342 | 3.9834 |
| Hsf1 | −1.3764 | −1.8832 | 0.0037 |
| Hspb1 | 28.3642 | 21.2732 | 19.3674 |
| Myc | −1.2534 | −1.1432 | 1.2874 |
| Trp53 | 1.3426 | −1.2653 | 1.2784 |
| NFAT signaling pathway genes | |||
| Cd5 | −1.0263 | −1.0924 | −1.1163 |
| Fas1 | 1.0564 | −1.2734 | 1.0932 |
| CREB pathway genes | |||
| Cyp19a1 | 2.7634 | 2.9834 | 1.8934 |
| Egr1 | 9.2634 | 6.2673 | 7.1863 |
| Fos | 3.9834 | 4.0152 | 3.6734 |
| JAK-STAT signaling pathway genes | |||
| Cxcl19 | −1.2654 | −2.0934 | −2.6143 |
| Li4ra | 7.3562 | 7.2263 | 6.7834 |
| Irf1 | −1.2734 | −1.0034 | −1.3564 |
| Mmp10 | 2.5643 | 2.456 | 1.9845 |
| Nos2 | 1.7834 | 1.4563 | −0.8934 |
| Estrogen regulator pathway genes | |||
| Bcl2 | −1.3674 | −1.5432 | −1.3763 |
| Brca1 | 1.0342 | 1.3654 | −1.3674 |
| Grb1 | 1.3452 | 1.2218 | 1.1783 |
| Igfbp4 | −4.1923 | −3.8934 | −4.0032 |
| Nrip1 | 1.6734 | 1.1178 | 1.2784 |
| Androgen regulatory pathway genes | |||
| Cdk2 | −1.3564 | −1.0094 | −1.3564 |
| Cdkn1a | −1.2754 | −1.4675 | −1.0034 |
| Ca2+ protein kinase pathway genes | |||
| Csf2 | 3.2613 | 2.9843 | 3.1178 |
| Fos | 4.1173 | 3.9845 | 4.1342 |
| II2ra | 1.7832 | 1.1563 | 1.2673 |
| Jun | 1.7342 | 1.5632 | 1.2343 |
| Myc | −1.1783 | −1.0035 | −1.5623 |
| Odc1 | 2.3413 | 1.7834 | 2.2173 |
| Tfrc | 2.2034 | 2.6723 | 1.8934 |
| Gene | AgNP | AgcPCNP | cPC |
|---|---|---|---|
| Phospolipase C signaling pathway genes | |||
| Bcl2 | −1.8404 | 1.2564 | −1.00934 |
| Egr1 | 9.3564 | 8.8934 | 6.8435 |
| Fos | 4.2673 | 4.8913 | 3.9803 |
| Icam1 | −1.3673 | −2.09245 | −1.8923 |
| Jun | 1.5632 | 1.0934 | 1.0023 |
| Nos2 | 1.4742 | 1.8723 | 1.0923 |
| Ptgs2 | 14.2262 | 13.0934 | 12.8723 |
| Vcam1 | −2.0934 | −2.9834 | −1.0934 |
| Insulin regulatory pathway genes | |||
| Cebpb | 1.5672 | 1.8934 | 1.0925 |
| Fasn | 1.2893 | 1.2229 | 1.1093 |
| Gys1 | 1.18923 | 1.9023 | 1.8992 |
| Hk2 | −1.2893 | 0.0943 | −1.9283 |
| Lep | 1.4044 | 1.3984 | 1.29984 |
| LDL pathway genes | |||
| Ccl2 | −3.2854 | −2.9453 | −3.4983 |
| Csf2 | 3.1903 | 3.0021 | 2.9834 |
| Sele | 3.6724 | 3.9913 | 3.0932 |
| Vcam1 | −2.2658 | −2.2093 | −1.0046 |
| Retinoic acid regulatory pathway genes | |||
| En1 | −1.5623 | −1.8934 | −1.0925 |
| Hoxa1 | 1.0425 | 1.8723 | 1.0936 |
| Rbpn1 | 4.724 | 3.7931 | 3.9982 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madhyastha, H.; Madhyastha, R.; Chakraborty, E.; Banerjee, K.; Shah, K.; Nakajima, Y.; Chauhan, N.S.; Sudhakaran, S.L.; Ohe, K.; Muthukaliannan, G.K.; et al. Fluro-Protein C-Phycocyanin Docked Silver Nanocomposite Accelerates Cell Migration through NFĸB Signaling Pathway. Int. J. Mol. Sci. 2023, 24, 3184. https://doi.org/10.3390/ijms24043184
Madhyastha H, Madhyastha R, Chakraborty E, Banerjee K, Shah K, Nakajima Y, Chauhan NS, Sudhakaran SL, Ohe K, Muthukaliannan GK, et al. Fluro-Protein C-Phycocyanin Docked Silver Nanocomposite Accelerates Cell Migration through NFĸB Signaling Pathway. International Journal of Molecular Sciences. 2023; 24(4):3184. https://doi.org/10.3390/ijms24043184
Chicago/Turabian StyleMadhyastha, Harishkumar, Radha Madhyastha, Eshika Chakraborty, Kaushita Banerjee, Kamal Shah, Yuichi Nakajima, Nagendra Singh Chauhan, Sajitha Lulu Sudhakaran, Kaoru Ohe, Gothandam Kodiveri Muthukaliannan, and et al. 2023. "Fluro-Protein C-Phycocyanin Docked Silver Nanocomposite Accelerates Cell Migration through NFĸB Signaling Pathway" International Journal of Molecular Sciences 24, no. 4: 3184. https://doi.org/10.3390/ijms24043184
APA StyleMadhyastha, H., Madhyastha, R., Chakraborty, E., Banerjee, K., Shah, K., Nakajima, Y., Chauhan, N. S., Sudhakaran, S. L., Ohe, K., Muthukaliannan, G. K., Gopalakrishnan, A. V., Maruyama, M., & Watanabe, N. (2023). Fluro-Protein C-Phycocyanin Docked Silver Nanocomposite Accelerates Cell Migration through NFĸB Signaling Pathway. International Journal of Molecular Sciences, 24(4), 3184. https://doi.org/10.3390/ijms24043184









