Human Brain Organoids in Migraine Research: Pathogenesis and Drug Development
Abstract
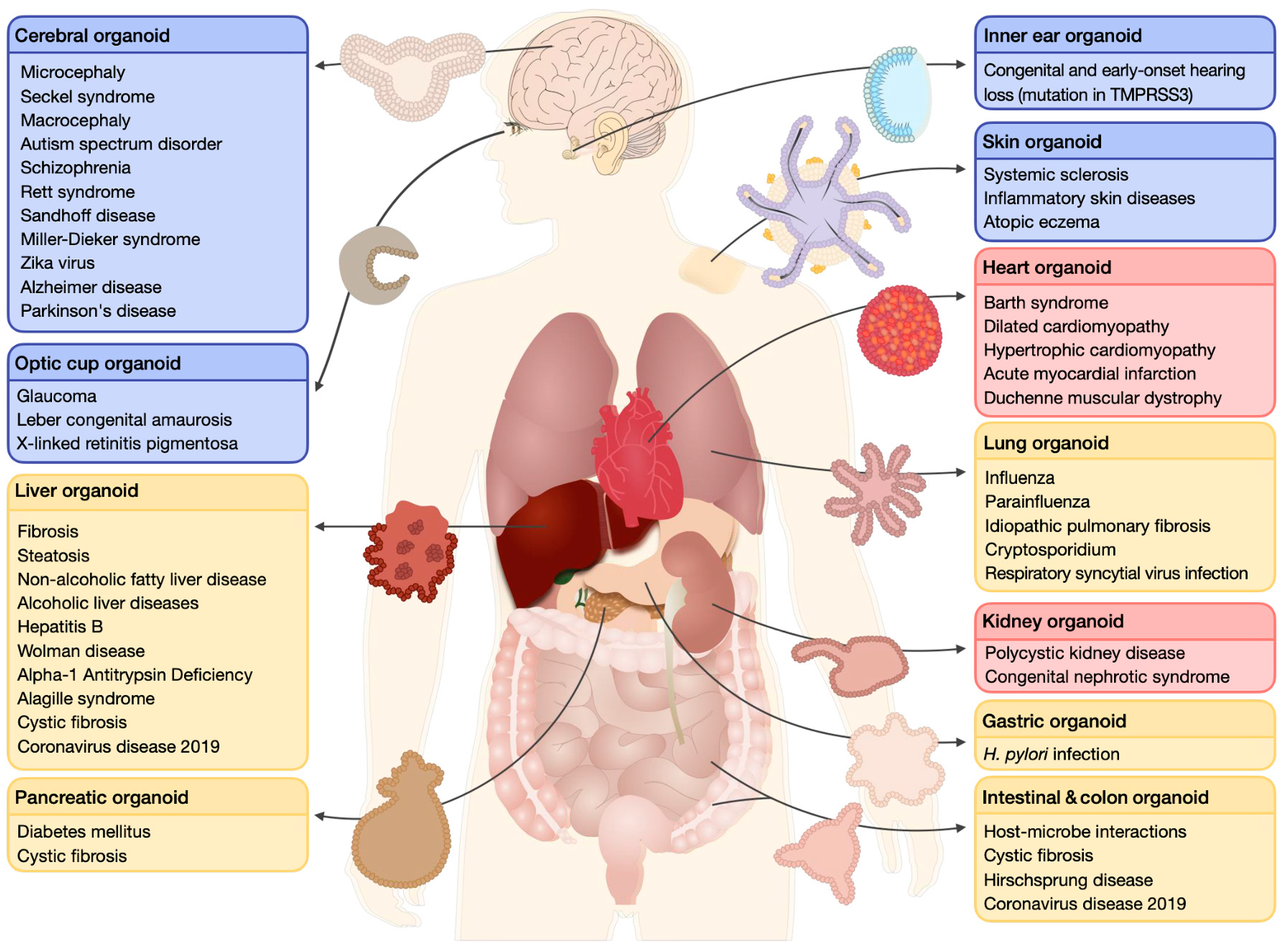
1. Human Organoids
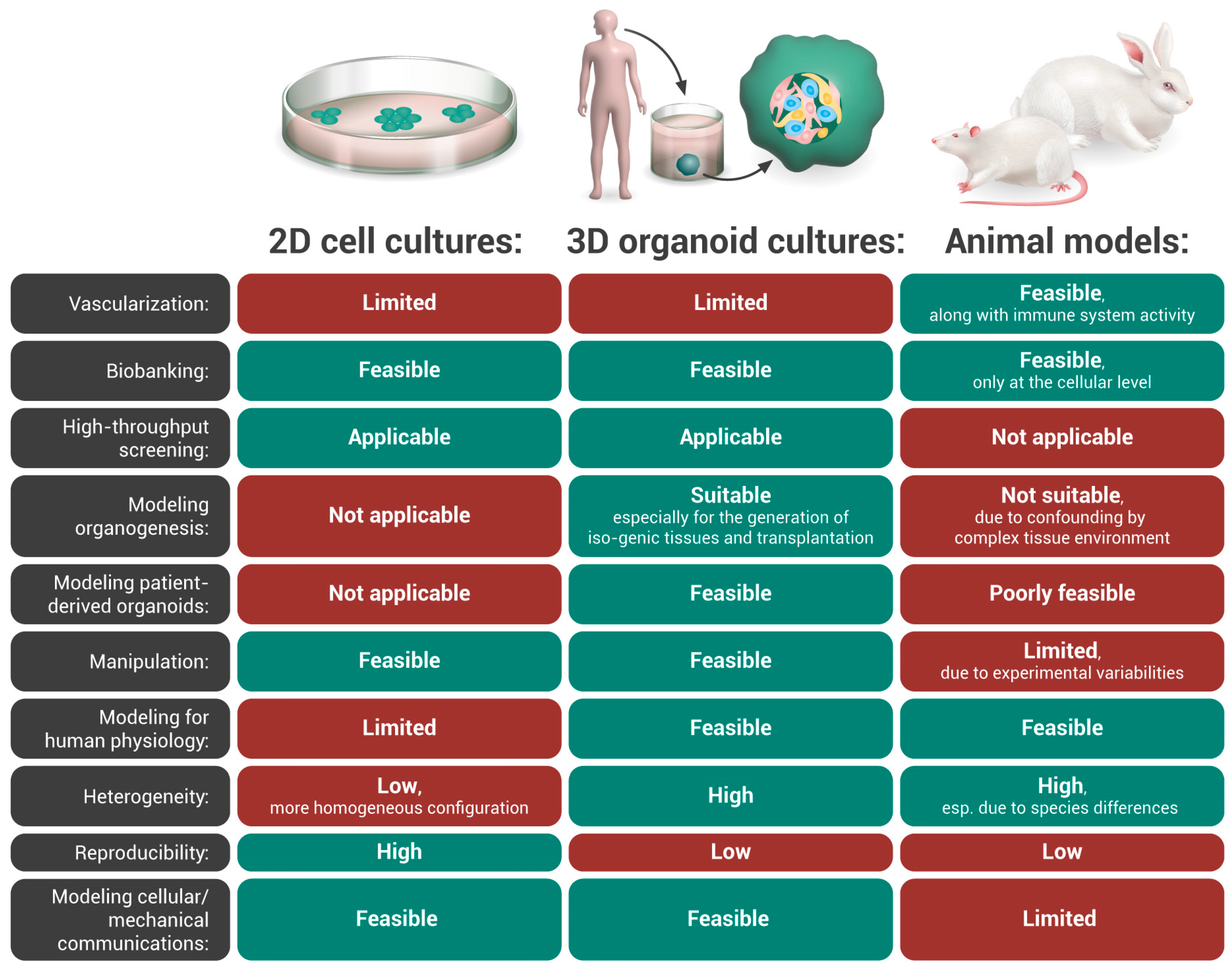
Disease Modeling and Drug Screening: From Animal Models to Human Organoids
2. Human Brain Organoids
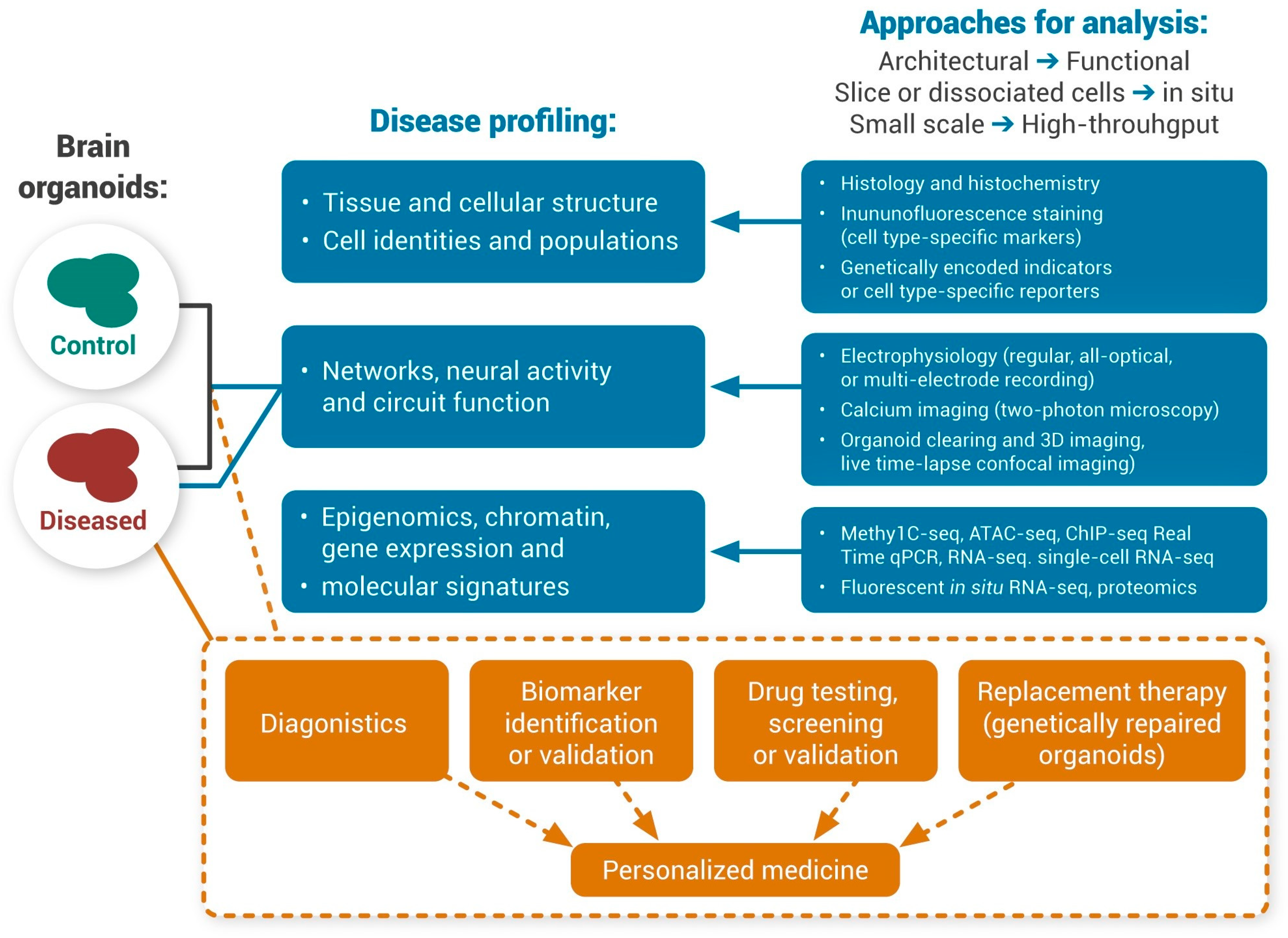
Human Brain Organoids for Neurological Disorders
3. Human Brain Organoids for Migraine
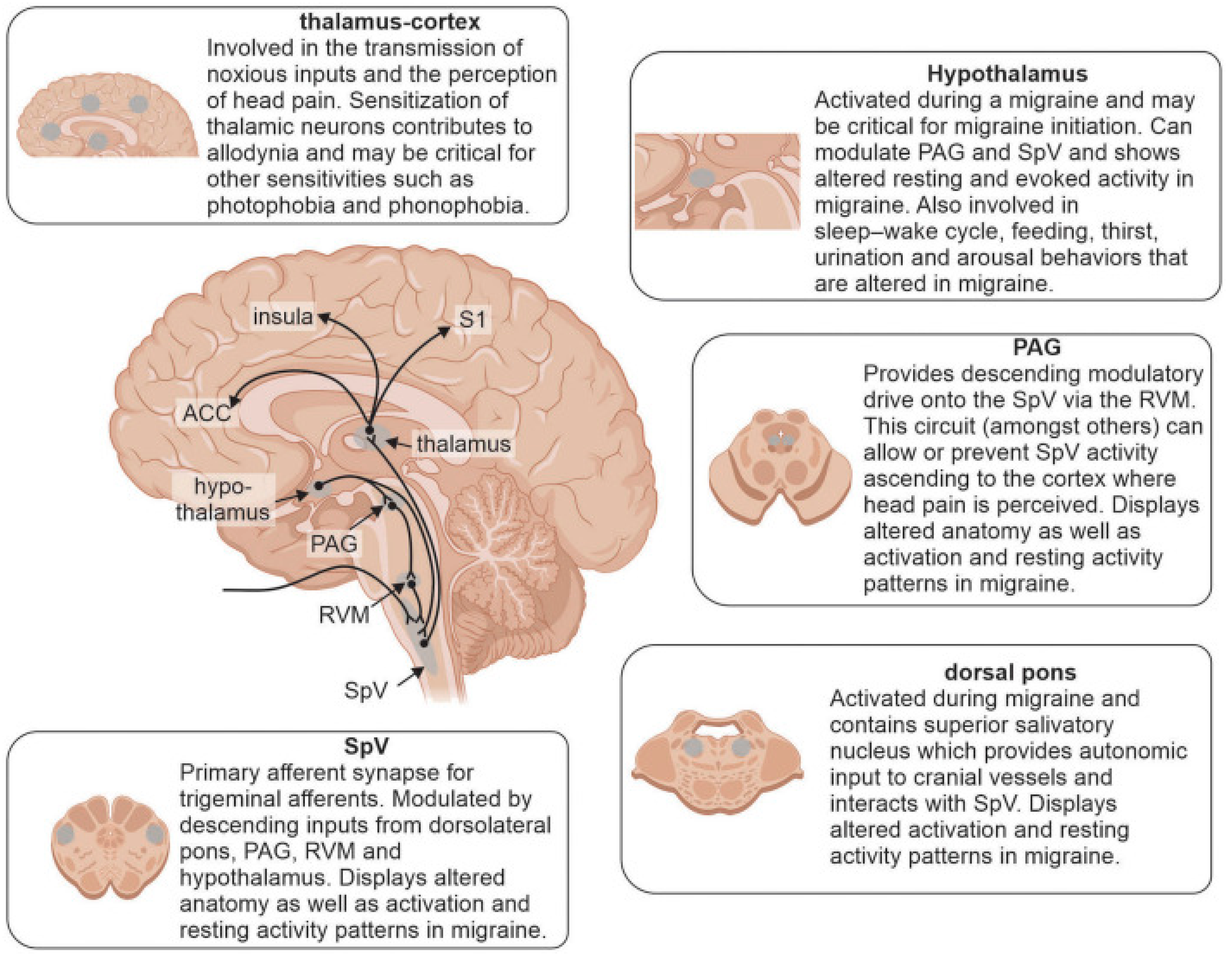
3.1. Migraine
3.2. Modeling Migraine
3.3. Can Human Brain Organoids Be Used as a Substitute Model for Migraine Disorder?
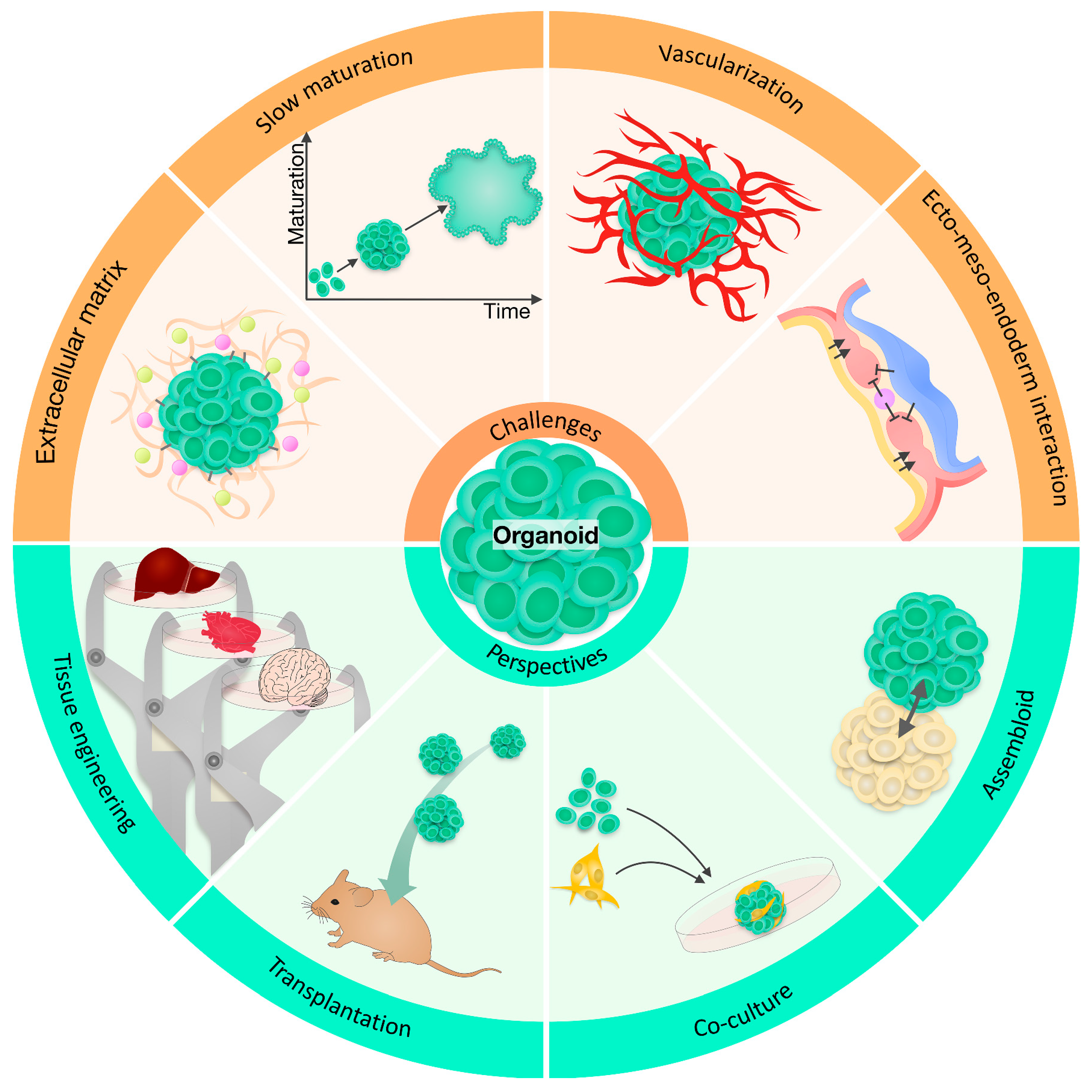
4. Potential and Limitations of Brain Organoids for Migraine
5. Drug Discovery and Development: Potential and Limitations of Brain Organoids
6. Neuroethical Considerations
7. Global Cooperation
8. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schutgens, F.; Clevers, H. Human organoids: Tools for understanding biology and treating diseases. Annu. Rev. Pathol. 2020, 15, 211–234. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.-Y.; Wu, S.; Wang, D.; Chu, C.; Hong, Y.; Tao, M.; Hu, H.; Xu, M.; Guo, X.; Liu, Y. Human organoids in basic research and clinical applications. Signal Transduct. Target. Ther. 2022, 7, 168. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Koo, B.-K.; Knoblich, J.A. Human organoids: Model systems for human biology and medicine. Nat. Rev. Mol. Cell Biol. 2020, 21, 571–584. [Google Scholar] [CrossRef]
- Clevers, H.C. Organoids: Avatars for personalized medicine. Keio J. Med. 2019, 68, 95. [Google Scholar] [CrossRef] [PubMed]
- Arjmand, B.; Rabbani, Z.; Soveyzi, F.; Tayanloo-Beik, A.; Rezaei-Tavirani, M.; Biglar, M.; Adibi, H.; Larijani, B. Advancement of organoid technology in regenerative medicine. Regen. Eng. Transl. Med. 2022, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Gania, Z.; Noorintan, S.T.; Septiari, N.P.D.P.; Fitriany, D.S.; Torizal, F.G. Strategies for generating human pluripotent stem cell-derived-organoid culture for disease modeling, drug screening, and regenerative therapy. Future Pharmacol. 2022, 2, 360–376. [Google Scholar] [CrossRef]
- Shariati, L.; Esmaeili, Y.; Haghjooy Javanmard, S.; Bidram, E.; Amini, A. Organoid technology: Current standing and future perspectives. Stem Cells 2021, 39, 1625–1649. [Google Scholar] [CrossRef]
- Shi, Y.; Inoue, H.; Wu, J.C.; Yamanaka, S. Induced pluripotent stem cell technology: A decade of progress. Nat. Rev. Drug Discov. 2017, 16, 115–130. [Google Scholar] [CrossRef]
- Shankaran, A.; Prasad, K.; Chaudhari, S.; Brand, A.; Satyamoorthy, K. Advances in development and application of human organoids. 3 Biotech. 2021, 11, 257. [Google Scholar] [CrossRef]
- Heydari, Z.; Moeinvaziri, F.; Agarwal, T.; Pooyan, P.; Shpichka, A.; Maiti, T.K.; Timashev, P.; Baharvand, H.; Vosough, M. Organoids: A novel modality in disease modeling. Bio-Des. Manuf. 2021, 4, 689–716. [Google Scholar] [CrossRef]
- Mukherjee, P.; Roy, S.; Ghosh, D.; Nandi, S.K. Role of animal models in biomedical research: A review. Lab. Anim. Res. 2022, 38, 18. [Google Scholar] [CrossRef]
- Singh, V.K.; Seed, T.M. How necessary are animal models for modern drug discovery? Expert Opin. Drug Discov. 2021, 16, 1391–1397. [Google Scholar] [CrossRef] [PubMed]
- Leenaars, C.H.C.; Kouwenaar, C.; Stafleu, F.R.; Bleich, A.; Ritskes-Hoitinga, M.; De Vries, R.B.M.; Meijboom, F.L.B. Animal to human translation: A systematic scoping review of reported concordance rates. J. Transl. Med. 2019, 17, 223. [Google Scholar] [CrossRef] [PubMed]
- Hubrecht, R.C.; Carter, E. The 3Rs and humane experimental technique: Implementing change. Animals 2019, 9, 754. [Google Scholar] [CrossRef]
- Zhao, X.; Bhattacharyya, A. Human models are needed for studying human neurodevelopmental disorders. Am. J. Hum. Genet. 2018, 103, 829–857. [Google Scholar] [CrossRef]
- Ingber, D.E. Human organs-on-chips for disease modelling, drug development and personalized medicine. Nat. Rev. Genet. 2022, 23, 467–491. [Google Scholar] [CrossRef]
- Liu, C.; Oikonomopoulos, A.; Sayed, N.; Wu, J.C. Modeling human diseases with induced pluripotent stem cells: From 2D to 3D and beyond. Development 2018, 145, dev156166. [Google Scholar] [CrossRef] [PubMed]
- Zakrzewski, W.; Dobrzyński, M.; Szymonowicz, M.; Rybak, Z. Stem cells: Past, present, and future. Stem Cell Res. Ther. 2019, 10, 68. [Google Scholar] [CrossRef]
- Thomson, J.A.; Itskovitz-Eldor, J.; Shapiro, S.S.; Waknitz, M.A.; Swiergiel, J.J.; Marshall, V.S.; Jones, J.M. Embryonic stem cell lines derived from human blastocysts. Science 1998, 282, 1145–1147. [Google Scholar] [CrossRef]
- Kim, C. iPSC technology--Powerful hand for disease modeling and therapeutic screen. BMB Rep. 2015, 48, 256–265. [Google Scholar] [CrossRef]
- Chang, E.A.; Jin, S.W.; Nam, M.H.; Kim, S.D. Human induced pluripotent stem cells: Clinical significance and applications in neurologic diseases. J. Korean Neurosurg. Soc. 2019, 62, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.Y.; Zhang, X.Y.; Zhu, P.; Ji, L. Development of clustered regularly interspaced short palindromic repeats/CRISPR-associated technology for potential clinical applications. World J. Clin. Cases 2022, 10, 5934–5945. [Google Scholar] [CrossRef]
- McTague, A.; Rossignoli, G.; Ferrini, A.; Barral, S.; Kurian, M.A. Genome Editing in iPSC-based neural systems: From disease models to future therapeutic strategies. Front. Genome Ed. 2021, 3, 630600. [Google Scholar] [CrossRef]
- Bédard, P.; Gauvin, S.; Ferland, K.; Caneparo, C.; Pellerin, È.; Chabaud, S.; Bolduc, S. Innovative human three-dimensional tissue-engineered models as an alternative to animal testing. Bioengineering 2020, 7, 115. [Google Scholar] [CrossRef]
- Kapałczyńska, M.; Kolenda, T.; Przybyła, W.; Zajączkowska, M.; Teresiak, A.; Filas, V.; Ibbs, M.; Bliźniak, R.; Łuczewski, Ł.; Lamperska, K. 2D and 3D cell cultures—A comparison of different types of cancer cell cultures. Arch. Med. Sci. 2018, 14, 910–919. [Google Scholar] [CrossRef] [PubMed]
- Jensen, C.; Teng, Y. Is it time to start transitioning from 2D to 3D cell culture? Front. Mol. Biosci. 2020, 7, 33. [Google Scholar] [CrossRef]
- Wu, X.; Su, J.; Wei, J.; Jiang, N.; Ge, X. Recent advances in three-dimensional stem cell culture systems and applications. Stem Cells Int. 2021, 2021, 9477332. [Google Scholar] [CrossRef]
- Zhao, Z.; Chen, X.; Dowbaj, A.M.; Sljukic, A.; Bratlie, K.; Lin, L.; Fong, E.L.S.; Balachander, G.M.; Chen, Z.; Soragni, A.; et al. Organoids. Nat. Rev. Methods Prim. 2022, 2, 94. [Google Scholar] [CrossRef]
- Suarez-Martinez, E.; Suazo-Sanchez, I.; Celis-Romero, M.; Carnero, A. 3D and organoid culture in research: Physiology, hereditary genetic diseases and cancer. Cell Biosci. 2022, 12, 39. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, G.; Ponnusamy, M.P.; Batra, S.K. Concise review: Current status of three-dimensional organoids as preclinical models. Stem Cells 2018, 36, 1329–1340. [Google Scholar] [CrossRef]
- Giandomenico, S.L.; Sutcliffe, M.; Lancaster, M.A. Generation and long-term culture of advanced cerebral organoids for studying later stages of neural development. Nat. Protoc. 2021, 16, 579–602. [Google Scholar] [CrossRef] [PubMed]
- Ergün, S.; Wörsdörfer, P. Organoids, assembloids and embryoids: New avenues for developmental biology, disease modeling, drug testing and toxicity assessment without animal experimentation. Organoids 2022, 1, 37–40. [Google Scholar] [CrossRef]
- Makrygianni, E.A.; Chrousos, G.P. From brain organoids to networking assembloids: Implications for neuroendocrinology and stress medicine. Front. Physiol. 2021, 12, 621970. [Google Scholar] [CrossRef]
- Vogt, N. Assembloids. Nat. Methods 2021, 18, 27. [Google Scholar] [CrossRef]
- Traversi, D.; Pulliero, A.; Izzotti, A.; Franchitti, E.; Iacoviello, L.; Gianfagna, F.; Gialluisi, A.; Izzi, B.; Agodi, A.; Barchitta, M.; et al. Precision medicine and public health: New challenges for effective and sustainable health. J. Pers. Med. 2021, 11, 135. [Google Scholar] [CrossRef]
- Rasool, M.; Malik, A.; Naseer, M.I.; Manan, A.; Ansari, S.; Begum, I.; Qazi, M.H.; Pushparaj, P.; Abuzenadah, A.M.; Al-Qahtani, M.H.; et al. The role of epigenetics in personalized medicine: Challenges and opportunities. BMC Med. Genom. 2015, 8 (Suppl. 1), S5. [Google Scholar] [CrossRef]
- Mansour, A.A.; Gonçalves, J.T.; Bloyd, C.W.; Li, H.; Fernandes, S.; Quang, D.; Johnston, S.; Parylak, S.L.; Jin, X.; Gage, F.H. An in vivo model of functional and vascularized human brain organoids. Nat. Biotechnol. 2018, 36, 432–441. [Google Scholar] [CrossRef]
- Chen, H.I.; Wolf, J.A.; Blue, R.; Song, M.M.; Moreno, J.D.; Ming, G.L.; Song, H. Transplantation of human brain organoids: Revisiting the science and ethics of brain chimeras. Cell Stem Cell 2019, 25, 462–472. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Song, H.; Ming, G.L. Brain organoids: Advances, applications and challenges. Development 2019, 146, dev166074. [Google Scholar] [CrossRef]
- Bassett, D.S.; Gazzaniga, M.S. Understanding complexity in the human brain. Trends Cogn. Sci. 2011, 15, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.T.; Bendriem, R.M.; Wu, W.W.; Shen, R.F. 3D brain Organoids derived from pluripotent stem cells: Promising experimental models for brain development and neurodegenerative disorders. J. Biomed. Sci. 2017, 24, 59. [Google Scholar] [CrossRef]
- Nowakowski, T.J.; Salama, S.R. Cerebral organoids as an experimental platform for human neurogenomics. Cells 2022, 11, 2803. [Google Scholar] [CrossRef] [PubMed]
- Rowe, R.G.; Daley, G.Q. Induced pluripotent stem cells in disease modelling and drug discovery. Nat. Rev. Genet. 2019, 20, 377–388. [Google Scholar] [CrossRef]
- Farcy, S.; Albert, A.; Gressens, P.; Baffet, A.D.; El Ghouzzi, V. Cortical organoids to model microcephaly. Cells 2022, 11, 2135. [Google Scholar] [CrossRef] [PubMed]
- Jacob, F.; Schnoll, J.G.; Song, H.; Ming, G.L. Building the brain from scratch: Engineering region-specific brain organoids from human stem cells to study neural development and disease. Curr. Top. Dev. Biol. 2021, 142, 477–530. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Shopit, A.; Wang, J. A comprehensive update of cerebral organoids between applications and challenges. Oxid. Med. Cell. Longev. 2022, 2022, 7264649. [Google Scholar] [CrossRef]
- Jang, H.; Kim, S.H.; Koh, Y.; Yoon, K.J. Engineering brain organoids: Toward mature neural circuitry with an intact cytoarchitecture. Int. J. Stem Cells 2022, 15, 41–59. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.Y.; Ju, X.C.; Li, Y.; Zeng, P.M.; Wu, J.; Zhou, Y.Y.; Shen, L.B.; Dong, J.; Chen, Y.J.; Luo, Z.G. Generation of vascularized brain organoids to study neurovascular interactions. eLife 2022, 11, e76707. [Google Scholar] [CrossRef] [PubMed]
- Bodnar, B.; Zhang, Y.; Liu, J.; Lin, Y.; Wang, P.; Wei, Z.; Saribas, S.; Zhu, Y.; Li, F.; Wang, X.; et al. Novel scalable and simplified system to generate microglia-containing cerebral organoids from human induced pluripotent stem cells. Front. Cell. Neurosci. 2021, 15, 682272. [Google Scholar] [CrossRef]
- Pașca, S.P.; Arlotta, P.; Bateup, H.S.; Camp, J.G.; Cappello, S.; Gage, F.H.; Knoblich, J.A.; Kriegstein, A.R.; Lancaster, M.A.; Ming, G.-L.; et al. A nomenclature consensus for nervous system organoids and assembloids. Nature 2022, 609, 907–910. [Google Scholar] [CrossRef]
- Andrey, P. The brain organoid technology: Diversity of protocols and challenges. In Organoid Bioengineering; Manash, K.P., Ed.; IntechOpen: Rijeka, UK, 2022. [Google Scholar] [CrossRef]
- Carroll, W.M. The global burden of neurological disorders. Lancet Neurol. 2019, 18, 418–419. [Google Scholar] [CrossRef] [PubMed]
- Feigin, V.L.; Vos, T.; Alahdab, F.; Amit, A.M.L.; Bärnighausen, T.W.; Beghi, E.; Beheshti, M.; Chavan, P.P.; Criqui, M.H.; Desai, R.; et al. Burden of neurological disorders across the US from 1990-2017: A global burden of disease study. JAMA Neurol. 2021, 78, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Savelieff, M.G.; Noureldein, M.H.; Feldman, E.L. Systems biology to address unmet medical needs in neurological disorders. Methods Mol. Biol. 2022, 2486, 247–276. [Google Scholar] [CrossRef]
- Bose, R.; Banerjee, S.; Dunbar, G.L. Modeling neurological disorders in 3d organoids using human-derived pluripotent stem cells. Front. Cell Dev. Biol. 2021, 9, 640212. [Google Scholar] [CrossRef]
- Fischer, J.; Heide, M.; Huttner, W.B. Genetic modification of brain organoids. Front. Cell. Neurosci. 2019, 13, 558. [Google Scholar] [CrossRef]
- Driehuis, E.; Clevers, H. CRISPR/Cas 9 genome editing and its applications in organoids. Am. J. Physiol.-Gastrointest. Liver Physiol. 2017, 312, G257–G265. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Feng, Y.; Tang, J.; Ma, S. Interfacing brain organoids with precision medicine and machine learning. Cell Rep. Phys. Sci. 2022, 3, 100974. [Google Scholar] [CrossRef]
- Eichmüller, O.L.; Knoblich, J.A. Human cerebral organoids—A new tool for clinical neurology research. Nat. Rev. Neurol. 2022, 18, 661–680. [Google Scholar] [CrossRef]
- Jalink, P.; Caiazzo, M. Brain organoids: Filling the need for a human model of neurological disorder. Biology 2021, 10, 740. [Google Scholar] [CrossRef]
- Chen, A.; Guo, Z.; Fang, L.; Bian, S. Application of fused organoid models to study human brain development and neural disorders. Front. Cell. Neurosci. 2020, 14, 133. [Google Scholar] [CrossRef]
- Gupta, J.; Gaurkar, S.S. Migraine: An underestimated neurological condition affecting billions. Cureus 2022, 14, e28347. [Google Scholar] [CrossRef] [PubMed]
- Agosti, R. Migraine burden of disease: From the patient’s experience to a socio-economic view. Headache 2018, 58 (Suppl. 1), 17–32. [Google Scholar] [CrossRef]
- Stovner, L.J.; Hagen, K.; Linde, M.; Steiner, T.J. The global prevalence of headache: An update, with analysis of the influences of methodological factors on prevalence estimates. J. Headache Pain 2022, 23, 34. [Google Scholar] [CrossRef] [PubMed]
- Al-Hassany, L.; Haas, J.; Piccininni, M.; Kurth, T.; Maassen Van Den Brink, A.; Rohmann, J.L. Giving researchers a headache—Sex and gender differences in migraine. Front. Neurol. 2020, 11, 549038. [Google Scholar] [CrossRef] [PubMed]
- Ashina, M. Migraine. N. Engl. J. Med. 2020, 383, 1866–1876. [Google Scholar] [CrossRef]
- Eigenbrodt, A.K.; Ashina, H.; Khan, S.; Diener, H.-C.; Mitsikostas, D.D.; Sinclair, A.J.; Pozo-Rosich, P.; Martelletti, P.; Ducros, A.; Lantéri-Minet, M.; et al. Diagnosis and management of migraine in ten steps. Nat. Rev. Neurol. 2021, 17, 501–514. [Google Scholar] [CrossRef]
- Andreou, A.P.; Edvinsson, L. Mechanisms of migraine as a chronic evolutive condition. J. Headache Pain 2019, 20, 117. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, H.G.; Albury, C.L.; Griffiths, L.R. Advances in genetics of migraine. J. Headache Pain 2019, 20, 72. [Google Scholar] [CrossRef]
- Gazerani, P. Current evidence on the role of epigenetic mechanisms in migraine: The way forward to precision medicine. OBM Genet. 2018, 02, 1804040. [Google Scholar] [CrossRef]
- Burstein, R.; Noseda, R.; Borsook, D. Migraine: Multiple processes, complex pathophysiology. J. Neurosci. 2015, 35, 6619–6629. [Google Scholar] [CrossRef]
- Mungoven, T.J.; Henderson, L.A.; Meylakh, N. Chronic migraine pathophysiology and treatment: A review of current perspectives. Front. Pain Res. 2021, 2, 705276. [Google Scholar] [CrossRef]
- Grech, O.; Mollan, S.P.; Wakerley, B.R.; Fulton, D.; Lavery, G.G.; Sinclair, A.J. The role of metabolism in migraine pathophysiology and susceptibility. Life 2021, 11, 415. [Google Scholar] [CrossRef] [PubMed]
- Spekker, E.; Tanaka, M.; Szabó, Á.; Vécsei, L. Neurogenic inflammation: The participant in migraine and recent advancements in translational research. Biomedicines 2021, 10, 76. [Google Scholar] [CrossRef]
- Shibata, Y. Migraine pathophysiology revisited: Proposal of a new molecular theory of migraine pathophysiology and headache diagnostic criteria. Int. J. Mol. Sci. 2022, 23, 13002. [Google Scholar] [CrossRef]
- Ashina, S.; Bentivegna, E.; Martelletti, P.; Eikermann-Haerter, K. Structural and functional brain changes in migraine. Pain Ther. 2021, 10, 211–223. [Google Scholar] [CrossRef]
- Ferrari, M.D.; Goadsby, P.J.; Burstein, R.; Kurth, T.; Ayata, C.; Charles, A.; Ashina, M.; van den Maagdenberg, A.M.J.M.; Dodick, D.W. Migraine. Nat. Rev. Dis. Prim. 2022, 8, 2. [Google Scholar] [CrossRef] [PubMed]
- Ong, J.J.Y.; De Felice, M. Migraine treatment: Current acute medications and their potential mechanisms of action. Neurotherapeutics 2018, 15, 274–290. [Google Scholar] [CrossRef] [PubMed]
- Sacco, S.; Bendtsen, L.; Ashina, M.; Reuter, U.; Terwindt, G.; Mitsikostas, D.-D.; Martelletti, P. European headache federation guideline on the use of monoclonal antibodies acting on the calcitonin gene related peptide or its receptor for migraine prevention. J. Headache Pain 2019, 20, 6. [Google Scholar] [CrossRef]
- Bron, C.; Sutherland, H.G.; Griffiths, L.R. Exploring the hereditary nature of migraine. Neuropsychiatr. Dis. Treat. 2021, 17, 1183–1194. [Google Scholar] [CrossRef]
- Erdener, S.E.; Dalkara, T. Modelling headache and migraine and its pharmacological manipulation. Br. J. Pharmacol. 2014, 171, 4575–4594. [Google Scholar] [CrossRef]
- Andreou, A.P.; Oshinsky, M.L. Animal models of migraine. In Pathophysiology of Headaches: From Molecule to Man; Ashina, M., Geppetti, P., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 31–66. [Google Scholar] [CrossRef]
- Harriott, A.M.; Strother, L.C.; Vila-Pueyo, M.; Holland, P.R. Animal models of migraine and experimental techniques used to examine trigeminal sensory processing. J. Headache Pain 2019, 20, 91. [Google Scholar] [CrossRef] [PubMed]
- Tardiolo, G.; Bramanti, P.; Mazzon, E. Migraine: Experimental models and novel therapeutic approaches. Int. J. Mol. Sci. 2019, 20, 2932. [Google Scholar] [CrossRef]
- Arendt-Nielsen, L.; Nielsen, T.A.; Gazerani, P. Translational pain biomarkers in the early development of new neurotherapeutics for pain management. Expert Rev. Neurother. 2014, 14, 241–254. [Google Scholar] [CrossRef]
- Okamoto, K.; Hasegawa, M.; Piriyaprasath, K.; Kakihara, Y.; Saeki, M.; Yamamura, K. Preclinical models of deep craniofacial nociception and temporomandibular disorder pain. Jpn. Dent. Sci. Rev. 2021, 57, 231–241. [Google Scholar] [CrossRef]
- Sessle, B.J. Chronic orofacial pain: Models, mechanisms, and genetic and related environmental influences. Int. J. Mol. Sci. 2021, 22, 7112. [Google Scholar] [CrossRef]
- Reddy, K.S.; Naidu, M.U.; Rani, P.U.; Rao, T.R. Human experimental pain models: A review of standardized methods in drug development. J. Res. Med. Sci. 2012, 17, 587–595. [Google Scholar]
- Olesen, A.E.; Andresen, T.; Staahl, C.; Drewes, A.M. Human experimental pain models for assessing the therapeutic efficacy of analgesic drugs. Pharmacol. Rev. 2012, 64, 722–779. [Google Scholar] [CrossRef] [PubMed]
- Argouarch, A.R.; Schultz, N.; Yang, A.C.; Jang, Y.; Garcia, K.; Cosme, C.G.; Corrales, C.I.; Nana, A.L.; Karydas, A.M.; Spina, S.; et al. Postmortem human dura mater cells exhibit phenotypic, transcriptomic and genetic abnormalities that impact their use for disease modeling. Stem Cell Rev. Rep. 2022, 18, 3050–3065. [Google Scholar] [CrossRef] [PubMed]
- Schulte, L.H.; May, A. The migraine generator revisited: Continuous scanning of the migraine cycle over 30 days and three spontaneous attacks. Brain 2016, 139, 1987–1993. [Google Scholar] [CrossRef]
- Ashina, M.; Hansen, J.M.; Bo, Á.D.; Olesen, J. Human models of migraine—Short-term pain for long-term gain. Nat. Rev. Neurol. 2017, 13, 713–724. [Google Scholar] [CrossRef]
- Villar-Martinez, M.D.; Goadsby, P.J. Pathophysiology and therapy of associated features of migraine. Cells 2022, 11, 2767. [Google Scholar] [CrossRef] [PubMed]
- Altamura, C.; Corbelli, I.; de Tommaso, M.; Di Lorenzo, C.; Di Lorenzo, G.; Di Renzo, A.; Filippi, M.; Jannini, T.B.; Messina, R.; Parisi, P.; et al. Pathophysiological bases of comorbidity in migraine. Front. Hum. Neurosci. 2021, 15, 640574. [Google Scholar] [CrossRef] [PubMed]
- Younis, S.; Hougaard, A.; Noseda, R.; Ashina, M. Current understanding of thalamic structure and function in migraine. Cephalalgia 2019, 39, 1675–1682. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.; Fu, Z.; Zeng, F.; Maleki, N.; Lan, L.; Li, Z.; Park, J.; Wilson, G.; Gao, Y.; Liu, M.; et al. Abnormal thalamocortical network dynamics in migraine. Neurology 2019, 92, e2706–e2716. [Google Scholar] [CrossRef]
- Ashina, M.; Terwindt, G.M.; Al-Karagholi, M.A.; de Boer, I.; Lee, M.J.; Hay, D.L.; Schulte, L.H.; Hadjikhani, N.; Sinclair, A.J.; Ashina, H.; et al. Migraine: Disease characterisation, biomarkers, and precision medicine. Lancet 2021, 397, 1496–1504. [Google Scholar] [CrossRef] [PubMed]
- Eising, E.; Datson, N.A.; van den Maagdenberg, A.M.; Ferrari, M.D. Epigenetic mechanisms in migraine: A promising avenue? BMC Med. 2013, 11, 26. [Google Scholar] [CrossRef]
- Skorobogatykh, K.; van Hoogstraten, W.S.; Degan, D.; Prischepa, A.; Savitskaya, A.; Ileen, B.M.; Bentivegna, E.; Skiba, I.; D’Acunto, L.; Ferri, L.; et al. Functional connectivity studies in migraine: What have we learned? J. Headache Pain 2019, 20, 108. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, S.N.; Xu, T.Y.; Miao, Z.W.; Su, D.F.; Miao, C.Y. Organoid technology for brain and therapeutics research. CNS Neurosci. Ther. 2017, 23, 771–778. [Google Scholar] [CrossRef]
- Agostoni, E.C.; Barbanti, P.; Calabresi, P.; Colombo, B.; Cortelli, P.; Frediani, F.; Geppetti, P.; Grazzi, L.; Leone, M.; Martelletti, P.; et al. Current and emerging evidence-based treatment options in chronic migraine: A narrative review. J. Headache Pain 2019, 20, 92. [Google Scholar] [CrossRef]
- Goadsby, P.J.; Holland, P.R.; Martins-Oliveira, M.; Hoffmann, J.; Schankin, C.; Akerman, S. Pathophysiology of migraine: A disorder of sensory processing. Physiol. Rev. 2017, 97, 553–622. [Google Scholar] [CrossRef]
- Jacobs, B.; Dussor, G. Neurovascular contributions to migraine: Moving beyond vasodilation. Neuroscience 2016, 338, 130–144. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, J.; Baca, S.M.; Akerman, S. Neurovascular mechanisms of migraine and cluster headache. J. Cereb. Blood Flow Metab. 2019, 39, 573–594. [Google Scholar] [CrossRef] [PubMed]
- Zeidler, M.; Kummer, K.K.; Kress, M. Towards bridging the translational gap by improved modeling of human nociception in health and disease. Pflug. Arch. 2022, 474, 965–978. [Google Scholar] [CrossRef]
- Albury, C.L.; Stuart, S.; Haupt, L.M.; Griffiths, L.R. Ion channelopathies and migraine pathogenesis. Mol. Genet. Genom. 2017, 292, 729–739. [Google Scholar] [CrossRef]
- Liu, T.H.; Wang, Z.; Xie, F.; Liu, Y.Q.; Lin, Q. Contributions of aversive environmental stress to migraine chronification: Research update of migraine pathophysiology. World J. Clin. Cases 2021, 9, 2136–2145. [Google Scholar] [CrossRef]
- Xu, J.; Wen, Z. Brain organoids: Studying human brain development and diseases in a dish. Stem Cells Int. 2021, 2021, 5902824. [Google Scholar] [CrossRef]
- Goto-Silva, L.; Junqueira, M. Single-cell proteomics: A treasure trove in neurobiology. Biochim. Biophys. Acta Proteins Proteom. 2021, 1869, 140658. [Google Scholar] [CrossRef] [PubMed]
- Bartsch, T.; Goadsby, P.J. The trigeminocervical complex and migraine: Current concepts and synthesis. Curr. Pain Headache Rep. 2003, 7, 371–376. [Google Scholar] [CrossRef]
- Yi, M.; Sherzai, A.Z.; Ani, C.; Shavlik, D.; Ghamsary, M.; Lazar, E.; Sherzai, D. Strong association between migraine and transient global amnesia: A national inpatient sample analysis. J. Neuropsychiatry Clin. Neurosci. 2019, 31, 43–48. [Google Scholar] [CrossRef]
- Waliszewska-Prosol, M.; Nowakowska-Kotas, M.; Bladowska, J.; Papier, P.; Budrewicz, S.; Pokryszko-Dragan, A. Transient global amnesia—Risk factors and putative background. Neurol India 2020, 68, 624–629. [Google Scholar] [CrossRef]
- Wang, Y.W.; Hu, N.; Li, X.H. Genetic and epigenetic regulation of brain organoids. Front. Cell Dev. Biol. 2022, 10, 948818. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-Y.; Ting, H.-C.; Su, H.-L.; Jeng, J.-R. Combining induced pluripotent stem cells and genome editing technologies for clinical applications. Cell Transplant. 2018, 27, 379–392. [Google Scholar] [CrossRef]
- Hofer, M.; Lutolf, M.P. Engineering organoids. Nat. Rev. Mater. 2021, 6, 402–420. [Google Scholar] [CrossRef] [PubMed]
- Mazzara, P.G.; Muggeo, S.; Luoni, M.; Massimino, L.; Zaghi, M.; Valverde, P.T.; Brusco, S.; Marzi, M.J.; Palma, C.; Colasante, G.; et al. Frataxin gene editing rescues Friedreich’s ataxia pathology in dorsal root ganglia organoid-derived sensory neurons. Nat. Commun. 2020, 11, 4178. [Google Scholar] [CrossRef]
- LaMontagne, E.; Muotri, A.R.; Engler, A.J. Recent advancements and future requirements in vascularization of cortical organoids. Front. Bioeng. Biotechnol. 2022, 10, 1048731. [Google Scholar] [CrossRef]
- Song, G.; Zhao, M.; Chen, H.; Zhou, X.; Lenahan, C.; Ou, Y.; He, Y. The application of brain organoid technology in stroke research: Challenges and prospects. Front. Cell. Neurosci. 2021, 15, 646921. [Google Scholar] [CrossRef]
- Nikolakopoulou, P.; Rauti, R.; Voulgaris, D.; Shlomy, I.; Maoz, B.M.; Herland, A. Recent progress in translational engineered in vitro models of the central nervous system. Brain 2020, 143, 3181–3213. [Google Scholar] [CrossRef]
- Matsui, T.K.; Tsuru, Y.; Hasegawa, K.; Kuwako, K.I. Vascularization of human brain organoids. Stem Cells 2021, 39, 1017–1024. [Google Scholar] [CrossRef]
- Cakir, B.; Xiang, Y.; Tanaka, Y.; Kural, M.H.; Parent, M.; Kang, Y.J.; Chapeton, K.; Patterson, B.; Yuan, Y.; He, C.S.; et al. Engineering of human brain organoids with a functional vascular-like system. Nat. Methods 2019, 16, 1169–1175. [Google Scholar] [CrossRef]
- Zhang, W.; Jiang, J.; Xu, Z.; Yan, H.; Tang, B.; Liu, C.; Chen, C.; Meng, Q. Microglia-containing human brain organoids for the study of brain development and pathology. Mol. Psychiatry 2022, 28, 96–107. [Google Scholar] [CrossRef] [PubMed]
- Porciúncula, L.O.; Goto-Silva, L.; Ledur, P.F.; Rehen, S.K. The age of brain organoids: Tailoring cell identity and functionality for normal brain development and disease modeling. Front. Neurosci. 2021, 15, 674563. [Google Scholar] [CrossRef] [PubMed]
- Butlen-Ducuing, F.; Pétavy, F.; Guizzaro, L.; Zienowicz, M.; Salmonson, T.; Haas, M.; Corruble, E.; Alteri, E. Challenges in drug development for central nervous system disorders: A European Medicines Agency perspective. Nat. Rev. Drug Discov. 2016, 15, 813–814. [Google Scholar] [CrossRef] [PubMed]
- Wang, H. Modeling neurological diseases with human brain organoids. Front. Synaptic Neurosci. 2018, 10, 15. [Google Scholar] [CrossRef]
- Groveman, B.R.; Ferreira, N.C.; Foliaki, S.T.; Walters, R.O.; Winkler, C.W.; Race, B.; Hughson, A.G.; Zanusso, G.; Haigh, C.L. Human cerebral organoids as a therapeutic drug screening model for Creutzfeldt–Jakob disease. Sci. Rep. 2021, 11, 5165. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Seong, H.; Li, X.; Yu, X.; Xu, S.; Li, Y. Human brain organoid: A versatile tool for modeling neurodegeneration diseases and for drug screening. Stem Cells Int. 2022, 2022, 2150680. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, L.; Bonfio, C.; Chadwick, J.; Begum, F.; Skehel, M.; Lancaster, M.A. Human CNS barrier-forming organoids with cerebrospinal fluid production. Science 2020, 369, eaaz5626. [Google Scholar] [CrossRef]
- Liu, F.; Huang, J.; Liu, Z. Vincristine impairs microtubules and causes neurotoxicity in cerebral organoids. Neuroscience 2019, 404, 530–540. [Google Scholar] [CrossRef]
- Park, J.-C.; Jang, S.-Y.; Lee, D.; Lee, J.; Kang, U.; Chang, H.; Kim, H.J.; Han, S.-H.; Seo, J.; Choi, M.; et al. A logical network-based drug-screening platform for Alzheimer’s disease representing pathological features of human brain organoids. Nat. Commun. 2021, 12, 280. [Google Scholar] [CrossRef]
- Sharlow, E.R.; Koseoglu, M.M.; Bloom, G.S.; Lazo, J.S. The promise and perils of compound discovery screening with inducible pluripotent cell-derived neurons. Assay Drug Dev. Technol. 2020, 18, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Do, T.P.; Guo, S.; Ashina, M. Therapeutic novelties in migraine: New drugs, new hope? J. Headache Pain 2019, 20, 37. [Google Scholar] [CrossRef]
- Yang, C.P.; Liang, C.S.; Chang, C.M.; Yang, C.C.; Shih, P.H.; Yau, Y.C.; Tang, K.T.; Wang, S.J. Comparison of new pharmacologic agents with triptans for treatment of migraine: A systematic review and meta-analysis. JAMA Netw. Open 2021, 4, e2128544. [Google Scholar] [CrossRef] [PubMed]
- Ramos, K.M.; Grady, C.; Greely, H.T.; Chiong, W.; Eberwine, J.; Farahany, N.A.; Johnson, L.S.M.; Hyman, B.T.; Hyman, S.E.; Rommelfanger, K.S.; et al. The NIH BRAIN initiative: Integrating neuroethics and neuroscience. Neuron 2019, 101, 394–398. [Google Scholar] [CrossRef] [PubMed]
- Niikawa, T.; Hayashi, Y.; Shepherd, J.; Sawai, T. Human brain organoids and consciousness. Neuroethics 2022, 15, 5. [Google Scholar] [CrossRef]
- Sawai, T.; Sakaguchi, H.; Thomas, E.; Takahashi, J.; Fujita, M. The ethics of cerebral organoid research: Being conscious of consciousness. Stem Cell Rep. 2019, 13, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Sawai, T.; Hayashi, Y.; Niikawa, T.; Shepherd, J.; Thomas, E.; Lee, T.L.; Erler, A.; Watanabe, M.; Sakaguchi, H. Mapping the ethical issues of brain organoid research and application. AJOB Neurosci. 2022, 13, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Lavazza, A.; Pizzetti, F.G. Human cerebral organoids as a new legal and ethical challenge†. J. Law Biosci. 2020, 7, lsaa005. [Google Scholar] [CrossRef]
- de Jongh, D.; Massey, E.K.; Berishvili, E.; Fonseca, L.M.; Lebreton, F.; Bellofatto, K.; Bignard, J.; Seissler, J.; Buerck, L.W.-V.; Honarpisheh, M.; et al. Organoids: A systematic review of ethical issues. Stem Cell Res. Ther. 2022, 13, 337. [Google Scholar] [CrossRef]
- Mollaki, V. Ethical challenges in organoid use. BioTech 2021, 10, 12. [Google Scholar] [CrossRef]
- Rosignoli, C.; Ornello, R.; Onofri, A.; Caponnetto, V.; Grazzi, L.; Raggi, A.; Leonardi, M.; Sacco, S. Applying a biopsychosocial model to migraine: Rationale and clinical implications. J. Headache Pain 2022, 23, 100. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gazerani, P. Human Brain Organoids in Migraine Research: Pathogenesis and Drug Development. Int. J. Mol. Sci. 2023, 24, 3113. https://doi.org/10.3390/ijms24043113
Gazerani P. Human Brain Organoids in Migraine Research: Pathogenesis and Drug Development. International Journal of Molecular Sciences. 2023; 24(4):3113. https://doi.org/10.3390/ijms24043113
Chicago/Turabian StyleGazerani, Parisa. 2023. "Human Brain Organoids in Migraine Research: Pathogenesis and Drug Development" International Journal of Molecular Sciences 24, no. 4: 3113. https://doi.org/10.3390/ijms24043113
APA StyleGazerani, P. (2023). Human Brain Organoids in Migraine Research: Pathogenesis and Drug Development. International Journal of Molecular Sciences, 24(4), 3113. https://doi.org/10.3390/ijms24043113







