Occupational Skin Dermatitis among Healthcare Workers Associated with the COVID-19 Pandemic: A Review of the Literature
Abstract
1. Introduction
2. General Characteristics of Occupational Skin Disorders
3. Healthcare Workers and Occupational Skin Disease
4. Occupational Skin Diseases among COVID-19-Engaged Healthcare Workers
4.1. The Types of Skin Eruption among COVID-19-Engaged Healthcare Workers
4.2. The Factors Influencing Occupational Skin Diseases in the COVID-19 Pandemic
4.2.1. The Influences of Hand Sanitizers and Handwashing
4.2.2. The Influences of Mask Wearing
4.2.3. Decreased QOL among COVID-19-Engaged Healthcare Workers
5. The Prevention of Occupational Skin Diseases among COVID-19-Engaged Healthcare Workers
6. Patch Testing and Causative Agents
6.1. Gloves and Causative Agents
6.2. Hand Sanitizer and Causative Agents
6.3. Masks and Causative Agents
7. Summary of, and Insights into, Dermatosis Associated with COVID-19-Engaged Work
8. Conclusions
Funding
Conflicts of Interest
References
- Sigmundsdottir, H.; Butcher, E.C. Environmental cues, dendritic cells and the programming of tissue-selective lymphocyte trafficking. Nat. Immunol. 2008, 9, 981–987. [Google Scholar] [CrossRef]
- Kabashima, K.; Honda, T.; Ginhoux, F.; Egawa, G. The immunological anatomy of the skin. Nat. Rev. Immunol. 2019, 19, 19–30. [Google Scholar] [CrossRef]
- Sawada, Y.; Nakatsuji, T.; Dokoshi, T.; Kulkarni, N.N.; Liggins, M.C.; Sen, G.; Gallo, R.L. Cutaneous innate immune tolerance is mediated by epigenetic control of MAP2K3 by HDAC8/9. Sci Immunol. 2021, 6, eabe1935. [Google Scholar] [CrossRef]
- Martora, F.; Battista, T.; Marasca, C.; Genco, L.; Fabbrocini, G.; Potestio, L. Cutaneous Reactions Following COVID-19 Vaccination: A Review of the Current Literature. Clin. Cosmet. Investig. Dermatol. 2022, 15, 2369–2382. [Google Scholar] [CrossRef]
- Picone, V.; Martora, F.; Fabbrocini, G.; Marano, L. “COVID arm”: Abnormal side effect after Moderna COVID-19 vaccine. Dermatol. Ther. 2022, 35, e15197. [Google Scholar] [CrossRef]
- Martora, F.; Villani, A.; Battista, T.; Fabbrocini, G.; Potestio, L. COVID-19 vaccination and inflammatory skin diseases. J. Cosmet. Dermatol. 2023, 22, 32–33. [Google Scholar] [CrossRef]
- Martora, F.; Fabbrocini, G.; Nappa, P.; Megna, M. Reply to ‘Development of severe pemphigus vulgaris following SARS-CoV-2 vaccination with BNT162b2’ by Solimani et al. J. Eur. Acad. Dermatol. Venereol. 2022, 36, e750–e751. [Google Scholar] [CrossRef] [PubMed]
- Huynh, T.; Sanchez-Flores, X.; Yau, J.; Huang, J.T. Cutaneous Manifestations of SARS-CoV-2 Infection. Am. J. Clin. Dermatol. 2022, 23, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Martora, F.; Picone, V.; Fornaro, L.; Fabbrocini, G.; Marasca, C. Can COVID-19 cause atypical forms of pityriasis rosea refractory to conventional therapies? J. Med. Virol. 2022, 94, 1292–1293. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Kaur, H.; Singh, K.; Sen, C.K. Cutaneous Manifestations of COVID-19: A Systematic Review. Adv. Wound Care 2021, 10, 51–80. [Google Scholar] [CrossRef] [PubMed]
- Olusegun, O.A.; Martincigh, B.S. Allergic contact dermatitis: A significant environmental and occupational skin disease. Int. J. Dermatol. 2021, 60, 1082–1091. [Google Scholar] [CrossRef] [PubMed]
- Kersh, A.E.; Johansen, M.; Ojeaga, A.; de la Feld, S. Hand Dermatitis in the Time of COVID-19: A Review of Occupational Irritant Contact Dermatitis. Dermatitis 2021, 32, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.; Marks, J.G., Jr.; Flamm, A. Occupational Contact Dermatitis: Common Occupational Allergens. Dermatol. Clin. 2020, 38, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.N.; Tan, J.C.; Li, J.; Li, S.; Cai, Y.; Wang, H. COVID-19 Pandemic: Experiences in China and Implications for its Prevention and Treatment Worldwide. Curr. Cancer Drug Targets 2020, 20, 410–416. [Google Scholar] [PubMed]
- Gostin, L.O.; Friedman, E.A.; Hossain, S.; Mukherjee, J.; Zia-Zarifi, S.; Clinton, C.; Rugege, U.; Buss, P.; Were, M.; Dhai, A. Human rights and the COVID-19 pandemic: A retrospective and prospective analysis. Lancet 2022, 401, 154–168. [Google Scholar] [CrossRef] [PubMed]
- Elston, D.M. Occupational skin disease among health care workers during the coronavirus (COVID-19) epidemic. J. Am. Acad. Dermatol. 2020, 82, 1085–1086. [Google Scholar] [CrossRef]
- Hadjieconomou, S.; Hughes, J.; Kamath, S. Occupational skin disease during the COVID-19 pandemic, as captured in a Dermatology staff clinic in the United Kingdom. J. Eur. Acad. Dermatol. Venereol. 2020, 34, e670–e671. [Google Scholar] [CrossRef]
- Zaino, M.L.; Huecker, M.R. The Impact of SARs-CoV-2 on Occupational Skin Disease Found in Physicians. Workplace Health Saf. 2021, 69, 485. [Google Scholar] [CrossRef]
- Darlenski, R.; Kazandjieva, J.; Tsankov, N. Prevention and occupational hazards for the skin during COVID-19 pandemic. Clin. Dermatol. 2021, 39, 92–97. [Google Scholar] [CrossRef]
- Šakić, F.; Babić, Ž.; Franić, Z.; Macan, J. Characteristics of hand eczema in final-year apprentice nurses during the COVID-19 pandemic. Contact Dermat. 2022, 86, 98–106. [Google Scholar] [CrossRef]
- Lee, H.C.; Goh, C.L. ‘Occupational dermatoses from Personal Protective Equipment during the COVID-19 pandemic in the tropics—A Review’. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 589–596. [Google Scholar] [CrossRef]
- Piapan, L.; Bramuzzo, D.; Rui, F.; Filon, F.L. Incidence of skin diseases in healthcare workers before and during the COVID-19 pandemic at Trieste hospitals (northeastern Italy). Contact Dermat. 2022, 87, 492–499. [Google Scholar] [CrossRef] [PubMed]
- Villani, A.; Fabbrocini, G.; Annunziata, M.C.; Potestio, L. Maskne prevalence and risk factors during the COVID-19 pandemic. J. Eur. Acad. Dermatol. Venereol. 2022, 36, e678–e680. [Google Scholar] [CrossRef]
- Lushniak, B.D. The importance of occupational skin diseases in the United States. Int. Arch. Occup. Environ. Health 2003, 76, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Mekonnen, T.H.; Yenealem, D.G.; Tolosa, B.M. Self-report occupational-related contact dermatitis: Prevalence and risk factors among healthcare workers in Gondar town, Northwest Ethiopia, 2018-a cross-sectional study. Environ. Health Prev. Med. 2019, 24, 11. [Google Scholar] [CrossRef]
- Boonchai, W.; Thanomkitti, K.; Kasemsarn, P. Occupational contact dermatitis in tertiary university hospital: A 5-year retrospective study. J. Med. Assoc. Thail. 2014, 97, 1182–1188. [Google Scholar]
- Larese Filon, F.; Pesce, M.; Paulo, M.S.; Loney, T.; Modenese, A.; John, S.M.; Kezic, S.; Macan, J. Incidence of occupational contact dermatitis in healthcare workers: A systematic review. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 1285–1289. [Google Scholar] [CrossRef]
- Schwensen, J.F.; Menné, T.; Sommerlund, M.; Andersen, K.E.; Mortz, C.G.; Zachariae, C.; Johansen, J.D. Contact Allergy in Danish Healthcare Workers: A Retrospective Matched Case-control Study. Acta Derm. Venereol. 2016, 96, 237–240. [Google Scholar] [CrossRef]
- Nichol, K.; Copes, R.; Kersey, K.; Eriksson, J.; Holness, D.L. Screening for hand dermatitis in healthcare workers: Comparing workplace screening with dermatologist photo screening. Contact Dermat. 2019, 80, 374–381. [Google Scholar] [CrossRef]
- Molin, S.; Bauer, A.; Schnuch, A.; Geier, J. Occupational contact allergy in nurses: Results from the Information Network of Departments of Dermatology 2003-2012. Contact Dermat. 2015, 72, 164–171. [Google Scholar] [CrossRef]
- Nethercott, J.R.; Holness, D.L.; Page, E. Occupational contact dermatitis due to glutaraldehyde in health care workers. Contact Dermat. 1988, 18, 193–196. [Google Scholar] [CrossRef] [PubMed]
- Omrane, A.; Khedher, A.; Harrathi, C.; Maoua, M.; Khalfallah, T.; Bouzgarrou, L.; Mrizak, N.; Henchi, M.A.; Ali, H.B.H. Quality of Life of Healthcare Workers Suffering from Occupational Contact Dermatitis. Recent Adv. Inflamm. Allergy Drug Discov. 2022, 15, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Rundle, C.W.; Presley, C.L.; Militello, M.; Barber, C.; Powell, D.L.; Jacob, S.E.; Atwater, A.R.; Watsky, K.L.; Yu, J.; Dunnick, C.A. Hand hygiene during COVID-19: Recommendations from the American Contact Dermatitis Society. J. Am. Acad. Dermatol. 2020, 83, 1730–1737. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.; Zhu, S.; Huang, Y.; Li, L.; Tao, J.; Lei, T.; Song, J.; Liu, D.; Chen, L.; Shi, Y.; et al. Adverse skin reactions among healthcare workers during the coronavirus disease 2019 outbreak: A survey in Wuhan and its surrounding regions. Br. J. Dermatol. 2020, 183, 190–192. [Google Scholar] [CrossRef] [PubMed]
- Kiely, L.F.; Moloney, E.; O’Sullivan, G.; Eustace, J.A.; Gallagher, J.; Bourke, J.F. Irritant contact dermatitis in healthcare workers as a result of the COVID-19 pandemic: A cross-sectional study. Clin. Exp. Dermatol. 2021, 46, 142–144. [Google Scholar] [CrossRef]
- Singh, M.; Bothra, A.; Pawar, M.; Maheswari, A.; Tiwari, A.; Adhicari, P. Prevalence of cheilitis in health care workers treating patients with COVID-19. J. Am. Acad. Dermatol. 2020, 83, e373–e374. [Google Scholar] [CrossRef]
- Bothra, A.; Das, S.; Singh, M.; Pawar, M.; Maheswari, A. Retroauricular dermatitis with vehement use of ear loop face masks during COVID-19 pandemic. J. Eur. Acad. Dermatol. Venereol. 2020, 34, e549–e552. [Google Scholar] [CrossRef]
- Hamnerius, N.; Pontén, A.; Bergendorff, O.; Bruze, M.; Björk, J.; Svedman, C. Skin Exposures, Hand Eczema and Facial Skin Disease in Healthcare Workers During the COVID-19 Pandemic: A Cross-sectional Study. Acta Derm. Venereol. 2021, 101, adv00543. [Google Scholar] [CrossRef]
- Proietti, I.; Borrelli, I.; Skroza, N.; Santoro, P.E.; Gualano, M.R.; Bernardini, N.; Mambrin, A.; Tolino, E.; Marchesiello, A.; Marraffa, F.; et al. Adverse skin reactions to personal protective equipment during COVID-19 pandemic in Italian health care workers. Dermatol. Ther. 2022, 35, e15460. [Google Scholar] [CrossRef]
- Seed, M.J.; Fowler, K.; Byrne, L.; Carder, M.; Daniels, S.; Iskandar, I.Y.K.; Feary, J.; Gawkrodger, D.J.; van Tongeren, M. Skin and respiratory ill-health attributed to occupational face mask use. Occup. Med. 2022, 72, 339–342. [Google Scholar] [CrossRef]
- Skiveren, J.G.; Ryborg, M.F.; Nilausen, B.; Bermark, S.; Philipsen, P.A. Adverse skin reactions among health care workers using face personal protective equipment during the coronavirus disease 2019 pandemic: A cross-sectional survey of six hospitals in Denmark. Contact Dermat. 2022, 86, 266–275. [Google Scholar] [CrossRef]
- Choi, S.Y.; Hong, J.Y.; Kim, H.J.; Lee, G.Y.; Cheong, S.H.; Jung, H.J.; Bang, C.H.; Lee, D.H.; Jue, M.S.; Kim, H.O.; et al. Mask-induced dermatoses during the COVID-19 pandemic: A questionnaire-based study in 12 Korean hospitals. Clin. Exp. Dermatol. 2021, 46, 1504–1510. [Google Scholar] [CrossRef] [PubMed]
- Justin, L.Y.S.; Yew, Y.W. Facial dermatoses induced by face masks: A systematic review and meta-analysis of observational studies. Contact Dermat. 2022, 87, 473–484. [Google Scholar] [CrossRef]
- Cosansu, N.C.; Yuksekal, G.; Kutlu, O.; Umaroglu, M.; Yaldız, M.; Dikicier, B.S. The change in the frequency and severity of facial dermatoses and complaints in healthcare workers during the COVID-19. J. Cosmet. Dermatol. 2022, 21, 3200–3205. [Google Scholar] [CrossRef] [PubMed]
- Nahm, W.J.; Nagler, A.R.; Milam, E.C. Association of perioral dermatitis with facial mask usage during the COVID-19 pandemic: A retrospective study. JAAD Int. 2023, 10, 86–87. [Google Scholar] [CrossRef]
- Keng, B.M.H.; Gan, W.H.; Tam, Y.C.; Oh, C.C. Personal protective equipment-related occupational dermatoses during COVID-19 among health care workers: A worldwide systematic review. JAAD Int. 2021, 5, 85–95. [Google Scholar] [CrossRef]
- Loh, E.W.; Yew, Y.W. Hand hygiene and hand eczema: A systematic review and meta-analysis. Contact Dermat. 2022, 87, 303–314. [Google Scholar] [CrossRef]
- Hamnerius, N.; Svedman, C.; Bergendorff, O.; Björk, J.; Bruze, M.; Pontén, A. Wet work exposure and hand eczema among healthcare workers: A cross-sectional study. Br. J. Dermatol. 2018, 178, 452–461. [Google Scholar] [CrossRef]
- Jindal, R.; Pandhi, D. Hand Hygiene Practices and Risk and Prevention of Hand Eczema during the COVID-19 Pandemic. Indian Dermatol. Online J. 2020, 11, 540–543. [Google Scholar] [PubMed]
- Kim, Y.C.; Park, J.H.; Ludovice, P.J.; Prausnitz, M.R. Synergistic enhancement of skin permeability by N-lauroylsarcosine and ethanol. Int. J. Pharm. 2008, 352, 129–138. [Google Scholar] [CrossRef]
- Merle, C.; Baillet-Guffroy, A. Physical and chemical perturbations of the supramolecular organization of the stratum corneum lipids: In vitro to ex vivo study. Biochim. Biophys. Acta 2009, 1788, 1092–1098. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Guio, D.A.; Díaz-Guio, Y.; Pinzón-Rodas, V.; Díaz-Gomez, A.S.; Guarín-Medina, J.A.; Chaparro-Zúñiga, Y.; Ricardo-Zapata, A.; Rodriguez-Morales, A.J. COVID-19: Biosafety in the Intensive Care Unit. Curr. Trop. Med. Rep. 2020, 7, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Hua, W.; Zuo, Y.; Wan, R.; Xiong, L.; Tang, J.; Zou, L.; Shu, X.; Li, L. Short-term skin reactions following use of N95 respirators and medical masks. Contact Dermat. 2020, 83, 115–121. [Google Scholar] [CrossRef]
- Hu, K.; Fan, J.; Li, X.; Gou, X.; Li, X.; Zhou, X. The adverse skin reactions of health care workers using personal protective equipment for COVID-19. Medicine 2020, 99, e20603. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Goldminz, A.; Chisolm, S.; Jacob, S.E.; Zippin, J.H.; Wu, P.A.; Hylwa, S.; Dunnick, C.A.; Chen, J.K.; Reeder, M.; et al. Facial Personal Protective Equipment: Materials, Resterilization Methods, and Management of Occupation-Related Dermatoses. Dermatitis 2021, 32, 78–85. [Google Scholar] [CrossRef]
- Damiani, G.; Gironi, L.C.; Pacifico, A.; Cristaudo, A.; Malagoli, P.; Allocco, F.; Bragazzi, N.L.; Linder, D.M.; Santus, P.; Buja, A.; et al. Masks use and facial dermatitis during COVID-19 outbreak: Is there a difference between CE and non-CE approved masks? Multi-center, real-life data from a large Italian cohort. Ital. J. Dermatol. Venerol. 2021, 156, 220–225. [Google Scholar] [CrossRef]
- Tatu, A.L.; Clatici, V.; Cristea, V. Isolation of Bacillus simplex strain from Demodex folliculorum and observations about Demodicosis spinulosa. Clin. Exp. Dermatol. 2016, 41, 818–820. [Google Scholar] [CrossRef]
- Tatu, A.L.; Ionescu, M.A.; Clatici, V.G.; Cristea, V.C. Bacillus cereus strain isolated from Demodex folliculorum in patients with topical steroid-induced rosaceiform facial dermatitis. An. Bras. De Dermatol. 2016, 91, 676–678. [Google Scholar] [CrossRef]
- Tatu, A.L.; Ionescu, M.A.; Cristea, V.C. Demodex folliculorum associated Bacillus pumilus in lesional areas in rosacea. Indian J. Dermatol. Venereol. Leprol. 2017, 83, 610–611. [Google Scholar] [CrossRef]
- Maher, A.; Staunton, K.; Kavanagh, K. Analysis of the effect of temperature on protein abundance in Demodex-associated Bacillus oleronius. Pathog. Dis. 2018, 76, fty032. [Google Scholar] [CrossRef]
- Paichitrojjana, A. Demodicosis Associated with Wearing a Face Mask: A Case Report. Case Rep. Dermatol. 2022, 14, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Nobeyama, Y.; Aihara, Y.; Asahina, A. Characteristics of Rosacea and Similar Diseases in Patients Wearing Face Masks. Ski. Appendage Disord. 2022, 8, 462–468. [Google Scholar] [CrossRef]
- Bui, A.N.; Yu, Z.; Lee, K.; Li, S.J.; Tsiaras, W.G.; Yu, S.H.; LeBoeuf, N.R.; Mostaghimi, A. A pilot study of the impact of facial skin protectants on qualitative fit testing of N95 masks. J. Am. Acad. Dermatol. 2021, 84, 554–556. [Google Scholar] [CrossRef]
- Kainth, G.S. Novel tip to prevent ear irritation with surgical face masks (FRSM) during the coronavirus (COVID-19) pandemic. Ann. R. Coll. Surg. Engl. 2020, 102, 470–471. [Google Scholar] [CrossRef]
- Loi, A.S.T.; Aribou, Z.M.; Fong, Y.T. Improving Recovery of Irritant Hand Dermatitis in Healthcare Workers with Workplace Interventions During the COVID-19 Pandemic. Front. Public Health 2022, 10, 844269. [Google Scholar] [CrossRef] [PubMed]
- Moldovan, H.R.; Manole, I.; Suru, A.; Butacu, A.I.; Tatu, A.L.; Lupu, A.; Dascalu, M.; Tiplica, G.S.; Salavastru, C.M. Prevention of Hand Eczema among Nurse Apprentice (PREVEDERM): An Interventional Study. Ann. Work. Expo. Health 2021, 65, 167–175. [Google Scholar] [CrossRef]
- Dejonckheere, G.; Herman, A.; Baeck, M. Allergic contact dermatitis caused by synthetic rubber gloves in healthcare workers: Sensitization to 1,3-diphenylguanidine is common. Contact Dermat. 2019, 81, 167–173. [Google Scholar] [CrossRef]
- Wentworth, A.B.; Yiannias, J.A.; Davis, M.D.; Killian, J.M. Benzalkonium Chloride: A Known Irritant and Novel Allergen. Dermatitis 2016, 27, 14–20. [Google Scholar] [CrossRef]
- Voller, L.M.; Schlarbaum, J.P.; Hylwa, S.A. Allergenic Ingredients in Health Care Hand Sanitizers in the United States. Dermatitis 2021, 32, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Kadivar, S.; Belsito, D.V. Occupational dermatitis in health care workers evaluated for suspected allergic contact dermatitis. Dermatitis 2015, 26, 177–183. [Google Scholar] [CrossRef]
- Navarro-Triviño, F.J.; Merida-Fernández, C.; Ródenas-Herranz, T.; Ruiz-Villaverde, R. Allergic contact dermatitis caused by elastic bands from FFP2 mask. Contact Dermat. 2020, 83, 168–169. [Google Scholar] [CrossRef]
- Aerts, O.; Dendooven, E.; Foubert, K.; Stappers, S.; Ulicki, M.; Lambert, J. Surgical mask dermatitis caused by formaldehyde (releasers) during the COVID-19 pandemic. Contact Dermat. 2020, 83, 172–173. [Google Scholar] [CrossRef]
- Yu, J.; Chen, J.K.; Mowad, C.M.; Reeder, M.; Hylwa, S.; Chisolm, S.; Dunnick, C.A.; Goldminz, A.M.; Jacob, S.E.; Wu, P.A.; et al. Occupational dermatitis to facial personal protective equipment in health care workers: A systematic review. J. Am. Acad. Dermatol. 2021, 84, 486–494. [Google Scholar] [CrossRef]
- Johansen, J.D.; Bonefeld, C.M.; Schwensen, J.F.B.; Thyssen, J.P.; Uter, W. Novel insights into contact dermatitis. J. Allergy Clin. Immunol. 2022, 149, 1162–1171. [Google Scholar] [CrossRef]
- Li, Y.; Li, L. Contact Dermatitis: Classifications and Management. Clin. Rev. Allergy Immunol. 2021, 61, 245–281. [Google Scholar] [CrossRef] [PubMed]
- Bains, S.N.; Nash, P.; Fonacier, L. Irritant Contact Dermatitis. Clin. Rev. Allergy Immunol. 2019, 56, 99–109. [Google Scholar] [CrossRef]
- Carøe, T.K.; Ebbehøj, N.; Agner, T. A survey of exposures related to recognized occupational contact dermatitis in Denmark in 2010. Contact Dermat. 2014, 70, 56–62. [Google Scholar] [CrossRef]
- Slodownik, D.; Lee, A.; Nixon, R. Irritant contact dermatitis: A review. Australas J. Dermatol. 2008, 49, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hunziker, T.; Brand, C.U.; Kapp, A.; Waelti, E.R.; Braathen, L.R. Increased levels of inflammatory cytokines in human skin lymph derived from sodium lauryl sulphate-induced contact dermatitis. Br. J. Dermatol. 1992, 127, 254–257. [Google Scholar] [CrossRef] [PubMed]
- Gawkrodger, D.J.; Carr, M.M.; McVittie, E.; Guy, K.; Hunter, J.A. Keratinocyte expression of MHC class II antigens in allergic sensitization and challenge reactions and in irritant contact dermatitis. J. Investig. Dermatol. 1987, 88, 11–16. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, X.; Wang, C.; Qu, L.; Deng, J.; Wang, H.; Qin, Z. Resolution of PMA-induced skin inflammation involves interaction of IFN-γ and ALOX15. Mediat. Inflamm. 2013, 2013, 930124. [Google Scholar] [CrossRef]
- Eberhard, Y.; Ortiz, S.; Ruiz Lascano, A.; Kuznitzky, R.; Serra, H.M. Up-regulation of the chemokine CCL21 in the skin of subjects exposed to irritants. BMC Immunol. 2004, 5, 7. [Google Scholar] [CrossRef] [PubMed]
- de Jongh, C.M.; Khrenova, L.; Verberk, M.M.; Calkoen, F.; van Dijk, F.J.; Voss, H.; John, S.M.; Kezic, S. Loss-of-function polymorphisms in the filaggrin gene are associated with an increased susceptibility to chronic irritant contact dermatitis: A case-control study. Br. J. Dermatol. 2008, 159, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Natsuaki, Y.; Egawa, G.; Nakamizo, S.; Ono, S.; Hanakawa, S.; Okada, T.; Kusuba, N.; Otsuka, A.; Kitoh, A.; Honda, T.; et al. Perivascular leukocyte clusters are essential for efficient activation of effector T cells in the skin. Nat. Immunol. 2014, 15, 1064–1069. [Google Scholar] [CrossRef] [PubMed]
- Sawada, Y.; Honda, T.; Hanakawa, S.; Nakamizo, S.; Murata, T.; Ueharaguchi-Tanada, Y.; Ono, S.; Amano, W.; Nakajima, S.; Egawa, G.; et al. Resolvin E1 inhibits dendritic cell migration in the skin and attenuates contact hypersensitivity responses. J. Exp. Med. 2015, 212, 1921–1930. [Google Scholar] [CrossRef] [PubMed]
- Cumberbatch, M.; Dearman, R.J.; Kimber, I. Langerhans cells require signals from both tumour necrosis factor-alpha and interleukin-1 beta for migration. Immunology 1997, 92, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Cumberbatch, M.; Kimber, I. Tumour necrosis factor-alpha is required for accumulation of dendritic cells in draining lymph nodes and for optimal contact sensitization. Immunology 1995, 84, 31–35. [Google Scholar] [PubMed]
- Kimber, I.; Kinnaird, A.; Peters, S.W.; Mitchell, J.A. Correlation between lymphocyte proliferative responses and dendritic cell migration in regional lymph nodes following skin painting with contact-sensitizing agents. Int. Arch. Allergy Appl. Immunol. 1990, 93, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Kripke, M.L.; Munn, C.G.; Jeevan, A.; Tang, J.M.; Bucana, C. Evidence that cutaneous antigen-presenting cells migrate to regional lymph nodes during contact sensitization. J. Immunol. 1990, 145, 2833–2838. [Google Scholar] [CrossRef]
- Honda, T.; Egawa, G.; Grabbe, S.; Kabashima, K. Update of immune events in the murine contact hypersensitivity model: Toward the understanding of allergic contact dermatitis. J. Investig. Dermatol. 2013, 133, 303–315. [Google Scholar] [CrossRef]
- Xu, H.; Banerjee, A.; Dilulio, N.A.; Fairchild, R.L. Development of effector CD8+ T cells in contact hypersensitivity occurs independently of CD4+ T cells. J. Immunol. 1997, 158, 4721–4728. [Google Scholar] [CrossRef] [PubMed]
- Kiely, L.F.; O’Connor, C.; O’Briain, G.; O’Briain, C.; Gallagher, J.; Bourke, J.F. Maskne prevalence and associated factors in Irish healthcare workers during the COVID-19 pandemic. J. Eur. Acad. Dermatol. Venereol. 2022, 36, e506–e508. [Google Scholar] [CrossRef]
- Hadžavdić, A.; Bukvić Mokos, Z. Maskne: A New Entity in the COVID-19 Pandemic. Acta Dermatovenerol. Croat. 2021, 29, 148–153. [Google Scholar] [PubMed]
- Bakhsh, R.A.; Saddeeg, S.Y.; Basaqr, K.M.; Alshammrani, B.M.; Zimmo, B.S. Prevalence and Associated Factors of Mask-Induced Acne (Maskne) in the General Population of Jeddah During the COVID-19 Pandemic. Cureus 2022, 14, e26394. [Google Scholar] [CrossRef]
- Nielsen, R.; Gwosdow, A.R.; Berglund, L.G.; DuBois, A.B. The effect of temperature and humidity levels in a protective mask on user acceptability during exercise. Am. Ind. Hyg. Assoc. J. 1987, 48, 639–645. [Google Scholar] [CrossRef]
- Tunçer Vural, A. The development of acne vulgaris due to face masks during the pandemic, risk awareness and attitudes of a group of university students. J. Cosmet. Dermatol. 2022, 21, 5306–5313. [Google Scholar] [CrossRef]
- Narang, I.; Sardana, K.; Bajpai, R.; Garg, V.K. Seasonal aggravation of acne in summers and the effect of temperature and humidity in a study in a tropical setting. J. Cosmet. Dermatol. 2019, 18, 1098–1104. [Google Scholar] [CrossRef] [PubMed]
- Brooks, J.K.; Sultan, A.S.; Jabra-Rizk, M.A. Prolonged facial mask wear is a concern for the development of dysbiotic microbiome. Respir. Med. Res. 2022, 81, 100877. [Google Scholar] [CrossRef]
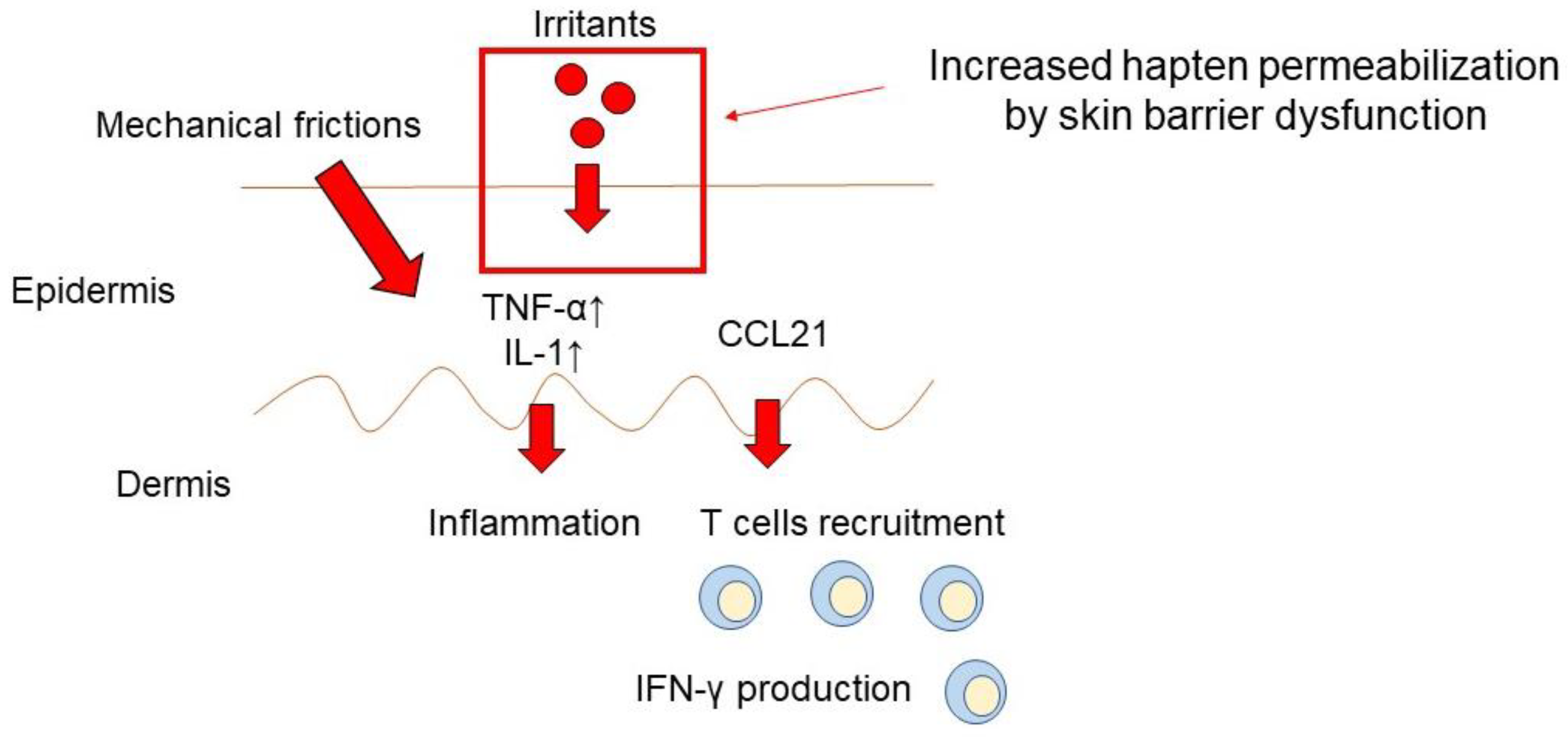
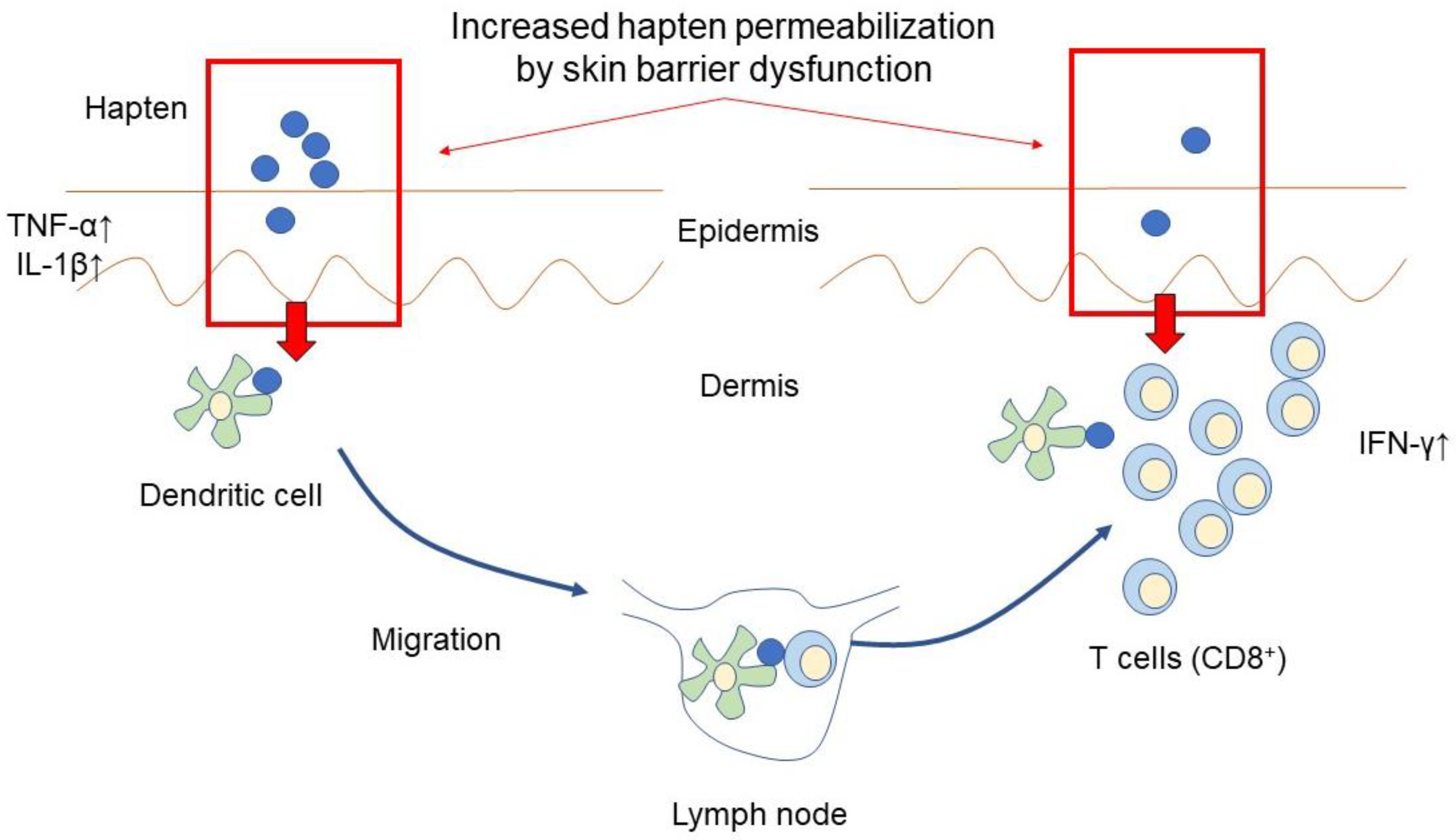
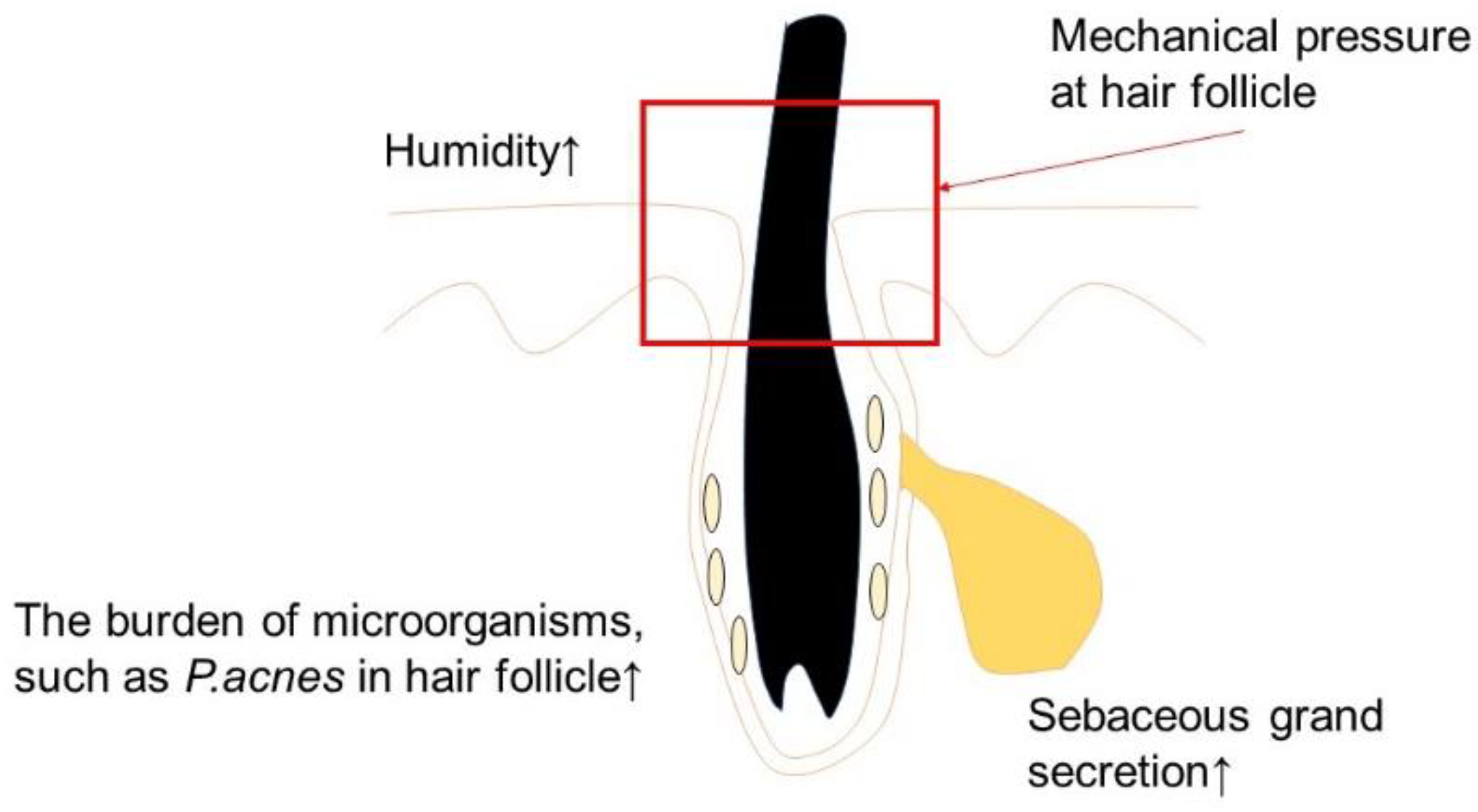
| Eruptions | Causative Factors | Possible Mechanism |
|---|---|---|
| Contact Dermatitis | Gloves [48] | Allergic hapten |
| Hand sanitizer [47]. | Allergic hapten Skin barrier dysfunction | |
| Mask [54] | Mechanical irritation Allergic hapten | |
| Acne Vulgaris | Mask [42] | Alteration in the local skin environment, e.g., the microbiome, temperature, moisture, mechanical follicular blocking |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sawada, Y. Occupational Skin Dermatitis among Healthcare Workers Associated with the COVID-19 Pandemic: A Review of the Literature. Int. J. Mol. Sci. 2023, 24, 2989. https://doi.org/10.3390/ijms24032989
Sawada Y. Occupational Skin Dermatitis among Healthcare Workers Associated with the COVID-19 Pandemic: A Review of the Literature. International Journal of Molecular Sciences. 2023; 24(3):2989. https://doi.org/10.3390/ijms24032989
Chicago/Turabian StyleSawada, Yu. 2023. "Occupational Skin Dermatitis among Healthcare Workers Associated with the COVID-19 Pandemic: A Review of the Literature" International Journal of Molecular Sciences 24, no. 3: 2989. https://doi.org/10.3390/ijms24032989
APA StyleSawada, Y. (2023). Occupational Skin Dermatitis among Healthcare Workers Associated with the COVID-19 Pandemic: A Review of the Literature. International Journal of Molecular Sciences, 24(3), 2989. https://doi.org/10.3390/ijms24032989






