Prevalence and Mechanisms of Skeletal Muscle Atrophy in Metabolic Conditions
Abstract
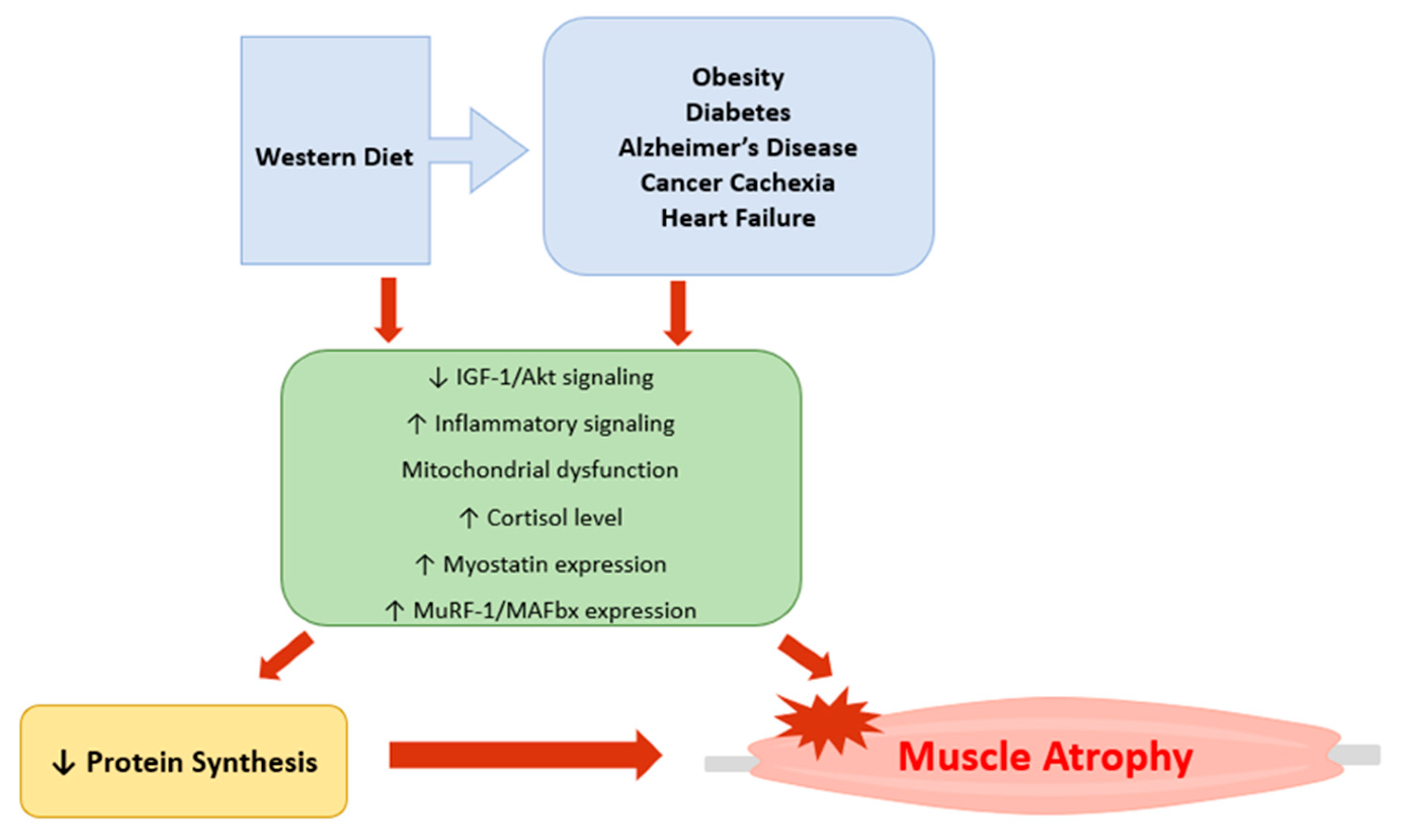
1. Introduction
2. Pathophysiology of Skeletal Muscle Atrophy
2.1. High-Fat Diet
2.2. Obesity
2.3. Diabetes Mellitus
2.3.1. Type 1 Diabetes Mellitus
2.3.2. Type 2 Diabetes Mellitus
2.4. Sarcopenia
2.5. Alzheimer’s Disease
2.6. Cancer Cachexia
2.7. Heart Failure
3. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Scott, W.; Stevens, J.; Binder-macleod, S.A. Human skeletal muscle fiber type classifications. Phys. Ther. 2001, 81, 1810–1816. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Pessin, J.E. Mechanisms for fiber-type specificity of skeletal muscle atrophy. Curr. Opin. Clin. Nutr. Metab. Care 2015, 16, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Bonaldo, P.; Sandri, M. Cellular and molecular mechanisms of muscle atrophy. Dis. Model. Mech. 2013, 6, 25–39. [Google Scholar] [CrossRef]
- Janssen, I.A.N.; Heymsfield, S.B.; Wang, Z.I.M.; Ross, R.; Heymsfield, S.B.; Wang, Z. Skeletal muscle mass and distribution in 468 men and women aged 18–88 yr. J. Appl. Physiol. 2021, 116, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Bachettini, N.P.; Bielemann, R.M.; Barbosa-Silva, T.G.; Menezes, A.M.B.; Tomasi, E.; Gonzalez, M.C. Sarcopenia as a mortality predictor in community-dwelling older adults: A comparison of the diagnostic criteria of the European Working Group on Sarcopenia in Older People. Eur. J. Clin. Nutr. 2020, 74, 573–580. [Google Scholar] [CrossRef]
- Johnson, R.J.; Segal, M.S.; Sautin, Y.; Nakagawa, T.; Feig, D.I.; Kang, D.-H.; Sersch, M.S.; Benner, S.; Sánchez-Lozada, L.G. Potential role of sugar (fructose) in the epidemic of hypertension, obesity and the metabolic syndrome, diabetes, kidney disease, and cardiovascular disease. Am. J. Clin. Nutr. 2007, 86, 899–906. [Google Scholar]
- Buckley, J. Availability of high-fat foods might drive the obesity epidemic. Nat. Rev. Endocrinol. 2018, 14, 574–575. [Google Scholar] [CrossRef]
- Ghibaudi, L.; Cook, J.; Farley, C.; Van Heek, M.; Hwa, J.J. Fat intake affects adiposity, cormorbidity factors, and energy metabolim of Sprague-Dawley rats. Obes. Res. 2012, 10, 956–963. [Google Scholar] [CrossRef]
- Lee, Y.S.; Li, P.; Huh, J.Y.; Hwang, I.J.; Lu, M.; Kim, J.I.; Ham, M.; Talukdar, S.; Chen, A.; Lu, W.J.; et al. Inflammation is necessary for long-term but not short-term high-fat diet-induced insulin resistance. Diabetes 2011, 60, 2474–2483. [Google Scholar] [CrossRef]
- Collins, K.H.; Hart, D.A.; Smith, I.C.; Issler, A.M.; Reimer, R.A.; Seerattan, R.A.; Rios, J.L.; Herzog, W. Acute and chronic changes in rat soleus muscle after high-fat high-sucrose diet. Physiol. Rep. 2017, 5, e13270. [Google Scholar] [CrossRef]
- Gomex-Perez, Y.; Capllonch-Amer, G.; Gianotti, M.; Llando, I.; Proenza, A.M. Long-term high-fat-diet feeding induces skeletal muscle mitochondrial biogenesis in rats in a sex-dependent and muscle-type specific manner. Nutr. Metab. 2012, 9, 15. [Google Scholar] [CrossRef] [PubMed]
- Messa, G.; Piasecki, M.; Hurst, J.; Hill, C.; Tallis, J.; Degens, H. The impact of a high-fat diet in mice is dependent on duration and age, and differs between muscles. J. Exp. Biol. 2020, 223, jeb217117. [Google Scholar] [CrossRef] [PubMed]
- Van den Broek, N.M.A.; Ciapaite, J.; De Feyter, H.M.M.L.; Houten, S.M.; Wanders, R.J.A.; Jeneson, J.A.L.; Nicolay, K.; Prompers, J.J. Increased mitochondrial content rescues in vivo muscle oxidative capacity in long-term high-fat diet-fed rats. FASEB J. 2009, 24, 1354–1364. [Google Scholar] [CrossRef] [PubMed]
- Sousa, D.L.G.O.; Marshall, A.G.; Norman, J.E.; Fuqua, J.D.; Lira, V.A.; Rutledge, J.C.; Bodine, S.C. The effects of diet composition and chronic obesity on muscle growth and function. J. Appl. Physiol. 2021, 130, 124–138. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-R.; Khamoui, A.V.; Jo, E.; Park, B.-S.; Zourdos, M.C.; Panton, L.B.; Ormsbee, M.J.; Kim, J.-S. Effects of chronic high-fat feeding on skeletal muscle mass and function in middle-aged mice. Aging Clin. Exp. Res. 2015, 27, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Eshima, H.; Tamura, Y.; Kakehi, S.; Kurebayashi, N. Long-term, but not short-term high-fat diet induces fiber composition changes and impaired contractile force in mouse fast-twitch skeletal muscle. Physiol. Rep. 2017, 5, e13250. [Google Scholar] [CrossRef]
- Roseno, S.L.; Davis, P.R.; Bollinger, L.M.; Powell, J.J.S.; Witczak, C.A.; Brault, J.J. Short-term, high-fat diet accelerates disuse atrophy and protein degradation in a muscle-specific manner in mice. Nutr. Metab. 2015, 12, 39. [Google Scholar] [CrossRef]
- Andrich, D.E.; Ou, Y.; Melbouci, L.; Leduc-Gaudet, J.-P.; Auclair, N.; Mercier, J.; Secco, B.; Tomaz, L.M.; Gouspillou, G.; Danialou, G.; et al. Altered lipid metabolism impairs skeletal muscle force in young rats submitted to a short-term high-fat diet. Front. Physiol. 2018, 9, 1327. [Google Scholar] [CrossRef]
- Rasool, S.; Geetha, T.; Broderick, T.L.; Babu, J.R.; Lambert, E. High Fat High Sucrose Diet Leads Obesity and Induces Myodegeneration. Front. Physiol. 2018, 9, 1054. [Google Scholar] [CrossRef]
- Hurst, J.; James, R.S.; Cox, V.M.; Hill, C.; Tallis, J. Investigating a dose-response relationship between high-fat diet consumption and the contractile performance of isolated mouse soleus, EDL and diaphragm muscles. Eur. J. Appl. Physiol. 2019, 119, 213–226. [Google Scholar] [CrossRef]
- Guerra, J.; Ferrer, B.; Giralt, M.; Comes, G.; Carrasco, J.; Molinero, A.; Serrano, A.L.; Hidalgo, J. Muscular interleukin-6 differentially regulates skeletal muscle adaptation to high-fat diet in a sex-dependent manner. Cytokine 2015, 74, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Campbell, T.L.; Mitchell, A.S.; McMillan, E.M.; Bloemberg, D.; Pavlov, D.; Messa, I.; Mielke, J.G.; Quadrilatero, J. High-fat feeding does not induce an autophagic or apoptotic phenotype in female rat skeletal muscle. Exp. Biol. Med. 2014, 240, 657–668. [Google Scholar] [CrossRef]
- Priego, T.; Sanchez, J.; Pico, C.; Palou, A. Sex-differential expression of metabolism-related genes in response to a high-fat diet. Obesity 2012, 16, 819–826. [Google Scholar] [CrossRef]
- Ferretti, R.; Moura, E.G.; Dos Santos, V.C.; Caldeira, E.J.; Conte, M.; Matsumura, C.Y.; Pertille, A.; Mosqueira, M. High-fat diet suppresses the positive effect of creatine supplementation on skeletal muscle function by reducing protein expression of IGF-PI3K-AKT-mTOR pathway. PLoS ONE 2018, 13, e0199728. [Google Scholar] [CrossRef]
- Sun, Y.; Huang, J.; Chen, Z.; Du, M.; Ren, F.; Luo, J.; Fang, B. Amyotrophy induced by a high-fat diet is closely related to inflammation and protein degradation determined by quantitative phosphoproteomic analysis in skeletal muscle of C57BL/6J mice. J. Nutr. 2020, 150, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Carlsen, H.; Haugen, F.; Zadelaar, S.; Drevon, C.; Blomhoff, R. Diet-induced obesity increases NF-κB signaling in reporter mice. Genes Nutr. 2009, 4, 215–222. [Google Scholar] [CrossRef]
- Tsai, S.-F.; Hung, H.-C.; Shih, M.M.-C.; Chang, F.-C.; Chung, B.; Wang, C.-Y.; Lin, Y.-L.; Kuo, Y.-M. High-fat diet-induced increases in glucocorticoids contribute to the development of non-alcoholic fatty liver disease in mice. FASEB J. 2021, 36, e22130. [Google Scholar] [CrossRef] [PubMed]
- Ramamoorthy, S.; Cidlowski, J.A. Corticosteroids-mechanisms of action in health and disease. Rheum. Dis. Clin. North Am. 2016, 42, 15. [Google Scholar] [CrossRef] [PubMed]
- Schoorlemmer, R.; Peeters, G.; Van Schoor, N.; Lips, P. Relationships between cortisol level, mortality and chronic diseases in older persons. Clin. Endocrinol. 2009, 71, 779–786. [Google Scholar] [CrossRef]
- Lee, M.-K.; Jeong, H.H.; Kim, M.-J.; Ryu, H.; Baek, J.; Lee, B. Nutrients against glucocorticoid-induced muscle atrophy. Foods 2022, 11, 687. [Google Scholar] [CrossRef]
- Seifu, C.N.; Fahey, P.P.; Hailemariam, T.G.; Frost, S.A.; Atlantis, E. Dietary patterns associated with obesity outcomes in adults: An umbrella review of systematic reviews. Public Health Nutr. 2021, 24, 6390–6414. [Google Scholar] [CrossRef]
- Pestoni, G.; Riedl, A.; Breuninger, T.A.; Wawro, N.; Krieger, J.-P.; Meisinger, C.; Pathmann, W.; Thorand, B.; Harris, C.; Peters, A.; et al. Association between dietary patterns and prediabetes, undetected diabetes or clinically diagnosed diabetes: Results from the KORA FF4 study. Eur. J. Nutr. 2021, 60, 2331–2341. [Google Scholar] [CrossRef] [PubMed]
- Mohseni, R.; Aliakbar, S.; Abdollahi, A.; Yekaninejad, M.S.; Maghbooli, Z.; Mirazaei, K. Relationship between major dietary patterns and sarcopenia among menopausal women. Aging Clin. Exp. Res. 2017, 29, 1241–1248. [Google Scholar] [CrossRef] [PubMed]
- Samuelsson, J.; Kern, S.; Zetterberg, H.; Blennow, K.; Rothenberg, E.; Wallengren, O.; Skoog, I.; Zettergren, A. A Western-style dietary pattern is associated with cerebrospinal fluid biomarker levels for preclinical Alzheimer’s disease-A population-based cross-sectional study among 70-year-olds. Alzheimer’s Dement. 2021, 7, e12183. [Google Scholar] [CrossRef]
- Niu, L.; Han, D.; Xu, R.; Han, B.; Zhou, X.; Li, S.; Qu, C.; Liu, M. A high-sugar high-fat diet induced metabolic syndrome shows some symptoms of Alzheimer’s disease in rats. J. Nutr. Health Aging 2016, 20, 509–513. [Google Scholar] [CrossRef]
- Najafi, M.; Mozaffari, H.; Jalilpiran, Y.; Mokhtari, P.; Teymouri, M.; Faghih, S. The associations between dietary patterns and cardiovascular risk factors among adults: A cross-sectional study. Clin. Nutr. ESPEN 2020, 40, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Beydoun, M.A. The obesity epidemic in the United States--gender, age, socioeconomic, racial/ethnic, and geographic characteristics: A systemic review and meta-regression analysis. Epidemiol. Rev. 2007, 29, 6–28. [Google Scholar] [CrossRef] [PubMed]
- Kelly, T.; Yang, W.; Chen, C.; Reynolds, K.; He, J. Global burden of obesity in 2005 and projections to 2030. Int. J. Obes. 2008, 32, 1431–1437. [Google Scholar] [CrossRef]
- Kinlen, D.; Cody, D.; O’Shea, D. Complications of obesity. Int. J. Med. 2018, 111, 437–443. [Google Scholar] [CrossRef]
- Pakhra, V.; Galappaththy, S.L.; Bulchandani, S.; Cabandugama, P.K. Obesity and the Western diet: How we got here. Mo Med. 2020, 117, 436–538. [Google Scholar]
- Mokdad, A.H.; Ford, E.S.; Bowman, B.A. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA 2003, 289, 76–79. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.; Leitzmann, M.; Freisling, H.; Bray, F.; Romieu, I.; Renehan, A.; Soerjomataram, I. Obesity and cancer: An update of the global impact. Cancer Epidemiol. 2016, 41, 8–15. [Google Scholar] [CrossRef]
- Brestoff, J.; Wilen, C.B.; Moley, J.R.; Li, Y.; Zou, W.; Malvin, N.P.; Rowen, M.N.; Saunders, B.; Ma, H.; Mack, M.R.; et al. Intercellular mitochondria transfer to macrophages regulates white adipose tissue homeostasis and is impaired in obesity. Cell Metab. 2021, 33, 270–282. [Google Scholar] [CrossRef] [PubMed]
- Unger, R.H.; Clark, G.O.; Scherer, P.E.; Orci, L. Lipid homeostasis, lipotoxicity and the metabolic syndrome. Biochim. Biophys. Acta 2010, 1801, 209–214. [Google Scholar] [CrossRef]
- Therkelsen, K.; Pedley, A.; Speliotes, E.; Massaro, J.; Murabito, J.; Hoffman, U.; Fox, C. Intramuscular fat and associations with metabolic risk factors in the Framingham Heart Study. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 863. [Google Scholar] [CrossRef] [PubMed]
- Michael, N.; Gupta, V.; Sadananthan, S.A.; Sampathkumar, A.; Chen, L.; Pan, H.; Tint, M.T.; Lee, K.J.; Loy, S.L.; Aris, I.M.; et al. Determinants of intramyocellular lipid accumulation in early childhood. Int. J. Obes. 2020, 44, 1141–1151. [Google Scholar] [CrossRef]
- Durschlag, R.; Layman, D. Skeletal muscle growth in lean and obese Zucker rats. Comp. Study 1983, 47, 282–291. [Google Scholar]
- Crettaz, M.; Prentki, M.; Zaninetti, D.; Jeanrenaud, B. Insulin resistance in soleus muscle from obese Zucker rats. Involv. Several Defective Sites. Biochem. J. 1980, 186, 525–534. [Google Scholar]
- Cuendet, G.S.; Loten, E.G.; Jeanrenaud, B.; Renold, A.E. Decreased basal, noninsulin-stimulated glucose uptake and metabolism by skeletal soleus muscle isolated from obese-hyperglycemic (ob/ob) mice. J. Clin. Investivation 1976, 58, 1078–1088. [Google Scholar] [CrossRef]
- Wang, X.; Hu, Z.; Hu, J.; Du, J.; Mitch, W.E. Insulin resistance accelerates muscle protein degradation: Activation of the ubiquitin-proteasome pathway by defects in muscle cell signaling. Endocrinology 2006, 147, 4160–4168. [Google Scholar] [CrossRef]
- Martinez-Gonzalez, M.A.; Martinez, J.A.; Hu, F.B.; Gibney, M.; Kearney, J. Physical inactivity, sedentary lifestyle and obesity in the European Union. Int. J. Obes. 1999, 23, 1192–1201. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Chen, X.; Fan, M. Signaling mechanims involved in disuse muscle atrophy. Med. Hypotheses 2007, 69, 310–321. [Google Scholar] [CrossRef] [PubMed]
- Tomlinson, D.J.; Erskine, R.M.; Morse, C.I.; Winwood, K.; Onambele-Pearson, G. The impact of obesity on skeletal muscle strength and structure through adolescence to old age. Biogerontology 2016, 17, 457–483. [Google Scholar] [CrossRef]
- Tallis, J.; Hill, C.; James, R.S.; Cox, V.M.; Seebacher, F. The effect of obesity on the contractile performance of isolated mouse soleus, EDL, and diaphragm muscles. J. Appl. Physiol. 2017, 122, 170–181. [Google Scholar] [CrossRef]
- Hill, C.; James, R.S.; Cox, V.M.; Tallis, J. Does dietary-induced obesity in old age impair the contractile performance of isolated mouse soleus, extensor digitorum longus and diaphragm skeletal muscles? Nutrients 2019, 11, 505. [Google Scholar] [CrossRef]
- Bollinger, L.M.; Powell, J.J.S.; Houmard, J.A.; Witczak, C.A.; Brault, J.J. Skeletal muscle myotubes in severe obesity exhibit altered ubiquitin-proteasome and autophagic/lysosomal proteolytic flux. Obesity 2015, 23, 1185–1193. [Google Scholar] [CrossRef]
- Sullivan, B.P.; Weiss, J.A.; Nie, Y.; Garner, R.T.; Drohan, C.J.; Kuang, S.; Stout, J.; Gavin, T. Skeletal muscle IGF-1 is lower at rest and after resistance exercise in humans with obesity. Eur. J. Appl. Physiol. 2020, 120, 2835–2846. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.A.; Lee, D.E.; Patton, J.F.; Perry Jr, R.A.; Brown, J.L.; Baum, J.I.; Smith-Blair, N.; Greene, N.P.; Washington, T.A. Diet-induced obesity alters anabolic signalling in mice at the onset of skeletal muscle regeneration. Acta Physiol. 2015, 215, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Murton, A.J.; Marimuthu, K.; Mallinson, J.E.; Selby, A.L.; Smith, K.; Rennie, M.J.; Greenhaff, P.L. Obesity appears to be associated with altered muscle protein synthetic and breakdown responses to increased nutrient delivery in older men, but not reduced muscle mass or contractile unction. Obes. Stud. 2015, 64, 3160–3171. [Google Scholar] [CrossRef] [PubMed]
- Akhmedov, D.; Berdeaux, R. The effects of obesity on skeletal muscle regeneration. Front. Physiol. 2013, 4, 371. [Google Scholar] [CrossRef]
- Hittle, D.S.; Berggren, J.R.; Shearer, J.; Boyle, K.; Houmard, J.A. Increased secretion and expression of myostatin in skeletal muscle from extremely obese women. Diabetes 2009, 58, 30–38. [Google Scholar] [CrossRef]
- Kurose, S.; Onishi, K.; Takao, N.; Miyauchi, T.; Takahashi, K.; Kimura, Y. Association of serum adiponectin and myostatin levels with skeletal muscle in patients with obesity: A cross-sectional study. PLoS ONE 2021, 16, e0245678. [Google Scholar] [CrossRef]
- Chatterjee, S.; Khunti, K.; Davies, M.J. Type 2 diabetes. Lancet 2017, 389, 2239–2251. [Google Scholar] [CrossRef]
- National Diabetes Statistics Report 2020. Available online: https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf (accessed on 28 December 2021).
- Shu, L.; Shen, X.-M.; Li, C.; Zhang, X.-Y.; Zheng, P.-F. Dietary patterns are associated with type 2 diabetes mellitus among middle-aged adults in Zhejiang Province, China. Nutr. J. 2017, 16, 81. [Google Scholar] [CrossRef]
- Neill, E.D.O.; Wilding, P.H.; Kahn, C.R.; Remmen, H.V.; McArdle, A.; Jackson, M.J.; Close, G.L. Absence of insulin signalling in skeletal muscle is associated with reduced muscle mass and function: Evidence for decreased protein synthesis and not increased degradation. Age 2010, 32, 209–222. [Google Scholar]
- Mahashabde, M.; Chauhary, G.; Kanchi, G.; Rohatgi, S.; Rao, P.; Patil, R.; Nallamothu, V. An unusual case of critical illness polyneuromyopathy. Indian J. Crit. Care Med. 2020, 24, 133–135. [Google Scholar] [PubMed]
- Maratova, K.; Soucek, O.; Matyskova, J.; Hlavka, Z.; Petruzelkova, L.; Obermannova, B.; Pruhova, S.; Kolouskova, S.; Sumnik, Z. Muscle functions and bone strength are impaired in adolescents with type 1 diabetes. Bone 2018, 106, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Li, M.; Wang, K.; Qi, G.; Liu, H.; Wei, W.; Ji, Y.; Chang, M.; Deng, C.; Xu, F.; et al. Diabetic muscular atrophy: Molecular mechanisms and promising therapies. Front. Endocrinol. 2021, 13, 917113. [Google Scholar] [CrossRef]
- Hiromine, Y.; Noso, S.; Rakugi, H.; Gugimoto, K.; Takata, Y.; Katsuya, T.; Fukuda, M.; Akasaka, H.; Osawa, H.; Tabara, Y.; et al. Poor glycemic control rather than types of diabetes is a risk factor for sarcopenia in diabetes mellitus: The MUSCLES-DM study. J. Diabetes Investig. 2022, 13, 1881–1888. [Google Scholar] [CrossRef]
- Monaco, C.M.; Hughes, M.C.; Ramos, S.V.; Varah, N.E.; Lamberz, C.; Rahman, F.A.; Mcglory, C.; Tarnopolsky, M.A.; Krause, M.P.; Laham, R.; et al. Altered mitochondrial bioenergetics and ultrastructure in the skeletal muscle of young adults with type 1 diabetes. Diabetologia 2018, 61, 1411–1423. [Google Scholar] [CrossRef]
- Kharroubi, A.; Darwish, H. Diabetes mellitus: The epidemic of the century. World J. Diabetes 2015, 6, 850–867. [Google Scholar] [CrossRef]
- Hirata, Y.; Kazuhiro, S.; Yoko, O.; Kenta, K.; Shiki, O.; Yasuhiko, M.; Imamura, M.; Takeda, S.; Hosooka, T.; Ogawa, W. Hyperglycemia induces skeletal muscle atrophy via a WWP1/KLF15 asix. JCI Insight 2019, 4, e124952. [Google Scholar] [CrossRef] [PubMed]
- Ishida, T.; Iizuka, M.; Ou, Y.; Morisawa, S.; Hirata, A. Juzentaihoto suppresses muscle atrophy in streptozotocin-induced diabetic mice. Biol. Pharm. Bull. 2019, 42, 1128–1133. [Google Scholar] [CrossRef] [PubMed]
- O’Neil, C.C.; Locke, E.J.; Sipf, D.A.; Thompson, J.H.; Drebushenko, E.; Berger, N.S.; Segich, B.S.; Kolwicz Jr, S.C. The effects of exercise training on glucose homeostasis and muscle metabolism in type 1 diabetic female mice. Metabolites 2022, 12, 948. [Google Scholar] [CrossRef]
- Park, S.W.; Goodpaster, B.H.; Lee, J.S.; Kuller, L.H.; Boudreau, R.; Rekeneire, N.; Harris, T.B.; Kritchevsky, S.; Tylavsky, F.A.; Nevitt, M.; et al. Excessive loss of skeletal muscle mass in older adults with type 2 diabetes. Diabetes Care 2009, 32, 1993–1997. [Google Scholar] [CrossRef]
- Kelley, D.E.; He, J.; Menshikova, E.V.; Ritov, V.B. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes 2002, 51, 2944–2950. [Google Scholar] [CrossRef] [PubMed]
- Minnock, D.; Annibaini, G.; Valli, G.; Saltarelli, R.; Krause, M.; Barbieri, E.; De Vito, G. Altered muscle mitochondrial, inflammatory and trophic markers, and reduced exercise training adaptations in type 1 diabetes. J. Physiol. 2022, 600, 1405–1418. [Google Scholar] [CrossRef]
- Sugimoto, K.; Ikegami, H.; Takata, Y.; Katsuya, T.; Fukuda, M.; Akasaka, H.; Tabara, Y.; Osawa, H.; Hiromine, Y.; Rakugi, H. Glycemic control and insulin improve muscle mass and gait speed in type 2 diabetes: The MUSCLES-DM Study. J. Am. Med. Dir. Assoc. 2021, 22, 834–838. [Google Scholar] [CrossRef]
- Li, R.; Zhang, Y.; Rasool, S.; Geetha, T.; Babu, J.R. Effects and underlying mechanisms of bioactive compounds on type 2 diabetes mellitus and Alzheimer’ s disease. Oxid. Med. Cell. Longev. 2019, 2019, 8165707. [Google Scholar] [CrossRef]
- Zierath, J.R.; Krook, A.; Wallberg-Henriksson, H. Insulin action and insulin resistance in human skeletal muscle. Diabetologia 2000, 43, 821–835. [Google Scholar] [CrossRef]
- Gaster, M.; Staehr, P.; Beck-nielsen, H.; Schrøder, H.D.; Handberg, A. GLUT4 is reduced in slow muscle fibers of type 2 diabetic patients: Is insulin resistance in type 2 diabetes a slow type 1 fiber disease? Comp. Study 2001, 50, 1324–1329. [Google Scholar]
- Albers, P.H.; Pedersen, A.J.T.; Birk, J.B.; Kristensen, D.E.; Vind, B.F.; Baba, O.; Nøhr, J.; Højlund, K.; Wojtaszewski, J. Human muscle fiber type-specific insulin signaling: Impact of obesity and type 2 diabetes. Diabetes 2014, 64, 485–497. [Google Scholar] [CrossRef] [PubMed]
- Oberbach, A.; Bossenz, Y.; Lehmann, S.; Niebauer, J.; Adams, V.; Paschke, R.; Schön, M.R.; BLuher, M.A.T.T.H.I.A.S.; Punkt, K. Altered fiber distribution and fiber-specific glycolytic and oxidative enzyme activity in skeletal muscle of patients with type 2 diabetes. Diabetes Care 2006, 29, 895–900. [Google Scholar] [CrossRef] [PubMed]
- Perry, B.D.; Caldow, M.K.; Brennan-Speranza, T.C.; Sbaraglia, M.; Jerums, G.; Garnham, A.; Wong, C.; Levinger, P.; Asrar, M. Muscle atrophy in patients with type 2 diabetes mellitus: Roles of inflammatory pathways, physical activity and exercise. Exerc. Immunol. Rev. 2017, 22, 94–109. [Google Scholar]
- Chiodini, I.; Adda, G.; Scillitani, A.; Coletti, F.; Morelli, V.; Lembo, S.D.; Epaminonda, P.; Masserini, B.; Beck-Peccoz, P.; Orsi, E.; et al. Cortisol secretion in patients with type 2 diabetes: Relationship with chronic complications. Diabetes Care 2007, 30, 83–88. [Google Scholar] [CrossRef]
- Chourpiliadis, C.; Aeddula, N.R. Physiology, Glucocorticoids; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Kamba, A.; Daimon, M.; Murakami, H.; Otaka, H.; Matsuki, K.; Sato, E.; Tanabe, J.; Takayasu, S.; Matsuhashi, Y.; Yanagimachi, M.; et al. Association between higher serum cortisol levels and decreased insulin secretion in a general population. PLoS ONE 2016, 11, e0166077. [Google Scholar] [CrossRef]
- Hu, Z.; Du, J.; Mitch, W.E.; Hu, Z.; Wang, H.; Lee, I.H.; Du, J.; Mitch, W.E. Endogenous glucocorticoids and impaired insulin signaling are both required to stimulate muscle wasting under pathophysiological conditions in mice. J. Clin. Investig. 2009, 119, 3059–3069. [Google Scholar] [CrossRef]
- Schakman, O.; Gilson, H.; Thissen, J.P. Mechanisms of glucocorticoid-induced myopathy. Soc. Endocrinol. 2008, 197, 1–10. [Google Scholar] [CrossRef]
- Pereira, R.M.R.; de Carvalho, J.F. Glucocorticoid-induced myopathy. Jt. Bone Spine 2011, 78, 41–44. [Google Scholar] [CrossRef]
- Wang, H.; Kubica, N.; Ellisen, L.; Jefferson, L.S.; Kimball, S.R. Dexamethasone represses signaling through the mammalian target of rapamycin in muscle cells by enhancing expression of REDD1. J. Biol. Chem. 2006, 281, P39128–P39134. [Google Scholar] [CrossRef] [PubMed]
- Stewart, P.; Krozowski, Z. 11 beta-hydroxysteroid dehydrogenase. Vitam. Horm. 1999, 57, 249–324. [Google Scholar] [PubMed]
- Gathercole, L.L.; Lavery, G.G.; Morgan, S.A.; Cooper, M.S.; Sinclair, A.J.; Tomlinson, J.W.; Stewart, P.M. 11β-Hydroxysteroid dehydrogenase 1: Translational and therapeutic aspects. Endocr. Rev. 2013, 34, 525–555. [Google Scholar] [CrossRef] [PubMed]
- Masuzaki, H.; Flier, J.S. Tissue-specific glucocorticoid reactivating enzyme, 11 beta-hydroxysteroid dehydrogenase type 1 (11 beta-HSD1)—A promising drug target for the treatment of metabolic syndrome. Curr. Drug Targets. Immun. Endocr. Metabol. Disord. 2003, 3, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Kilgour, A.H.; Gallagher, I.J.; MacLullich, A.M.; Andrew, R.; Gray, C.D.; Hyde, P.; Wackerhage, H.; Husi, H.; Ross, J.A.; Starr, J.M.; et al. Increased skeletal muscle 11βHSD1 mRNA is associated with lower muscle strength in ageing. PLoS ONE 2013, 8, e84057. [Google Scholar] [CrossRef]
- Morgan, S.A.; McCabe, E.L.; Gathercole, L.L.; Hassan-Smith, Z.K.; Larner, D.P.; Bujalska, I.J.; Stewart, P.M.; Tomlinson, J.W.; Lavery, G.G. 11β-HSD1 is the major regulator of the tissue-specific effects of circulating glucocorticoid excess. Proc. Natl. Acad. Sci. USA 2014, 111, E2482–E2491. [Google Scholar] [CrossRef] [PubMed]
- Morgan, S.A.; Hassan-Smith, Z.K.; Doig, C.; Sherlock, M.; Stewart, P.M.; Lavery, G.G. Glucocorticoids and 11β-HSD1 are major regulators of intramyocellular protein metabolism. J. Endocrinol. 2016, 229, 277–286. [Google Scholar] [CrossRef]
- Hardy, R.S.; Doig, C.L.; Hussain, Z.; O’Leary, M.; Morgan, S.A.; Pearson, M.J.; Naylor, A.; Jones, S.W.; Filer, A.; Stewart, P.M.; et al. 11β-Hydroxysteroid dehydrogenase type 1 within muscle protects against the adverse effects of local inflammation. J. Pathol. 2016, 240, 472–483. [Google Scholar] [CrossRef]
- Lee, J.; Auyeung, T.; Kwok, T.; Leung, P.; Woo, J. The effect of diabetes mellitus on age-associated lean mass loss in 3153 older adults. Diabet. Med. 2010, 27, 1366–1371. [Google Scholar] [CrossRef]
- Jakobsen, J.; Reske-Nielsen, E. Diffuse muscle fiber atrophy in newly diagnosed diabetes. Clin. Neuropathol. 1986, 5, 73–77. [Google Scholar]
- Andersen, H.; Nielsen, S.; Mogensen, C.E.; Jakobsen, J. Muscle strength in type 2 diabetes. Diabetes 2004, 53, 1543–1548. [Google Scholar] [CrossRef]
- Kalyani, R.R.; Tra, Y.; Yeh, H.-C.; Egan, J.M.; Ferrucci, L.; Brancati, F.L. Quadriceps strength, quadriceps power, and gait speed in older U.S. adults with diabetes mellitus: Results from the National Health and Nutrition Examination Survey, 1999–2002. J. Am. Geriatr. Soc. 2013, 61, 769–775. [Google Scholar] [CrossRef] [PubMed]
- Andreassen, C.S.; Jakobsen, J.; Andersen, H. Muscle weakness: A progressive late complication in diabetic distal symmetric polyneuropathy. Diabetes 2006, 55, 806–812. [Google Scholar] [CrossRef] [PubMed]
- Said, G.; Goulon-Goeau, C.; Slama, G.; Tchobroutsky, G. Severe early-onset polyneuropathy in insulin-dependent diabetes mellitus. N. Engl. J. Med. 1992, 326, 1257–1263. [Google Scholar] [CrossRef] [PubMed]
- Rabol, R.; Larsen, S.; Hojberg, M.; Almdal, T.; Boushel, R.; Haugaard, S.; Andersen, J.L.; Madsbad, S.; Dela, F. Regional anatomic differences in skeletal muscle mitochondrial respiration in type 2 diabetes and obesity. J. Clin. Endocrinol. Metab. 2010, 95, 857–863. [Google Scholar] [CrossRef] [PubMed]
- Park, S.W.; Goodpaster, B.H.; Strotmeyer, E.S.; Rekeneire, N.D.; Harris, T.B.; Schwartz, A.V.; Tylavsky, F.A.; Newman, A.B. Decreased muscle strength and quality in older adults: The health, aging, and body composition study. Diabetes 2006, 55, 1813–1818. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.J.; Moon, J.W.; Lee, J.O.; Kim, J.H.; Jung, E.J.; Kim, S.J.; Oh, J.Y.; Wu, S.W.; Lee, P.R.; Park, S.H.; et al. Metformin induces muscle atrophy by transcriptional regulation of myostatin via HDAC6 and FoxO3a. J. Cachexia. Sarcopenia Muscle 2022, 13, 605–620. [Google Scholar] [CrossRef]
- Hasan, M.M.; Shalaby, S.M.; El-Gendy, J.; Abdelghany, E.M.A. Beneficial effects of metformin on muscle atrophy induced by obesity in rats. J. Cell. Biochem. 2018, 120, 5677–5686. [Google Scholar] [CrossRef]
- Deschenes, M.R. Effects of aging on muscle fibre type and size. Sport Med. 2004, 34, 809–824. [Google Scholar] [CrossRef]
- Morley, J.E.; Anker, S.D.; Haehling, S. Von Prevalence, incidence, and clinical impact of sarcopenia: Facts, numbers, and epidemiology—update 2014. J. Cachexia Sarcopenia Muscle 2014, 5, 253–259. [Google Scholar] [CrossRef]
- Kirkendall, D.T.; Garrett, W.E. The effect of aging and training on skeletal muscle. Am. J. Sport Med. 1998, 26, 598–602. [Google Scholar] [CrossRef]
- Goodpaster, B.H.; Park, S.W.; Harris, T.B.; Kritchevsky, S.B.; Nevitt, M.; Schwartz, A.V.; Simonsick, E.M.; Tylavsky, F.A.; Visser, M.; Newman, A.B.; et al. The loss of skeletal muscle strength, mass, and quality in older adults: The health, aging and body composition study. J. Gerontol. 2006, 61, 1059–1064. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.-P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis Report of the European Working Group on Sarcopenia in Older People. Age Aging 2010, 39, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Kallman, D.A.; Plato, C.C.; Tobin, J.D. The role of muscle loss in the age-related decline of grip strength: Cross-sectional and longitudinal perspectives. J. Gerontol. 1990, 45, M82–M88. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.R. Loss of skeletal muscle mass in aging: Examining the relationship of starvation, sarcopenia and cachexia. Clin. Nutr. 2007, 26, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Picard, M.; Ritchie, D.; Thomas, M.M.; Wright, K.J.; Hepple, R.T. Alterations in intrinsic mitochondrial function with aging are fiber type-specific and do not explain differential atrophy between muscles. Aging Cell 2011, 10, 1047–1055. [Google Scholar] [CrossRef]
- Kim, H.-J.; Kim, S.-W.; Lee, S.-H.; Jung, D.-W.; Williams, D.R. Inhibiting 5-lipoxygenase prevents skeletal muscle atrophy by targeting organogenesis signalling and insulin-like growth factor-1. J. Cachexia. Sarcopenia Muscle 2022, 13, 3062–3077. [Google Scholar] [CrossRef]
- Belizário, J.E.; Oliveira, C.C.F.; Borges, J.P.; Kashiabara, J.A.; Vannier, E. Skeletal muscle wasting and renewal: A pivotal role of myokine IL-6. Springerplus 2016, 5, 619. [Google Scholar] [CrossRef]
- Kadi, F.; Charifi, N.; Denis, C.; Lexell, J. Satellite cells and myonuclei in young and elderly women and men. Muscle Nerve 2003, 29, 120–127. [Google Scholar] [CrossRef]
- Vechetti Jr, I.; Valentino, T.; Mobley, B.; McCarthy, J. The role of extracellular vesicles in skeletal muscle and systemic adaptation to exercise. J. Physiol. 2021, 599, 845–861. [Google Scholar] [CrossRef]
- Shao, X.; Gong, W.; Wang, Q.; Wang, P.; Shi, T.; Mahmut, A.; Qin, J.; Yao, Y.; Yan, W.; Chen, D.; et al. Atrophic skeletal muscle fibre-derived small extracellular vesicle miR-690 inhibits satellite cell differentiation during ageing. J. Cachexi. Sarcopenia Muscle 2022, 13, 3163–3180. [Google Scholar] [CrossRef]
- Fanzani, A.; Conraads, V.M. Molecular and cellular mechanisms of skeletal muscle atrophy: An update. J. Cachexia Sarcopenia Muscle 2012, 3, 163–179. [Google Scholar] [CrossRef]
- Aagaard, P.; Suetta, C.; Caserotti, P.; Magnusson, S.P.; Kjær, M. Role of the nervous system in sarcopenia and muscle atrophy with aging: Strength training as a countermeasure. Scand. J. Med. Sci. Sport 2010, 20, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Ryan, A.S.; Li, G. Skeletal muscle myostatin gene expression and sarcopenia in overweight and obese middle-aged and older adults. JCSM Clin. Rep. 2021, 6, 137–142. [Google Scholar] [CrossRef]
- Schaap, L.A.; Pluijm, S.M.F.; Deeg, D.J.H.; Visser, M. Inflammatory markers and loss of muscle mass (sarcopenia) and strength. Am. J. Med. 2006, 119, 526-e9. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Datzkiw, D.; Rudnicki, M.A. Satellite cells in ageing: Use it or lose it. Open Biol. 2020, 10, 200048. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, R.E.; O’Mahony, S.; Calver, B.L.; Woodhouse, K.W. Nutrition, inflammation, and leptin levels in aging and frailty. J. Am. Geriatr. Soc. 2008, 56, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, R.; Motlagh, A.D.; Heshmat, R.; Esmaillzadeh, A.; Payab, M.; Yousefinia, M.; Siassi, F.; Pasalar, P.; Baygi, F. Diet and its relationship to sarcopenia in community dwelling Iranian elderly: A cross sectional study. Nutrition 2015, 31, 97–104. [Google Scholar] [CrossRef]
- Rasaei, N.; Kashavarz, S.A.; Yekaninejad, M.S.; Mirzaei, K. The association between sarcopenic obesity (SO) and major dietary patterns in overweight and obese adult women. Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 2519–2524. [Google Scholar] [CrossRef]
- Smee, D.; Pumpa, K.; Falchi, M.; Lithander, F.E. The relationship between diet quality and falls risk, physical function and body composition in older adults. J. Nutr. Health Aging 2015, 19, 1037–1042. [Google Scholar] [CrossRef]
- Dupont-Versteegden, E.E. Apoptosis in muscle atrophy: Relevance to sarcopenia. Exp. Gerontol. 2005, 40, 473–481. [Google Scholar] [CrossRef]
- Buford, T.W.; Anton, S.D.; Judge, A.R.; Marzetti, E.; Wohlgemuth, S.E.; Carter, C.S.; Leeuwenburgh, C.; Pahor, M.; Manini, T.M. Models of accelerated sarcopenia: Critical pieces for solving the puzzle of age-related muscle atrophy. Ageing Res. Rev. 2010, 9, 369–383. [Google Scholar] [CrossRef] [PubMed]
- Dodson, S.; Baracos, V.E.; Jatoi, A.; Evans, W.J.; Cella, D.; Dalton, J.T.; Steiner, M.S. Muscle wasting in cancer cachexia: Clinical implications, diagnosis, and emerging treatment strategies. Annu. Rev. Med. 2011, 62, 265–279. [Google Scholar] [CrossRef] [PubMed]
- Monteiro-cardoso, V.F.; Castro, M.; Oliveira, M.M.; Moreira, P.I.; Peixoto, F.; Videira, R.A. Age-dependent biochemical dysfunction in skeletal muscle of triple-transgenic mouse model of Alzheimer’s disease. Curr. Alzheimer Res. 2015, 12, 100–115. [Google Scholar] [CrossRef]
- Trierweiler, H.; Kisielewicz, G.; Jonasson, T.H.; Petterle, R.R.; Moreira, C.A.; Zeghbi, V.; Borba, C. Sarcopenia: A chronic complication of type 2 diabetes mellitus. Diabetol. Metab. Syndr. 2018, 10, 25. [Google Scholar] [CrossRef]
- Chen, X.; Afghah, Z. Development of AD-like pathology in skeletal muscle. J. Park. Dis. Alzheimers Dis. 2020, 6, 10. [Google Scholar] [CrossRef]
- Torcinaro, A.; Ricci, V.; Strimpakos, G.; Santa, F.D.; Middei, S. Peripheral nerve impairment in a mouse model of Alzheimer’s disease. Brain Sci. 2021, 11, 1245. [Google Scholar] [CrossRef]
- Burns, J.M.; Johnson, D.K.; Watts, A.; Swerdlow, R.H.; Brooks, W.M. Lean mass is reduced in early Alzheimer’s disease and associated with brain atrophy. Arch. Neurol. 2010, 67, 428–433. [Google Scholar] [CrossRef]
- Boyle, P.A.; Buchman, A.S.; Wilson, R.S.; Sue, E.; Bennett, D.A. Association of muscle strength with the risk of Alzheimer’s disease and the rate of cognitive decline in community-dwelling older persons. Arch. Neurol. 2009, 66, 1339–1344. [Google Scholar] [CrossRef]
- Low, S.; Ng, T.P.; Lim, C.L.; Moh, A.; Ang, S.F.; Wang, J.; Goh, K.S.; Ang, K.; Tang, W.E.; Kwan, P.Y.; et al. Mass and impaired cognitive function in type 2 diabetes. Sci. Rep. 2020, 10, 2956. [Google Scholar] [CrossRef]
- Moon, Y.; Choi, Y.; Kim, J.O.; Han, S. Muscle profile and cognition in patients with Alzheimer’ s disease dementia. Neurol. Sci. 2018, 39, 1861–1866. [Google Scholar] [CrossRef]
- Ogawa, Y.; Kaneko, Y.; Sato, T.; Shimizu, S.; Kanetaka, H. Sarcopenia and muscle functions at various stages of Alzheimer disease. Front. Neurol. 2018, 9, 710. [Google Scholar] [CrossRef] [PubMed]
- Oudbier, S.J.; Goh, J.; Looijaard, S.M.L.M.; Reijnierse, E.M.; Meskers, C.G.M.; Maier, A.B. Pathophysiological mechanisms explaining the association between low skeletal muscle mass and cognitive function. J. Gerontol. 2022, 77, 1959–1968. [Google Scholar] [CrossRef] [PubMed]
- Chong, J.; Aston, N.; Karikari, T.; Tanaka, T.; Schöll, M.; Zetterberg, H.; Blennow, K.; Chen, C.; Lai, M. Blood-based high sensitivity measurements of beta-amyloid and phosphorylated tau as biomarkers of Alzheimer’s disease: A focused review on recent advances. J. Neurol. Neurosurg. Psychiatry 2021, 92, 1231–1241. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.H.; Lee, K.H.; Chang, Y.; Choe, Y.S.; Kim, J.P.; Jang, H.; Shin, H.Y.; Kim, H.J.; Koh, S.B.; Na, D.L.; et al. Gender-specific relationship between thigh muscle and fat mass and brain amyloid-β positivity. Alzheimers. Res. Ther. 2022, 14, 145. [Google Scholar] [CrossRef]
- Kuo, Y.-M.; Kokjohn, T.A.; Watson, M.D.; Woods, A.S.; Cotter, R.J.; Sue, L.I.; Kalback, W.M.; Emmerling, M.R.; Beach, T.G.; Roher, A.E. Elevated abeta42 in skeletal muscle of Alzheimer disease patients suggests peripheral alterations of AbetaPP metabolism. Am. J. Pathol. 2000, 156, 797–805. [Google Scholar] [CrossRef]
- Lin, Y.; Lin, F.; Hsiao, Y. Myostatin is associated with cognitive decline in an animal model of Alzheimer’s disease. Mol. Neurobiol. 2019, 56, 1984–1991. [Google Scholar] [CrossRef]
- Schuh, R.A.; Jackson, K.C.; Schlappal, A.E.; Spangenburg, E.E.; Ward, C.W.; Park, J.H.; Dugger, N.; Shi, G.L.; Fishman, P.S. Mitochondrial oxygen consumption deficits in skeletal muscle isolated from an Alzheimer’s disease-relevant murine model. BMC Neurosci. 2014, 15, 24. [Google Scholar] [CrossRef]
- Schmidt, J.; Barthel, K.; Wrede, A.; Salajegheh, M.; Bähr, M.; Dalakas, M.C. Interrelation of inflammation and APP in sIBM: IL-1β induces accumulation of β-amyloid in skeletal muscle. Brain 2008, 131, 1228–1240. [Google Scholar] [CrossRef]
- Yoo, S.-M.; Park, J.; Kim, S.-H.; Jung, Y.-K. Emerging perspectives on mitochondrial dysfunction and inflammation in Alzheimer’s disease. BMB Rep. 2020, 53, 35–46. [Google Scholar] [CrossRef]
- Scisciola, L.; Fontanella, R.A.; Surina; Cataldo, V.; Paolisso, G.; Barbieri, M. Sarcopenia and cognitive function: Role of myokines in muscle brain cross-talk. Life 2021, 11, 173. [Google Scholar] [CrossRef]
- Gupta, R.; Khan, R.; Cortes, C.J. Forgot to exercise? Exercise derived circulating myokines in Alzheimer’s disease: A perspective. Front. Neurol. 2021, 12, 649452. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Jun, H. Role of myokines in regulating skeletal muscle mass and function. Front. Physiol. 2019, 10, 42. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, P.; Castillo-García, A.; Morales, J.; Villa, P.; Hampel, H.; Emanuele, E.; Lista, S.; Lucia, A. Exercise benefits on Alzheimer’s disease: State-of-the-science. Ageing Res. Rev. 2020, 62, 101108. [Google Scholar] [CrossRef]
- Pedersen, B.K. Exercise-induced myokines and their role in chronic diseases. Brain. Behav. Immun. 2011, 25, 811–816. [Google Scholar] [CrossRef] [PubMed]
- Nagase, T.; Tohda, C. Skeletal muscle atrophy-induced hemopexin accelerates onset of cognitive impairement in Alzheimer’s disease. J. Cachexia Sarcopenia Muscle 2021, 12, 2199–2210. [Google Scholar] [CrossRef]
- Zhang, X.; He, Q.; Huang, T.; Zhao, N.; Liang, F.; Xu, B.; Chen, X.; Li, T.; Bi, J. Treadmill exercise decreases Aβ deposition and counteracts cognitive decline in APP/PS1 mice, possibly via hippocampal microglia modifications. Front. Aging Neurosci. 2018, 11, 78. [Google Scholar] [CrossRef]
- López-Ortiz, S.; Pinto-Fraga, J.; Valenzuela, P.; Martín-Hernández, J.; Seisdedos, M.M.; García-López, O.; Toschi, N.; Giuliano, F.D.; Garaci, G.; Mercuri, N.B.; et al. Physical exercise and Alzheimer’s Disease: Effects on pathophysiolocial molecular pathways of the disease. Int. J. Mol. Sci. 2021, 22, 2897. [Google Scholar] [CrossRef]
- Lee, B.; Shin, M.; Park, Y.; Won, W.-Y.; Cho, K.S. Physical exercise-induced myokines in neurodegenerative diseases. Int. J. Mol. Sci. 2021, 22, 5795. [Google Scholar] [CrossRef]
- Penet, M.; Bhujwalla, Z.M. Cancer cachexia, recent advances, and future directions. Cancer J. 2016, 21, 117–122. [Google Scholar] [CrossRef]
- Lenk, K.; Schuler, G.; Adams, V. Skeletal muscle wasting in cachexia and sarcopenia: Molecular pathophysiology and impact of exercise training. J. Cachexia Sarcopenia Muscle 2010, 1, 9–21. [Google Scholar] [CrossRef]
- Johns, N.; Hatakeyama, S.; Stephens, N.A.; Degen, M.; Degen, S.; Frieauff, W.; Lambert, C.; Ross, J.A.; Roubenoff, R.; Glass, D.J.; et al. Clinical classification of cancer cachexia: Phenotypic correlates in human skeletal muscle. PLoS ONE 2014, 9, e83618. [Google Scholar] [CrossRef] [PubMed]
- Loritel, M.J.; Thompson, M.G.; Drake, J.L.; Carling, G.; Tisdale, M.J. Mechanism of muscle protein degradation induced by a cancer cachectic factor. Br. J. Cancer 1998, 78, 850–856. [Google Scholar] [CrossRef] [PubMed]
- Acharyya, S.; Swoap, S.; Guttridge, D.C.; Acharyya, S.; Ladner, K.J.; Nelsen, L.L.; Damrauer, J.; Reiser, P.J.; Swoap, S.; Guttridge, D.C. Cancer cachexia is regulated by selective targeting of skeletal muscle gene products. J. Clin. Investig. 2004, 114, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Tisdale, M.J. Cachexia in cancer patients. Nat. Rev. Cancer 2002, 2, 862–871. [Google Scholar] [CrossRef]
- Hasselgren, P.; Fischer, J.E. Muscle cachexia: Current concepts of intracellular mechanisms and molecular regulation. Ann. Surg. 2001, 233, 9–17. [Google Scholar] [CrossRef]
- Vander Heiden, M.; Cantley, L.; Thompson, C. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef]
- Porporato, P. Understanding cachexia as a cancer metabolism syndrome. Oncogenesis 2016, 5, e200. [Google Scholar] [CrossRef]
- Tisdale, M.J. Molecular pathways leading to cancer cachexia. Physiology 2005, 20, 340–348. [Google Scholar] [CrossRef]
- Bonetto, A.; Aydogdu, T.; Kunzevitzky, N.; Guttridge, D.C.; Khuri, S. STAT3 Activation in Skeletal Muscle Links Muscle Wasting and the Acute Phase Response in Cancer Cachexia. PLoS ONE 2011, 6, e22538. [Google Scholar] [CrossRef]
- Fan, M.; Gu, X.; Zhang, W.; Shen, Q.; Zhang, R.; Fang, Q.; Wang, Y.; Guo, X.; Zhang, X.; Liu, X. Atractylenolide I ameliorates cancer cachexia through inhibiting biogenesis of IL-6 and tumor-derived extracellular vesicles. J. Cachexi. Sarcopenia Muscle 2022, 13, 2724–2739. [Google Scholar] [CrossRef]
- Liu, D.; Qiao, X.; Ge, Z.; Shang, Y.; Li, Y.; Wang, W.; Chen, M.; Si, S.; Chen, S.-Z. IMB0901 inhibits muscle atrophy induced by cancer cachexia through MSTN signaling pathway. Skelet. Muscle 2019, 9, 8. [Google Scholar] [CrossRef] [PubMed]
- Aparicio, H.J.; Benjamin, E.J.; Callaway, C.W.; Carson, A.P.; Cheng, S.; Elkind, M.S.V.; Evenson, K.R.; Ferguson, J.F.; Knutson, K.L.; Lee, C.D.; et al. Heart Disease and Stroke Statistics—2021 Update A Report From the American Heart Association. Circulation 2021, 143, e254–e743. [Google Scholar]
- Schocken, D.D.; Arrieta, M.I.; Leaverton, P.E.; Ros, E.A. Prevalence and mortality rate of congestive heart failure in the United States. JACC J. 1992, 20, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Anker, S.D.; Ponikowski, P.; Varney, S.; Chua, T.P.; Clark, A.L.; Webb-peploe, K.M.; Harrington, D.; Kox, W.J.; Poole-wilson, P.A.; Coats, A.J.S. Wasting as independent risk factor for mortality in chronic heart failure. Lancet 1997, 349, 1050–1053. [Google Scholar] [CrossRef] [PubMed]
- Mancini, D.M.; Walter, G.; Reichek, N.; Lenkinski, R.; Mccully, K.K.; Mullen, J.L.; Wilson, J.R. Contribution of skeletal muscle atrophy to exercise intolerance and altered muscle metabolism in heart failure. Circulation 1992, 85, 1364–1373. [Google Scholar] [CrossRef] [PubMed]
- Buller, N.P.; Jones, D.; Poole-wilson, P.A. Direct measurement of skeletal muscle fatigue in patients with chronic heart failure. Br. Heart J. 1991, 65, 20–24. [Google Scholar] [CrossRef]
- Fulster, S.; Tacke, M.; Sandek, A.; Ebner, N.; Tscho, C.; Doehner, W.; Anker, S.D.; Haehling, S. Von Muscle wasting in patients with chronic heart failure: Results from the studies investigating co-morbidities aggravating heart failure (SICA-HF). Eur. Soc. Cardiol. 2013, 34, 512–519. [Google Scholar] [CrossRef]
- Schaufelberger, M.; Erikssonf, B.O.; Grimbyi, G.; Held, P.; Swedberg, K. Skeletal muscle alterations in patients with chronic heart failure. Eur. Heart J. 1997, 18, 971–980. [Google Scholar] [CrossRef] [PubMed]
- Drexler, H.; Riede, U.; Miinzel, T.; Kdnig, H. Alterations of skeletal muscle in chronic heart failure. Circulation 1992, 85, 1751–1759. [Google Scholar] [CrossRef]
- Suzuki, T.; Palus, S.; Springer, J. Skeletal muscle wasting in chronic heart failure. ESC Hear. Fail. 2018, 5, 1099–1107. [Google Scholar] [CrossRef]
- Sullivan, M.J.; Green, H.J.; Cobb, F.R. Skeletal muscle biochemistry and histology in ambulatory patients with long-term heart failure. Circulation 1900, 81, 518–527. [Google Scholar] [CrossRef]
- Heineke, J.; Auger-Messier, M.; Xu, J.; Sargent, M.; York, A.; Welle, S.; Molkentin, J.D. Genetic deletion of myostatin from the heart prevents skeletal muscle atrophy in heart failure. Circulation 2010, 121, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Doehner, W.; Turhan, G.; Leyva, F.; Rauchhaus, M.; Sandek, A.; Jankowska, E.A.; Haehling, S.V.; Anker, S.D. Skeletal muscle weakness is related to insulin resistance in patients with chronic heart failure. ESC Hear. Fail. 2015, 2, 85–89. [Google Scholar] [CrossRef]
- Warmington, S.A.; Tolan, R.; Mcbennett, S. Functional and histological characteristics of skeletal muscle and the effects of leptin in the genetically obese (ob/ob) mouse. Int. J. Obes. 2000, 24, 1040–1050. [Google Scholar] [CrossRef]
- Pompeani, N.; Rybalka, E.; Latchman, H.; Murphy, R.M.; Croft, K.; Hayes, A. Skeletal muscle atrophy in sedentary Zucker obese rats is not caused by calpain-mediated muscle damage or lipid peroxidation induced by oxidative stress. J. Negat. Results Biomed. 2014, 13, 19. [Google Scholar] [CrossRef] [PubMed]
- Park, S.W.; Goodpaster, B.H.; Strotmeyer, E.S.; Kuller, L.H.; Broudeau, R.; Kammerer, C.; Rekeneire, N.; Harris, T.B.; Schwartz, A.V.; Tylavsky, F.A.; et al. Accelerated loss of skeletal muscle strength in older adults with type 2 diabetes. Diabetes Care 2007, 30, 1507–1512. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.G.; Boyko, E.J.; Barrett-Connor, E.; Milijkovic, I.; Hoffman, A.R.; Everson-Rose, S.A.; Lewis, C.E.; Cawthon, P.M.; Strotmeyer, E.S.; Orwoll, E.S. Insulin Sensitizers may attenuate lean mass loss in older men with diabetes. Diabetes Care 2011, 34, 2381–2386. [Google Scholar] [CrossRef]
- Keller, K.; Engelhardt, M. Strength and muscle mass loss with aging process. Age strength loss. Muscles Ligaments Tendons J. 2013, 3, 346–350. [Google Scholar] [CrossRef]
- Patti, M.E.; Butte, A.J.; Crunkhorn, S.; Cusi, K.; Berria, R.; Kashyap, S.; Miyazaki, Y.; Kohane, I.; Costello, M.; Saccone, R.; et al. Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: Potential role of PGC1 and NRF1. Proc. Natl. Acad. Sci. USA 2003, 100, 8466–8471. [Google Scholar] [CrossRef]
- Smith, K.L.; Tisdale, M.J. Increased protein degradation and decreased protein synthesis in skeletal muscle during cancer cachexia. Br. J. Cancer 1993, 67, 680–685. [Google Scholar] [CrossRef]
| Condition | Affected Muscle | Main Findings | References |
|---|---|---|---|
| High-Fat Diet | EDL muscle | More than 12-week HFD induced degradation of fast-twitch muscle fibers (EDL). | [16,20] |
| Gastrocnemius muscle | Long-term HFD decreased mitochondrial enzyme gene expression and activity in the gastrocnemius muscle. | [19] | |
| Soleus muscle | Three-week HFD induced denervation muscle atrophy in soleus muscle. | [17] | |
| EDL and soleus muscles | Shift from type IIb fiber to type IIa fiber was observed in the ob/ob group in the EDL muscles, whereas the opposite was observed in the soleus muscles. | [186] | |
| Obesity | Soleus and EDL muscles | The absolute isometric force of the obese soleus muscles was significantly greater than that of lean controls; however, the maximal isometric stress and normalized power output of both obese soleus and EDL muscles were reduced. | [54] |
| Soleus and EDL muscles | Despite the increased muscle mass in the HFD-induced obese soleus and EDL muscles, muscle strength remained unchanged. | [55] | |
| EDL, gastrocnemius, and plantaris muscles | EDL, gastrocnemius, and plantaris muscles were significantly smaller in the obese animals compared to lean counterparts. | [47] | |
| EDL and soleus muscles | EDL and soleus muscle mass were lower in the obese Zucker rats than lean controls, concomitant with reduced fiber area. | [187] | |
| Diabetes Mellitus | Leg and appendicular muscles | T2DM was associated with accelerated loss of leg muscle strength and quality in older adults. | [100,188] |
| Appendicular, trunk, and thigh muscles | Older adults with either diagnosed or undiagnosed T2DM showed excessive loss of muscle mass. | [76] | |
| Total and appendicular muscles | There was a greater loss in total and appendicular lean muscle mass in older men with untreated DM compared to normoglycemic counterparts. | [189] | |
| Gastrocnemius | Mice with T1DM phenotype showed a significantly lower gastrocnemius muscle fiber cross-sectional area. | [74] | |
| Ankle flexors, ankle extensors, knee flexors, elbow, and wrist | Significant reduction was observed in the muscle strength of the ankle flexors (17%), ankle extensors (14%), and knee flexors (14%) in patients with T2DM. Elbow and wrist muscle strengths were preserved. | [102] | |
| Quadriceps | DM status was significantly associated with reduced gait speed. Insulin-dependent older adults had significantly reduced quadricep strength and power. | [103] | |
| Hand grip and knee extensor | Older adults with T2DM had lower muscle strength, but not muscle mass compared with non-diabetic counterparts. | [107] | |
| Sarcopenia | Gastrocnemius, EDL, and soleus muscles | Muscle atrophy was greatest in aging gastrocnemius muscles and intermediate in aging EDL and soleus muscles. | [117] |
| Leg circumference | The leg circumference of >40 years of age was less than that of <40 years of age. | [190] | |
| Abdomen, lower extremities | Muscle thickness in the abdomen and lower extremities decreased significantly in Japanese men aged 60 years and older. | [191] | |
| Hand grip and upper and lower extremities | Decreased hand grip strength and low gait speed were observed in elderly individuals with cognitive impairment and dementia. Reductions in muscle strength of both upper and lower extremities were higher in the AD group. | [143] | |
| Tibialis anterior muscle | There was a reduction in axonal innervation of transgenic mice compared to the wild-type control. | [138] | |
| Cancer Cachexia | Soleus and gastrocnemius muscles | Muscles of MAC16 injected mice showed an increased lysosomal protease activity. | [164] |
| Quadriceps | Injection of IL-6 in mice with colon cancer induced systemic muscle wasting. | [171] | |
| Gastrocnemius muscle | There was a significant nitrogen loss with a depressed muscle protein synthesis and increased protein degradation during cancer cachexia. | [192] | |
| Rectus muscle | Mean muscle fiber diameter in cachectic cancer patients was reduced by about 15% compared to non-cachectic cancer patients. | [163] | |
| Heart Failure | Type I fibers | Patients with chronic HF developed significant abnormalities in skeletal muscle, reflecting a decreased oxidative capacity in type I fibers. | [181] |
| Handgrip and quadriceps | Patients with chronic HF presented reduced muscle mass and decreased exercise capacity in treadmill performance and walking exercise tests. | [179] | |
| Quadriceps | Myofibril contractile function was strongly related to insulin sensitivity in HF patients, independent of muscle size. | [185] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jun, L.; Robinson, M.; Geetha, T.; Broderick, T.L.; Babu, J.R. Prevalence and Mechanisms of Skeletal Muscle Atrophy in Metabolic Conditions. Int. J. Mol. Sci. 2023, 24, 2973. https://doi.org/10.3390/ijms24032973
Jun L, Robinson M, Geetha T, Broderick TL, Babu JR. Prevalence and Mechanisms of Skeletal Muscle Atrophy in Metabolic Conditions. International Journal of Molecular Sciences. 2023; 24(3):2973. https://doi.org/10.3390/ijms24032973
Chicago/Turabian StyleJun, Lauren, Megan Robinson, Thangiah Geetha, Tom L. Broderick, and Jeganathan Ramesh Babu. 2023. "Prevalence and Mechanisms of Skeletal Muscle Atrophy in Metabolic Conditions" International Journal of Molecular Sciences 24, no. 3: 2973. https://doi.org/10.3390/ijms24032973
APA StyleJun, L., Robinson, M., Geetha, T., Broderick, T. L., & Babu, J. R. (2023). Prevalence and Mechanisms of Skeletal Muscle Atrophy in Metabolic Conditions. International Journal of Molecular Sciences, 24(3), 2973. https://doi.org/10.3390/ijms24032973






