Fluphenazine-Induced Neurotoxicity with Acute Almost Transient Parkinsonism and Permanent Memory Loss: Lessons from a Case Report
Abstract
1. Introduction
2. Case Presentation
2.1. Clinical Assessment
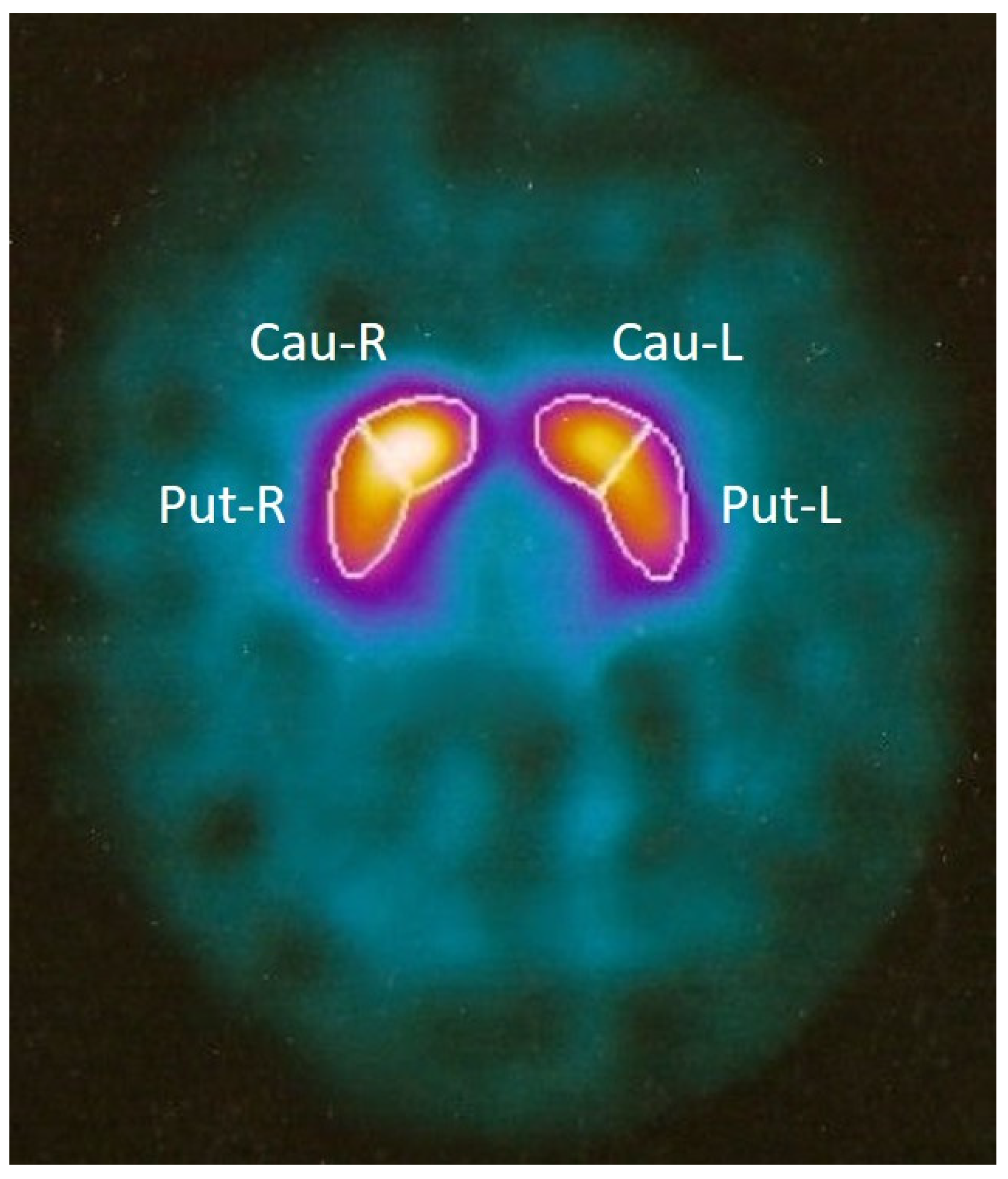
2.2. MRI Assessment
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shuaib, U.A.; Rajput, A.H.; Robinson, C.A.; Rajput, A. Neuroleptic-induced Parkinsonism: Clinicopathological study. Mov. Disord. 2016, 31, 360–365. [Google Scholar] [CrossRef] [PubMed]
- de Rijk, M.C.; Rocca, W.A.; Anderson, D.W.; Melcon, M.O.; Breteler, M.M.; Maraganore, D.M. A population perspective on diagnostic criteria for Parkinson’s disease. Neurology 1997, 48, 1277–1281. [Google Scholar] [CrossRef] [PubMed]
- Hughes, A.J.; Daniel, S.E.; Kilford, L.; Lees, A.J. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: A clinico pathological study of 100 cases. J. Neurol. Neurosurg. Psychiatry 1992, 55, 181e4. [Google Scholar] [CrossRef] [PubMed]
- Bower, J.H.; Dickson, D.W.; Taylor, L.; Maraganore, D.M.; Rocca, W.A. Clinical correlates of the pathology underlying parkinsonism: A population perspective. Mov. Disord. 2002, 17, 910–916. [Google Scholar] [CrossRef]
- Reynolds, L.M.; Flores, C. Mesocorticolimbic Dopamine Pathways across Adolescence: Diversity in Development. Front. Neural Circuits 2021, 15, 735625. [Google Scholar] [CrossRef]
- Battaglia, S.; Garofalo, S.; di Pellegrino, G.; Starita, F. Revaluing the Role of vmPFC in the Acquisition of Pavlovian Threat Conditioning in Humans. J. Neurosci. 2020, 40, 8491–8500. [Google Scholar] [CrossRef]
- Bidwell, L.C.; McClernon, F.J.; Kollins, S.H. Cognitive enhancers for the treatment of ADHD. Pharmacol. Biochem. Behav. 2011, 99, 262–274. [Google Scholar] [CrossRef]
- Vaiman, E.E.; Shnayder, N.A.; Novitsky, M.A.; Dobrodeeva, V.S.; Goncharova, P.S.; Bochanova, E.N.; Sapronova, M.R.; Popova, T.E.; Tappakhov, A.A.; Nasyrova, R.F. Candidate Genes Encoding Dopamine Receptors as Predictors of the Risk of Antipsychotic-Induced Parkinsonism and Tardive Dyskinesia in Schizophrenic Patients. Biomedicines 2021, 9, 879. [Google Scholar] [CrossRef]
- Shin, H.W.; Chung, S.J. Drug-induced parkinsonism. J. Clin. Neurol. 2012, 8, 15–21. [Google Scholar] [CrossRef]
- Posso, M.C.; Domingues, F.C.; Ferreira, S.; Silvestre, S. Development of Phenothiazine Hybrids with Potential Medicinal Interest: A Review. Molecules 2022, 27, 276. [Google Scholar] [CrossRef]
- Varga, B.; Csonka, Á.; Csonka, A.; Molnár, J.; Amaral, L.; Spengler, G. Possible Biological and Clinical Applications of Phenothiazines. Anticancer Res. 2017, 37, 5983–5993. [Google Scholar] [PubMed]
- Goyette, M.A.; Cusseddu, R.; Elkholi, I.; Abu-Thuraia, A.; El-Hachem, N.; Haibe-Kains, B.; Gratton, J.P.; Côté, J.F. AXL knockdown gene signature reveals a drug repurposing opportunity for a class of antipsychotics to reduce growth and metastasis of triple-negative breast cancer. Oncotarget 2019, 10, 2055–2067. [Google Scholar] [CrossRef] [PubMed]
- Jamora, D.; Lim, S.H.; Pan, A.; Tan, L.; Tan, E.K. Valproate-induced parkinsonism in epilepsy patients. Mov. Disord. 2007, 22, 130–133. [Google Scholar] [CrossRef] [PubMed]
- Siragusa, S.; Bistas, K.G.; Saadabadi, A. Fluphenazine. Available online: https://www.ncbi.nlm.nih.gov/books/NBK459194/ (accessed on 8 May 2022).
- Sykes, D.A.; Moore, H.; Stott, L.; Holliday, N.; Javitch, J.A.; Lane, J.R.; Charlton, S.J. Extrapyramidal side effects of antipsychotics are linked to their association kinetics at dopamine D2 receptors. Nat. Commun. 2017, 8, 763. [Google Scholar] [CrossRef]
- Chakos, M.H.; Mayerhoff, D.I.; Loebel, A.D.; Alvir, J.M.; Liebermann, J.A. Incidence and correlates of acute extrapiramidal symptoms in first episode of schizophrenia. Psychofarmacol. Bull. 1992, 28, 81–86. [Google Scholar]
- Levinson, D.F.; Simpson, G.M.; Singh, H.; Yadalam, K.; Jain, A.; Stephanos, M.J.; Silver, P. Fluphenazine dose, clinical response, and extrapyramidal symptoms during acute treatment. Arch. Gen. Psychiatry 1990, 47, 761–768. [Google Scholar] [CrossRef]
- Koreen, A.R.; Liebermann, J.; Alvir, J.; Chakos, M.; Loebel, A.; Cooper, T.; Kane, J. Relation of plasma fluphenazine levels to treatment response and extrapyramidal side effects in first episode schizophrenic patients. Am. J. Psychiatry 1994, 151, 35–39. [Google Scholar]
- Hassin-Baer, S.; Sirota, P.; Korczyn, A.D.; Treves, T.A.; Epstein, B.; Shabtai, H.; Martin, T.; Litvinjuk, Y.; Giladi, N. Clinical characteristics of neuroleptic-induced parkinsonism. J. Neural Transm. 2001, 108, 1299–1308. [Google Scholar] [CrossRef]
- Calabresi, P.; Picconi, B.; Tozzi, A.; Ghiglieri, V.; Di Filippo, M. Direct and indirect pathways of basal ganglia: A critical reappraisal. Nat. Neurosci. 2014, 17, 1022–1030. [Google Scholar] [CrossRef]
- Maneuf, Y.P.; Mitchell, I.J.; Crossman, A.R.; Brotchie, J.M. On the role of enkephalin cotransmission in the GABAergic striatal efferents to the globus pallidus. Exp. Neurol. 1994, 125, 65–71. [Google Scholar] [CrossRef]
- Johannessen, C.U. Mechanisms of action of valproate: A commentatory. Neurochem. Int. 2000, 37, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Athauda, D.; Batley, R.; Ellis, C. Clinically silent idiopathic Parkinson’s disease unmasked by valproate use: A brief report. Aging Clin. Exp. Res. 2015, 27, 387–390. [Google Scholar] [CrossRef] [PubMed]
- Brugger, F.; Bhatia, K.P.; Besag, F.M. Valproate-Associated Parkinsonism: A Critical Review of the Literature. CNS Drugs 2016, 30, 527–540. [Google Scholar] [CrossRef]
- Silva, M.F.; Aires, C.C.; Luis, P.B.; Ruiter, J.P.; IJlst, L.; Duran, M.; Wanders, R.J.; Tavares de Almeida, I. Valproic acid metabolism and its effects on mitochondrial fatty acid oxidation: A review. J. Inherit. Metab. Dis. 2008, 31, 205–216. [Google Scholar] [CrossRef]
- Cheung, H.K.; Yu, E.C. Effect of 1050 mg fluphenazine decanoate given intramuscularly over six days. Br. Med. J. (Clin. Res. Ed.) 1983, 286, 1016–1017. [Google Scholar] [CrossRef]
- Susatia, F.; Fernandez, H.H. Drug-Induced Parkinsonism. Curr. Treat. Options Neurol. 2009, 11, 162–169. [Google Scholar] [CrossRef]
- Martel, J.C.; Gatti McArthur, S. Dopamine Receptor Subtypes, Physiology and Pharmacology: New Ligands and Concepts in Schizophrenia. Front. Pharmacol. 2020, 11, 1003. [Google Scholar] [CrossRef]
- Kapur, S.; Seeman, P. Does fast dissociation from the dopamine d(2) receptor explain the action of atypical antipsychotics?: A new hypothesis. Am. J. Psychiatry 2001, 158, 360–369. [Google Scholar] [CrossRef]
- Stahl, S.M. “Hit-and-Run” actions at dopamine receptors, part 2: Illustrating fast dissociation from dopamine receptors that typifies atypical antipsychotics. J. Clin. Psychiatry 2001, 62, 747–748. [Google Scholar] [CrossRef]
- Wieland, S.; Du, D.; Oswald, M.J.; Parlato, R.; Kohr, G.; Kelsch, W. Phasic dopaminergic activity exerts fast control of cholinergic interneuron firing via sequential NMDA, D2, and D1 receptor activation. J. Neurosci. 2014, 34, 11549–11559. [Google Scholar] [CrossRef]
- Battaglini, M.; Gentile, G.; Luchetti, L.; Giorgio, A.; Vrenken, H.; Barkhof, F.; Cover, K.S.; Bakshi, R.; Chu, R.; Sormani, M.P.; et al. Lifespan normative data on rates of brain volume changes. Neurobiol. Aging 2019, 81, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Pearce, J.M. Wallerian degeneration. J. Neurol. Neurosurg. Psychiatry 2000, 69, 791. [Google Scholar] [CrossRef] [PubMed]
- Nasrallah, H.A.; Chen, A.T. Multiple neurotoxic effects of haloperidol resulting in neuronal death. Ann. Clin. Psychiatry 2017, 29, 195–202. [Google Scholar]
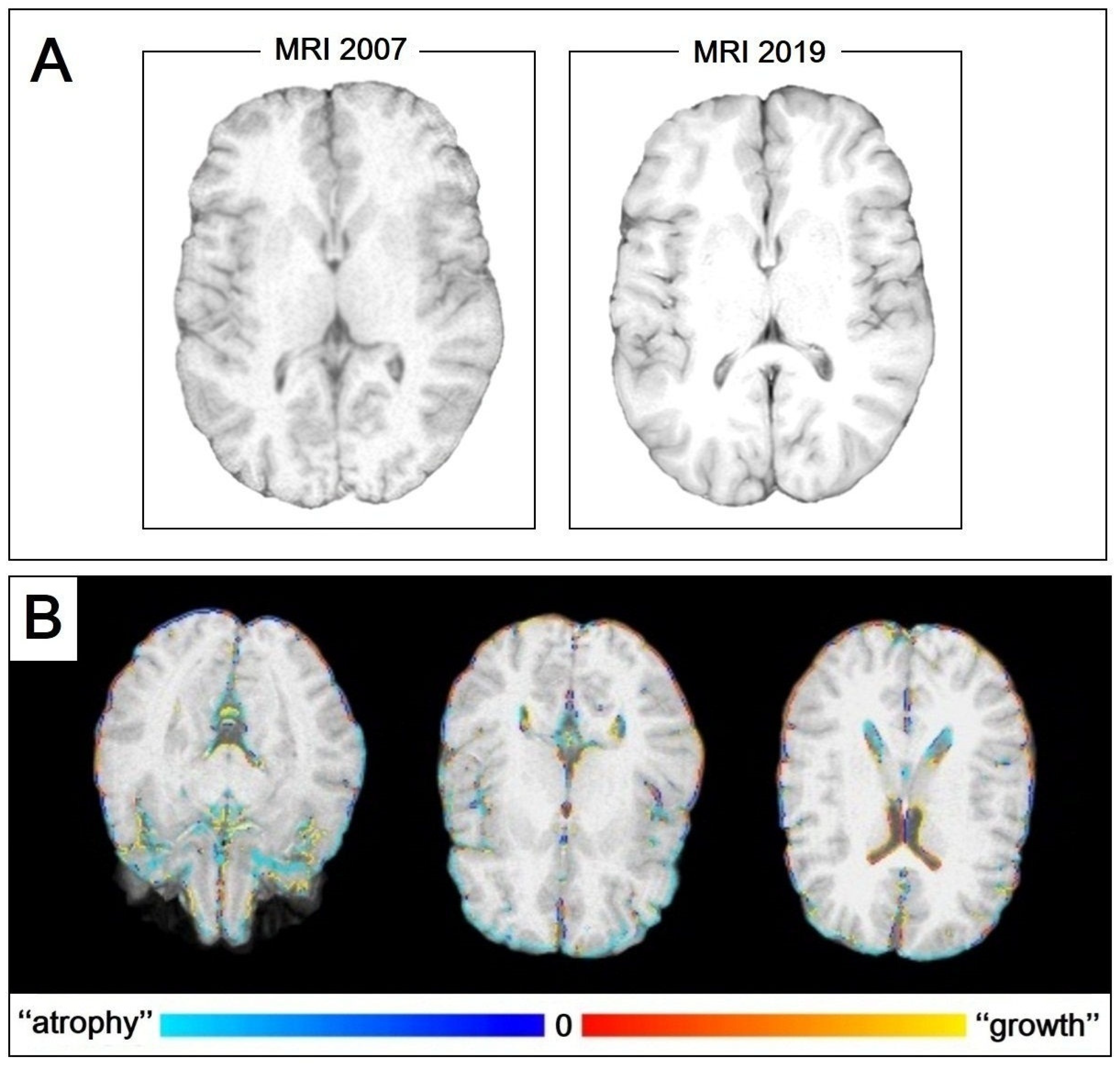
| MRI 2007 | MRI 2019 | |
|---|---|---|
| GM | 589.09 | 576.43 |
| WM | 617.71 | 628.64 |
| CSF | 204.15 | 250.23 |
| TBV | 1242.18 | 1161.44 |
| pGM | 461.82 | 428.11 |
| vCSF | 23.90 | 18.79 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Masi, R.; Orlando, S.; Toni, V.; Costa, M.C. Fluphenazine-Induced Neurotoxicity with Acute Almost Transient Parkinsonism and Permanent Memory Loss: Lessons from a Case Report. Int. J. Mol. Sci. 2023, 24, 2968. https://doi.org/10.3390/ijms24032968
De Masi R, Orlando S, Toni V, Costa MC. Fluphenazine-Induced Neurotoxicity with Acute Almost Transient Parkinsonism and Permanent Memory Loss: Lessons from a Case Report. International Journal of Molecular Sciences. 2023; 24(3):2968. https://doi.org/10.3390/ijms24032968
Chicago/Turabian StyleDe Masi, Roberto, Stefania Orlando, Vincenzo Toni, and Maria Carmela Costa. 2023. "Fluphenazine-Induced Neurotoxicity with Acute Almost Transient Parkinsonism and Permanent Memory Loss: Lessons from a Case Report" International Journal of Molecular Sciences 24, no. 3: 2968. https://doi.org/10.3390/ijms24032968
APA StyleDe Masi, R., Orlando, S., Toni, V., & Costa, M. C. (2023). Fluphenazine-Induced Neurotoxicity with Acute Almost Transient Parkinsonism and Permanent Memory Loss: Lessons from a Case Report. International Journal of Molecular Sciences, 24(3), 2968. https://doi.org/10.3390/ijms24032968








