Argonaute 2 Restores Erectile Function by Enhancing Angiogenesis and Reducing Reactive Oxygen Species Production in Streptozotocin (STZ)-Induced Type-1 Diabetic Mice
Abstract
1. Introduction
2. Results
2.1. Physiologic and Metabolic Parameters
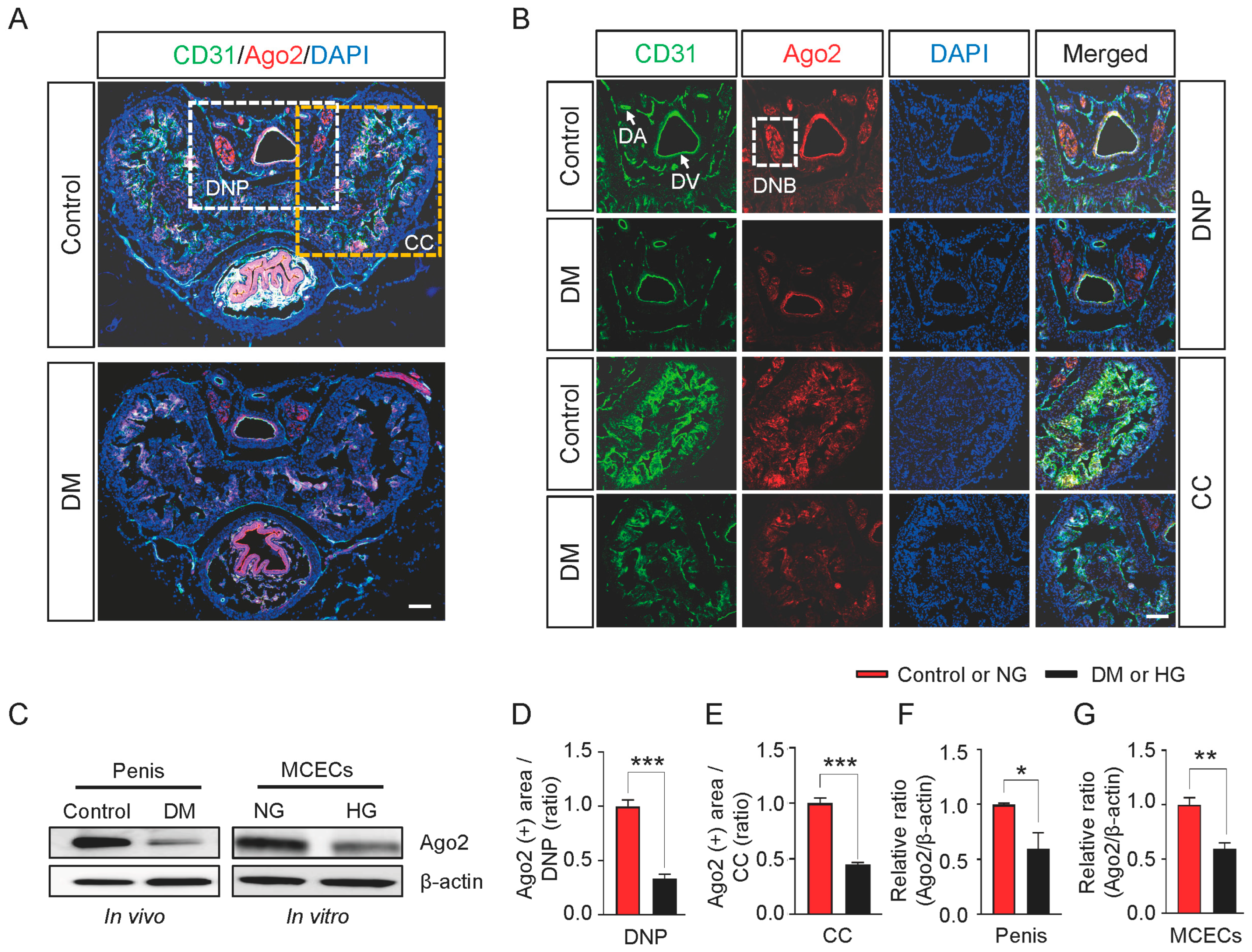
2.2. Ago2 Expression Decreases in Diabetic and High-Glucose Conditions
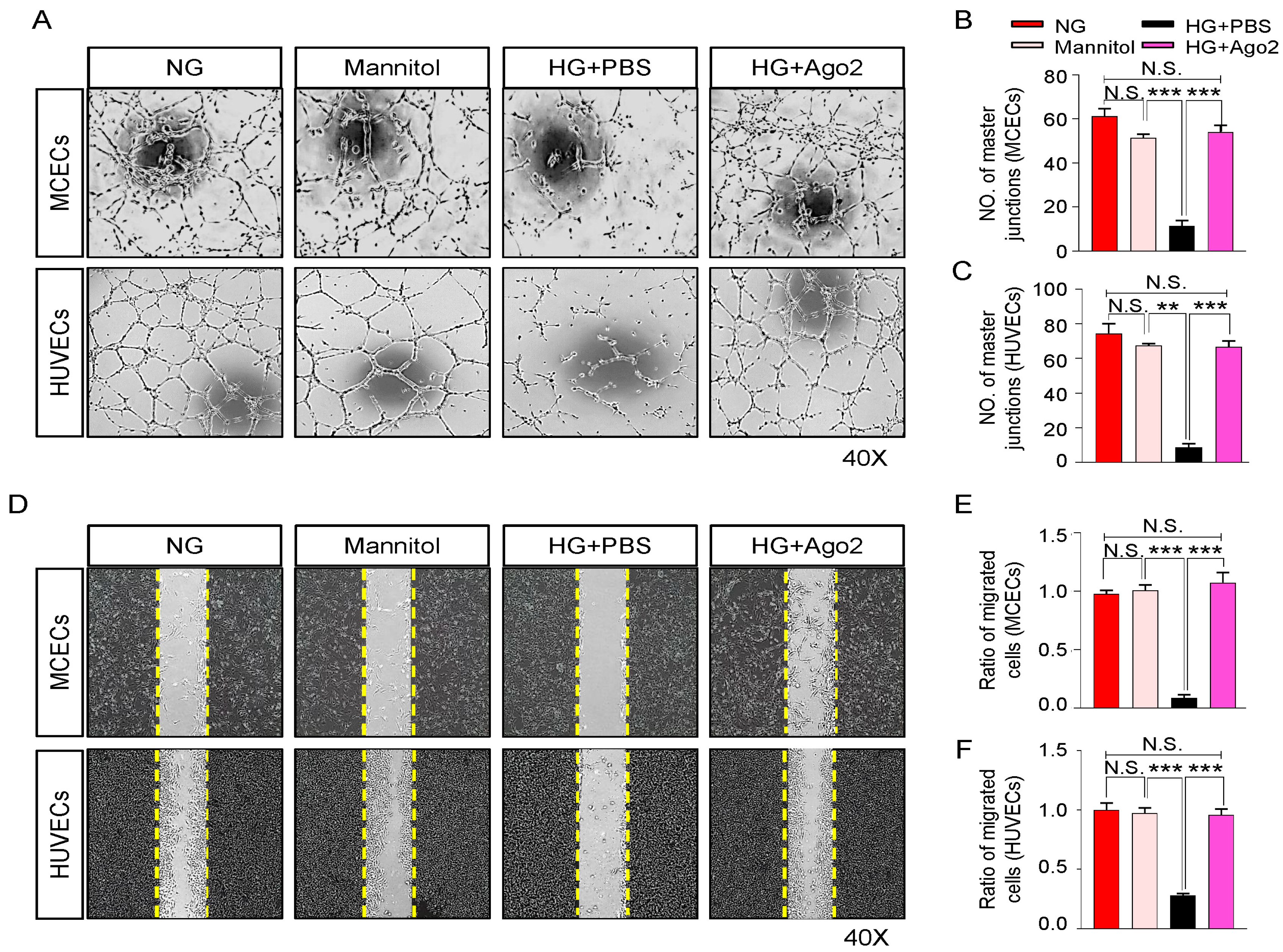
2.3. Ago2 Improves Angiogenesis under High-Glucose Conditions
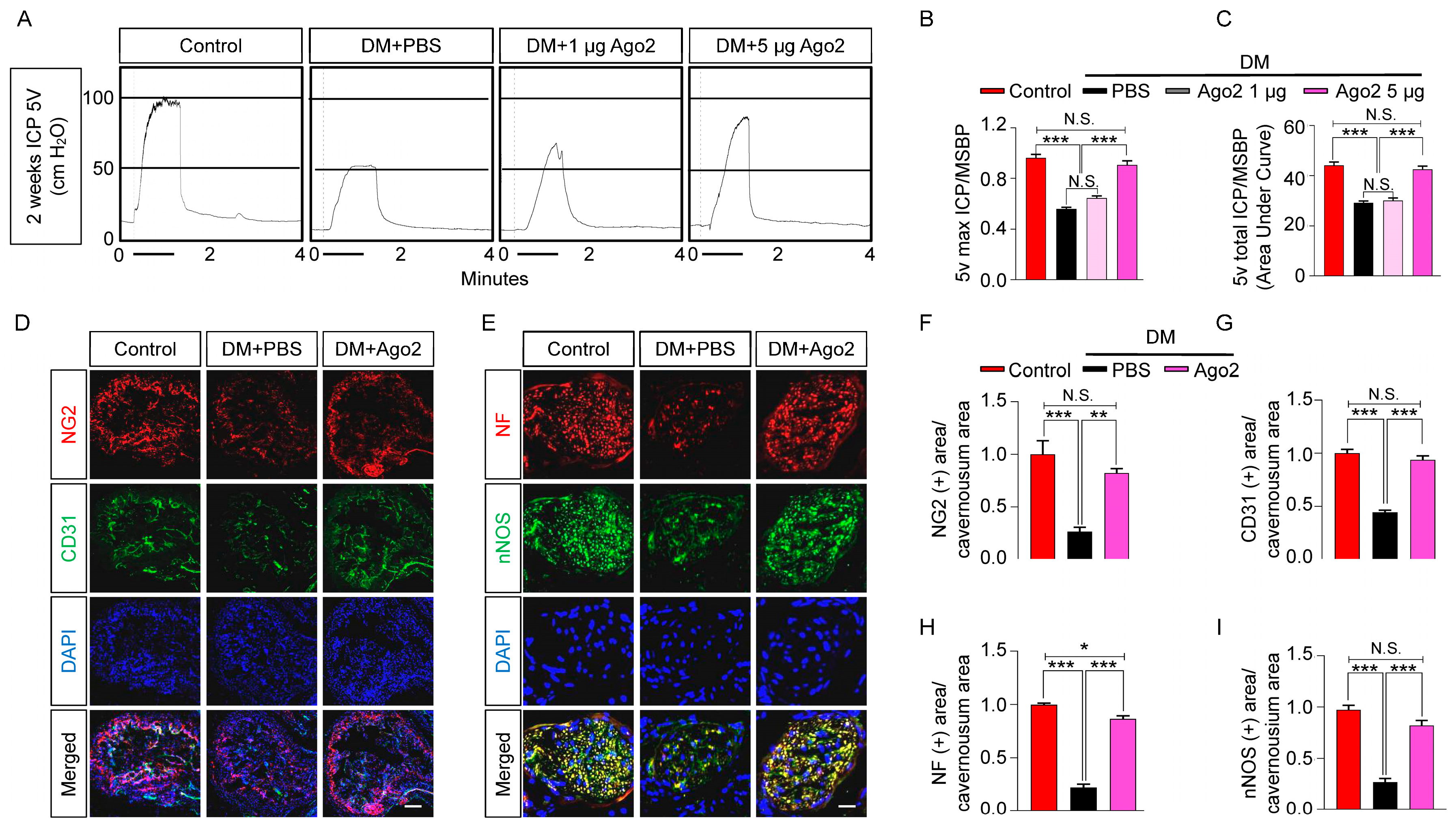
2.4. Exogenous Injection of Ago2 Improves Erectile Function in Diabetic Mice
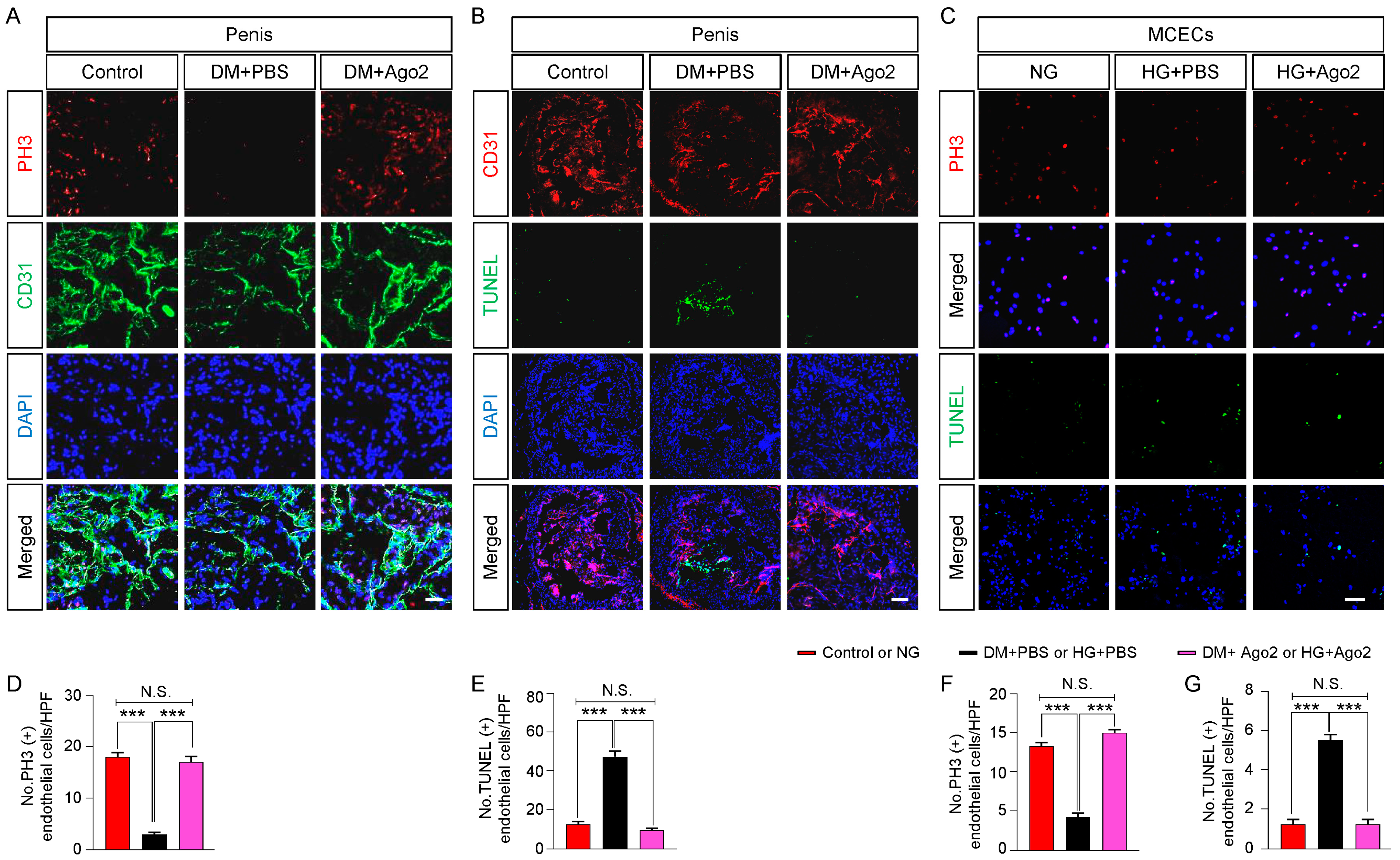
2.5. Ago2 Induces Endothelial Proliferation and Reduces Apoptosis under Diabetic Conditions In Vivo and In Vitro
2.6. Ago2 Reduces Reactive Oxygen Species in Cavernous Tissue of Diabetic Mice
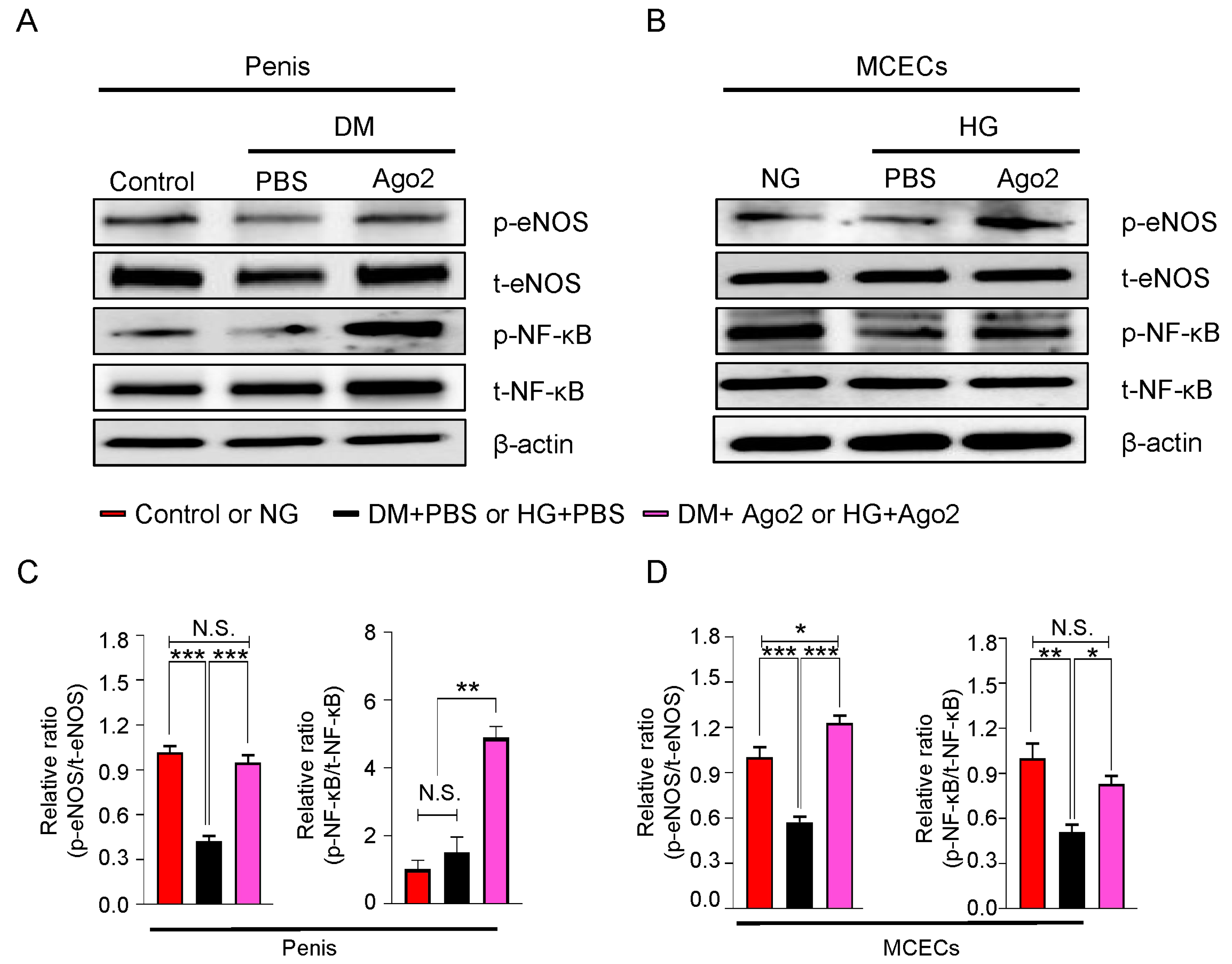
2.7. Ago2 Induces Cavernous eNOS Ser1177 and NF-κB Ser536 Phosphorylation under Diabetic Conditions Mouse Penis Tissues and MCECs
3. Discussion
4. Materials and Methods
4.1. Animals Study Design and Ethics Statement
4.2. Cell Culture
4.3. In Vitro Tube Formation Assay
4.4. Cell Migration Assay
4.5. Measurement of Erectile Function
4.6. TUNEL Assay
4.7. Histologic Examinations
4.8. In Situ Detection of Superoxide Anion
4.9. Western Blot
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kolluru, G.K.; Bir, S.C.; Kevil, C.G. Endothelial dysfunction and diabetes: Effects on angiogenesis, vascular remodeling, and wound healing. Int. J. Vasc. Med. 2012, 2012, 918267. [Google Scholar] [CrossRef]
- Gur, S.; Peak, T.C.; Kadowitz, P.J.; Sikka, S.C.; Hellstrom, W.J. Review of erectile dysfunction in diabetic animal models. Curr. Diabetes Rev. 2014, 10, 61–73. [Google Scholar] [CrossRef]
- Angulo, J.; Gonzalez-Corrochano, R.; Cuevas, P.; Fernandez, A.; La Fuente, J.M.; Rolo, F.; Allona, A.; Saenz de Tejada, I. Diabetes exacerbates the functional deficiency of NO/cGMP pathway associated with erectile dysfunction in human corpus cavernosum and penile arteries. J. Sex Med. 2010, 7, 758–768. [Google Scholar] [CrossRef] [PubMed]
- Cayetano-Alcaraz, A.A.; Tharakan, T.; Chen, R.; Sofikitis, N.; Minhas, S. The management of erectile dysfunction in men with diabetes mellitus unresponsive to phosphodiesterase type 5 inhibitors. Andrology 2022, 11, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Yin, G.N.; Choi, M.J.; Kim, W.J.; Kwon, M.H.; Song, K.M.; Park, J.M.; Das, N.D.; Kwon, K.D.; Batbold, D.; Oh, G.T.; et al. Inhibition of Ninjurin 1 restores erectile function through dual angiogenic and neurotrophic effects in the diabetic mouse. Proc. Natl. Acad. Sci. USA 2014, 111, E2731–E2740. [Google Scholar] [CrossRef] [PubMed]
- Bennett, N.E.; Kim, J.H.; Wolfe, D.P.; Sasaki, K.; Yoshimura, N.; Goins, W.F.; Huang, S.; Nelson, J.B.; de Groat, W.C.; Glorioso, J.C.; et al. Improvement in erectile dysfunction after neurotrophic factor gene therapy in diabetic rats. J. Urol. 2005, 173, 1820–1824. [Google Scholar] [CrossRef] [PubMed]
- Burchardt, M.; Burchardt, T.; Anastasiadis, A.G.; Buttyan, R.; de la Taille, A.; Shabsigh, A.; Frank, J.; Shabsigh, R. Application of angiogenic factors for therapy of erectile dysfunction: Protein and DNA transfer of VEGF 165 into the rat penis. Urology 2005, 66, 665–670. [Google Scholar] [CrossRef]
- Hu, L.; Qi, S.; Zhang, K.; Fu, Q. Essential role of brain-derived neurotrophic factor (BDNF) in diabetic erectile dysfunction. Andrologia 2018, 50, e12924. [Google Scholar] [CrossRef]
- Israeli, J.M.; Lokeshwar, S.D.; Efimenko, I.V.; Masterson, T.A.; Ramasamy, R. The potential of platelet-rich plasma injections and stem cell therapy for penile rejuvenation. Int. J. Impot. Res. 2022, 34, 375–382. [Google Scholar] [CrossRef]
- Manfredi, C.; Castiglione, F.; Fode, M.; Lew-Starowicz, M.; Romero-Otero, J.; Bettocchi, C.; Corona, G.; ESSM Scientific Collaboration and Partnership (ESCAP). News and future perspectives of non-surgical treatments for erectile dysfunction. Int. J. Impot. Res. 2022. [Google Scholar] [CrossRef]
- Yin, G.N.; Jin, H.R.; Choi, M.J.; Limanjaya, A.; Ghatak, K.; Minh, N.N.; Ock, J.; Kwon, M.H.; Song, K.M.; Park, H.J.; et al. Pericyte-Derived Dickkopf2 Regenerates Damaged Penile Neurovasculature Through an Angiopoietin-1-Tie2 Pathway. Diabetes 2018, 67, 1149–1161. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Carmell, M.A.; Rivas, F.V.; Marsden, C.G.; Thomson, J.M.; Song, J.J.; Hammond, S.M.; Joshua-Tor, L.; Hannon, G.J. Argonaute2 is the catalytic engine of mammalian RNAi. Science 2004, 305, 1437–1441. [Google Scholar] [CrossRef] [PubMed]
- Arroyo, J.D.; Chevillet, J.R.; Kroh, E.M.; Ruf, I.K.; Pritchard, C.C.; Gibson, D.F.; Mitchell, P.S.; Bennett, C.F.; Pogosova-Agadjanyan, E.L.; Stirewalt, D.L.; et al. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc. Natl. Acad. Sci. USA 2011, 108, 5003–5008. [Google Scholar] [CrossRef]
- Jagannathan, R.; Thapa, D.; Nichols, C.E.; Shepherd, D.L.; Stricker, J.C.; Croston, T.L.; Baseler, W.A.; Lewis, S.E.; Martinez, I.; Hollander, J.M. Translational Regulation of the Mitochondrial Genome Following Redistribution of Mitochondrial MicroRNA in the Diabetic Heart. Circ. Cardiovasc. Genet. 2015, 8, 785–802. [Google Scholar] [CrossRef]
- Pozzi, A.; Dowling, D.K. New Insights into Mitochondrial-Nuclear Interactions Revealed through Analysis of Small RNAs. Genome Biol. Evol. 2022, 14, evac023. [Google Scholar] [CrossRef]
- Zhang, X.; Zuo, X.; Yang, B.; Li, Z.; Xue, Y.; Zhou, Y.; Huang, J.; Zhao, X.; Zhou, J.; Yan, Y.; et al. MicroRNA directly enhances mitochondrial translation during muscle differentiation. Cell 2014, 158, 607–619. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.L.; Huang, Y.; Li, L.F.; Zhu, H.L.; Gao, H.X.; Liu, H.; Lv, S.Q.; Xu, Z.H.; Zheng, L.N.; Liu, T.; et al. Argonaute 2 promotes angiogenesis via the PTEN/VEGF signaling pathway in human hepatocellular carcinoma. Acta Pharmacol. Sin. 2015, 36, 1237–1245. [Google Scholar] [CrossRef] [PubMed]
- Machado-Pereira, M.; Saraiva, C.; Bernardino, L.; Cristovao, A.C.; Ferreira, R. Argonaute-2 protects the neurovascular unit from damage caused by systemic inflammation. J. Neuroinflamm. 2022, 19, 11. [Google Scholar] [CrossRef] [PubMed]
- Tattikota, S.G.; Rathjen, T.; McAnulty, S.J.; Wessels, H.H.; Akerman, I.; van de Bunt, M.; Hausser, J.; Esguerra, J.L.; Musahl, A.; Pandey, A.K.; et al. Argonaute2 mediates compensatory expansion of the pancreatic beta cell. Cell Metab. 2014, 19, 122–134. [Google Scholar] [CrossRef] [PubMed]
- Iosue, I.; Quaranta, R.; Masciarelli, S.; Fontemaggi, G.; Batassa, E.M.; Bertolami, C.; Ottone, T.; Divona, M.; Salvatori, B.; Padula, F.; et al. Argonaute 2 sustains the gene expression program driving human monocytic differentiation of acute myeloid leukemia cells. Cell Death Dis. 2013, 4, e926. [Google Scholar] [CrossRef] [PubMed]
- Neppl, R.L.; Kataoka, M.; Wang, D.Z. Crystallin-alphaB regulates skeletal muscle homeostasis via modulation of argonaute2 activity. J. Biol. Chem. 2014, 289, 17240–17248. [Google Scholar] [CrossRef]
- Kim, B.S.; Jung, J.S.; Jang, J.H.; Kang, K.S.; Kang, S.K. Nuclear Argonaute 2 regulates adipose tissue-derived stem cell survival through direct control of miR10b and selenoprotein N1 expression. Aging Cell 2011, 10, 277–291. [Google Scholar] [CrossRef]
- Florijn, B.W.; Duijs, J.; Levels, J.H.; Dallinga-Thie, G.M.; Wang, Y.; Boing, A.N.; Yuana, Y.; Stam, W.; Limpens, R.; Au, Y.W.; et al. Diabetic Nephropathy Alters the Distribution of Circulating Angiogenic MicroRNAs Among Extracellular Vesicles, HDL, and Ago-2. Diabetes 2019, 68, 2287–2300. [Google Scholar] [CrossRef]
- Wu, S.; Yu, W.; Qu, X.; Wang, R.; Xu, J.; Zhang, Q.; Xu, J.; Li, J.; Chen, L. Argonaute 2 promotes myeloma angiogenesis via microRNA dysregulation. J. Hematol. Oncol. 2014, 7, 40. [Google Scholar] [CrossRef]
- Cheng, N.; Li, Y.; Han, Z.G. Argonaute2 promotes tumor metastasis by way of up-regulating focal adhesion kinase expression in hepatocellular carcinoma. Hepatology 2013, 57, 1906–1918. [Google Scholar] [CrossRef] [PubMed]
- Naoghare, P.K.; Tak, Y.K.; Kim, M.J.; Han, E.; Song, J.M. Knock-down of argonaute 2 (AGO2) induces apoptosis in myeloid leukaemia cells and inhibits siRNA-mediated silencing of transfected oncogenes in HEK-293 cells. Basic Clin. Pharmacol. Toxicol. 2011, 109, 274–282. [Google Scholar] [CrossRef]
- Volpe, C.M.O.; Villar-Delfino, P.H.; Dos Anjos, P.M.F.; Nogueira-Machado, J.A. Cellular death, reactive oxygen species (ROS) and diabetic complications. Cell Death Dis. 2018, 9, 119. [Google Scholar] [CrossRef] [PubMed]
- Carmell, M.A.; Xuan, Z.; Zhang, M.Q.; Hannon, G.J. The Argonaute family: Tentacles that reach into RNAi, developmental control, stem cell maintenance, and tumorigenesis. Genes Dev. 2002, 16, 2733–2742. [Google Scholar] [CrossRef] [PubMed]
- Cerutti, L.; Mian, N.; Bateman, A. Domains in gene silencing and cell differentiation proteins: The novel PAZ domain and redefinition of the Piwi domain. Trends Biochem. Sci. 2000, 25, 481–482. [Google Scholar] [CrossRef] [PubMed]
- Muller, M.; Fazi, F.; Ciaudo, C. Argonaute Proteins: From Structure to Function in Development and Pathological Cell Fate Determination. Front. Cell Dev. Biol. 2019, 7, 360. [Google Scholar] [CrossRef]
- Li, J.; Kim, T.; Nutiu, R.; Ray, D.; Hughes, T.R.; Zhang, Z. Identifying mRNA sequence elements for target recognition by human Argonaute proteins. Genome Res. 2014, 24, 775–785. [Google Scholar] [CrossRef] [PubMed]
- Cefalu, W.T. Animal models of type 2 diabetes: Clinical presentation and pathophysiological relevance to the human condition. ILAR J. 2006, 47, 186–198. [Google Scholar] [CrossRef]
- Anita, L.; Yin, G.N.; Hong, S.S.; Kang, J.H.; Gho, Y.S.; Suh, J.K.; Ryu, J.K. Pericyte-derived extracellular vesicle-mimetic nanovesicles ameliorate erectile dysfunction via lipocalin 2 in diabetic mice. Int. J. Biol. Sci. 2022, 18, 3653–3667. [Google Scholar] [CrossRef]
- Choi, J.S.Y.; de Haan, J.B.; Sharma, A. Animal models of diabetes-associated vascular diseases: An update on available models and experimental analysis. Br. J. Pharmacol. 2022, 179, 748–769. [Google Scholar] [CrossRef] [PubMed]
- Ghatak, K.; Yin, G.N.; Hong, S.S.; Kang, J.H.; Suh, J.K.; Ryu, J.K. Heat Shock Protein 70 in Penile Neurovascular Regeneration Requires Cystathionine Gamma-Lyase. World J. Mens. Health 2022, 40, 580–599. [Google Scholar] [CrossRef] [PubMed]
- Parikh, J.; Zemljic-Harpf, A.; Fu, J.; Giamouridis, D.; Hsieh, T.C.; Kassan, A.; Murthy, K.S.; Bhargava, V.; Patel, H.H.; Rajasekaran, M.R. Altered Penile Caveolin Expression in Diabetes: Potential Role in Erectile Dysfunction. J. Sex Med. 2017, 14, 1177–1186. [Google Scholar] [CrossRef]
- Purvis, G.S.D.; Chiazza, F.; Chen, J.; Azevedo-Loiola, R.; Martin, L.; Kusters, D.H.M.; Reutelingsperger, C.; Fountoulakis, N.; Gnudi, L.; Yaqoob, M.M.; et al. Annexin A1 attenuates microvascular complications through restoration of Akt signalling in a murine model of type 1 diabetes. Diabetologia 2018, 61, 482–495. [Google Scholar] [CrossRef]
- Yin, G.N.; Kim, D.K.; Kang, J.I.; Im, Y.; Lee, D.S.; Han, A.R.; Ock, J.; Choi, M.J.; Kwon, M.H.; Limanjaya, A.; et al. Latrophilin-2 is a novel receptor of LRG1 that rescues vascular and neurological abnormalities and restores diabetic erectile function. Exp. Mol. Med. 2022, 54, 626–638. [Google Scholar] [CrossRef]
- Bobbie, M.W.; Roy, S.; Trudeau, K.; Munger, S.J.; Simon, A.M.; Roy, S. Reduced connexin 43 expression and its effect on the development of vascular lesions in retinas of diabetic mice. Investig. Ophthalmol. Vis. Sci. 2010, 51, 3758–3763. [Google Scholar] [CrossRef]
- Eftekhari, A.; Vahed, S.Z.; Kavetskyy, T.; Rameshrad, M.; Jafari, S.; Chodari, L.; Hosseiniyan, S.M.; Derakhshankhah, H.; Ahmadian, E.; Ardalan, M. Cell junction proteins: Crossing the glomerular filtration barrier in diabetic nephropathy. Int. J. Biol. Macromol. 2020, 148, 475–482. [Google Scholar] [CrossRef]
- Li, A.F.; Roy, S. High glucose-induced downregulation of connexin 43 expression promotes apoptosis in microvascular endothelial cells. Investig. Ophthalmol. Vis. Sci. 2009, 50, 1400–1407. [Google Scholar] [CrossRef]
- Tien, T.; Barrette, K.F.; Chronopoulos, A.; Roy, S. Effects of high glucose-induced Cx43 downregulation on occludin and ZO-1 expression and tight junction barrier function in retinal endothelial cells. Investig. Ophthalmol. Vis. Sci. 2013, 54, 6518–6525. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.R.; Kim, W.J.; Song, J.S.; Piao, S.; Choi, M.J.; Tumurbaatar, M.; Shin, S.H.; Yin, G.N.; Koh, G.Y.; Ryu, J.K.; et al. Intracavernous delivery of a designed angiopoietin-1 variant rescues erectile function by enhancing endothelial regeneration in the streptozotocin-induced diabetic mouse. Diabetes 2011, 60, 969–980. [Google Scholar] [CrossRef]
- Musicki, B.; Kramer, M.F.; Becker, R.E.; Burnett, A.L. Inactivation of phosphorylated endothelial nitric oxide synthase (Ser-1177) by O-GlcNAc in diabetes-associated erectile dysfunction. Proc. Natl. Acad. Sci. USA 2005, 102, 11870–11875. [Google Scholar] [CrossRef] [PubMed]
- Griendling, K.K.; Sorescu, D.; Ushio-Fukai, M. NAD(P)H oxidase: Role in cardiovascular biology and disease. Circ. Res. 2000, 86, 494–501. [Google Scholar] [CrossRef]
- Luczak, K.; Balcerczyk, A.; Soszynski, M.; Bartosz, G. Low concentration of oxidant and nitric oxide donors stimulate proliferation of human endothelial cells in vitro. Cell Biol. Int. 2004, 28, 483–486. [Google Scholar] [CrossRef]
- Stone, J.R.; Collins, T. The role of hydrogen peroxide in endothelial proliferative responses. Endothelium 2002, 9, 231–238. [Google Scholar] [CrossRef]
- Morgan, M.J.; Liu, Z.G. Crosstalk of reactive oxygen species and NF-kappaB signaling. Cell Res. 2011, 21, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Noort, A.R.; van Zoest, K.P.; Weijers, E.M.; Koolwijk, P.; Maracle, C.X.; Novack, D.V.; Siemerink, M.J.; Schlingemann, R.O.; Tak, P.P.; Tas, S.W. NF-kappaB-inducing kinase is a key regulator of inflammation-induced and tumour-associated angiogenesis. J. Pathol. 2014, 234, 375–385. [Google Scholar] [CrossRef]
- Taniguchi, K.; Karin, M. NF-kappaB, inflammation, immunity and cancer: Coming of age. Nat. Rev. Immunol. 2018, 18, 309–324. [Google Scholar] [CrossRef]
- Jeremy, J.Y.; Jones, R.A.; Koupparis, A.J.; Hotston, M.; Persad, R.; Angelini, G.D.; Shukla, N. Reactive oxygen species and erectile dysfunction: Possible role of NADPH oxidase. Int. J. Impot. Res. 2007, 19, 265–280. [Google Scholar] [CrossRef]
- Tirichen, H.; Yaigoub, H.; Xu, W.; Wu, C.; Li, R.; Li, Y. Mitochondrial Reactive Oxygen Species and Their Contribution in Chronic Kidney Disease Progression Through Oxidative Stress. Front. Physiol. 2021, 12, 627837. [Google Scholar] [CrossRef] [PubMed]
- Yin, G.N.; Shin, T.Y.; Ock, J.; Choi, M.J.; Limanjaya, A.; Kwon, M.H.; Liu, F.Y.; Hong, S.S.; Kang, J.H.; Gho, Y.S.; et al. Pericyte-derived extracellular vesicles-mimetic nanovesicles improves peripheral nerve regeneration in mouse models of sciatic nerve transection. Int. J. Mol. Med. 2022, 49, 18. [Google Scholar] [CrossRef] [PubMed]
- Yin, G.N.; Ryu, J.K.; Kwon, M.H.; Shin, S.H.; Jin, H.R.; Song, K.M.; Choi, M.J.; Kang, D.Y.; Kim, W.J.; Suh, J.K. Matrigel-based sprouting endothelial cell culture system from mouse corpus cavernosum is potentially useful for the study of endothelial and erectile dysfunction related to high-glucose exposure. J. Sex Med. 2012, 9, 1760–1772. [Google Scholar] [CrossRef]
- Yin, G.N. Pericyte-derived heme-binding protein 1 promotes angiogenesis and improves erectile function in diabetic mice. Investig. Clin. Urol. 2022, 63, 464–474. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, F.-Y.; Yin, G.N.; Ock, J.; Fridayana, F.R.; Niloofar, L.; Huang, Y.; Vo, M.N.; Suh, J.-K.; Hong, S.-S.; Kang, J.-H.; et al. Argonaute 2 Restores Erectile Function by Enhancing Angiogenesis and Reducing Reactive Oxygen Species Production in Streptozotocin (STZ)-Induced Type-1 Diabetic Mice. Int. J. Mol. Sci. 2023, 24, 2935. https://doi.org/10.3390/ijms24032935
Liu F-Y, Yin GN, Ock J, Fridayana FR, Niloofar L, Huang Y, Vo MN, Suh J-K, Hong S-S, Kang J-H, et al. Argonaute 2 Restores Erectile Function by Enhancing Angiogenesis and Reducing Reactive Oxygen Species Production in Streptozotocin (STZ)-Induced Type-1 Diabetic Mice. International Journal of Molecular Sciences. 2023; 24(3):2935. https://doi.org/10.3390/ijms24032935
Chicago/Turabian StyleLiu, Fang-Yuan, Guo Nan Yin, Jiyeon Ock, Fitri Rahma Fridayana, Lashkari Niloofar, Yan Huang, Minh Nhat Vo, Jun-Kyu Suh, Soon-Sun Hong, Ju-Hee Kang, and et al. 2023. "Argonaute 2 Restores Erectile Function by Enhancing Angiogenesis and Reducing Reactive Oxygen Species Production in Streptozotocin (STZ)-Induced Type-1 Diabetic Mice" International Journal of Molecular Sciences 24, no. 3: 2935. https://doi.org/10.3390/ijms24032935
APA StyleLiu, F.-Y., Yin, G. N., Ock, J., Fridayana, F. R., Niloofar, L., Huang, Y., Vo, M. N., Suh, J.-K., Hong, S.-S., Kang, J.-H., & Ryu, J.-K. (2023). Argonaute 2 Restores Erectile Function by Enhancing Angiogenesis and Reducing Reactive Oxygen Species Production in Streptozotocin (STZ)-Induced Type-1 Diabetic Mice. International Journal of Molecular Sciences, 24(3), 2935. https://doi.org/10.3390/ijms24032935







