Insights into Cell-Specific Functions of Microtubules in Skeletal Muscle Development and Homeostasis
Abstract
1. Introduction
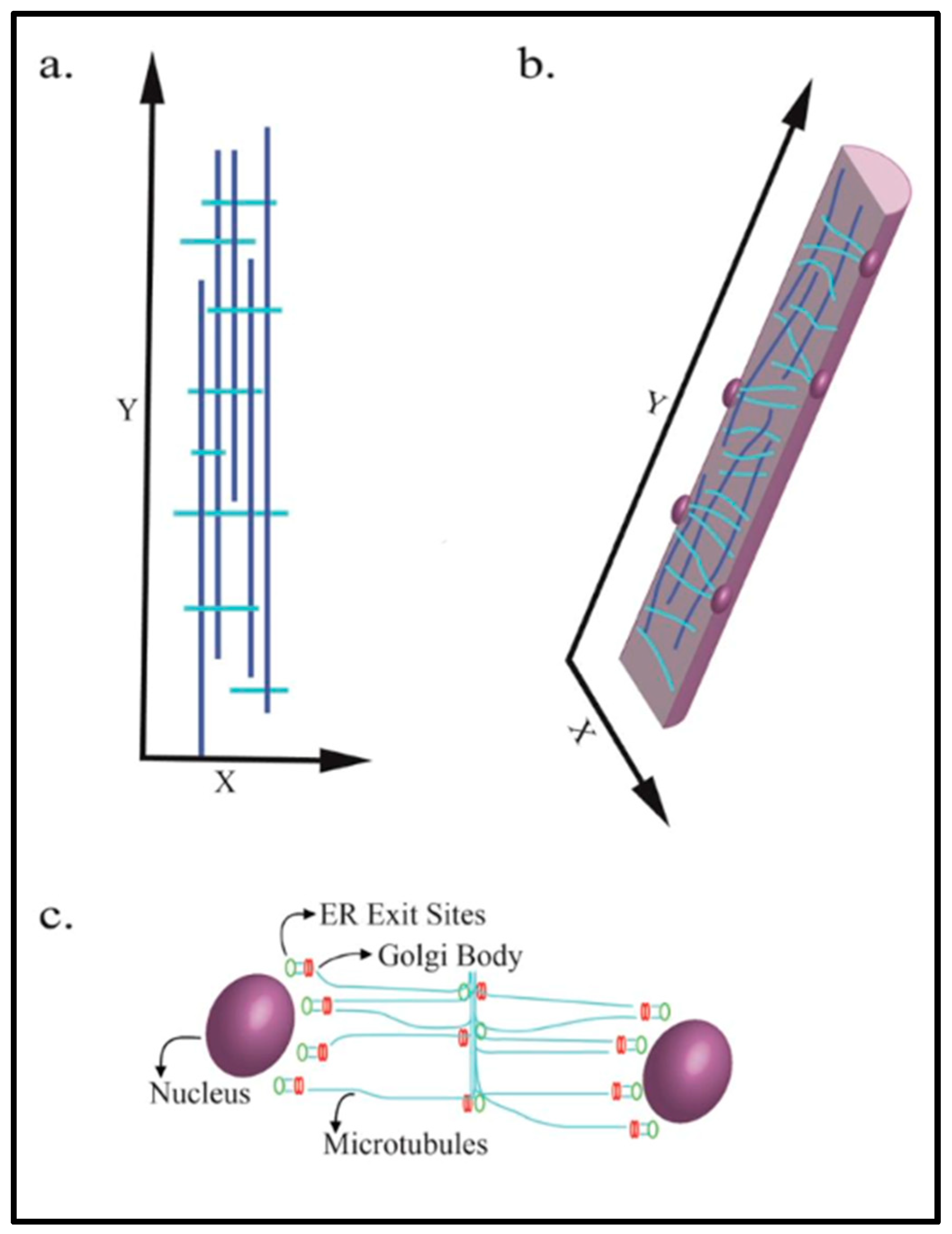
2. Microtubule Arrangement on Skeletal Muscle Cells
3. Microtubule Functions during Myoblast Differentiation
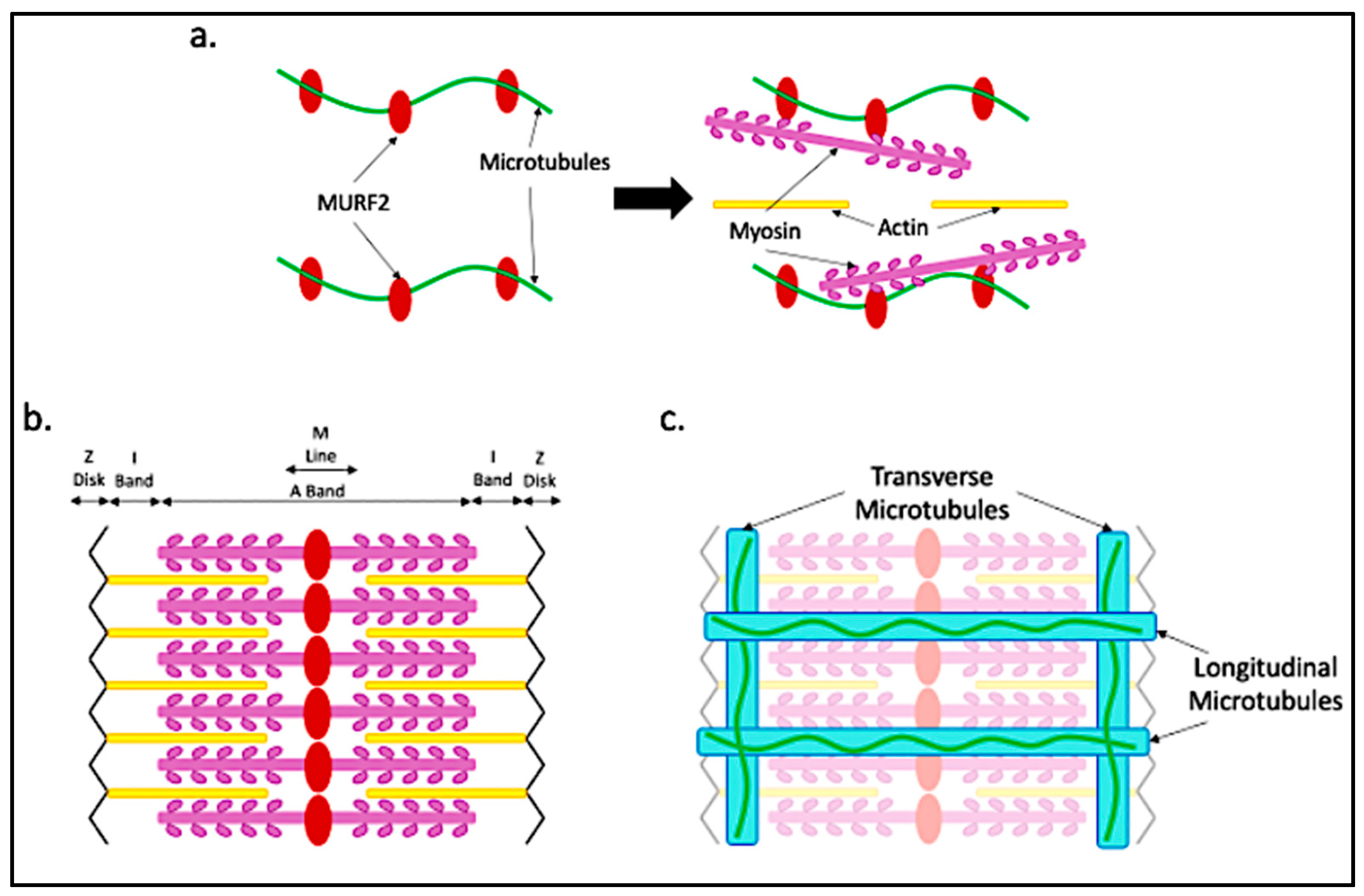
4. Microtubule Functions in Maturing Myofibers
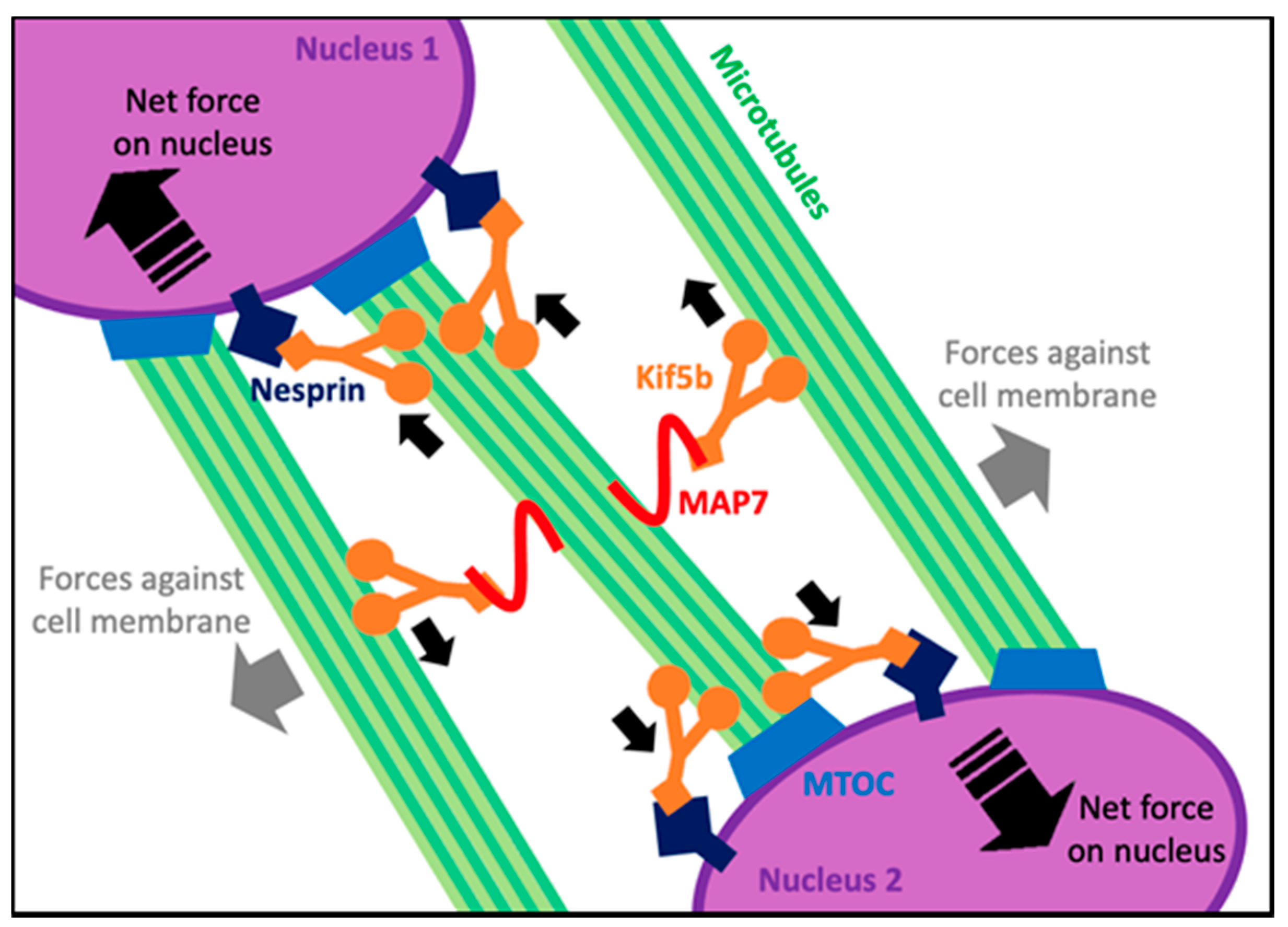
5. Microtubule Form and Function in Adult Myofibers
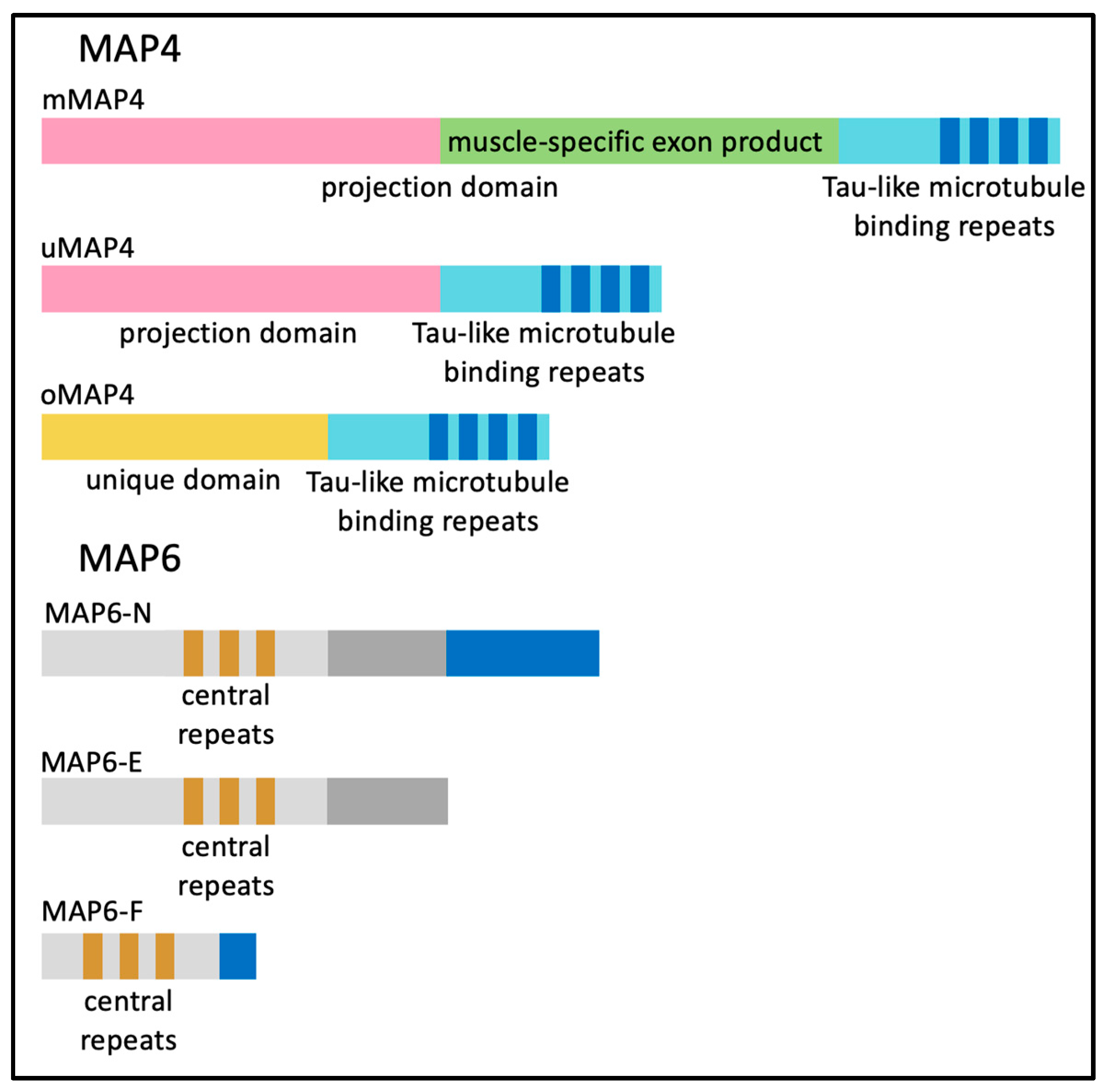
6. Microtubule-Associated Proteins (MAPs) and End-Binding (EB) Proteins in Skeletal Muscle Myofibers
7. Diseases Related to Skeletal Muscle Microtubule Abnormalities
| Gene Name | Protein Name | Disease Defective in: | Role in Relation to Microtubules | Possible Disease Pathogenesis * |
|---|---|---|---|---|
| DNM2 | dynamin 2 | Centronuclear Myopathy (CNM) | Coordinates centrosome localization [70] | Improper microtubule function in myoblasts and/or myofibers |
| DMD | dystrophin | Duchenne Muscular Dystrophy (DMD) | Organizes microtubules within myofibers [46] | Improper alignment of microtubules weakens and subjects myofibers to damage |
| RAC1 | Ras-related C3 botulinum toxin substrate 1 | Duchenne Muscular Dystrophy (DMD) | Activates X-ROS signaling pathway during microtubule stretching [73] | Increased ROS production as a result of dense microtubule structure damages myofibers |
| TUBB6 | Tubulin Beta Class V | Duchenne Muscular Dystrophy (DMD) | Β-tubulin isoform typically expressed during myoblast differentiation [75] | Tubb6 upregulation in DMD myofibers results in microtubule disorganization |
8. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Abbreviation | Description |
| ATP | adenosine triphosphate |
| C2C12 | mouse myoblast cell line |
| CNM | centronuclear myopathies |
| COPII | coat protein complex II |
| Cryo-EM | cryo-electron microscopy |
| DAPC | dystrophin-associated protein complex |
| DMD | Duchenne muscular dystrophy |
| DNM2 | dynamin 2 |
| Drp1 | dynamin-related protein 1 |
| EB | end-binding protein |
| EB1 | end-binding protein 1 |
| EB2 | end-binding protein 2 |
| EB3 | end-binding protein 3 |
| ER | endoplasmic reticulum |
| FCM | fusion competent myoblast |
| FDB | flexor digitorum brevis |
| GFP-EB3 | green fluorecent protein—end-binding protein 3 |
| Kif5b | kinesin-1 heavy chain |
| KO | knock-out |
| L6 | rat myoblast cell line |
| LEWD | lysine, glutamic acid, an acidic amino acid, aspartic acid |
| LKB1 | liver kinase B1 |
| MAP4 | microtubule-associated protein 4 |
| MAP6 | microtubule-associated protein 6 |
| MAP6-E | MAP6—embryonic |
| MAP6-F | MAP6—fibroblast |
| MAP6-N | MAP6—neuronal |
| MAP7 | microtubule-associated protein 7 |
| MAPs | microtubule-associated proteins |
| mdx | dystrophin-depleted mouse model of DMD |
| mMAP4 | muscle-specific MAP4 isoform |
| mRNA | messenger ribose nucleic acid |
| MTOC | microtubule-organizing center |
| MURF2 | Muscle RING-finger 2 |
| NOX-2 | NADPH oxidase-2 |
| oMAP4 | organizing MAP4 isoform |
| Par6β | partitioning defective 6 homolog beta |
| PCM | pericentriolar matrix |
| PCM1 | pericentriolar material 1 |
| Rac1 | ras-related C3 botulinum toxin substrate 1 |
| ROS | reactive oxygen species |
| skCIP | skeletal muscle-specific cardiac islet-1 interaction protein |
| SUN | Sad1 and UNC-84 |
| Tubb6 | beta-tubulin beta 6 class V |
| uMAP4 | ubiquitously expressed MAP4 isoform |
References
- Oddoux, S.; Zaal, K.; Tate, V.; Kenea, A.; Nandkeolyar, S.; Reid, E.; Liu, W.; Ralston, E. Microtubules that form the stationary lattice of muscle fibers are dynamic and nucleated at Golgi elements. J. Cell Biol. 2013, 203, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Starr, D.A. Muscle Development: Nucleating Microtubules at the Nuclear Envelope. Curr. Biol. 2017, 27, R1071–R1073. [Google Scholar] [CrossRef] [PubMed]
- Kollman, J.M.; Merdes, A.; Mourey, L.; Agard, D.A. Microtubule nucleation by γ-tubulin complexes. Nat. Rev. Mol. Cell Biol. 2011, 12, 709–721. [Google Scholar] [CrossRef] [PubMed]
- Teixido-Travesa, N.; Roig, J.; Luders, J. The where, when and how of microtubule nucleation—One ring to rule them all. J. Cell Sci. 2012, 125, 4445–4456. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.C.; Neuner, A.; Schiebel, E. Targeting of γ-tubulin complexes to microtubule organizing centers: Conservation and divergence. Trends Cell Biol. 2015, 25, 296–307. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, A.D.; Feldman, J.L. Microtubule-organizing centers: From the centrosome to non-centrosomal sites. Curr. Opin. Cell Biol. 2017, 44, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Woodruff, J.B.; Wueseke, O.; Hyman, A. Pericentriolar material structure and dynamics. Phil. Trans. R. Soc. B 2014, 369, 20130459. [Google Scholar] [CrossRef]
- Bannykh, S.I.; Rowe, T.; Balch, W. The organization of endoplasmic reticulum export complexes. J. Cell Biol. 1996, 135, 19–35. [Google Scholar] [CrossRef]
- Lu, Z.; Joseph, D.; Bugnard, E.; Zaal, K.J.M.; Ralston, E. Golgi Complex Reorganization during Muscle Differentiation: Visualization in Living Cells and Mechanism. mBoC 2001, 12, 795–808. [Google Scholar] [CrossRef]
- Rahkila, P.; Väänänen, K.; Saraste, J.; Metsikkö, K. Endoplasmic Reticulum to Golgi Trafficking in Multinucleated Skeletal Muscle Fibers. Exp. Cell Res. 1997, 234, 452–464. [Google Scholar] [CrossRef]
- Srsen, V.; Fant, X.; Heald, R.; Rabouille, C.; Merdes, A. Centrosome proteins form an insoluble perinuclear matrix during muscle cell differentiation. BMC Cell Biol. 2009, 10, 28. [Google Scholar] [CrossRef] [PubMed]
- Tassin, A.M.; Maro, B.; Bornens, M. Fate of microtubule-organizing centers during myogenesis in vitro. J. Cell BioL. 1985, 100, 35–46. [Google Scholar] [CrossRef]
- Becker, R.; Leone, M.; Engel, F.B. Microtubule Organization in Striated Muscle Cells. MDPI Cells 2020, 9, 1395. [Google Scholar] [CrossRef] [PubMed]
- Bugnard, E.; Zaal, K.J.M.; Ralston, E. Reorganization of microtubule nucleation during muscle differentiation. Cell Motil. Cytoskelet. 2005, 60, 1–13. [Google Scholar] [CrossRef]
- Nadkarni, A.V.; Heald, R. Reconstitution of muscle cell microtubule organization in vitro. Cytoskeleton (Hoboken) 2021, 78, 492–502. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Huang, Z.; Nie, M.; Wang, G.; Silva, W.; Yang, O.; Freire, P.; Hu, X.; Chen, H.; Deng, Z.; et al. Regulation of myonuclear positioning and muscle function by the skeletal muscle-specific CIP protein. Proc. Natl. Acad. Sci. USA 2020, 117, 19254–19265. [Google Scholar] [CrossRef]
- Mian, I.; Pierre-Louis, W.S.; Dole, N.; Gilberti, R.M.; Dodge-Kafka, K. LKB1 Destabilizes Microtubules in Myoblasts and Contributes to Myoblast Differentiation. PLoS ONE 2012, 7, e31583. [Google Scholar] [CrossRef] [PubMed]
- Warren, R.H. Microtubular organization in elongating myogenic cells. J. Cell Biol. 1974, 63, 550–566. [Google Scholar] [CrossRef] [PubMed]
- Clark, P.; Dunn, G.; Knibbs, A.; Peckham, M. Alignment of myoblasts on ultrafine gratings inhibits fusion in vitro. Int. J. Biochem. Cell Biol. 2002, 34, 816–825. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Jin, P.; Duan, R.; Chen, E. Mechanisms of myoblast fusion during muscle development. 2015 Curr. Opin. Genet. Dev. 2015, 32, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Brukman, N.; Uygur, B.; Podbilewicz, B.; Chernomordik, L. How cells fuse. J. Cell Biol. 2019, 218, 1436–1451. [Google Scholar] [CrossRef] [PubMed]
- Geuens, G.; Gundersen, G.; Nuydens, R.; Cornelissen, F.; Bulinski, J.; DeBrabander, M. Ultrastructural colocalization of tyrosinated and detyrosinated alpha-tubulin in interphase and mitotic cells. J. Cell Biol. 1986, 103, 1883–1893. [Google Scholar] [CrossRef] [PubMed]
- Gundersen, G.; Bulinski, J. Microtubule arrays in differentiated cells contain elevated levels of a post-translationally modified form of tubulin. Eur. J. Cell Biol. 1986, 42, 288–294. [Google Scholar]
- Gundersen, G.G.; Khawaja, S.; Bulinski, J. Generation of a stable, posttranslationally modified microtubule array is an early event in myogenic differentiation. J. Cell Biol. 1989, 109, 2275–2288. [Google Scholar] [CrossRef]
- Mangan, M.E.; Olmsted, J.B. A muscle-specific variant of microtubule-associated protein 4 (MAP4) is required in myogenesis. Development 1996, 122, 771–781. [Google Scholar] [CrossRef]
- Saitoh, O.; Arai, T.; Obinata, T. Distribution of microtubules and other cytoskeletal filaments during myotube elongation as revealed by fluorescence microscopy. Cell Tissue Res. 1988, 252, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Cadot, B.; Gache, V.; Vasyutina, E.; Falcone, S.; Birchmeier, C.; Gomes, E. Nuclear movement during myotube formation is microtubule and dynein dependent and is regulated by Cdc42, Par6 and Par3. EMBO 2012, 13, 741–749. [Google Scholar] [CrossRef]
- Burakov, A.; Nadezhdina, E.; Slepchenko, B.; Rodionov, V. Centrosome positioning in interphase cells. J. Cell Biol. 2003, 162, 963–969. [Google Scholar] [CrossRef]
- Gönczy, P.; Pichler, S.; Kirkham, M.; Hyman, A. Cytoplasmic Dynein Is Required for Distinct Aspects of Mtoc Positioning, Including Centrosome Separation, in the One Cell Stage Caenorhabditis elegans Embryo. J. Cell Biol. 1999, 147, 135–150. [Google Scholar] [CrossRef]
- Metzger, T.; Gache, V.; Xu, M.; Cadot, B.; Folker, E.; Richardson, B.; Gomes, E.; Baylies, M. MAP and kinesin-dependent nuclear positioning is required for skeletal muscle function. Nature 2012, 484, 120–124. [Google Scholar] [CrossRef]
- Wilson, M.H.; Holzbaur, E.L.F. Opposing microtubule motors drive robust nuclear dynamics in developing muscle cells. J. Cell Sci. 2012, 125, 4158–4169. [Google Scholar] [CrossRef]
- Wilson, M.H.; Holzbaur, E.L.F. Nesprins anchor kinesin-1 motors to the nucleus to drive nuclear distribution in muscle cells. Development 2015, 142, 218–228. [Google Scholar] [CrossRef]
- Manhart, A.; Windner, S.; Baylies, M.; Mogilner, A. Mechanical positioning of multiple nuclei in muscle cells. PLoS Comput. Biol. 2018, 14, e1006208. [Google Scholar] [CrossRef] [PubMed]
- Roman, W.; Martins, J.; Carvalho, F.; Voituriez, R.; Abella, J.; Santos, N.; Cadot, B.; Way, M.; Gomes, E. Myofibril contraction and crosslinking drive nuclear movement to the periphery of skeletal muscle. Nat. Cell Biol. 2017, 19, 1189–1201. [Google Scholar] [CrossRef] [PubMed]
- Bruusgaard, J.C.; Liestøl, K.; Gundersen, K. Distribution of myonuclei and microtubules in live muscle fibers of young, middle-aged, and old mice. J. Appl. Physiol. 2006, 100, 2024–2030. [Google Scholar] [CrossRef] [PubMed]
- Shaw, N.M.; Rios-Monterrosa, J.L.; Fedorchak, G.R.; Ketterer, M.R.; Coombs, G.S.; Lammerding, J.; Wallrath, L.L. Effects of mutant lamins on nucleo-cytoskeletal coupling in Drosophilamodels of LMNA muscular dystrophy. Front Cell Dev. Biol. 2022, 10, 934586. [Google Scholar] [CrossRef]
- Earle, A.J.; Kirby, T.J.; Fedorchak, G.R.; Isermann, P.; Patel, J.; Iruvanti, S.; Moore, S.A.; Bonne, G.; Wallrath, L.L.; Lammerding, J. Mutant lamins cause nuclear envelope rupture and DNA damage in skeletal muscle cells. Nat. Mater. 2020, 19, 464–473. [Google Scholar] [CrossRef]
- Dhanyasi, N.; VijayRaghavan, K.; Shilo, B.; Schejter, E. Microtubules provide guidance cues for myofibril and sarcomere assembly and growth. Dev. Dyn. 2021, 250, 60–73. [Google Scholar] [CrossRef] [PubMed]
- Trinick, J. Cytoskeleton: Titin as a scaffold and spring. Curr. Biol. 1996, 6, 258–260. [Google Scholar] [CrossRef]
- Pizon, V. Transient association of titin and myosin with microtubules in nascent myofibrils directed by the MURF2 RING-finger protein. J. Cell Sci. 2002, 115, 4469–4482. [Google Scholar] [CrossRef]
- Pizon, V.; Gerbal, F.; Diaz, C.; Karsenti, E. Microtubule-dependent transport and organization of sarcomeric myosin during skeletal muscle differentiation. EMBO J. 2005, 24, 3781–3792. [Google Scholar] [CrossRef] [PubMed]
- Ralston, E.; Lu, Z.; Ploug, T. The Organization of the Golgi Complex and Microtubules in Skeletal Muscle Is Fiber Type-Dependent. J. Neurosci. 1999, 19, 10694–10705. [Google Scholar] [CrossRef]
- Ralston, E.; Ploug, T.; Kalhovde, J.; Lømo, T. Golgi Complex, Endoplasmic Reticulum Exit Sites, and Microtubules in Skeletal Muscle Fibers Are Organized by Patterned Activity. J. Neurosci. 2001, 21, 875–883. [Google Scholar] [CrossRef]
- Barlowe, C. COPII and selective export from the endoplasmic reticulum. Biochim. Et Biophys. Acta (BBA)–-Mol. Cell Res. 1998, 1404, 67–76. [Google Scholar] [CrossRef]
- Demonbreun, A.R.; McNally, E.M. DNA Electroporation, Isolation and Imaging of Myofibers. J. Vis. Exp. 2015, 106, e53551. [Google Scholar]
- Prins, K.W.; Humston, J.; Mehta, A.; Tate, V.; Ralston, E.; Ervasti, J. Dystrophin is a microtubule-associated protein. J. Cell Biol. 2009, 186, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, S.; Hood, D.A. Cytoskeletal regulation of mitochondrial movements in myoblasts. Cytoskeleton (Hoboken) 2014, 71, 564–572. [Google Scholar] [CrossRef]
- Anesti, V.; Scorrano, L. The relationship between mitochondrial shape and function and the cytoskeleton. Biochim. Biophys. Acta. 2006, 1757, 692–699. [Google Scholar] [CrossRef]
- Milner, D.J.; Mavroidis, M.; Weisleder, N.; Capetanaki, Y. Desmin cytoskeleton linked to muscle mitochondrial distribution and respiratory function. J. Cell Biol. 2000, 150, 1283–1298. [Google Scholar] [CrossRef] [PubMed]
- Dalpe, G.; Mathieu, M.; Comtois, A.; Zhu, E.; Wasiak, S.; De Repen-tigny, Y. Dystonin-deficient mice exhibit an intrinsic muscle weakness and an instability of skeletal muscle cytoarchitecture. Dev. Biol. 1999, 210, 367–380. [Google Scholar] [CrossRef] [PubMed]
- Winter, L.; Kuznetsov, A.V.; Grimm, M.; Zeold, A.; Fischer, I.; Wiche, G. Plectin isoform P1b and P1d deficiencies differentially affect mitochondrial morphology and function in skeletal muscle. Hum. Mol. Genet. 2015, 24, 4530–4544. [Google Scholar] [CrossRef] [PubMed]
- Giovarelli, M.; Zecchini, S.; Martini, E.; Garrè, M.; Barozzi, S.; Ripolone, M.; Napoli, L.; Coazzoli, M.; Vantaggiato, C.; Roux-Biejat, P.; et al. Drp1 overexpression induces desmin disassembling and drives kinesin-1 activation promoting mitochondrial trafficking in skeletal muscle. Cell Death Differ. 2020, 27, 2383–2401. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, H.; Pimentel, M.R.; Sequeira, C.; Oliveira, L.M.; Pezzarossa, A.; Roman, W.; Gomes, E.R. mRNA distribution in skeletal muscle is associated with mRNA size. J. Cell Sci. 2021, 15, jcs256388. [Google Scholar] [CrossRef] [PubMed]
- Denes, L.T.; Kelley, C.P.; Wang, E.T. Microtubule-based transport is essential to distribute RNA and nascent protein in skeletal muscle. Nat, Commun. 2021, 27, 6079. [Google Scholar] [CrossRef]
- Shigematsu, H.; Imasaki, T.; Doki, C.; Sumi, T.; Aoki, M.; Uchikubo-Kamo, T.; Sakamoto, A.; Tokuraku, K.; Shirouzu, M.; Nitta, R. Structural insight into microtubule stabilization and kinesin inhibition by Tau family MAPs. J. Cell Biol. 2018, 217, 4155–4163. [Google Scholar] [CrossRef] [PubMed]
- Aizawa, H.; Emori, Y.; Mori, A.; Murofushi, H.; Sakai, H.; Suzuki, K. Functional analyses of the domain structure of microtubule-associated protein-4 (MAP-U). J. Biol. Chem. 1991, 266, 9841–9846. [Google Scholar] [CrossRef] [PubMed]
- West, R.R.; Tenbarge, K.M.; Olmsted, J.B. A model for microtubule-associated protein 4 structure. Domains defined by comparisons of human, mouse, and bovine sequences. J. Biol. Chem. 1991, 266, 21886–21896. [Google Scholar] [CrossRef]
- Mogessie, B.; Roth, D.; Rahil, Z.; Straube, A. A novel isoform of MAP4 organises the paraxial microtubule array required for muscle cell differentiation. eLife 2015, 4, e05697. [Google Scholar] [CrossRef]
- Baratier, J.; Peris, L.; Brocard, J.; Gory-Fauré, S.; Dufour, F.; Bosc, C.; Fourest-Lieuvin, A.; Blanchoin, L.; Salin, P.; Job, D.; et al. Phosphorylation of Microtubule-associated Protein STOP by Calmodulin Kinase II. J. Biol. Chem. 2006, 281, 19561–19569. [Google Scholar] [CrossRef]
- Deloulme, J.-C.; Gory-Fauré, S.; Mauconduit, F.; Chauvet, S.; Jonckheere, J.; Boulan, B.; Mire, E.; Xue, J.; Jany, M.; Maucler, C.; et al. Microtubule-associated protein 6 mediates neuronal connectivity through Semaphorin 3E-dependent signalling for axonal growth. Nat. Commun. 2015, 6, 7246. [Google Scholar] [CrossRef]
- Sébastien, M.; Giannesini, B.; Aubin, P.; Brocard, J.; Chivet, M.; Pietrangelo, L.; Boncompagni, S.; Bosc, C.; Brocard, J.; Rendu, J.; et al. Deletion of the microtubule-associated protein 6 (MAP6) results in skeletal muscle dysfunction. Skelet. Muscle 2018, 8, 30. [Google Scholar] [CrossRef] [PubMed]
- Roth, D.; Fitton, B.; Chmel, N.; Wasiluk, N.; Straube, A. Spatial positioning of EB family proteins at microtubule tips involves distinct nucleotide-dependent binding properties. J. Cell Sci. 2019, 132, jcs219550. [Google Scholar] [CrossRef] [PubMed]
- Su, L.-K.; Qi, Y. Characterization of Human MAPRE Genes and Their Proteins. Genomics 2001, 71, 142–149. [Google Scholar] [CrossRef]
- Straube, A.; Merdes, A. EB3 Regulates Microtubule Dynamics at the Cell Cortex and Is Required for Myoblast Elongation and Fusion. Curr. Biol. 2007, 17, 1318–1325. [Google Scholar] [CrossRef]
- Zhang, T.; Zaal, K.J.; Sheridan, J.; Mehta, A.; Gundersen, G.G.; Ralston, E. Microtubule plus-end binding protein EB1 is necessary for muscle cell differentiation, elongation and fusion. J. Cell Sci. 2009, 122, 1401–1409. [Google Scholar] [CrossRef] [PubMed]
- Jungbluth, H.; Wallgren-Pettersson, C.; Laporte, J. Centronuclear (myotubular) myopathy. Orphanet J. Rare Dis. 2008, 3, 26. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, K.; Takei, K. Dynamic instability of microtubules requires dynamin 2 and is impaired in a Charcot-Marie-Tooth mutant. J. Cell Biol. 2009, 185, 939–948. [Google Scholar] [CrossRef] [PubMed]
- Warnock, D.E.; Baba, T.; Schmid, S. Ubiquitously Expressed Dynamin-II Has a Higher Intrinsic GTPase Activity and a Greater Propensity for Self-assembly Than Neuronal Dynamin-I. mBoC 1997, 8, 2553–2562. [Google Scholar] [CrossRef]
- Bitoun, M.; Maugenre, S.; Jeannet, P.-Y.; Lacène, E.; Ferrer, X.; Laforêt, P.; Martin, J.-J.; Laporte, J.; Lochmüller, H.; Beggs, A.H.; et al. Mutations in dynamin 2 cause dominant centronuclear myopathy. Nat. Genet. 2005, 37, 1207–1209. [Google Scholar] [CrossRef] [PubMed]
- Thompson, H.M.; Cao, H.; Chen, J.; Euteneuer, U.; McNiven, M.A. Dynamin 2 binds γ-tubulin and participates in centrosome cohesion. Nat. Cell Biol. 2004, 6, 335–342. [Google Scholar] [CrossRef]
- Hoffman, E.P.; Brown, R.H.; Kunkel, L.M. Dystrophin: The protein product of the duchennee muscular dystrophy locus. Cell 1987, 51, 919–928. [Google Scholar] [CrossRef] [PubMed]
- Blake, D.J.; Weir, A.; Newey, S.; Davies, K.E. Function and Genetics of Dystrophin and Dystrophin-Related Proteins in Muscle. Physiol. Rev. 2002, 82, 291–329. [Google Scholar] [CrossRef]
- Khairallah, R.J.; Shi, G.; Sbrana, F.; Prosser, B.; Borroto, C.; Mazaitis, M.; Hoffman, E.; Mahurkar, A.; Sachs, F.; Sun, Y.; et al. Microtubules Underlie Dysfunction in Duchenne Muscular Dystrophy. Sci. Signal. 2012, 5, ra56. [Google Scholar] [CrossRef] [PubMed]
- Percival, J.M.; Gregorevic, P.; Odom, G.L.; Banks, G.B.; Chamberlain, J.S.; Froehner, S.C. rAAV6-Microdystrophin Rescues Aberrant Golgi Complex Organization in mdx Skeletal Muscles. Traffic 2007, 8, 1424–1439. [Google Scholar] [CrossRef] [PubMed]
- Randazzo, D.; Khalique, U.; Belanto, J.; Kenea, A.; Talsness, D.; Olthoff, J.; Tran, M.; Zaal, K.; Pak, K.; Pinal-Fernandez, I.; et al. Persistent upregulation of the β-tubulin tubb6, linked to muscle regeneration, is a source of microtubule disorganization in dystrophic muscle. Hum. Mol. Genet. 2019, 28, 1117–1135. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lucas, L.; Cooper, T.A. Insights into Cell-Specific Functions of Microtubules in Skeletal Muscle Development and Homeostasis. Int. J. Mol. Sci. 2023, 24, 2903. https://doi.org/10.3390/ijms24032903
Lucas L, Cooper TA. Insights into Cell-Specific Functions of Microtubules in Skeletal Muscle Development and Homeostasis. International Journal of Molecular Sciences. 2023; 24(3):2903. https://doi.org/10.3390/ijms24032903
Chicago/Turabian StyleLucas, Lathan, and Thomas A. Cooper. 2023. "Insights into Cell-Specific Functions of Microtubules in Skeletal Muscle Development and Homeostasis" International Journal of Molecular Sciences 24, no. 3: 2903. https://doi.org/10.3390/ijms24032903
APA StyleLucas, L., & Cooper, T. A. (2023). Insights into Cell-Specific Functions of Microtubules in Skeletal Muscle Development and Homeostasis. International Journal of Molecular Sciences, 24(3), 2903. https://doi.org/10.3390/ijms24032903





