Regulatory Mechanism between Ferritin and Mitochondrial Reactive Oxygen Species in Spinal Ligament-Derived Cells from Ossification of Posterior Longitudinal Ligament Patient
Abstract
1. Introduction
2. Results
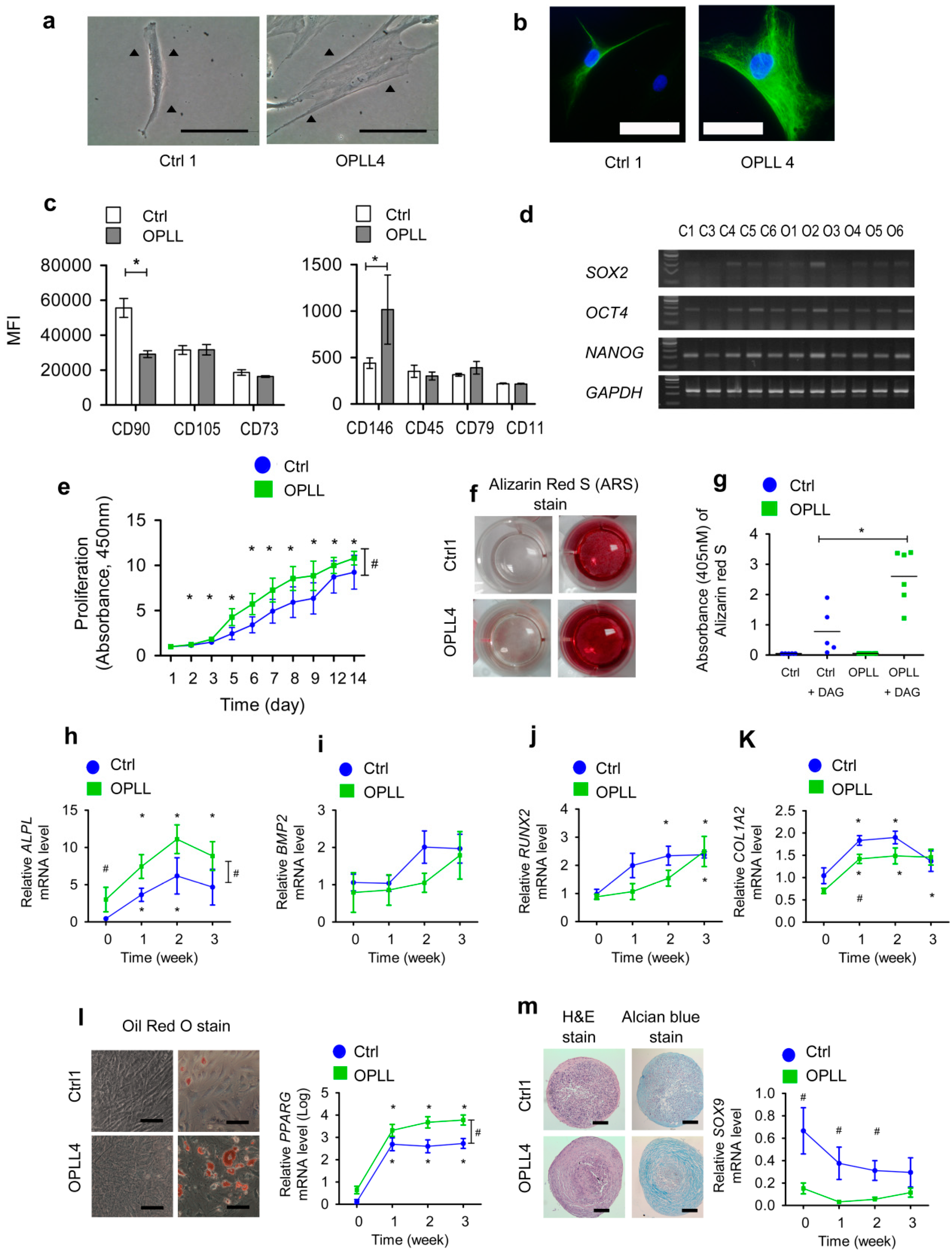
2.1. SLDCs Exhibited Properties of Mesenchymal Stem/Stromal Cells
2.2. SLDCs from the OPLL Group Showed Enhanced Osteogenic Differentiation
2.3. Enhanced TCA Cycle and Oxidative Phosphorylation Pathway in the OPLL Group
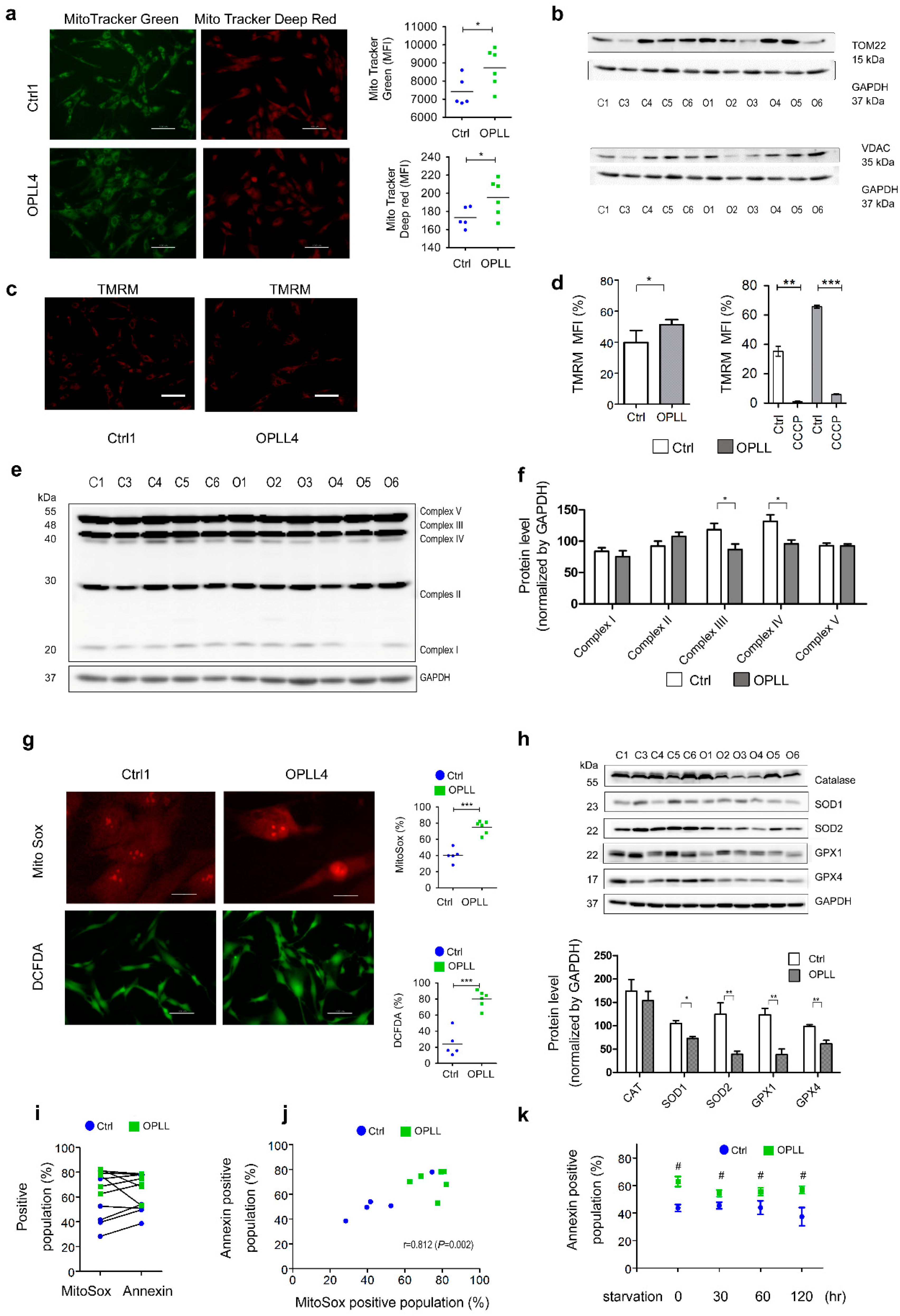
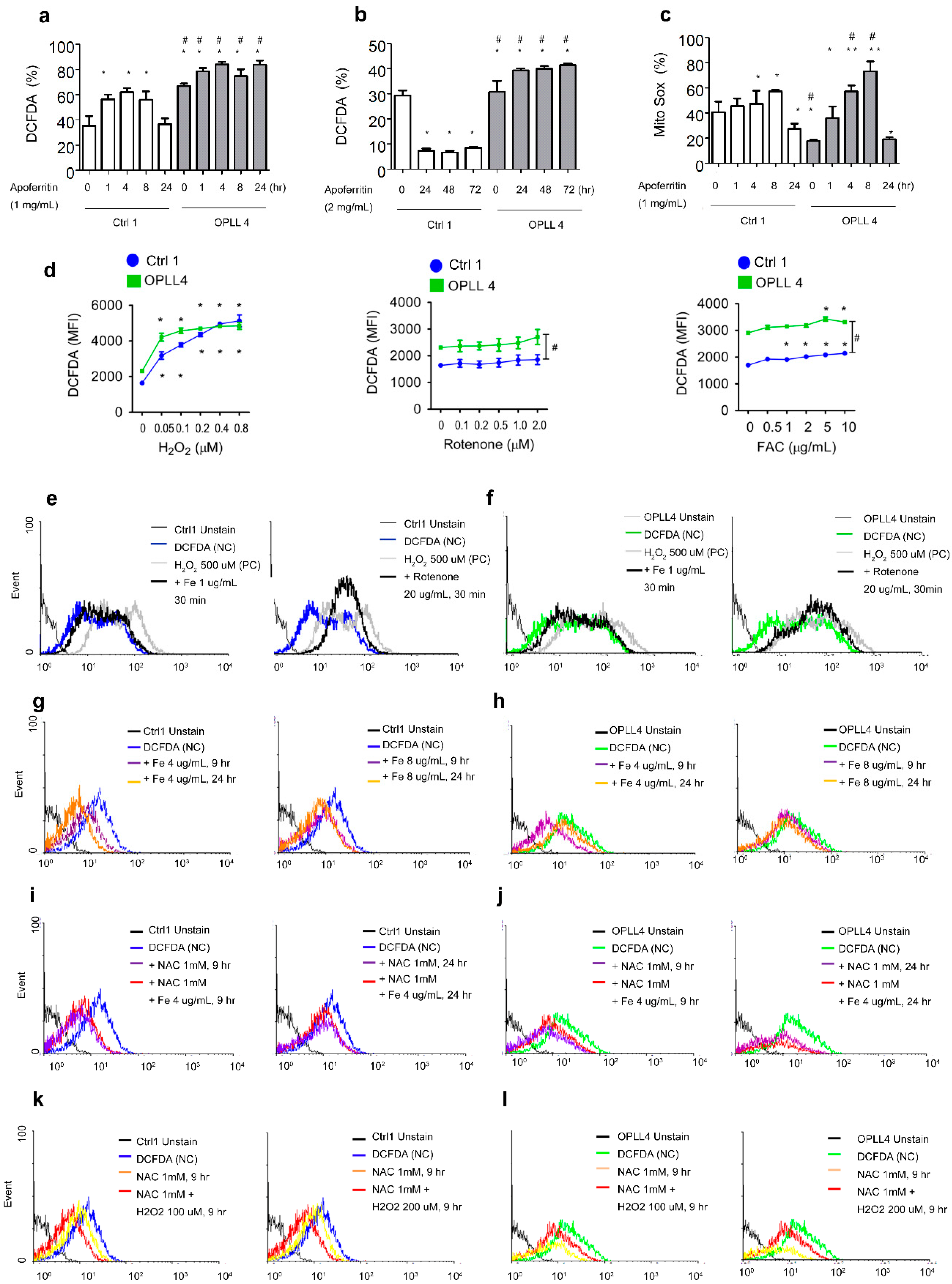
2.4. Mitochondrial Characteristics and ROS Status
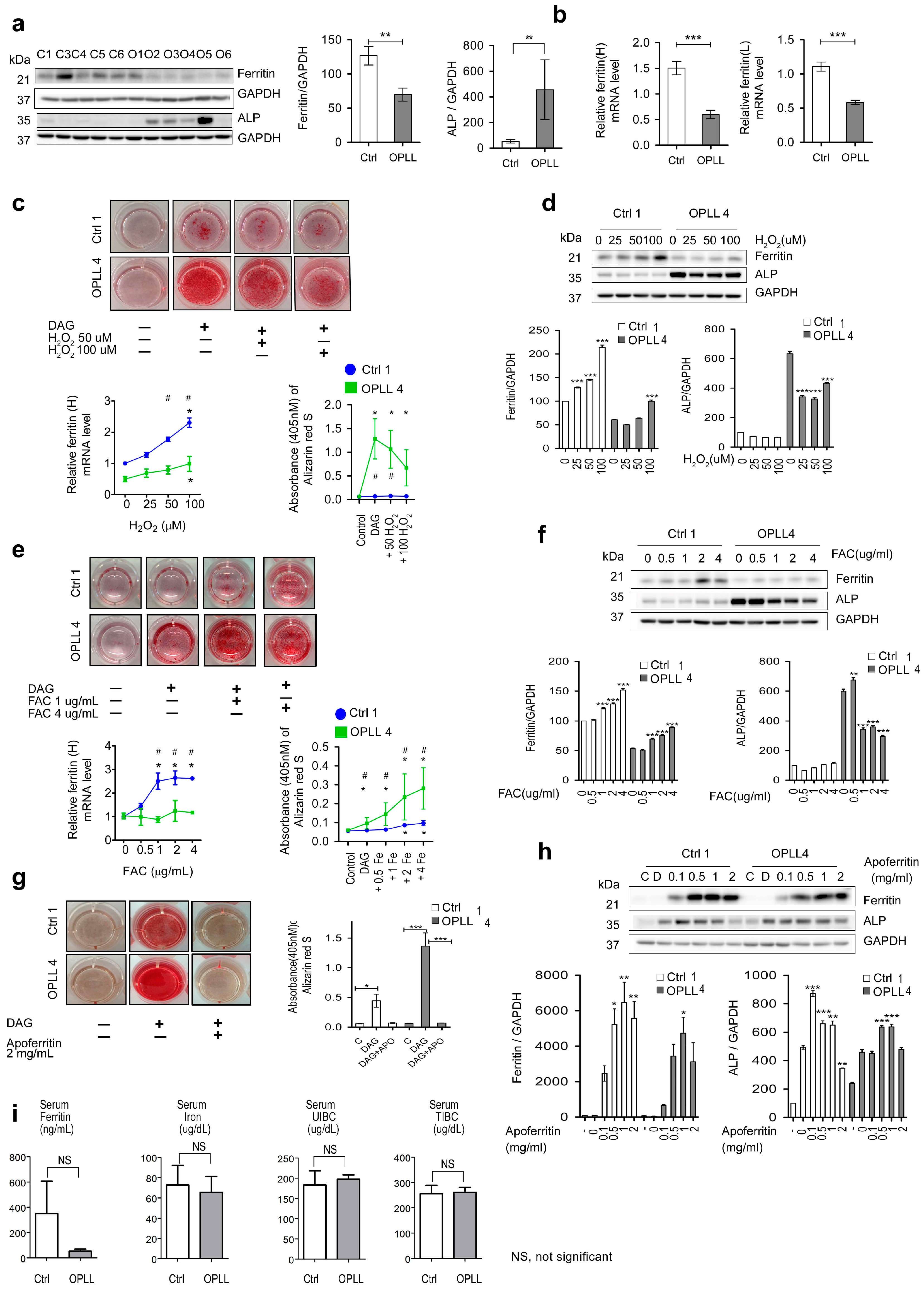
2.5. Relationship between ROS and Ferritin in SLDC
2.6. Effects of Fe, H2O2 and Apoferritin on Ferritin and ALP
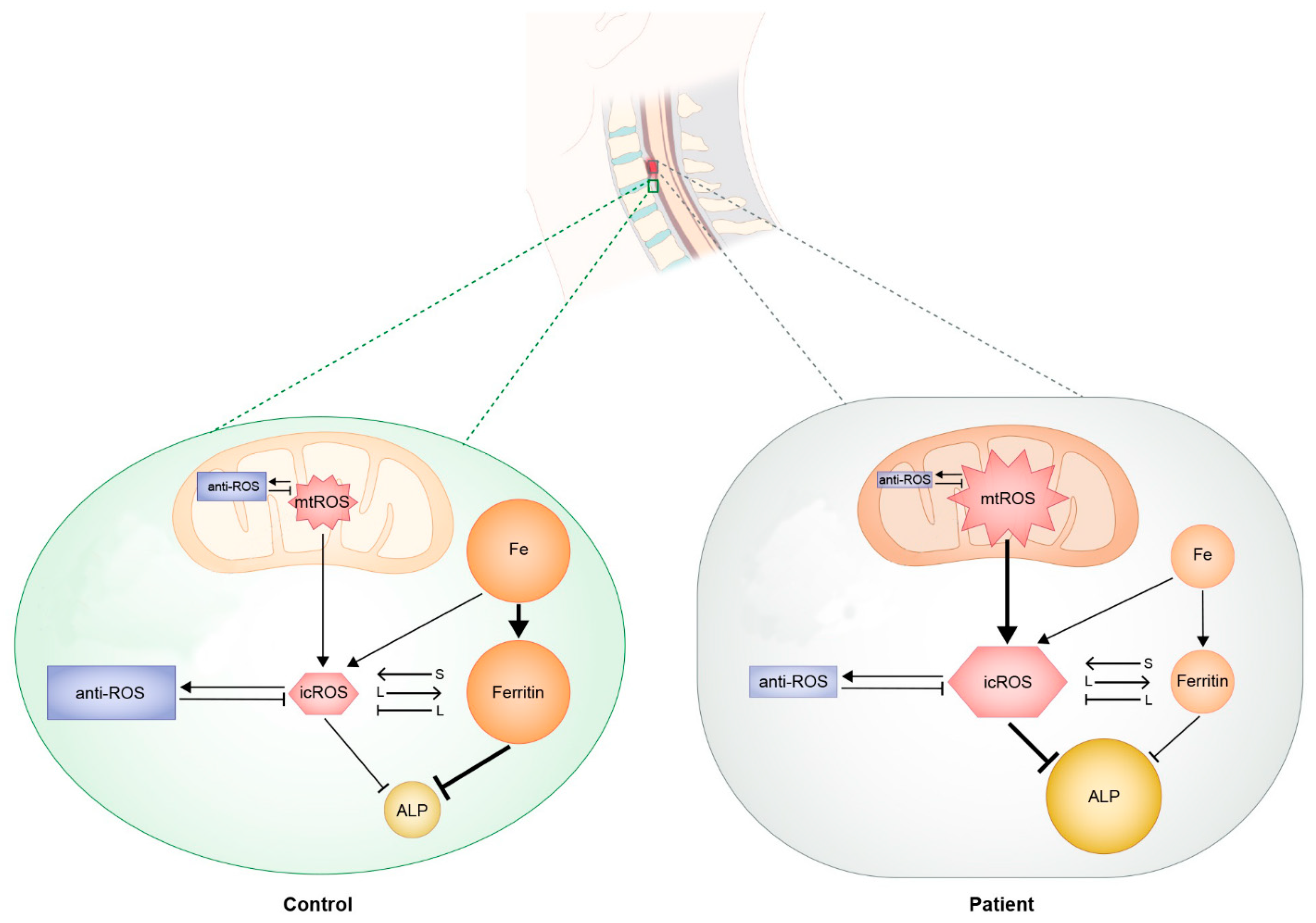
2.7. Suggested Regulatory Mechanism
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Cell Culture
4.3. In Vitro Differentiation
4.4. Cell Proliferation Assay
4.5. Real-Time Quantitative Polymerase Chain Reaction (qRT-PCR) and Conventional PCR
4.6. Flow Cytometric Analysis
4.7. ALP Concentration Assessment
4.8. Western Blot Analysis
4.9. Live Cell Imaging
4.10. RNA-Seq Analysis
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Inamasu, J.; Guiot, B.; Sachs, D. Ossification of the posterior longitudinal ligament: An update on its biology, epidemiology, and natural history. Neurosurgery 2006, 56, 1027–1038. [Google Scholar] [CrossRef]
- Matunaga, S.; Sakou, T. Ossification of the posterior longitudinal ligament of the cervical spine. Spine 2012, 32, E309–E314. [Google Scholar] [CrossRef]
- Sohn, S.; Chung, C.K.; Yun, T.J.; Sohn, C.H. Epidemiological survey of ossification of the posterior longitudinal ligament in an adult Korean population: Three-dimensional computed tomographic observation of 3,240 Cases. Calcif. Tissue Int. 2014, 94, 613–620. [Google Scholar] [CrossRef]
- Kim, T.; Bae, K.; Uhm, W.; Kim, T.; Joo, K.; Jun, J. Prevalence of ossification of the posterior longitudinal ligament of the cervical spine. Jt. Bone Spine 2008, 75, 471–474. [Google Scholar] [CrossRef]
- Kwon, S.Y.; Shin, J.J.; Lee, J.H.; Cho, W.H. Prognostic factors for surgical outcome in spinal cord injury associated with ossification of the posterior longitudinal ligament (OPLL). J. Orthop. Surg. Res. 2015, 10, 94. [Google Scholar] [CrossRef]
- Head, J.; Rymarczuk, G.; Stricsek, G.; Velagapudi, L.; Maulucci, C.; Hoelscher, C.; Harrop, J. Ossification of the posterior longitudinal ligament: Surgical approaches and associated complications. Neurospine 2019, 16, 517–529. [Google Scholar] [CrossRef]
- Huang, W.; Yang, S.; Shao, J.; Li, Y.P. Signaling and transcriptional regulation in osteoblast commitment and differentiation. Front. Biosci. 2007, 1, 3068–3092. [Google Scholar] [CrossRef]
- Stapleton, C.J.; Pham, M.H.; Attenello, F.J.; Hsieh, P.C. Ossification of the posterior longitudinal ligament: Genetics and pathophysiology. Neurosurg. Focus. 2011, 30, E6. [Google Scholar] [CrossRef]
- Karasugi, T.; Nakajima, M.; Ikari, K.; Tsuji, T.; Matsumoto, M.; Chiba, K.; Uchida, K.; Kawaguchi, Y.; Mizuta, H.; Ogata, N.; et al. A genome-wide sib-pair linkage analysis of ossification of the posterior longitudinal ligament of the spine. J. Bone Miner. Metab. 2013, 31, 136–143. [Google Scholar] [CrossRef]
- Jekarl, D.W.; Paek, C.; An, Y.J.; Kim, Y.J.; Kim, M.; Kim, Y.; Lee, J.; Sung, C.H. TGFBR2 gene polymorphism is associated with ossification of the posterior longitudinal ligament. J. Clin. Neurosci. 2013, 20, 453–456. [Google Scholar] [CrossRef]
- Nakajima, M.; Takahashi, A.; Tsuji, T.; Karasugi, T.; Baba, H.; Uchida, K.; Kawabata, S.; Okawa, A.; Shindo, S.; Takeuchi, K.; et al. A genome-wide association study identifies susceptibility loci for ossification of the posterior longitudinal ligament of the spine. Nat. Genet. 2014, 46, 1012–1016. [Google Scholar] [CrossRef]
- Harada, Y.; Furukawa, K.; Asari, T.; Chin, S.; Ono, A.; Tanaka, T.; Mizukami, H.; Murakami, M.; Yagihashi, S.; Motomura, S.; et al. Osteogenic lineage commitment of mesenchymal stem cells from patients with ossification of the posterior longitudinal ligament. Biochem. Biophys. Res. Commun. 2014, 443, 1014–1020. [Google Scholar] [CrossRef]
- Kudo, H.; Furukawa, K.-I.; Yokoyama, T.; Ono, A.; Numasawa, T.; Wada, K.; Tanaka, S.; Asari, T.; Ueyama, K.; Motomura, S.; et al. Genetic Differences in the Osteogenic Differentiation Potency According to the Classification of Ossification of the Posterior Longitudinal Ligament of the Cervical Spine. Spine 2011, 36, 951–957. [Google Scholar] [CrossRef]
- Atashi, F.; Modarressi, A.; Pepper, M. The role of reactive oxygen species in mesenchymal stem cell adipogenic and osteogenic differentiation: A review. Stem Cells Dev. 2015, 24, 1150–1163. [Google Scholar] [CrossRef]
- Imhoff, B.R.; Hansen, H.M. Differentiation redox potential profiles during adipogenesis and osteogenesis. Cell Mol. Biol. Lett. 2011, 16, 149–161. [Google Scholar] [CrossRef]
- Lee, K.J.; Clegg, P.D.; Comerford, E.J.; Canty-Laird, E.G. Ligament-derived stem cells: Identification, characterization and therapeutic application. Stem Cells Int. 2017, 2017, 1919845. [Google Scholar] [CrossRef]
- Kristjansson, B.; Limthongkul, W.; Yingsakmongkol, W.; Thantiworasit, P.; Jirathanathornnukul, N.; Honsawek, S. Isolation and characterization of human mesenchymal stem cells from facet joints and interspinous ligaments. Spine 2016, 41, E1–E7. [Google Scholar] [CrossRef]
- Bianco, P.; Cao, X.; Frenette, P.S.; Mao, J.J.; Robey, P.G.; Simmons, P.J.; Wang, C. The meaning, the sense and the significance: Translating the science of mesenchymal stem cells into medicine. Nat. Med. 2013, 19, 35–42. [Google Scholar] [CrossRef]
- Sacchetti, B.; Funari, A.; Remoli, C.; Giannicola, G.; Kogler, G.; Liedtke, S.; Cossu, G.; Serafini, M.; Sampaolesi, M.; Tagliafico, E.; et al. No Identical “Mesenchymal Stem Cells” at Different Times and Sites: Human Committed Progenitors of Distinct Origin and Differentiation Potential Are Incorporated as Adventitial Cells in Microvessels. Stem Cell Rep. 2016, 6, 897–913. [Google Scholar] [CrossRef]
- Ray, P.D.; Huang, B.W.; Tsuji, Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal 2012, 24, 981–990. [Google Scholar] [CrossRef]
- Murphy, M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009, 417, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Korshunov, S.S.; Skulachev, V.P.; Starkov, A.A. High protonic potential actuates a mechanism of production of reactive oxygen species in mitochondria. FEBS Lett. 1997, 416, 15–18. [Google Scholar] [CrossRef] [PubMed]
- Zorov, D.B.; Juhaaszova, M.; Sollott, S.J. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol. Rev. 2014, 94, 909–950. [Google Scholar] [CrossRef] [PubMed]
- Dunn, J.D.; Alvarez, L.A.; Zhang, X.; Soldati, T. Reactive oxygen species and mitochondria: A nexus of cellular homeostasis. Redox. Biol. 2015, 6, 472–485. [Google Scholar] [CrossRef]
- Schulz, E.; Wenzel, P.; Munzel, T.; Daiber, A. Mitochondrial redox signaling: Interaction of mitochondrial reactive oxygen species with other source of oxidative stress. Antioxid. Redox Signal 2014, 20, 308–324. [Google Scholar] [CrossRef]
- Kane, M.S.; Paris, A.; Codron, P.; Cassereau, J.; Procaccio, V.; Lenaers, G.; Reynier, P.; Chevrollier, A. Current mechanistic insights into the CCCP-induced cell survival response. Biochem. Pharmacol. 2018, 148, 100–110. [Google Scholar] [CrossRef]
- Kwon, K.; Viollet, B.; Yoo, O. CCCP induces autophagy in an AMPK-independent manner. Biochem. Biophys. Res. Commun. 2011, 416, 343–348. [Google Scholar] [CrossRef]
- Bresgen, N.; Eckl, P. Oxidative stress and the homeodynamics of iron metabolism. Biomolecules 2015, 5, 808–847. [Google Scholar] [CrossRef]
- Dikic, I.; Elazar, Z. Mechanism and medical implications of mammalian autophagy. Nat. Rev. Mol. Cell Biol. 2018, 19, 349–364. [Google Scholar] [CrossRef]
- Wang, Y.; Nartiss, Y.; Steipe, B.; McQuibban, A.; Kim, P.K. ROS-induced mitochondrial depolarization initiates RARK2/PARKIN-dependent mitochondrial degradation by autophagy. Autphagy 2012, 8, 1462–1476. [Google Scholar] [CrossRef]
- Levine, B.; Kroemer, G. Biological functions of autophagy genes: A disease perspective. Cell 2019, 176, 11–42. [Google Scholar] [CrossRef] [PubMed]
- Klionsky, D.J.; Abdel-Aziz, A.K.; Abdelfatah, S.; Abdellatif, M.; Abdoli, A.; Abel, S.; Abeliovich, H.; Abildgaard, M.H.; Abudu, Y.P.; Acevedo-Arozena, A.; et al. Guidelines for the use and interpretation of assays for monitoring autophagy (4th edition). Autophagy 2021, 17, 1–382. [Google Scholar] [CrossRef] [PubMed]
- Filomeni, G.; De Zio, D.; Cecconi, F. Oxidative stress and autophagy: The clash between damage and metabolic needs. Cell Death Differ. 2015, 22, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Frank, M.; Duvezin-Caubet, S.; Koob, S.; Occhipinti, A.; Jagasia, R.; Petcherski, A.; Ruonala, M.O.; Priault, M.; Salin, B.; Reichert, A.S. Mitophagy is triggered by mild oxidative stress in a mitochondrial fission dependent manner. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2012, 1823, 2297–2310. [Google Scholar] [CrossRef]
- Youle, R.J.; Narendra, D.P. Mechanism of mitophagy. Nat. Rev. Mol. Cell Biol. 2011, 12, 9–14. [Google Scholar] [CrossRef] [PubMed]
- White, E. Autophagy and p53. Cold Spring Harb. Perspect. Med. 2015, 6, a026120. [Google Scholar] [CrossRef]
- Bou-Abdallah, F. The iron redox and hydrolysis chemistry of the ferritins. Biochim. Biophys. Acta 2018, 1800, 719–731. [Google Scholar] [CrossRef]
- Zhao, G.; Arosio, P.; Chasteen, N.D. Iron(II) and hydrogen peroxide detoxification by human H-chain ferritin. An EPR spin-trapping study. Biochemistry 2006, 45, 3429–3436. [Google Scholar] [CrossRef]
- Tori, F.M.; Tori, S.V. Regulation of ferritin genes and protein. Blood 2012, 99, 3505–3516. [Google Scholar] [CrossRef]
- Theil, E.C. Ferritin: The protein nanocage and iron biomineral in health and in disease. Inorg. Chem. 2013, 52, 12223–12233. [Google Scholar] [CrossRef]
- Zarjou, A.; Jeney, V.; Arosio, P.; Poli, M.; Zavaczki, E.; Balla, G.; Balla, J. Ferritin ferroxidase activity: A potent inhibitor of osteogenesis. J. Bone Miner. Res. 2010, 25, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Zarjou, A.; Jeney, V.; Arosio, P.; Poli, M.; Antal-Szalmás, P.; Agarwal, A.; Balla, G.; Balla, J. Ferritin prevents calcification and osteoblastic differentiation of vascular smooth muscle cells. J. Am. Soc. Nephrol. 2009, 20, 1254–1263. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Gerstein, S.M. RNA-Seq: A revolutionary tool for transcriptomics. Nat. Rev. Genet. 2009, 10, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Stark, R.; Grzelak, M.; Hadfield, J. RNA sequencing: The teenage years. Nat. Rev. Genet. 2019, 20, 631–656. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Seo, J.-S.; Rhie, A.; Kim, J.; Lee, S.; Sohn, M.-H.; Kim, C.-U.; Hastie, A.; Cao, H.; Yun, J.-Y.; Kim, J.; et al. De novo assembly and phasing of a Korean human genome. Nature 2016, 538, 243–247. [Google Scholar] [CrossRef]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Systemic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef]
- Luo, W.; Friedman, M.S.; Shedden, K.; Hankenson, K.D.; Woolf, P.J. GAGE: Generally applicable gene set enrichment for pathway analysis. BMC Bioinform. 2009, 10, 161. [Google Scholar] [CrossRef]
- Mandal, S.; Lindgren, A.G.; Srivastava, A.S.; Clark, A.T.; Banderjee, U. Mitochondrial function controls proliferation and early differentiation potential of embryonic stem cells. Stem Cells 2010, 29, 486–495. [Google Scholar] [CrossRef] [PubMed]
- Sivandzade, F.; Bhalerao, A.; Cucullo, L. Analysis of the mitochondrial membrane potential using the cationic JC-1 dyes as a sensitive fluorescent probe. Bio Protoc. 2019, 9, e3128. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, L.L.; Davanzo, G.G.; de Aguiar, C.F.; Moraes-Vieria, P.M.M. Using flow cytometry for mitochondrial assays. MethodsX 2020, 7, 100938. [Google Scholar] [CrossRef] [PubMed]
- Perry, S.W.; Norman, J.P.; Barbieri, J.; Brown, E.B.; Gelbard, H.A. Mitochondrial membrane potential probes and the proton gradient: A practical usage guide. Biotechniques 2011, 50, 98–115. [Google Scholar] [CrossRef]
- Dolman, N.J.; Chambers, K.M.; Mandavilli, B.; Batchelor, R.H.; Janes, M.J. Tools and techniques to measure mitophagy using fluorescence microscopy. Autophagy 2013, 9, 1653–16613. [Google Scholar] [CrossRef] [PubMed]
- Kohen, R.; Nyska, A. Oxidation of biological system: Oxidative stress phenomena, antioxidants, redox reactions, and methods for their quantification. Tox. Pathol. 2002, 30, 620–650. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef]
- Rader, B.A. Alkaline phosphatase, an unconventional immune protein. Front. Immunol. 2017, 8, 897. [Google Scholar] [CrossRef]
- Sacchetti, B.; Augello, A.; Bari, D.C. The regulation of differentiation in mesenchymal stem cells. Hum. Gene Ther. 2010, 21, 1226–1238. [Google Scholar]
- Pittenger, M.F.; Discher, D.E.; Peault, B.M.; Phinney, D.G.; Hare, J.M.; Caplan, A.I. Mesencymal stem cell perspective: Cell biology to clinical progress. Regen. Med. 2019, 4, 22. [Google Scholar]
- Anthony, B.A.; Link, D.C. Regulation of hematopoietic stem cells by bone marrow stromal cells. Trends Immunol. 2014, 35, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Kim, C.; Choi, Y.S.; Kim, M.; Park, C.; Suh, Y. Age-related alterations in mesenchymal stem cells related to shift in differentiation from osteogenic to adipogenic potential: Implication to age-associated bone diseases and defects. Mech. Ageing Dev. 2012, 133, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Lin, Z.; Chen, J.; Ding, S.; Mao, W.; Shi, S.; Liang, B. Autophagy in spinal ligament fibroblasts: Evidence and possible implications for ossification of the posterior longitudinal ligament. J. Orthop. Surg. Res. 2020, 15, 490. [Google Scholar] [CrossRef] [PubMed]
- Palmer, C.S.; Osellame, L.D.; Stojanovski, D.; Ryan, M.T. The regulation of mitochondrial morphology: Intricate mechanisms and dynamic machinery. Cell. Signal. 2011, 23, 1534–1545. [Google Scholar] [CrossRef] [PubMed]
- Chistiakov, D.A.; Sobenin, I.A.; Revin, V.V.; Orekhove, A.N.; Bobryshev, Y.V. New insights into vascular aging: Emerging role of mitochondria function. Biomed. Res. Int. 2014, 2014, 238463. [Google Scholar] [PubMed]
- Bryniarska, N.; Kubiak, A.; Labedz-Maslowska, A.; Zuba-Surma, E. Impact of developmental origin, niche mechanics and oxygen availability on osteogenic differentiation capacity of mesenchymal stem/stromal cells. Acta Biochim. Pol. 2019, 66, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Iatridis, J.C.; Nicoll, S.B.; Michalek, A.J.; Walter, B.A.; Gupta, M.S. Role of biomechanics in intervertebral disc degeneration and regenerative therapies: What needs repairing in the disc and what are promising biomaterials for its repair? Spine J. 2013, 13, 243–262. [Google Scholar] [CrossRef]
- Inoue, Z.; Orias, A.A.E. Biomechanics of intervertebral disk degeneration. Orthop. Clin. N. Am. 2011, 42, 487–499. [Google Scholar] [CrossRef]
- Lee, S.; Park, B.-J.; Kim, J.Y.; Jekarl, D.; Choi, H.Y.; Lee, S.Y.; Kim, M.; Kim, Y.; Park, M.-S. The effect of fibroblast growth factor on distinct differentiation potential of cord blood–derived unrestricted somatic stem cells and Wharton’s jelly–derived mesenchymal stem/stromal cells. Cytotherapy 2015, 17, 1723–1731. [Google Scholar] [CrossRef]
- Kwon, A.; Kim, Y.; Kim, M.; Kim, J.; Choi, H.; Jekarl, D.W.; Lee, S.; Kim, J.M.; Shin, J.-C.; Park, I.Y. Tissue-specific Differentiation Potency of Mesenchymal Stromal Cells from Perinatal Tissues. Sci. Rep. 2016, 6, 23544. [Google Scholar] [CrossRef]
- Gregory, C.A.; Gunn, W.G.; Peister, A.; Prockop, D. An Alizarin red-based assay of mineralization by adherent cells in culture: Comparison with cetylpyridinium choride extraction. Anal. Biochem. 2004, 329, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Scott, M.A.; Nguyen, V.T.; Levi, B.; James, A.W. Current methods of adipogenic differentiation of mesenchymal stem cells. Stem Cells Dev. 2011, 20, 1793–1804. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, P.; Rajesh, M.; Haskó, G.; Hawkins, B.J.; Madesh, M.; Pacher, P. Simultaneous detection of apoptosis and mitochondrial superoxide production in live cells by flow cytometry and confocal microscopy. Nat. Protoc. 2007, 2, 2295–2301. [Google Scholar] [CrossRef] [PubMed]
- Gassmann, M.; Grenacher, B.; Rohde, B.; Vogel, J. Quantifying western blots: Pitfalls of densitometry. Electrophoresis 2009, 30, 1845–1855. [Google Scholar] [CrossRef]
- Butler, T.A.; Paul, J.W.; Chan, E.; Smith, R.; Tolosa, J.M. Misleading westerns: Common quantification mistakes in Western blot densitometry and proposed corrective measures. Biomed. Res. Int. 2019, 2019, 5214821. [Google Scholar] [CrossRef]

| No | Age | Sex | Involved Spine | Treatment |
|---|---|---|---|---|
| Ctrl1 | 39 | Female | C6,7 | Total disc replacement |
| Ctrl3 | 36 | Male | C5,6 | Total disc replacement |
| Ctrl4 | 32 | Female | C5,6 | Total disc replacement |
| Ctrl5 | 46 | Female | C4,5, C5,6 | Anterior cervical interbody fusion |
| Ctrl6 | 62 | Female | C5,6 | Anterior cervical interbody fusion |
| OPLL1 | 65 | Female | C4,5,6,7 | Laminoplasty |
| OPLL2 | 61 | Male | C4,5,6,7 | Laminoplasty |
| OPLL3 | 66 | Female | C2,3,4,5,6,7 | Laminoplasty, Partial hemilaminectomy |
| OPLL4 | 61 | Male | C4,5,6 | Laminoplasty |
| OPLL5 | 52 | Female | C2,3,4,5,6,7 | Laminoplasty, Partial hemilaminectomy |
| OPLL6 | 67 | Male | C2,3,4,5,6,7 | Laminoplasty |
| Primer | Sequence | Amplicon (bp) | |
|---|---|---|---|
| Differentiation | |||
| GAPDH | F | 5′-GGA GTC CAC TGG CGT CTT CA-3′ | 123 |
| R | 5′-TGG TTC ACA CCC ATG ACG AA-3′ | ||
| BMP2 | F | 5′-TTCGGCCTGAAACAGAGAC-3′ | 199 |
| R | 5′-TCCCACTCGTTTCTGGTAGT-3′ | ||
| RUNX2 | F | 5′-AGATGACGTCCCCGTCCATC-3′ | 215 |
| R | 5′-TGAAATGCTTGGGAACTGCC-3′ | ||
| Osteocalcin | F | 5′-CCTCACAC CCTCGCCCATT-3′ | 117 |
| R | 5′-CCCTCCTGCTTGGACACAAA-3′ | ||
| ALP | F | 5′-CCCACCTACAGCATGTCCTA-3′ | 195 |
| R | 5′-AATTCTGCCTCCTTCCACCA-3′ | ||
| PPARG | F | 5′-TGACCCAGAAAGCGATTCCT-3′ | 100 |
| R | 5′-AAA GTT GGT GGG CCA GAA TG-3′ | ||
| SOX9 | F | 5′-GTGCTCAAAGGCTACGACTG-3′ | 200 |
| R | 5′-AGAAGTCTCCAGAGCTTGCC-3′ | ||
| COL2A1 | F | 5′-ACATGCCGTGACTTGAGACTCA-3′ | 152 |
| R | 5′-GGGATGTTTTCAGGTTGGGC-3′ | ||
| MSX1 | F | 5′-CTCTCAAGCTGCCAGAAGAT-3′ | 150 |
| R | 5′-GTTCGTCTTGTGTTTGCGGA-3′ | ||
| FTH | F | 5′-GCTGAATGCAATGGAGTGTG-3′ | 335 |
| R | 5′-CAGGGTGTGCTTGTCAAAGA-3′ | ||
| FTL | F | 5′-AGAAGATGGGTGACCACCTG-3′ | 85 |
| R | 5′-TGGTCCAAGGCTTGTTAGGA-3′ | ||
| Stemness | |||
| GAPDH | F | 5′-ACAACTTTGGTATCGTGGAA-3′ | 456 |
| R | 5-AAATTCGTTGTCATACCAGG-3′ | ||
| OCT4 | F | 5-CGTGAAGCTGGAGAAGGAGAAGCTG-3′ | 245 |
| R | 5′-CAAGGGCCGCAGCTCACACATGTT-3′ | ||
| SOX2 | F | 5′-GCCGAGTGGAAACTTTTGTC-3′ | 264 |
| R | 5′-GTTCATGTGCGCGTAACTGT-3′ | ||
| NANOG | F | 5′-AGAAGGCCTCAGCACCTAC-3′ | 250 |
| R | 5′-GGCCTGATTGTTCCAGGATT-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.T.; Kim, Y.; Kim, J.Y.; Lee, S.; Kim, M.; Jekarl, D.W. Regulatory Mechanism between Ferritin and Mitochondrial Reactive Oxygen Species in Spinal Ligament-Derived Cells from Ossification of Posterior Longitudinal Ligament Patient. Int. J. Mol. Sci. 2023, 24, 2872. https://doi.org/10.3390/ijms24032872
Kim JT, Kim Y, Kim JY, Lee S, Kim M, Jekarl DW. Regulatory Mechanism between Ferritin and Mitochondrial Reactive Oxygen Species in Spinal Ligament-Derived Cells from Ossification of Posterior Longitudinal Ligament Patient. International Journal of Molecular Sciences. 2023; 24(3):2872. https://doi.org/10.3390/ijms24032872
Chicago/Turabian StyleKim, Jong Tae, Yonggoo Kim, Ji Yeon Kim, Seungok Lee, Myungshin Kim, and Dong Wook Jekarl. 2023. "Regulatory Mechanism between Ferritin and Mitochondrial Reactive Oxygen Species in Spinal Ligament-Derived Cells from Ossification of Posterior Longitudinal Ligament Patient" International Journal of Molecular Sciences 24, no. 3: 2872. https://doi.org/10.3390/ijms24032872
APA StyleKim, J. T., Kim, Y., Kim, J. Y., Lee, S., Kim, M., & Jekarl, D. W. (2023). Regulatory Mechanism between Ferritin and Mitochondrial Reactive Oxygen Species in Spinal Ligament-Derived Cells from Ossification of Posterior Longitudinal Ligament Patient. International Journal of Molecular Sciences, 24(3), 2872. https://doi.org/10.3390/ijms24032872






