Abstract
The serine/threonine kinase Akt modulates the functions of numerous substrates, many of them being involved in cell proliferation and growth, metabolism, angiogenesis, resistance to hypoxia and migration. Akt is frequently deregulated in many types of human cancers, its overexpression or abnormal activation being associated with the increased proliferation and survival of cancer cells. A promising avenue for turning off the functionality of Akt is to either interfere with the K63-linked ubiquitination that is necessary for Akt membrane recruitment and activation or increase the K48-linked polyubiquitination that aims to target Akt to the proteasome for its degradation. Recent evidence indicates that targeting the ubiquitin proteasome system is effective for certain cancer treatments. In this review, the functions and roles of Akt in human cancer will be discussed, with a main focus on molecules and compounds that target various elements of the ubiquitination processes that regulate the activation and inactivation of Akt. Moreover, their possible and attractive implications for cancer therapy will be discussed.
1. The Akt Signaling Pathway in Cancer
The PI3K/Akt/mTOR pathway has been shown to be aberrantly activated in many human cancers, including breast, lung, ovarian, pancreatic and gastric carcinomas [1]. In all these cases, Akt is responsible for driving cell growth and tumor progression [2,3,4,5,6]. Indeed, phosphorylated Akt (pAkt) translocates to a plethora of intracellular locations, where it modulates the function of numerous substrates, many of them being involved in cell proliferation and growth, metabolism, angiogenesis, resistance to hypoxia and migration [7,8] (Figure 1). All three Akt isoforms (Akt1, Akt2 and Akt3) were found to be overexpressed in human cancers [9,10,11,12,13,14,15,16,17,18,19], where, besides promoting tumor progression, they are also involved in conferring acquired resistance to many conventional chemotherapeutic agents [20,21,22], mainly for their contribution to the Epithelial-Mesenchymal Transition (EMT) occurring in drug-resistant and metastatic cancer cells [23,24,25]. In this regard, it is worth noting that either the overexpression or amplification of Akt1 and Akt2 is associated with ovarian cancer cells’ acquired resistance to paclitaxel [26]. The Cancer Genome Atlas (TCGA) pan-cancer datasets have profiled a comprehensive catalog of PI3K/Akt/mTOR-associated variants across 10,000 tumors and represent a valuable resource for both understanding the PI3K/Akt/mTOR pathway deregulation in cancers and translating this information in clinical utility for personalized treatments [27,28,29]. Although the Akt pathway is unquestionably believed to be a gold target for cancer therapy, to date, no drugs targeting Akt have been approved for any cancer treatments. For this reason, the discovery of a K63-linked ubiquitination of Akt, specifically involved in its activation, has focused the attention on the appealing opportunity of studying this ubiquitin-mediated regulation as a druggable target in future molecular investigations.
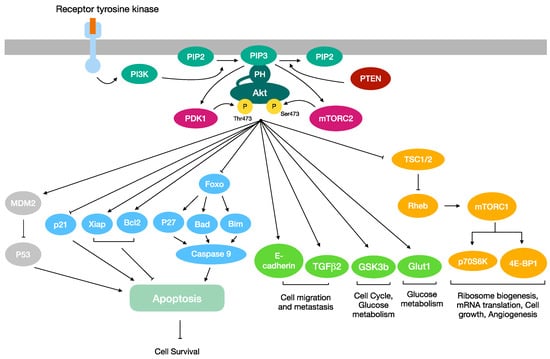
Figure 1.
Overview of the PI3K/AKT/mTOR signaling pathway. Once phosphorylated, Akt translocates to a plethora of intracellular locations, where it modulates the function of numerous substrates. Akt may promote cell survival by regulating proteins involved in the activation of the apoptosis cascade. Akt activation stimulates the cell migration, cell cycle and glucose metabolism. Furthermore, it is a key regulator of angiogenesis, ribosome biogenesis, mRNA translation and cell growth.
1.1. Akt: An Overview
The serine/threonine kinase Akt, alias Protein Kinase B (PKB), is activated by differ-ent kinds of stimuli, including cytokines, hormones, stresses and growth factors like Vascular Endothelial Growth Factor (VEGF), Fibroblast Growth Factor (FGF), Nerve Growth Factor (NGF), Epidermal Growth Factor (EGF), Platelet-Derived Growth Factor (PDGF) and Insulin-like Growth Factor (IGF) [30]. All the Akt isoforms are composed of three domains: an N-terminal Pleckstrin Homology (PH) domain, a central kinase domain separated by a hinge region and a C-terminal regulatory domain. All three Akt kinases show a highly conserved primary structure, sharing 90–95% of the homology in the kinase region and 60% of the homology in their PH domain [31,32]. The main Akt isoform is encoded by the Akt1 gene; it was shown that each Akt isoform is differentially expressed at both the mRNA and protein levels, and each one exerts different roles not only in a physiological context but also in cancer pathogenesis [33]. Studies of Akt isoforms performed in knockout mice have documented that the different Akt isoforms may exert tissue-specific and not completely overlapping functions; indeed, Akt1 knockout mice turned out to be smaller than their wild-type counterparts, indicating that Akt1 has a critical role in the tight regulation of cell survival and cell growth [34]; Akt2 knockout mice turned out to be prone to developing a type 2 diabetes-like phenotype, suggesting a main role for Akt2 in the maintenance of glucose homeostasis [35]; Akt3 knockout mice, instead, displayed an impaired brain development, thus demonstrating a major role for Akt3 in neurogenesis [36]. Furthermore, several studies addressed differential roles for Akt1 and Akt2 in the regulation of cell cycle progression and cell migration [37,38].
Akt kinase activity is finely tuned by post-translational modifications such as phosphorylation, ubiquitination, acetylation, palmitoylation, hydroxylation and methylation [39,40,41], which may also be relevant for the Akt hyperactivation observed in cancers [42]. Akt activation requires its translocation to the cell membrane, which is mediated by the binding of the PH domain to the phosphatidylinositol 3,4,5-trisphosphate (PIP3) [43]. PIP3 is a phospholipid generated by Phosphatidylinositol 3 Kinase (PI3K), which is able to act as a second messenger for the recruitment to the membrane of a subset of signaling proteins with PH domains, including not only Akt but also the 3-Phosphoinositide-Dependent Protein Kinase 1 (PDK1) [44]. In the OFF state, the PH induces the inhibition of Akt activation, by interacting with the kinase domain [45]. Instead, when the PH domain is engaged by PIP3, it triggers the membrane translocation of Akt and its subsequent phosphorylation by PDK1 and mTOR complex 2 (mTORC2) [46]. Indeed, the binding of PIP3 to the PH domain of Akt causes a conformational change which makes Akt prone for the PDK1-mediated phosphorylation of T308. Even if the phosphorylation mediated by PDK1 is pivotal, it is still not sufficient for Akt activation; indeed, a second phosphorylation at S473 is required, mediated by the kinase mammalian Target Of Rapamycin (mTOR), which acts as a part of two complexes called mTORC1 and mTORC2, the latter one being the responsible kinase for S473 phosphorylation [47]. Besides directly phosphorylating Akt, mTORC2 phosphorylates and activates the IGF receptor, thus indirectly promoting Akt activation [48,49]. mTORC1, instead, up-regulates protein synthesis by phosphorylating the Eukaryotic translation Initiation Factor 4E Binding Protein 1 (EIF4EBP1) and the Ribosomal S6 Kinase family members RPS6KB1 and RPS6KB2, thereby leading to an increased protein synthesis [50]. The above-described PI3K/Akt/mTOR signaling pathway is regulated by the Phosphatase and Tensin homolog (PTEN) protein, a phosphatase that is able to suppress the PI3K/Akt signaling pathway by de-phosphorylating PIP3 (Figure 1) [51,52,53]. Last but not least, Akt activation may also be promoted by a K63-linked ubiquitination. As will be deeply explained in the next chapter, this specific non-degrading ubiquitination acts as a critical step for Akt membrane recruitment and phosphorylation [54,55,56,57].
The Akt kinase can directly phosphorylate a plethora of proteins (to date, more than one hundred Akt substrates have been identified [58], most of them being involved in cell survival, proliferation, migration and metabolism [7,8,9]). The Akt pathway transmits signals from upstream regulatory proteins, such as PTEN and PI3K, to many downstream effectors, such as Glycogen Synthase Kinase 3 (GSK3), Forkhead transcription factor (FOXO) and Murine Double Minute 2 (MDM2) [59,60,61]. Each Akt isoform has been demonstrated to play different and specific roles in cancer cells signaling; indeed, Akt1 and Akt2 displayed opposite roles in cell cycle progression, migration and invasion among different types of human cancers [62,63].
1.2. Akt in Proliferation and Cell Survival
Akt may promote cell proliferation and survival by regulating many different targets. First of all, Akt exerts its major oncogenic role by modulating the PI3K/Akt signaling pathway, which is responsible for promoting cell proliferation and survival and preventing apoptosis [64].
When activated by growth factors, IGF and EGF receptors dimerize and undergo autophosphorylation, thus activating a cascade of phosphorylations that leads to PI3K activation and PIP2 conversion to PIP3. PIP3 recruits to the plasma membrane both Akt and PDK1, which phosphorylate Akt. The activated Akt, in turn, induces the release of the inhibitory Tuberous Sclerosis Complex (TSC, composed of TSC1 and 2) from the Ras-related small G protein Rheb-GTPase (Rheb), a step required for the activation of both Rheb and mTORC1 [65]. mTORC1 acts as a regulator of cell survival and growth by stimulating translation initiation and ribosome biogenesis, by activating the transcription of genes involved in cell survival [66] and by catalyzing the phosphorylation of multiple targets, such as ribosomal protein S6 Kinase (p70S6K) and eukaryotic translation initiation factor 4E binding Protein 1 (4E-BP1) (Figure 1) [49].
Akt oncogenic activation confers resistance to apoptosis [67] due to its crosstalk with the tumor suppressor p53. Indeed, the Akt-mediated phosphorylation of MDM2 results in MDM2 import in the nucleus, where it promotes the ubiquitination and consequent proteasomal degradation of p53 [68,69]. Furthermore, the PI3K/Akt pathway activation in tumors may be accompanied by the tumor suppressor PTEN inactivating mutations, thus boosting survival advantages and the uncontrolled proliferation of cancer cells, lacking the PTEN-mediated cell cycle arrest in the G1 phase [70].
Akt may also act as an inhibitor of cell cycle arrest by phosphorylating the tumor suppressor p21; briefly, Akt-mediated phosphorylation induces the cytoplasmic localization of p21 and its sequestration mediated by the bind with 14-3-3 proteins [71,72]. The activation of the Akt pathway may promote the expression of anti-apoptotic proteins such as B-cell Lymphoma 2 (BCL2) [73]. In addiction, Akt has also been found to phosphorylate and inhibit the BCL2-Associated agonist of cell Death protein (BAD) [74], p27 and Bim, the latter two through the phosphorylation and nuclear export of FOXO, which is essential for their transcription, promoting cell proliferation and survival overall [75,76]. Furthermore, Akt promotes cell survival and suppresses apoptotic death by inducing the X-linked inhibitor of apoptosis protein (XIAP) [77]. In addition, Akt directly phosphorylates human procaspase-9, thus leading to a decrease in its protease activity (Figure 1) [78,79].
1.3. Akt in Cell Migration and Metastasis
Akt, despite promoting tumor growth, also promotes cell migration and metastasis. Metastasis is a multistep process starting from the loss of cell adhesion, the invasion into local vessels and tissues and, lastly, the colonization of distant sites. Akt plays a key role in both metastasis and invasion, its expression being higher in distant metastasis than in the primary tumor [80].
When activated by Vascular Endothelial Growth Factor (VEGF), Akt plays a key role in the angiogenesis required for tumor growth, promoting endothelial cell survival, growth and proliferation. VEGF may enhance the Akt signaling pathway to regulate the expression of not only VEGF itself and its receptor [81] but also Hypoxia Inducible Factor 1α (HIF-1α), heme oxygenase 1, inducible Nitric Oxide Synthase (iNOS) and several glycolytic enzymes required for angiogenesis induction [82]. In tumors, the sustained activity of Akt stimulates both the endothelial cells migration and the formation of structurally abnormal blood vessels; moreover, it promotes tumor angiogenesis by activating angiopoietins and endothelial nitric oxide [83,84]. Furthermore, Akt up-regulates Transforming Growth Factor-β2 (TGFβ2) expression, thus promoting cancer metastasis [85].
The PI3K/Akt/mTOR signaling pathway plays a critical role in EMT, a hallmark of which is the downregulation of E-cadherin [86]. EMT is a biological process that plays a key role in tumor cell invasion, metastasis and chemoresistance. It can be induced either directly or indirectly by PI3K/Akt/mTOR proteins in cooperation with other signaling pathways, such as TGFβ, NF-kB, Ras and Wnt/β-catenin [23].
The remodeling of the extracellular matrix (ECM) is required for cell migration and invasion. In this process, invadopodia, which are actin-rich structures associated with the plasma membrane, exert a relevant role [87]. Both invadopodia formation and activity are triggered by the PI3K/Akt signaling. In this context, the balance between the formation of PIP3 and PI34P2 PI3Ks has been shown to modulate the metastatic potential of many cancer cell lines [88]. Indeed, in MDA-MB-231 breast cancer cells, the knockdown of the catalytic subunit of PI3K p110α and the knockdown of Akt are able to attenuate invadopodia formation [89].
1.4. Akt in Cancer Metabolism Regulation
The PI3K/Akt signaling network has a complex and critical role in cancer metabolism [90,91]. While the metabolic functions of Akt support its physiological functions in cell survival, growth and proliferation, de-regulated Akt function instead supports the abnormal proliferation and survival of cancer cells by deregulating the control of the metabolism.
PI3K/Akt signaling may affect cell metabolism both directly and indirectly through the phosphorylation-mediated regulation of metabolic enzymes or regulators of nutrient transport or through the activation of key downstream effectors involved in cellular metabolic reprogramming, such as the mTORC1, GSK3 and FOXO family members [91,92].
An altered glucose metabolism is a common metabolic hallmark distinguishing cancer cells. In response to growth factors, PI3K/Akt signaling controls nutrient uptake and metabolism, exerting its most relevant role in regulating glucose metabolism by trafficking the cellular uptake of glucose and by inducing gene expression. Akt may alter the expression of Glucose Transporter 1 (GLUT1) by increasing the translation of its mRNA through mTORC1 and 4E-BP1 [93].
GSK3 acts as a negative regulator of glucose metabolism since it is the kinase responsible for inhibiting glycogen synthase by phosphorylating this enzyme at Ser652 [94]. Insulin stimulation reverses the GSK3-mediated phosphorylation, thus promoting glycogen synthesis [95]. Indeed, in response to insulin, Akt, which inhibits both GSK3α and GSK3β [96], may stimulate glycogen synthesis [97].
2. Two Distinct Ubiquitination Processes Regulate the Activation and Inactivation of Akt Kinase
Ubiquitin is an 8.6 kDa regulatory protein originally suggested to mark tagged proteins to the degradation via 26S proteasome in a process defined as ubiquitination. Over the years, the role of the ubiquitination has been enlarged to multiple functions, including the trafficking, stabilization and activation of the target protein.
The covalent addition of ubiquitin to the substrate protein is mediated by the concerted action of three enzyme families, called E1, E2 and E3. First, a ubiquitin-activating enzyme (E1) activates the ubiquitin through an ATP-dependent reaction. The activated ubiquitin is hence transferred to a ubiquitin-conjugating enzyme (E2). Finally, a ubiquitin-protein ligase E3 mediates the transfer of the ubiquitin from E2 to a specific lysine side chain of a target protein [98,99]. In the human genome, there are over 500 genes codifying for a specific E3 ligase [100]. The E3 ubiquitin ligase involved in the ubiquitination cascade is crucial to defining the substrate specificity and the spatiotemporal nature of the pathway.
Since the amino acid chain of ubiquitin contains seven lysine residues (K6, K11, K27, K29, K33, K48 and K63), in the poly-ubiquitination process, the ubiquitin molecules can be linked to each other through each of these seven different sites. The diverse ways to assemble ubiquitin chains create a ubiquitin code that is not completely deciphered yet [101]. K48- and K63-linked polyubiquitination represent the two most common mechanisms of ubiquitin linkage to protein substrates. The former is usually related to the proteolysis of the target protein via the ubiquitin-proteasome system (UPS) [101]. Conversely, the latter is usually involved in modulating the activity, interaction and intracellular trafficking of substrate proteins, participating in multiple biological processes, including cell growth and proliferation, apoptosis, DNA damage response, inflammation, neurodegenerative diseases and cancer [102].
Both of these distinct polyubiquitination mechanisms have been reported for Akt. K48-linked ubiquitination promotes the proteasomal degradation of Akt, thus turning off its activity and downstream responses. On the other hand, regarding the K63-linked polyubiquitination of Akt, multiple studies define its importance in the activation of Akt. Indeed, K63-linked ubiquitination serves as a regulatory signal that induces the plasma membrane recruitment, serine/threonine phosphorylation and activation of Akt (Figure 2).
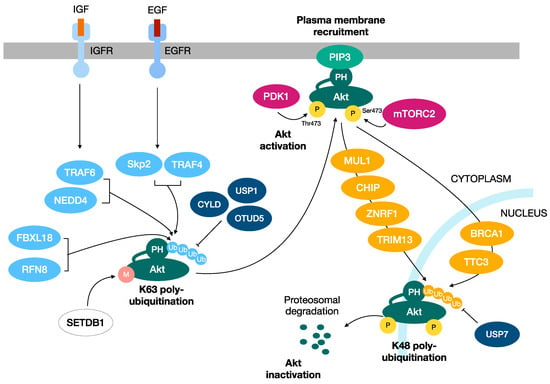
Figure 2.
Two distinct ubiquitination processes regulate the activation and inactivation of Akt kinase. K48-linked ubiquitination promotes the proteasomal degradation of Akt, turning off its activity and downstream responses. Instead, the K63-linked polyubiquitination of Akt has a role in the activation of Akt, functioning as a regulatory signal that induces the plasma membrane recruitment, serine/threonine phosphorylation and activation of Akt.
2.1. K63-Linked Polyubiquitination of Akt
In 2009, Yang and collaborators showed the first evidence that Akt undergoes a K63-linked ubiquitination at the K8 and K14 residues within its PH domain in response to IGF-1 and cytokine Il-1 stimulation. TNF Receptor-Associated Factor 6 (TRAF6) has represented the first example of an E3 ligase that orchestrates Akt activation by inducing its K63-linked ubiquitination [54].
Since then, the list of E3-ubiquitin ligases promoting the K63-linked ubiquitination of Akt has been extended, suggesting that different extracellular signals utilize distinct E3-ubiquitin ligases to orchestrate Akt signaling pathway activation. These studies have also further demonstrated the involvement of K63-linked ubiquitination in several steps of cancerogenesis, including tumor onset, progression and invasion. In this regard, two distinct groups showed that S-Phase Kinase Associated Protein 2 (Skp2) and TNF Receptor Associated Factor 4 (TRAF4) promote Akt activation by mediating its K63-linked ubiquitination upon EGF stimulation. Notably, the ablation of E3-ubiquitin ligases of Akt TRAF6, Skp2 and TRAF4 impairs tumor progression in vivo [54,55,57]. Along this line, Skp2 depletion downregulates Akt activity, thus repressing Neu overexpression-driven breast cancer development [58]. Moreover, the subunit of SCF complex F-Box and Leucine Rich Repeat Protein 18 (FBXL18) promotes the glioma progression by inducing the K63-linked ubiquitination of Akt [103]. In agreement with the involvement of the K63-linked ubiquitination of Akt in cancerogenesis, the E3 ubiquitin ligase Ring Finger Protein 8 (RNF8) induces Akt activation by K63-linked ubiquitination, thus leading to lung cancer cell proliferation and chemotherapy resistance [103]. Furthermore, the SET domain bifurcate 1 (SETDB1)-mediated Akt K64 methylation induces K63-linked ubiquitination by several E3-ubiquitin ligases, including TRAF6 and Skp2-SCF complex, contributing to cancerogenesis [104]. The interaction of Lysine Demethylase 4B (KDM4B) with Akt stimulates the TRAF6-mediated K63-linked ubiquitination and activation of Akt, facilitating glucose metabolism and colorectal cancer growth [105]. Recent findings indicate that Cockayne Syndrome group A (CSA) is a novel Akt interacting protein that promotes its K63-linked ubiquitination and subsequent plasma membrane recruitment and activation. CSA depletion attenuates Akt phosphorylation and activation, thus leading to the diminished tumorigenic capabilities of breast cancer cells [106].
K63-linked ubiquitination seems to also have a critical role in regulating breast cancer invasion. Indeed, Ubiquitin-conjugating enzyme complex Ubc13-Uev1A promotes Akt signaling activation via K63-linked ubiquitination, thus leading to increased Cancer/Testis Antigen Family 45 Member A (CT45A) expression, cell migration and EMT signaling in breast cancer cells [107].
The physiological relevance of the K63-linked ubiquitination of Akt in cancerogenesis is further highlighted by the observation that the constitutively active and cancer-associated Akt E17K mutant is more effectively ubiquitinated by NEDD4-1 and shows a higher basal level of K63-linked ubiquitination than wild-type, thus explaining the increased activity and oncogenic potential of this mutant [54,108,109].
2.2. K48-Linked Polyubiquitination of Akt
Conversely, the involvement of the K48-linked ubiquitination of Akt in cancer can be attributed to its role in promoting its proteasomal degradation, thus resulting in shutting off the Akt pathway signaling. In this context, BReast CAncer gene 1 (BRCA1) plays a crucial role in negatively regulating Akt. Indeed, BRCA1 binds phosphorylated Akt and triggers its K48-linked ubiquitination and degradation. In agreement with this, BRCA1 deficiency, a condition commonly reported in several tumors, leads to the oncogenic activation of Akt. This emphasizes the involvement of the BRCA1-Akt interplay in tumorigenesis, suggesting the BRCA1-Akt pathway as a promising target in chemotherapies directed against BRCA1-deficient cancers [110]. Other E3 ligases have been further described to modify Akt by K48-linked ubiquitination, thus promoting the degradation and suppression of pro-tumorigenic Akt signaling [111,112,113,114,115]. TRIM13, for example, is an E3 ubiquitin ligase that has been found to be de-regulated in several tumor types, including B-cell chronic lymphocytic leukemia, multiple myeloma and non-small-cell lung carcinoma. TRIM13 may act as a tumor suppressor function by promoting Akt degradation, thus inducing p53 stabilization and apoptosis [112]. Moreover, MUL1 binds to Akt in its phosphorylated and active form to induce its degradation. Hence, MUL1 negatively modulates Akt signaling, regulating multiple cellular processes, including cell proliferation and migration [111]. Along this line, CHIP and TTC3 preferentially bind phosphorylated Akt and target it for proteasomal degradation, thus turning off Akt signaling and the Akt-related oncogenic signal transduction downstream [113,114]. In addition, the overexpression of ZNRF1, another E3 ubiquitin-ligase that has been described to induce the ubiquitination and degradation of Akt, has been associated with diminished protein levels of Akt and the subsequent inhibition of the proliferation and stemness properties of leukemia NB4 cells [115,116].
2.3. The Role of the Deubiquitination of Akt
Several studies also described the crucial role of different deubiquitinating enzymes (DUBs) in regulating the Akt signaling pathway. Indeed, DUBs such as CYLD, OTUD5 and USP1 can reverse the K63-linked ubiquitination of Akt and turn off its signaling activation [117,118,119]. Accordingly, the enhanced Akt ubiquitination and activation caused by the downregulation of OTUD5 gives rise to radioresistance in cervical cancer [119]. In line with this, the loss of CYLD promotes Akt hyperubiquitination and activation, as well as cell proliferation, survival and prostate tumorigenesis [120]. Noteworthily, the CYLD-mediated deubiquitination of Akt induced by bisdemethoxycurcumin has been shown to inhibit hepatocellular carcinoma cell growth [121]. Finally, a recent work suggests stimulating the de-ubiquitinating action of USP1, which is frequently de-regulated in multiple tumors, toward Akt as a putative therapeutic treatment of cancer [117].
3. Akt as a Target for Cancer Therapy
Due to its crucial involvement in carcinogenesis and drug resistance, Akt represents an attractive potential drug target for the development of anticancer therapies.
3.1. Targeting Akt Kinase
Nonselective Akt kinase inhibitors have been initially exploited, and medicinal chemistry efforts have been made to improve their pharmaceutical properties [122]. Nevertheless, the side effects observed in animal models limited their potential therapeutic application. More recently, many efforts have been made to achieve ATP-binding pockets structure-based Akt inhibitors with improved efficacy, selectivity and safety. Two ATP-competitive compounds, capivasertib and ipatasertib, have been extensively tested in clinical trials, and, to date, phase III clinical trials are under way for prostate and breast cancer [123,124].
In addition to directly targeting the kinase functionality of Akt, alternative therapeutic approaches have been pursued to control its aberrant activation. Among them, the phospholipid-containing molecule perifosine, by inhibiting the association of the PH domain with PIP3, has been shown to block Akt plasma membrane localization and its subsequent activation and phosphorylation [125]. Extensive phase I and II clinical programs on a large panel of cancer types are currently under way [126].
Additionally, the action of allosteric Akt inhibitors acting on the PH domain of Akt is exploited to maintain it in the inactive conformation. Currently, the structure-based design of small molecule agents that interact with various residues in the PH domains of Akt isoforms is exploited to allow for the isoform-specific inhibition of Akt [127]. In this context, an allosteric inhibitor named SC66 has been described, which interferes with the PIP3 binding function of the PH domain and, additionally, determines a robust K48-linked ubiquitination of Akt, which is finally targeted at the pericentrosomal region for proteasomal degradation. Along this line, the SC66 compound has shown anticancer activity in vitro and in vivo [128].
3.2. Targeting the Ubiquitin Pathway
A promising avenue for turning off the functionality of Akt is to interfere with the ubiquitination processes that target Akt. As we have seen in the previous paragraph, two distinct polyubiquitination modifications have been reported to regulate Akt signaling: K63-linked polyubiquitination, which is necessary for Akt membrane recruitment, phosphorylation and activation, and K48-linked polyubiquitination, which instead triggers the proteasomal degradation of phosphorylated Akt in order to silence its activity.
In light of this, a promising way to suppress the oncogenic activation of Akt might be to interfere with the K63 polyubiquitination. TRAF6 is the main E3 ligase that, in response to IGF-1 stimulation, promotes the K63 polyubiquitination of Akt. Interestingly, TRAF6 overexpression appears to be closely related to tumorigenesis and tumor development, and the analysis of TCGA and Gene Expression Omnibus (GEO) data indicates that the high expression of TRAF6 is significantly related to a poor prognosis compared with the low expression of TRAF6 [129]. In corroboration with these findings, the suppression of TRAF6 by shRNA impairs Akt activation in prostate cancer cells, suppresses tumor formation in the xenograft tumor model and potentiates apoptosis induced by chemotherapy agents [54]. Along this line, mir-124, miR-145 and miR-146 have been shown to repress TRAF6 protein translation and determine tumor suppressive effects on both primary and metastatic breast cancer [130,131,132,133,134,135]. Interestingly, it was shown that TRAF6, together with p62, K63-polyubiquitinates and activates mTOR [136]. Overall, TRAF6 can be considered an ideal therapeutic target for human cancer, and small molecules targeting TRAF6 may be considered as potential or adjuvant agents for cancer therapy. Targeting TRAF6 by inhibitors has been extensively studied. Proteasome inhibitors, such as bortezomib and MG132, have been shown to inhibit TRAF6 expression in myeloma and pancreatic cancer [137,138]. Small molecule inhibitors targeting TRAF6 have been developed: among them, C25-140 has been shown to inhibit, both in vitro and ex vivo, the production of K63-linked ubiquitin chains [139]. Epigallocate-chin-3-gallate (EGCG) is a novel E3 ubiquitin ligase inhibitor targeting TRAF6 assessed in melanoma [140]. Resveratrol has been shown to mediate the degradation of TRAF6 and decrease the proliferation and migration of prostate cancer cells [141]. Finally, the Chincona alkaloid has been shown to bind to the RING domain of TRAF6 and to promote the apoptosis of cancer cells [142].
Skp2 is the E3 ligase for ErbB/EGF2 receptor-mediated Akt K63-polyubiquitination, and its deficiency correlates with a decreased activation of Akt [56]. In corroboration, either the genetic or pharmacological targeting of Skp2 has been shown to hit cancer development in diverse genetic tumor models [143,144,145], thus pointing out that the Skp2 factor might represent a potential target for human cancer treatment. Consistently, one study shows that a small molecule (compound SZL-P1-41) that binds to Skp2 and prevents its binding with Skp1 disrupts Skp2 E3-ligase activity toward Akt, leading to the suppression of cancer progression in mouse models [146]. Interestingly, several substances, such as Longikaurin A, quercetin, Curcumin, lycopene, Rottlerin, nitidine chloride, Flavokawain and dioscin, have been shown to repress the expression of Skp2 in various types of human malignancies, thus arresting their proliferation [147].
3.3. Proteolysis-Targeting Chimeras
An alternative approach might be to increase K48-polyubiquitination to target Akt to the proteasome. The protein degraders Proteolysis Targeting Chimeras (PROTACs) are able to bind a E3 ligase protein and a target protein leading to its ubiquitination and proteasomal degradation [148]. These degraders could be exploited to degrade Akt as a potential therapy for cancer treatment. Along this line, the PROTAC degrader INY-03-041 has shown to degrade all the three Akt isoforms [149]. Other Akt de-graders, MS21 and MS143, which are von Hippel–Lindau (VHL)-recruiting PROTACs, shown to induce rapid and massive Akt degradation, thereby leading to the suppression of both cancer cells and tumor growth in vivo in a xenograft model [150].
4. Conclusions
The Akt pathway, one of the most frequently deregulated pathways in human cancer, affects a plethora of aspects of cancer malignancy. Mounting studies have provided deep knowledge of the mechanisms by which Akt is activated and inactivated as well as the diverse involvements of Akt in both tumor progression and drug resistance. This review was aimed to describe the multiple regulatory aspects of this broad signaling pathway with a particular focus on the multiubiquitination processes devoted to the Akt activation/deactivation cycles. A comprehensive understanding of this complex network will help to fully exploit the potential of small molecules acting on the inhibition or activation of this pathway. For example, an inhibitor of K63-linked Akt ubiquitination may be able to inhibit plasma membrane recruitment of Akt thus blocking its activation (Figure 3). On the other hand, activation of those E3 ligases that mediate K48-linked Akt ubiquitination might contribute to its inhibition. Therefore, targeting this pathway represents a unique and promising strategy that will push the field from science-based hypotheses to clinical applications and thereby hopefully reduce cancer-related mortality.
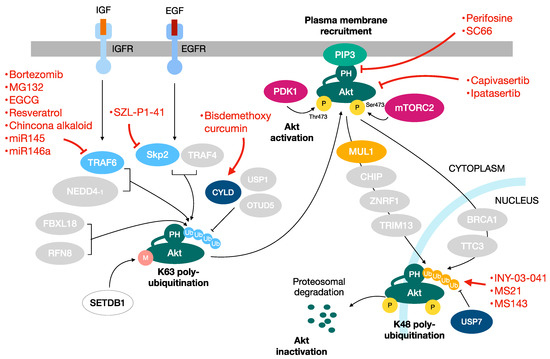
Figure 3.
Targeting the Akt pathway in cancer. Akt is an attractive potential target for the development of anticancer therapies. Besides the drugs that directly target Akt functioning, other useful approaches are represented by the targeting of the ubiquitination pathways involved in Akt regulation. In red the drugs targeting the Akt pathway.
Author Contributions
Conceptualization, E.P., A.B. and L.P.-D.-S.; writing—original draft preparation, E.P., A.B. and L.P.-D.-S.; writing—review and editing, E.P., A.B. and L.P.-D.-S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Shi, Y.; Liu, X.; Han, E.K.; Guan, R.; Shoemaker, A.R.; Oleksijew, A.; Woods, K.W.; Fisher, J.P.; Klinghofer, V.; Lasko, L.; et al. Optimal classes of chemotherapeutic agents sensitized by specific small-molecule inhibitors of Akt in vitro and in vivo. Neoplasia 2005, 7, 992–1000. [Google Scholar] [CrossRef] [PubMed]
- Carracedo, A.; Pandolfi, P.P. The PTEN–PI3K pathway: Of feedbacks and cross-talks. Oncogene 2008, 27, 5527–5541. [Google Scholar] [CrossRef] [PubMed]
- Fruman, D.A.; Rommel, C. PI3K and cancer: Lessons, challenges and opportunities. Nat. Rev. Drug Discov. 2014, 13, 140–156. [Google Scholar] [CrossRef]
- Fruman, D.A.; Chiu, H.; Hopkins, B.D.; Bagrodia, S.; Cantley, L.C.; Abraham, R.T. The PI3K pathway in human disease. Cell 2017, 170, 605–635. [Google Scholar] [CrossRef]
- Thorpe, L.M.; Yuzugullu, H.; Zhao, J.J. PI3K in cancer: Divergent roles of isoforms, modes of activation and therapeutic targeting. Nat. Rev. Cancer 2015, 15, 7–24. [Google Scholar] [CrossRef]
- Manning, B.D.; Toker, A. Akt/PKB signaling: Navigating the network. Cell 2017, 169, 381–405. [Google Scholar] [CrossRef] [PubMed]
- Al-Bazz, Y.O.; Underwood, J.C.; Brown, B.L.; Dobson, P.R. Prognostic significance of Akt, phospho-Akt and BAD expression in primary breast cancer. Eur. J. Cancer 2009, 45, 694–704. [Google Scholar] [CrossRef]
- Xue, G.; Hemmings, B.A. PKB/Akt-dependent regulation of cell motility. J. Natl. Cancer Inst. 2013, 105, 393–404. [Google Scholar] [CrossRef]
- Staal, S.P. Molecular cloning of the Akt oncogene and its human homologues Akt1 and Akt2: Amplification of Akt1 in a primary human gastric adenocarcinoma. Proc. Natl. Acad. Sci. USA 1987, 84, 5034–5037. [Google Scholar] [CrossRef]
- Altomare, D.A.; Tanno, S.; De Rienzo, A.; Klein-Szanto, A.J.; Tanno, S.; Skele, K.L.; Hoffman, J.P.; Testa, J.R. Frequent activation of Akt2 kinase in human pancreatic carcinomas. J. Cell Biochem. 2002, 87, 470–476. [Google Scholar] [CrossRef]
- Dobashi, Y.; Kimura, M.; Matsubara, H.; Endo, S.; Inazawa, J.; Ooi, A. Molecular alterations in Akt and its protein activation in human lung carcinomas. Hum. Pathol. 2012, 43, 2229–2240. [Google Scholar] [CrossRef] [PubMed]
- Ruggeri, B.A.; Huang, L.; Wood, M.; Cheng, J.Q.; Testa, J.R. Amplification and overexpression of the Akt2 oncogene in a subset of human pancreatic ductal adenocarcinomas. Mol. Carcinog. 1998, 21, 81–86. [Google Scholar] [CrossRef]
- Bellacosa, A.; De Feo, D.; Godwin, A.K.; Bell, D.W.; Cheng, J.Q.; Altomare, D.A.; Wan, M.; Dubeau, L.; Scambia, G.; Masciullo, V.; et al. Molecular alterations of the Akt2 oncogene in ovarian and breast carcinomas. Int. J. Cancer 1995, 64, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Turner, K.M.; Sun, Y.; Ji, P.; Granberg, K.J.; Bernard, B.; Hu, L.; Cogdell, D.E.; Zhou, X.; Yli-Harja, O.; Nykter, M.; et al. Genomically amplified Akt3 activates DNA repair pathway and promotes glioma progression. Proc. Natl. Acad. Sci. USA. 2015, 112, 3421–3426. [Google Scholar] [CrossRef]
- Stahl, J.M.; Sharma, A.; Cheung, M.; Zimmerman, M.; Cheng, J.Q.; Bosenberg, M.W.; Kester, M.; Sandirasegarane, L.; Robertson, G.P. Deregulated Akt3 activity promotes development of malignant melanoma. Cancer Res. 2004, 64, 7002–7010. [Google Scholar] [CrossRef]
- Bellacosa, A.; Testa, J.R.; Moore, R.; Larue, L. A portrait of Akt kinases: Human cancer and animal models depict a family with strong individualities. Cancer Biol. Ther. 2004, 3, 268–275. [Google Scholar] [CrossRef]
- Cheng, J.Q.; Lindsley, C.W.; Cheng, G.Z.; Yang, H.; Nicosia, S.V. The Akt/PKB pathway: Molecular target for cancer drug discovery. Oncogene 2005, 24, 7482–7492. [Google Scholar] [CrossRef]
- Altomare, D.A.; Testa, J.R. Perturbations of the Akt signaling pathway in human cancer. Oncogene 2005, 24, 7455–7464. [Google Scholar] [CrossRef]
- Bellacosa, A.; Kumar, C.C.; Di Cristofano, A.; Testa, J.R. Activation of Akt kinases in cancer: Implications for therapeutic targeting. Adv. Cancer Res. 2005, 94, 29–86. [Google Scholar]
- Sobočan, M.; Bračič, S.; Knez, J.; Takač, I.; Haybaeck, J. The communication between the PI3K/Akt/mTOR pathway and Y-box binding protein-1 in gynecological cancer. Cancers 2020, 12, 205. [Google Scholar] [CrossRef]
- Ma, X.L.; Shen, M.N.; Hu, B.; Wang, B.L.; Yang, W.J.; Lv, L.H.; Wang, H.; Zhou, Y.; Jin, A.-L.; Sun, Y.-F.; et al. CD73 promotes hepatocellular carcinoma progression and metastasis via activating PI3K/Akt signaling by inducing Rap1-mediated membrane localization of P110β and predicts poor prognosis. J. Hematol. Oncol. 2019, 12, 37. [Google Scholar] [CrossRef] [PubMed]
- Gallyas, F., Jr.; Sumegi, B.; Szabo, C. Role of Akt activation in PARP inhibitor resistance in cancer. Cancers 2020, 12, 532. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Yang, Z.; Lu, N. A new role for the PI3K/Akt signaling pathway in the epithelial-mesenchymal transition. Cell Adhes. Migr. 2015, 9, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.-G.; Ai, Y.-W.; Yu, L.-L.; Zhou, X.-D.; Liu, J.; Li, J.-H.; Xu, X.-M.; Liu, S.; Chen, J.; Liu, F.; et al. Phosphoinositide 3-kinase/Akt pathway plays an important role in chemoresistance of gastric cancer cells against etoposide and doxorubicin induced cell death. Int. J. Cancer 2008, 122, 433–443. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.L.; Dai, N.; Yu, H.G.; Sun, L.M.; Si, J.M. Akt associates with nuclear factor κB and plays an important role in chemoresistance of gastric cancer cells. Oncol. Rep. 2010, 24, 113–119. [Google Scholar] [CrossRef]
- Page, C.; Lin, H.J.; Jin, Y.; Castle, V.P.; Nunez, G.; Huang, M.; Lin, J. Overexpression of Akt/Akt can modulate chemotherapy-induced apoptosis. Anticancer Res. 2000, 20, 407–416. [Google Scholar]
- Zhang, Y.; Kwok-Shing Ng, P.; Kucherlapati, M.; Chen, F.; Liu, Y.; Tsang, Y.H.; De Velasco, G.; Jeong, K.J.; Akbani, R.; Hadjipanayis, A.; et al. A Pan-Cancer Proteogenomic Atlas of PI3K/Akt/mTOR Pathway Alterations. Cancer Cell. 2017, 12, 820–832. [Google Scholar] [CrossRef]
- Alwhaibi, A.; Kolhe, R.; Gao, F.; Cobran, E.K.; Somanath, P.R. Genome atlas analysis based profiling of Akt pathway genes in the early and advanced human prostate cancer. Oncoscience 2019, 6, 317–336. [Google Scholar] [CrossRef]
- Xu, J.; Wang, X.; Ke, Q.; Liao, K.; Wan, Y.; Zhang, K.; Zhang, G.; Wang, X. Combined bioinformatics technology to explore pivot genes and related clinical prognosis in the development of gastric cancer. Sci. Rep. 2021, 11, 15412. [Google Scholar] [CrossRef]
- Alessi, D.R.; Andjelkovic, M.; Caudwell, B.; Cron, P.; Morrice, N.; Cohen, P.; Hemmings, B.A. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 1996, 15, 6541–6551. [Google Scholar] [CrossRef]
- Datta, K.; Franke, T.F.; Chan, T.O.; Makris, A.; Yang, S.I.; Kaplan, D.R.; Morrison, D.K.; Golemis, E.A.; Tsichlis, P.N. AH/PH domain-mediated interaction between Akt molecules and its potential role in Akt regulation. Mol. Cell Biol. 1995, 15, 2304–2310. [Google Scholar] [CrossRef] [PubMed]
- Morrow, J.K.; Du-Cuny, L.; Chen, L.; Meuillet, E.J.; Mash, E.A.; Powis, G.; Zhang, S. Recent development of anticancer therapeutics targeting Akt. Recent Pat. Anticancer Drug Discov. 2011, 6, 146–159. [Google Scholar] [CrossRef] [PubMed]
- Bellacosa, A.; Franke, T.F.; Gonzalez-Portal, M.E.; Datta, K.; Taguchi, T.; Gardner, J.; Cheng, J.Q.; Testa, J.R.; Tsichlis, P.N. Structure, expression and chromosomal mapping of c-Akt: Relationship to v-Akt and its implications. Oncogene 1993, 8, 745–754. [Google Scholar]
- Chen, W.S.; Xu, P.Z.; Gottlob, K.; Chen, M.L.; Sokol, K.; Shiyanova, T.; Roninson, I.; Weng, W.; Suzuki, R.; Tobe, K.; et al. Growth retardation and increased apoptosis in mice with homozygous disruption of the Akt1 gene. Genes Dev. 2001, 15, 2203–2208. [Google Scholar] [CrossRef] [PubMed]
- Garofalo, R.S.; Orena, S.J.; Rafidi, K.; Torchia, A.J.; Stock, J.L.; Hildebrandt, A.L.; Coskran, T.; Black, S.C.; Brees, D.J.; Wicks, J.R.; et al. Severe diabetes, age-dependent loss of adipose tissue, and mild growth deficiency in mice lacking Akt2/PKBβ. J. Clin. Investig. 2003, 112, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Tschopp, O.; Yang, Z.Z.; Brodbeck, D.; Dummler, B.A.; Hemmings-Mieszczak, M.; Watanabe, T.; Michaelis, T.; Frahm, J.; Hemmings, B.A. Essential role of protein kinase Bγ (PKBγ/Akt3) in postnatal brain development but not in glucose homeostasis. Development 2005, 132, 2943–2954. [Google Scholar] [CrossRef]
- Gonzalez, E.; McGraw, T.E. The Akt kinases: Isoform specificity in metabolism and cancer. Cell Cycle 2009, 8, 2502–2508. [Google Scholar] [CrossRef]
- Stambolic, V.; Woodgett, J.R. Functional distinctions of protein kinase B/Akt isoforms defined by their influence on cell migration. Trends Cell Biol. 2006, 16, 461–466. [Google Scholar] [CrossRef]
- Chu, N.; Salguero, A.L.; Liu, A.Z.; Chen, Z.; Dempsey, D.R.; Ficarro, S.B.; Alexander, W.M.; Marto, J.A.; Li, Y.; Amzel, L.M.; et al. Akt kinase activation mechanisms revealed using protein semisynthesis. Cell 2018, 174, 897–907. [Google Scholar] [CrossRef]
- Guo, J.; Chakraborty, A.A.; Liu, P.; Gan, W.; Zheng, X.; Inuzuka, H.; Wang, B.; Zhang, J.; Zhang, L.; Yuan, M.; et al. pVHL suppresses kinase activity of Akt in a proline-hydroxylation-dependent manner. Science 2016, 353, 929–932. [Google Scholar] [CrossRef]
- Guo, J.; Dai, X.; Laurent, B.; Zheng, N.; Gan, W.; Zhang, J.; Guo, A.; Yuan, M.; Liu, P.; Asara, J.M.; et al. AKT methylation by SETDB1 promotes AKT kinase activity and oncogenic functions. Nat. Cell Biol. 2019, 21, 226–237. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.-H.; Jo, U.; Kohrman, A.; Rezaeian, A.H.; Chou, P.-C.; Logothetis, C.; Lin, H.-K. Posttranslational regulation of Akt in human cancer. Cell Biosci. 2014, 4, 59. [Google Scholar] [CrossRef] [PubMed]
- Alessi, D.R.; Kozlowski, M.T.; Weng, Q.P.; Morrice, N.; Avruch, J. Avruch 3-Phosphoinositide-dependent protein kinase 1 (PDK1) phosphorylates and activates the p70 S6 kinase in vivo and in vitro. Curr. Biol. 1998, 8, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Hunter, T. Signaling—2000 and beyond. Cell 2000, 100, 113–127. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.I.; Voegtli, W.C.; Sturgis, H.L.; Dizon, F.P.; Vigers, G.P.; Brandhuber, B.J. Crystal structure of human Akt1 with an allosteric inhibitor reveals a new mode of kinase inhibition. PLoS ONE 2010, 5, e12913. [Google Scholar] [CrossRef]
- James, S.R.; Downes, C.P.; Gigg, R.; Grove, S.J.; Holmes, A.B.; Alessi, D.R. Specific binding of the Akt-1 protein kinase to phosphatidylinositol 3,4,5-trisphosphate without subsequent activation. Biochem. J. 1996, 315, 709–713. [Google Scholar] [CrossRef]
- Sarbassov, D.D.; Guertin, D.A.; Ali, S.M.; Sabatini, D.M. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 2005, 307, 1098–1101. [Google Scholar] [CrossRef]
- Yin, Y.; Hua, H.; Li, M.; Liu, S.; Kong, Q.; Shao, T.; Wang, J.; Luo, Y.; Wang, Q.; Luo, T.; et al. mTORC2 promotes type I insulin-like growth factor receptor and insulin receptor activation through the tyrosine kinase activity of mTOR. Cell Res. 2016, 26, 46–65. [Google Scholar] [CrossRef]
- Hua, H.; Kong, Q.; Zhang, H.; Wang, J.; Luo, T.; Jiang, Y. Targeting mTOR for cancer therapy. J. Hematol. Oncol. 2019, 12, 71. [Google Scholar] [CrossRef]
- Singh, S.S.; Yap, W.N.; Arfuso, F.; Kar, S.; Wang, C.; Cai, W.; Dharmarajan, A.M.; Sethi, G.; Kumar, A.P. Targeting the PI3K/Akt signaling pathway in gastric carcinoma: A reality for personalized medicine? World J. Gastroenterol. 2015, 21, 12261–12273. [Google Scholar] [CrossRef]
- Chen, C.Y.; Chen, J.; He, L.; Stiles, B.L. PTEN: Tumor Suppressor and Metabolic Regulator. Front. Endocrinol. 2018, 9, 338. [Google Scholar] [CrossRef]
- Gkountakos, A.; Sartori, G.; Falcone, I.; Piro, G.; Ciuffreda, L.; Carbone, C.; Tortora, G.; Scarpa, A.; Bria, E.; Milella, M.; et al. PTEN in lung cancer: Dealing with the problem, building on new knowledge and turning the game around. Cancers 2019, 11, 1141. [Google Scholar] [CrossRef] [PubMed]
- Luongo, F.; Colonna, F.; Calapà, F.; Vitale, S.; Fiori, M.E.; De Maria, R. PTEN tumor-suppressor: The dam of stemness in cancer. Cancers 2019, 11, 1076. [Google Scholar] [CrossRef]
- Yang, W.L.; Wang, J.; Chan, C.H.; Lee, S.W.; Campos, A.D.; Lamothe, B.; Hur, L.; Grabiner, B.C.; Lin, X.; Darnay, B.G.; et al. The E3 ligase TRAF6 regulates Akt ubiquitination and activation. Science 2009, 325, 1134–1138. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.-L.; Wu, C.-Y.; Wu, J.; Lin, H.-K. Regulation of Akt signalling activation by ubiquitination. Cell Cycle 2010, 9, 486–497. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.H.; Li, C.F.; Yang, W.L.; Gao, Y.; Lee, S.W.; Feng, Z.; Huang, H.-Y.; Tsai, K.K.; Flores, L.G.; Shao, Y.; et al. The Skp2-SCF E3 ligase regulates Akt ubiquitination, glycolysis, herceptin sensitivity, and tumorigenesis. Cell 2012, 149, 1098–1111. [Google Scholar] [CrossRef]
- Li, W.; Peng, C.; Lee, M.H.; Lim, D.; Zhu, F.; Fu, Y.; Yang, G.; Sheng, Y.; Xiao, L.; Dong, X.; et al. TRAF4 is a critical molecule for Akt activation in lung cancer. Cancer Res. 2013, 73, 6938–6950. [Google Scholar] [CrossRef]
- Manning, B.D.; Cantley, L.C. Akt/PKB signaling: Navigating downstream. Cell 2007, 129, 1261–1274. [Google Scholar] [CrossRef]
- Maurer, U.; Charvet, C.; Wagman, A.S.; Dejardin, E.; Green, D.R. Glycogen synthase kinase-3 regulates mitochondrial outer membrane permeabilization and apoptosis by destabilization of MCL-1. Mol. Cell 2006, 21, 749–760. [Google Scholar] [CrossRef] [PubMed]
- Mayo, L.D.; Donner, D.B. A phosphatidylinositol 3-kinase/Akt pathway promotes translocation of Mdm2 from the cytoplasm to the nucleus. Proc. Natl. Acad. Sci. USA 2001, 25, 11598–11603. [Google Scholar] [CrossRef]
- Zhou, B.P.; Liao, Y.; Xia, W.; Zou, Y.; Spohn, B.; Hung, M.C. HER-2/neu induces p53 ubiquitination via Akt-mediated MDM2 phosphorylation. Nat Cell Biol. 2001, 3, 973–982. [Google Scholar] [CrossRef] [PubMed]
- Heron-Milhavet, L.; Franckhauser, C.; Rana, V.; Berthenet, C.; Fisher, D.; Hemmings, B.A.; Fernandez, A.; Lamb, N.J.C. Only Akt1 is required for proliferation, while Akt2 promotes cell cycle exit through p21 binding. Mol. Cell Biol. 2006, 26, 8267–8280. [Google Scholar] [CrossRef] [PubMed]
- Dillon, R.L.; Marcotte, R.; Hennessy, B.T.; Woodgett, J.R.; Mills, G.B.; Muller, W.J. Akt1 and Akt2 play distinct roles in the initiation and metastatic phases of mammary tumor progression. Cancer Res. 2009, 69, 5057–5064. [Google Scholar] [CrossRef] [PubMed]
- Kauffmann-Zeh, A.; Rodriguez-Viciana, P.; Ulrich, E.; Gilbert, C.; Coffer, P.; Downward, J.; Evan, G. Suppression of c-Myc-induced apoptosis by Ras signalling through PI(3)K and PKB. Nature 1997, 385, 544. [Google Scholar] [CrossRef]
- Deng, L.; Chen, L.; Zhao, L.; Xu, Y.; Peng, X.; Wang, X.; Ding, L.; Jin, J.; Teng, H.; Wang, Y.; et al. Ubiquitination of Rheb governs growth factor-induced mTORC1 activation. Cell Res. 2019, 29, 136–150. [Google Scholar] [CrossRef]
- Gentilella, A.; Kozma, S.C.; Thomas, G. A liaison between mTOR signaling, ribosome biogenesis and cancer. Biochim. Biophys. Acta (BBA) Gene Regul. Mech. 2015, 1849, 812–820. [Google Scholar] [CrossRef]
- Jiang, N.; Dai, Q.; Su, X.; Fu, J.; Feng, X.; Peng, J. Role of PI3K/Akt pathway in cancer: The framework of malignant behavior. Mol. Biol. Rep. 2020, 47, 4587–4629. [Google Scholar] [CrossRef]
- Wee, K.B.; Aguda, B.D. Akt versus p53 in a network of oncogenes and tumour suppressor genes regulating cell survival and death. Biophys. J. 2006, 91, 857–865. [Google Scholar] [CrossRef]
- Singh, S.; Ramamoorthy, M.; Vaughan, C.; Yeudall, W.A.; Deb, S. Human oncoprotein MDM2 activates the Akt signalling pathway through an interaction with the repressor element-1 silencing transcription factor conferring a survival advantage to cancer cells. Cell Death Differ. 2013, 20, 558. [Google Scholar] [CrossRef]
- Georgescu, M.M. PTEN Tumor Suppressor Network in PI3K-Akt Pathway Control. Genes Cancer 2010, 1, 1170–1177. [Google Scholar] [CrossRef]
- Zhou, B.P.; Hung, M.C. Novel targets of Akt, p21(Cipl/WAF1), and MDM2. Semin. Oncol. 2002, 29, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, X.; Wang, H.; Liu, S.; Hu, N.; Li, X. Akt Regulated Phosphorylation of GSK-3β/Cyclin D1, p21 and p27 Contributes to Cell Proliferation Through Cell Cycle Progression From G1 to S/G2M Phase in Low-Dose Arsenite Exposed HaCat Cells. Front Pharmacol. 2019, 11, 1176. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.C.; Yuan, T.; Rui, B.Y.; Zhu, Z.Z.; Guo, S.C.; Zhang, C.Q. Exosomes derived from human platelet-rich plasma prevent apoptosis induced by glucocorticoid-associated endoplasmic reticulum stress in rat osteonecrosis of the femoral head via the Akt/Bad/Bcl-2 signal pathway. Theranostics 2017, 15, 733–750. [Google Scholar] [CrossRef]
- Datta, S.R.; Dudek, H.; Tao, X.; Masters, S.; Fu, H.; Gotoh, Y.; Greenberg, M.E. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell 1997, 91, 231–241. [Google Scholar] [CrossRef]
- Tran, H.; Brunet, A.; Griffith, E.C.; Greenberg, M.E. The many forks in FOXO’s road. Sci. STKE 2003, 2003, RE5. [Google Scholar] [CrossRef]
- Herold, M.J.; Rohrbeck, L.; Lang, M.J.; Grumont, R.; Gerondakis, S.; Tai, L.; Bouillet, P.; Kaufmann, T.; Strasser, A. Foxo-mediated Bim transcription is dispensable for the apoptosis of hematopoietic cells that is mediated by this BH3-only protein. EMBO Rep. 2013, 14, 992–998. [Google Scholar] [CrossRef]
- Dan, H.C.; Sun, M.; Kaneko, S.; Feldman, R.I.; Nicosia, S.V.; Wang, H.-G.; Tsang, B.K.; Cheng, J.Q. Akt phosphorylation and stabilization of X-linked inhibitor of apoptosis protein (XIAP). J. Biol. Chem. 2004, 279, 5405–5412. [Google Scholar] [CrossRef] [PubMed]
- Cardone, M.H.; Roy, N.; Stennicke, H.R.; Salvesen, G.S.; Franke, T.F.; Stanbridge, E.; Frisch, S.; Reed, J.C. Regulation of cell death protease caspase-9 by phosphorylation. Science 1998, 282, 1318–1321. [Google Scholar] [CrossRef] [PubMed]
- Sangawa, A.; Shintani, M.; Yamao, N.; Kamoshida, S. Phosphorylation status of Akt and caspase-9 in gastric and colorectal carcinomas. Int. J. Clin. Exp. Pathol. 2014, 7, 3312–3317. [Google Scholar]
- Chin, Y.R.; Toker, A. Function of Akt/PKB signaling to cell motility, invasion and the tumor stroma in cancer. Cell. Signal. 2009, 21, 470–476. [Google Scholar] [CrossRef]
- Olsson, A.K.; Dimberg, A.; Kreuger, J.; Claesson-Welsh, L. VEGF receptor signalling in control of vascular function. Nat. Rev. Mol. Cell Biol. 2006, 7, 359–371. [Google Scholar] [CrossRef]
- Jiang, B.H.; Liu, L.Z. Akt signaling in regulating angiogenesis. Curr. Cancer Drug Targets 2008, 8, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Dimmeler, S.; Fleming, I.; Fisslthaler, B.; Hermann, C.; Busse, R.; Zeiher, A.M. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature 1999, 399, 601–605. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, A.; Miyakoda, G.; Hirose, Y.; Mori, T. Activation of endothelial nitric oxide synthase by cilostazol via a cAMP/protein kinase A- and phosphatidylinositol 3-kinase/Akt-dependent mechanism. Atherosclerosis 2006, 189, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Xue, G.; Restuccia, D.F.; Lan, Q.; Hynx, D.; Dirnhofer, S.; Hess, D.; Ruegg, C.; Hemmings, B.A. Akt/ PKB-mediated phosphorylation of Twist1 promotes tumor metastasis via mediating cross-talk between PI3K/Akt and TGF-β signaling axes. Cancer Discov. 2012, 2, 248–259. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; Weinberg, R.A. The basics of epithelial-mesenchymal transition. J. Clin. Investig. 2009, 119, 1420–1428. [Google Scholar] [CrossRef]
- Eddy, R.J.; Weidmann, M.D.; Sharma, V.P.; Condeelis, J.S. Tumor cell invadopodia: Invasive protrusions that orchestrate metastasis. Trends Cell Biol. 2017, 27, 595–607. [Google Scholar] [CrossRef]
- Fukumoto, M.; Ijuin, T.; Takenawa, T. PI(3,4)P2 plays critical roles in the regulation of focal adhesion dynamics of MDA-MB-231 breast cancer cells. Cancer Sci. 2017, 108, 941–951. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Yoshida, S.; Muroi, E.; Yoshida, N.; Kawamura, M.; Kouchi, Z.; Nakamura, Y.; Sakai, R.; Fukami, K. Phosphoinositide 3-kinase signaling pathway mediated by p110α regulates invadopodia formation. J. Cell Biol. 2011, 193, 1275–1288. [Google Scholar] [CrossRef]
- Revathidevi, S.; Munirajan, A.K. Akt in cancer: Mediator and more. Semin. Cancer Biol. 2019, 59, 80–91. [Google Scholar] [CrossRef]
- Hoxhaj, G.; Manning, B.D. The PI3K-Akt network at the interface of oncogenic signalling and cancer metabolism. Nat. Rev. Cancer 2020, 20, 74–88. [Google Scholar] [CrossRef] [PubMed]
- Papadopoli, D.; Pollak, M.; Topisirovic, I. The role of GSK3 in metabolic pathway perturbations in cancer. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2021, 1868, 119059. [Google Scholar] [CrossRef] [PubMed]
- Taha, C.; Liu, Z.; Jin, J.; Al-Hasani, H.; Sonenberg, N.; Klip, A. Opposite Translational Control of glut1 and glut4 glucose transporter mRNAs in response to insulin role of mammalian target of rapamycin, protein kinase b, and phosphatidylinositol 3-kinase in glut1 mRNA TRANSLATION. J. Biol. Chem. 1999, 274, 33085–33091. [Google Scholar] [CrossRef] [PubMed]
- Picton, C.; Woodgett, J.; Hemmings, B.; Cohen, P. Multisite phosphorylation of glycogen synthase from rabbit skeletal muscle. Phosphorylation of site 5 by glycogen synthase kinase-5 (casein kinase-II) is a prerequisite for phosphorylation of sites 3 by glycogen synthase kinase-3. FEBS Lett. 1982, 150, 191–196. [Google Scholar] [CrossRef]
- Hunter, R.W.; Treebak, J.T.; Wojtaszewski, J.F.; Sakamoto, K. Molecular mechanism by which AMP-activated protein kinase activation promotes glycogen accumulation in muscle. Diabetes 2011, 60, 766–774. [Google Scholar] [CrossRef]
- Cross, D.A.; Alessi, D.R.; Cohen, P.; Andjelkovich, M.; Hemmings, B.A. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature 1995, 378, 785–789. [Google Scholar] [CrossRef]
- Frame, S.; Cohen, P.; Biondi, R.M. A common phosphate binding site explains the unique substrate specificity of GSK3 and its inactivation by phosphorylation. Mol. Cell 2001, 7, 1321–1327. [Google Scholar] [CrossRef]
- Hochstrasser, M. Evolution and function of ubiquitin-like protein-conjugation systems. Nat. Cell Biol. 2000, 2, E153–E157. [Google Scholar] [CrossRef]
- Pickart, C.M.; Eddins, M.J. Ubiquitin: Structures, functions, mechanisms. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2004, 1695, 55–72. [Google Scholar] [CrossRef]
- Merbl, Y.; Refour, P.; Patel, H.; Springer, M.; Kirschner, M.W. Profiling of ubiquitin-like modifications reveals features of mitotic control. Cell 2013, 152, 1160. [Google Scholar] [CrossRef]
- Kwon, Y.T.; Ciechanover, A. The Ubiquitin Code in the Ubiquitin-Proteasome System and Autophagy. Trends Biochem. Sci. 2017, 42, 873–886. [Google Scholar] [CrossRef]
- Swatek, K.N.; Komander, D. Ubiquitin modifications. Cell Res. 2016, 26, 399–422. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yang, Z.; Ou, J.; Xia, X.; Zhi, F.; Cui, J. The F-box protein FBXL18 promotes glioma progression by promoting K63-linked ubiquitination of Akt. FEBS Lett. 2017, 591, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Long, J.; Gao, Y.; Zhang, W.; Han, F.; Xu, C.; Sun, L.; Yang, S.C.; Lan, J.; Hou, Z.; et al. SETDB1-mediated methylation of Akt promotes its K63-linked ubiquitination and activation leading to tumorigenesis. Nat. Cell Biol. 2019, 21, 214–225. [Google Scholar] [CrossRef]
- Li, H.; Lan, J.; Wang, G.; Guo, K.; Han, C.; Li, X.; Hu, J.; Cao, Z.; Luo, X. KDM4B facilitates colorectal cancer growth and glucose metabolism by stimulating TRAF6-mediated AKT activation. J. Exp. Clin. Cancer Res. 2020, 39, 12–13. [Google Scholar] [CrossRef] [PubMed]
- Balzerano, A.; Pisani, A.; Paccosi, E.; Filippi, S.; Sugoni, C.; Balajee, A.S.; Proietti-De-Santis, L. The novel role of E3-ubiquitin ligase CSA in Akt activation highlights the implication of CSA-Akt axis in premature aging and cancer. Proc. Natl. Acad. Sci. USA 2023. submitted for publication. [Google Scholar]
- Niu, T.; Wu, Z.; Xiao, W. Uev1A promotes breast cancer cell migration by up-regulating CT45A expression via the AKT pathway. BMC Cancer 2021, 21, 1012. [Google Scholar] [CrossRef]
- Carpten, J.D.; Faber, A.L.; Horn, C.; Donoho, G.P.; Briggs, S.L.; Robbins, C.M.; Hostetter, G.; Boguslawski, S.; Moses, T.Y.; Savage, S.; et al. A transforming mutation in the pleckstrin homology domain of AKT1 in cancer. Nature 2007, 448, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.D.; Lum, M.A.; Xu, C.; Black, J.D.; Wang, X. Ubiquitin-dependent regulation of phospho-AKT dynamics by the ubiquitin E3 ligase, NEDD4-1, in the insulin-like growth factor-1 response. J. Biol. Chem. 2013, 288, 1674–1684. [Google Scholar] [CrossRef]
- Xiang, T.; Ohashi, A.; Huang, Y.; Pandita, T.K.; Ludwig, T.; Powell, S.N.; Yang, Q. Negative Regulation of AKT Activation by BRCA1. Cancer Res. 2008, 68, 10040–10044. [Google Scholar] [CrossRef]
- Bae, S.; Kim, S.Y.; Jung, J.H.; Yoon, Y.; Cha, H.J.; Lee, H.; Kim, K.; Kim, J.; An, I.S.; Kim, J.; et al. Akt is negatively regulated by the MULAN E3 ligase. Cell Res. 2012, 22, 873–885. [Google Scholar] [CrossRef] [PubMed]
- Joo, H.M.; Kim, J.Y.; Jeong, J.B.; Seong, K.M.; Nam, S.Y.; Yang, K.H.; Kim, C.S.; Kim, H.S.; Jeong, M.; An, S.; et al. Ret finger protein 2 enhances ionizing radiation-induced apoptosis via degradation of AKT and MDM2. Eur. J. Cell Biol. 2011, 90, 420–431. [Google Scholar] [CrossRef] [PubMed]
- Su, C.H.; Wang, C.Y.; Lan, K.H.; Li, C.P.; Chao, Y.; Lin, H.C.; Lee, S.D.; Lee, W.P. Akt phosphorylation at Thr308 and Ser473 is required for CHIP-mediated ubiquitination of the kinase. Cell. Signal. 2011, 23, 1824–1830. [Google Scholar] [CrossRef] [PubMed]
- Suizu, F.; Hiramuki, Y.; Okumura, F.; Matsuda, M.; Okumura, A.J.; Hirata, N.; Narita, M.; Kohno, T.; Yokota, J.; Bohgaki, M.; et al. The E3 Ligase TTC3 Facilitates Ubiquitination and Degradation of Phosphorylated Akt. Dev. Cell 2009, 17, 800–810. [Google Scholar] [CrossRef]
- Wakatsuki, S.; Saitoh, F.; Araki, T. ZNRF1 promotes Wallerian degeneration by degrading AKT to induce GSK3B-dependent CRMP2 phosphorylation. Nat. Cell Biol. 2011, 13, 1415–1423. [Google Scholar] [CrossRef]
- Li, Y.; Xu, Y.; Gao, C.; Sun, Y.; Zhou, K.; Wang, P.; Cheng, J.; Guo, W.; Ya, C.; Fan, J.; et al. USP1 Maintains the Survival of Liver Circulating Tumor Cells by Deubiquitinating and Stabilizing TBLR1. Front. Oncol. 2020, 25, 554809. [Google Scholar] [CrossRef]
- Goldbraikh, D.; Neufeld, D.; Eid-Mutlak, Y.; Lasry, I.; Gilda, J.E.; Parnis, A.; Cohen, S. USP1 deubiquitinates Akt to inhibit PI3K-Akt-FoxO signaling in muscle during prolonged starvation. EMBO Rep. 2020, 21, e48791. [Google Scholar] [CrossRef]
- Lim, J.H.; Jono, H.; Komatsu, K.; Woo, C.H.; Lee, J.; Miyata, M.; Matsuno, T.; Xu, X.; Huang, Y.; Zhang, W.; et al. CYLD negatively regulates transforming growth factor-β-signalling via deubiquitinating Akt. Nat. Commun. 2012, 3, 771. [Google Scholar] [CrossRef]
- Yin, F.; He, H.; Zhang, B.; Zheng, J.; Wang, M.; Zhang, M.; Cui, H. Effect of Deubiquitinase Ovarian Tumor Domain-Containing Protein 5 (OTUD5) on Radiosensitivity of Cervical Cancer by Regulating the Ubiquitination of Akt and its Mechanism. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2019, 25, 3469–3475. [Google Scholar] [CrossRef]
- Yang, W.L.; Jin, G.; Li, C.F.; Jeong, Y.S.; Moten, A.; Xu, D.; Feng, Z.; Chen, W.; Cai, Z.; Darnay, B.; et al. Cycles of ubiquitination and deubiquitination critically regulate growth factor-mediated activation of Akt signaling. Sci. Signal. 2013, 6, ra3. [Google Scholar] [CrossRef]
- Qiu, C.; Liu, K.; Zhang, S.; Gao, S.; Chen, W.; Li, D.; Huang, Y. Bisdemethoxycurcumin Inhibits Hepatocellular Carcinoma Proliferation Through Akt Inactivation via CYLD-Mediated Deubiquitination. Drug Des. Dev. Ther. 2020, 14, 993–1001. [Google Scholar] [CrossRef]
- Garcia-Echeverria, C.; Sellers, W.R. Drug discovery approaches targeting the PI3K/Akt pathway in cancer. Oncogene 2008, 27, 5511–5526. [Google Scholar] [CrossRef]
- Hua, H.; Zhang, H.; Chen, J.; Wang, J.; Liu, J.; Jiang, Y. Targeting Akt in cancer for precision therapy. J. Hematol. Oncol. 2021, 14, 128. [Google Scholar] [CrossRef]
- Tsai, P.J.; Lai, Y.H.; Manne, R.K.; Tsai, Y.S.; Sarbassov, D.; Lin, H.K. Akt: A key transducer in cancer. J. Biomed. Sci. 2022, 29, 76. [Google Scholar] [CrossRef] [PubMed]
- Kondapaka, S.B.; Singh, S.S.; Dasmahapatra, G.P.; Sausville, E.A.; Roy, K.K. Perifosine, a novel alkylphospholipid, inhibits protein kinase B activation. Mol. Cancer Ther. 2003, 2, 1093–1103. [Google Scholar]
- Song, Z.; Tu, X.; Zhou, Q.; Huang, J.; Chen, Y.; Liu, J.; Lee, S.; Kim, W.; Nowsheen, S.; Luo, K.; et al. A novel UCHL3 inhibitor, perifosine, enhances PARP inhibitor cytotoxicity through inhibition of homologous recombination-mediated DNA double strand break repair. Cell Death Dis. 2019, 10, 398. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, N.; Jabeen, I. Pharmacoinformatic Approaches to Design Novel Inhibitors of Protein Kinase B Pathways in Cancer. Curr. Cancer Drug Targets 2018, 18, 830–846. [Google Scholar] [CrossRef] [PubMed]
- Jo, H.; Lo, P.K.; Li, Y.; Loison, F.; Green, S.; Wang, J.; Silberstein, L.E.; Ye, K.; Chen, H.; Luo, H.R. Deactivation of Akt by a small molecule inhibitor targeting pleckstrin homology domain and facilitating Akt ubiquitination. Proc. Natl. Acad. Sci. USA 2011, 108, 6486–6491. [Google Scholar] [CrossRef]
- Li, J.; Liu, N.; Tang, L.; Yan, B.; Chen, X.; Zhang, J.; Peng, C. The relationship between TRAF6 and tumors. Cancer Cell Int. 2020, 20, 429. [Google Scholar] [CrossRef]
- Hou, J.; Wang, P.; Lin, L.; Liu, X.; Ma, F.; An, H.; Wang, Z.; Cao, X. MicroRNA-146a feedback inhibits RIG-I-dependent Type I IFN production in macrophages by targeting TRAF6, IRAK1, and IRAK2. J. Immunol. 2009, 183, 2150–2158. [Google Scholar] [CrossRef]
- Hurst, D.R.; Edmonds, M.D.; Scott, G.K.; Benz, C.C.; Vaidya, K.S.; Welch, D.R. Breast cancer metastasis suppressor 1 up-regulates miR-146, which suppresses breast cancer metastasis. Cancer Res. 2009, 69, 1279–1283. [Google Scholar] [CrossRef] [PubMed]
- Starczynowski, D.T.; Kuchenbauer, F.; Argiropoulos, B.; Sung, S.; Morin, R.; Muranyi, A.; Hirst, M.; Hogge, D.; Marra, M.; Wells, R.A.; et al. Identification of miR-145 and miR-146a as mediators of the 5q-syndrome phenotype. Nat. Med. 2010, 16, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xu, J.; Li, H.; Sun, C.; Yu, L.; Li, Y.; Shi, C.; Zhou, X.; Bian, X.; Ping, Y.; et al. miR-146b-5p functions as a tumor suppressor by targeting TRAF6 and predicts the prognosis of human gliomas. Oncotarget 2015, 6, 29129–29142. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Zhang, W.; Xu, X.; Li, J.; Mu, H.; Liu, X.; Qin, L.; Zhu, X.; Zheng, M. The effects of TRAF6 on proliferation, apoptosis and invasion in osteosarcoma are regulated by miR-124. Int. J. Mol. Med. 2018, 41, 2968–2976. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Lei, L.; Hui, H.; Tao, Z. MicroRNA-124 regulates TRAF6 expression and functions as an independent prognostic factor in colorectal cancer. Oncol Lett. 2019, 18, 856–863. [Google Scholar] [CrossRef]
- Linares, J.F.; Duran, A.; Yajima, T.; Pasparakis, M.; Moscat, J.; Diaz-Meco, M.T. K63 polyubiquitination and activation of mTOR by the p62-TRAF6 complex in nutrient-activated cells. Mol. Cell. 2013, 51, 283–296. [Google Scholar] [CrossRef]
- Hongming, H.; Jian, H. Bortezomib inhibits maturation and function of osteoclasts from PBMCs of patients with multiple myeloma by downregulating TRAF6. Leuk. Res. 2009, 33, 115–122. [Google Scholar] [CrossRef]
- Chiu, H.W.; Lin, S.W.; Lin, L.C.; Hsu, Y.H.; Lin, Y.F.; Ho, S.Y.; Wu, Y.H.; Wang, Y.J. Synergistic antitumor effects of radiation and proteasome inhibitor treatment in pancreatic cancer through the induction of autophagy and the downregulation of TRAF6. Cancer Lett. 2015, 365, 229–239. [Google Scholar] [CrossRef]
- Brenke, J.K.; Popowicz, G.M.; Schorpp, K.; Rothenaigner, I.; Roesner, M.; Meininger, I.; Kalinski, C.; Ringelstetter, L.; R’kyek, O.; Jürjens, G.; et al. Targeting TRAF6 E3 ligase activity with a small-molecule inhibitor combats autoimmunity. J. Biol. Chem. 2018, 293, 13191–13203. [Google Scholar] [CrossRef]
- Zhang, J.; Lei, Z.; Huang, Z.; Zhang, X.; Zhou, Y.; Luo, Z.; Zeng, W.; Su, J.; Peng, C.; Chen, X. Epigallocatechin-3-gallate(EGCG) suppresses melanoma cell growth and metastasis by targeting TRAF6 activity. Oncotarget 2016, 7, 79557–79571. [Google Scholar] [CrossRef]
- Khusbu, F.Y.; Zhou, X.; Roy, M.; Chen, F.Z.; Cao, Q.; Chen, H.C. Resveratrol induces depletion of TRAF6 and suppresses prostate cancer cell proliferation and migration. Int. J. Biochem. Cell Biol. 2020, 118, 105644. [Google Scholar] [CrossRef]
- Qi, Z.; Xu, Y.; Wang, X.; Wang, S.; Zhang, Q.; Wang, Z.; Gao, Q. TLR13, TLR22, TRAF6, and TAK1 in the soiny mullet (Liza haematocheila): Molecular characterization and expression profiling analysis. Dev. Comp. Immunol. 2020, 112, 103774. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.K.; Chen, Z.; Wang, G.; Nardella, C.; Lee, S.W.; Chan, C.H.; Yang, W.L.; Wang, J.; Egia, A.; Nakayama, K.I.; et al. Skp2 targeting suppresses tumorigenesis by Arf-p53-independent cellular senescence. Nature 2010, 464, 374–379. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Iqbal, N.J.; Sukrithan, V.; Nicholas, C.; Xue, Y.; Yu, C.; Locker, J.; Zou, J.; Schwartz, E.L.; Zhu, L. Targeted Inhibition of the E3 Ligase SCFSkp2/Cks1 Has Antitumor Activity in RB1-Deficient Human and Mouse Small-Cell Lung Cancer. Cancer Res. 2020, 80, 2355–2367. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Bauzon, F.; Fu, H.; Lu, Z.; Cui, J.; Nakayama, K.; Nakayama, K.I.; Locker, J.; Zhu, L. Skp2 deletion unmasks a p27 safeguard that blocks tumorigenesis in the absence of pRb and p53 tumor suppressors. Cancer Cell. 2013, 24, 645–659. [Google Scholar] [CrossRef]
- Chan, C.H.; Morrow, J.K.; Li, C.F.; Gao, Y.; Jin, G.; Moten, A.; Stagg, L.J.; Ladbury, J.E.; Cai, Z.; Xu, D.; et al. Pharmacological inactivation of Skp2 SCF ubiquitin ligase restricts cancer stem cell traits and cancer progression. Cell 2013, 154, 556–568. [Google Scholar] [CrossRef]
- Jiang, W.; Lin, M.; Wang, Z. Dioscin: A new potential inhibitor of Skp2 for cancer therapy. EBioMedicine 2020, 51, 102593. [Google Scholar] [CrossRef]
- Burslem, G.M.; Crews, C.M. Proteolysis-Targeting Chimeras as Therapeutics and Tools for Biological Discovery. Cell 2020, 181, 102–114. [Google Scholar] [CrossRef]
- You, I.; Erickson, E.C.; Donovan, K.A.; Eleuteri, N.A.; Fischer, E.S.; Gray, N.S.; Toker, A. Discovery of an Akt Degrader with Prolonged Inhibition of Downstream Signaling. Cell Chem Biol. 2020, 27, 66–73. [Google Scholar] [CrossRef]
- Yu, X.; Xu, J.; Shen, Y.; Cahuzac, K.M.; Park, K.S.; Dale, B.; Liu, J.; Parsons, R.E.; Jin, J. Discovery of Potent, Selective, and In Vivo Efficacious Akt Kinase Protein Degraders via Structure-Activity Relationship Studies. J. Med. Chem. 2022, 65, 3644–3666. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).