PEDF Protects Endothelial Barrier Integrity during Acute Myocardial Infarction via 67LR
Abstract
1. Introduction
2. Results
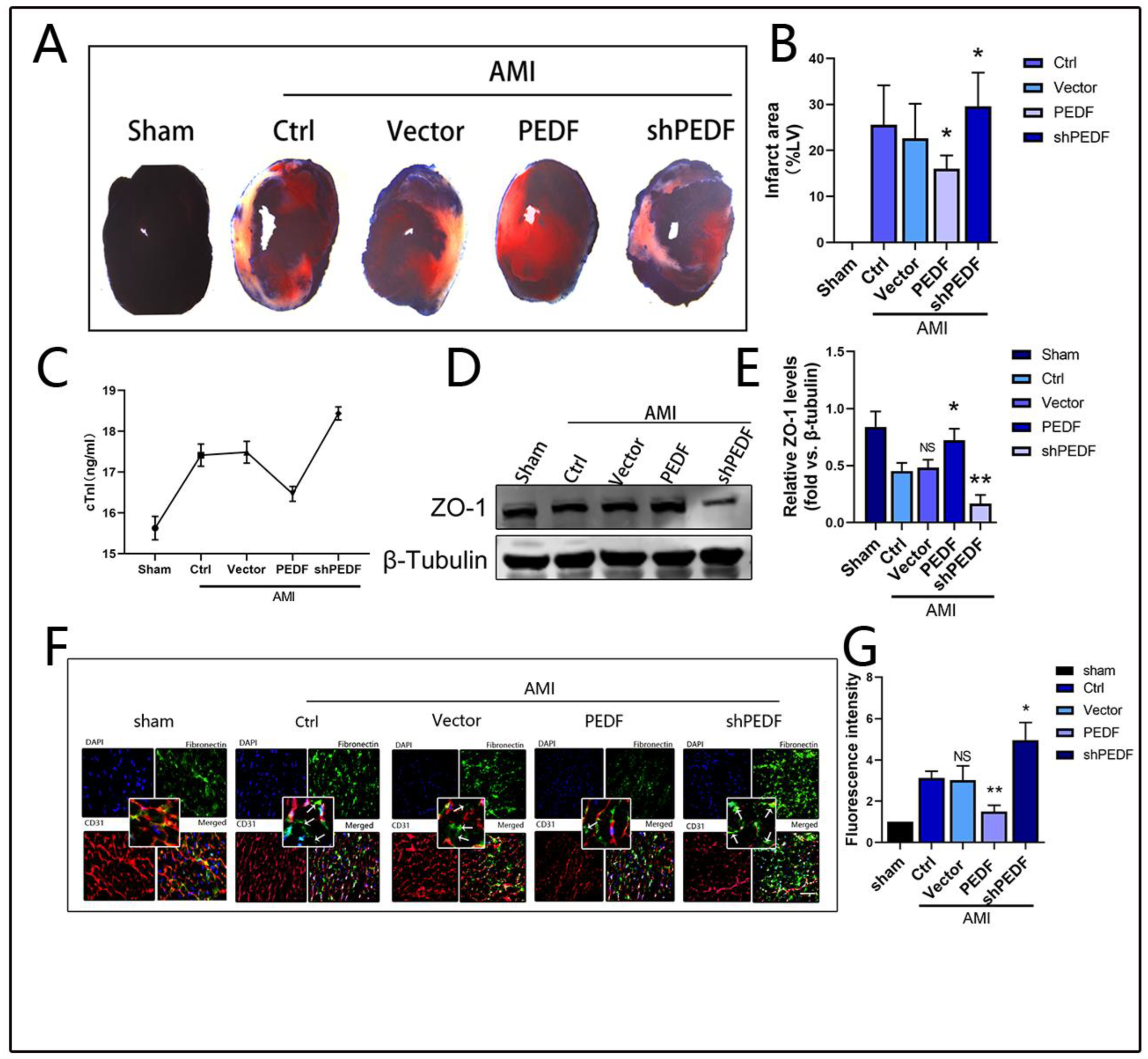
2.1. PEDF Reduces Myocardial Infarct Area and Vascular Endothelial Permeability in AMI Rats
2.2. PEDF Maintains the Stability of Vascular Endothelial TJs under OGD Conditions
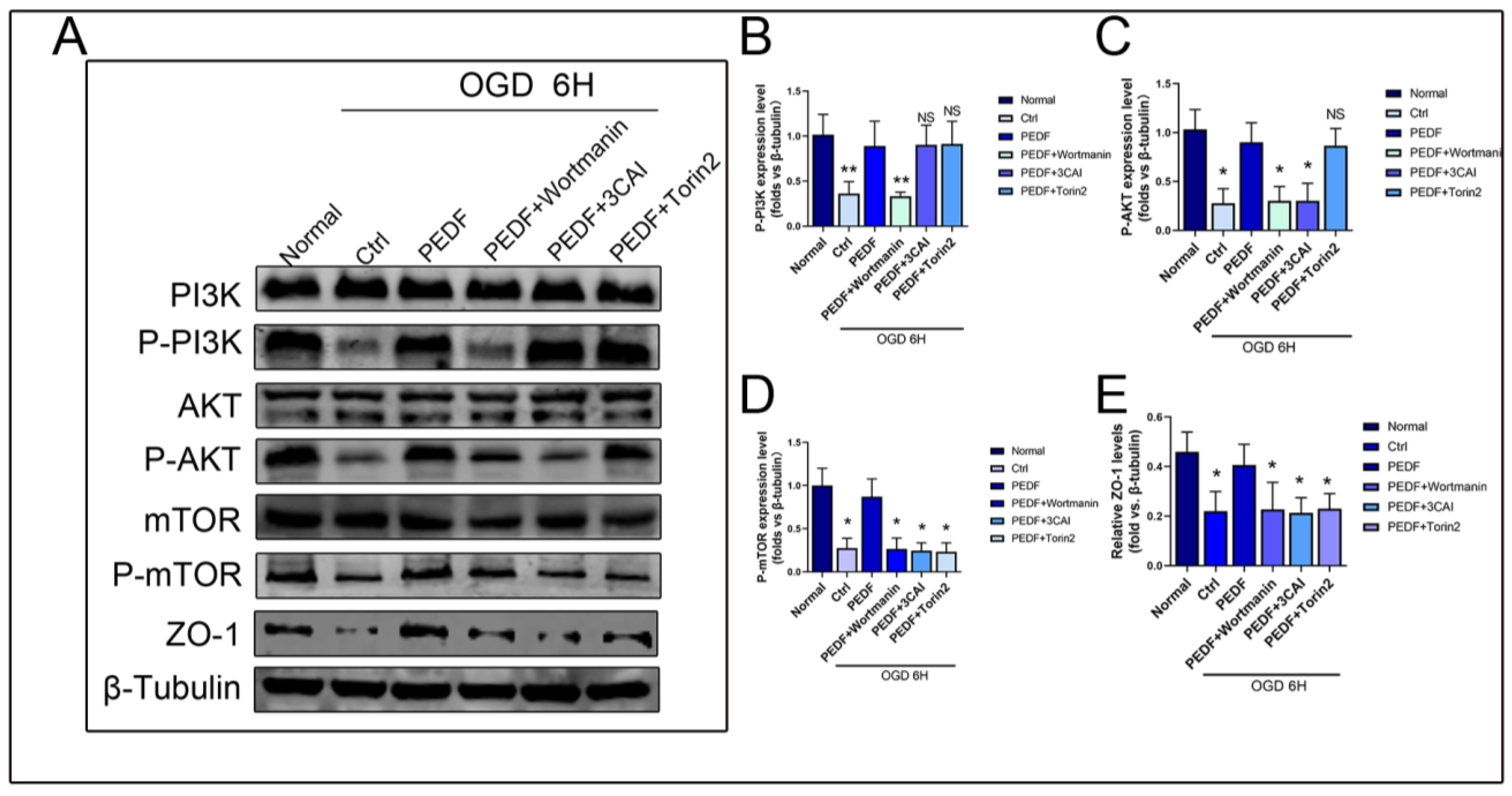
2.3. PEDF Regulates the Expression of ZO-1 in Endothelial Cells through the PI3K-AKT-mTOR Pathway
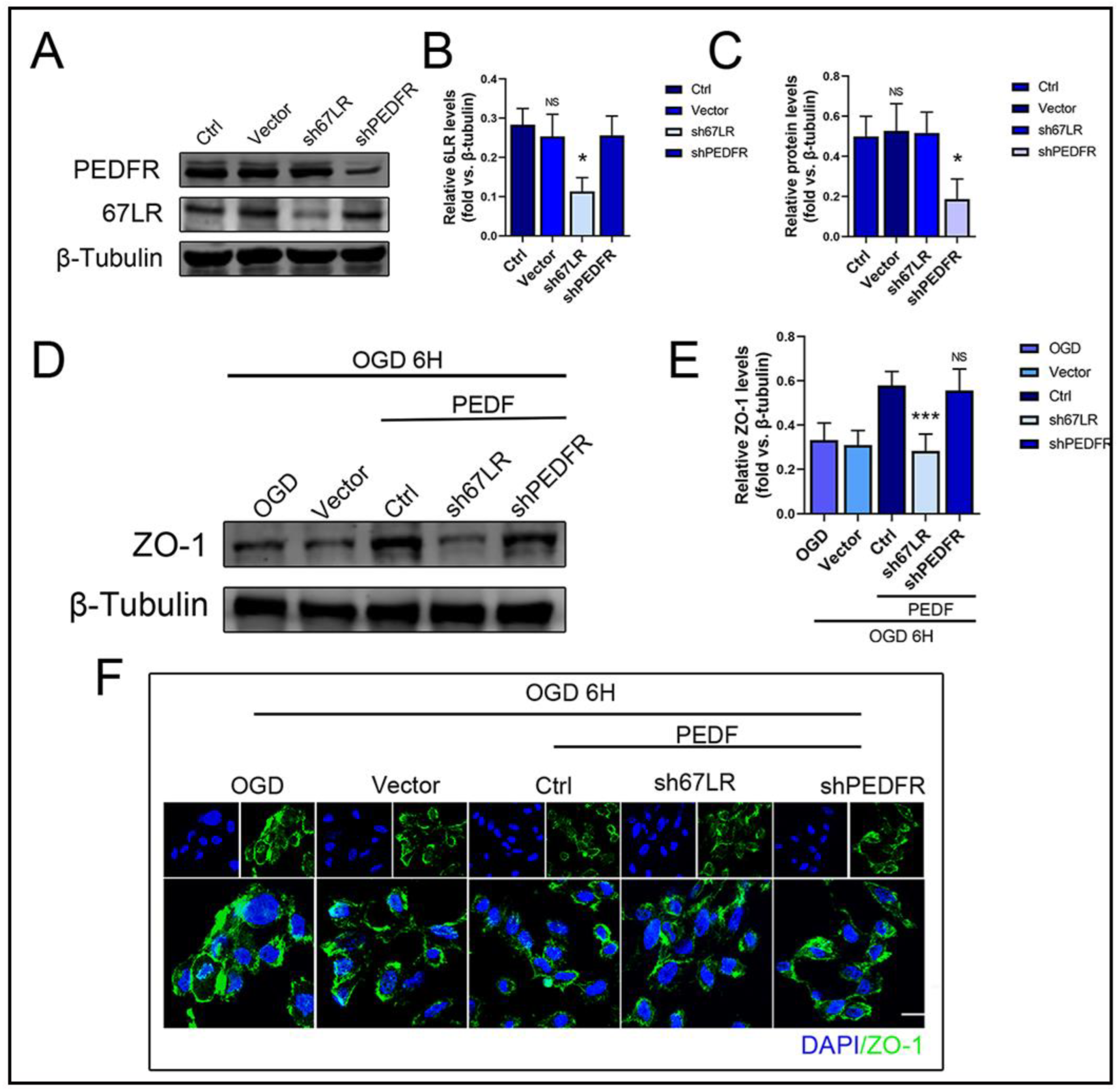
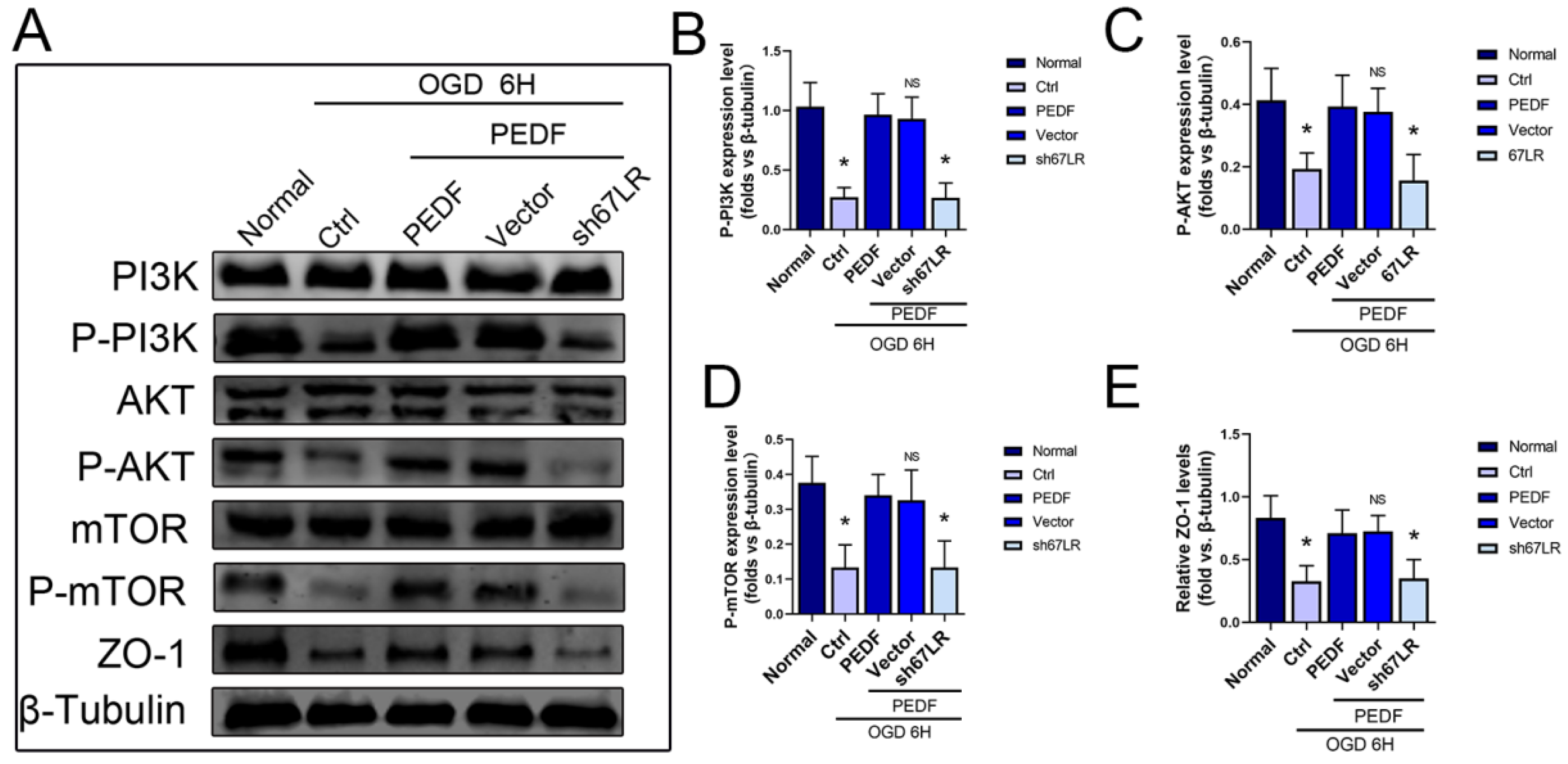
2.4. PEDF Maintains the Stability of Vascular Endothelial TJs by Activating the PI3K-AKT-mTOR Pathway via 67LR
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Reagents and Antibodies
4.3. Lentivirus (LV) Preparation
4.4. Immunofluorescence Assay
4.5. Cell Culture and Treatment
4.6. Western Blot Analysis
4.7. AMI Model Generation
4.8. Transwell Assay
4.9. Evans Blue/TTC Staining
4.10. Enzyme-Linked Immune Sorbent Assay (ELISA)
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Wang, Y.; Leifheit, E.C.; Krumholz, H.M. Trends in 10-Year Outcomes Among Medicare Beneficiaries Who Survived an Acute Myocardial Infarction. JAMA Cardiol. 2022, 7, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Khera, R.; Secemsky, E.A.; Wang, Y.; Desai, N.R.; Krumholz, H.M.; Maddox, T.M.; Shunk, K.A.; Virani, S.S.; Bhatt, D.L.; Curtis, J.; et al. Revascularization Practices and Outcomes in Patients with Multivessel Coronary Artery Disease Who Presented With Acute Myocardial Infarction and Cardiogenic Shock in the US, 2009–2018. JAMA Intern. Med. 2020, 180, 1317–1327. [Google Scholar] [CrossRef] [PubMed]
- Galley, H.F.; Webster, N.R. Randomized trial of red cell washing for the prevention of transfusion-associated organ injury in cardiac surgery. BJA Br. J. Anaesth. 2017, 118, 689–698. [Google Scholar]
- Huveneers, S.; Daemen, M.J.; Hordijk, P.L. Between Rho(k) and a Hard Place The Relation Between Vessel Wall Stiffness, Endothelial Contractility, and Cardiovascular Disease. Circ. Res. 2015, 116, 895–908. [Google Scholar] [CrossRef] [PubMed]
- Kushner, F.G.; Hand, M.; Smith, S.C., Jr.; King, S.B., III; Anderson, J.L.; Antman, E.M.; Bailey, S.R.; Bates, E.R.; Blankenship, J.C.; Casey, D.E., Jr.; et al. 2009 focused updates: ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction (updating the 2004 guideline and 2007 focused update) and ACC/AHA/SCAI guidelines on percutaneous coronary intervention (updating the 2005 guideline and 2007 focused update): A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2009, 120, 2271–2306. [Google Scholar]
- Deffieu, M.S.; Clément, C.M.H.; Dorobantu, C.M. Occludin stalls HCV particle dynamics apart from hepatocyte tight junctions, promoting virion internalization. Hepatology 2022, 76, 1164–1179. [Google Scholar] [CrossRef] [PubMed]
- Komarova, Y.A.; Kruse, K.; Mehta, D. Protein Interactions at Endothelial Junctions and Signaling Mechanisms Regulating Endothelial Permeability. Circ. Res. 2017, 120, 179–206. [Google Scholar] [CrossRef]
- Quan, X.; Liu, X.; Qin, X. The role of LR-TIMAP/PP1c complex in the occurrence and development of no-reflow. EBioMedicine 2021, 65, 103251. [Google Scholar] [CrossRef]
- Tombran-Tink, J.; Barnstable, C.J. PEDF: A multifaceted neurotrophic factor. Nat. Rev. Neurosci. 2003, 4, 628–636. [Google Scholar] [CrossRef]
- Parmryd, I.; Andersson, B.; Dallner, G. Protein prenylation in spinach chloroplasts. Proc. Natl. Acad. Sci. USA 1999, 96, 10074–10079. [Google Scholar] [CrossRef]
- Wang, M.; Casey, P.J. Protein prenylation: Unique fats make their mark on biology. Nat. Rev. Mol. Cell Bio. 2016, 17, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Boratko, A.; Csortos, C. PKC mediated phosphorylation of TIMAP regulates PP1c activity and endothelial barrier function. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2017, 1864, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Boratko, A.; Peters, M.; Csortos, C. Regulation of merlin by protein phosphatase 1-TIMAP and EBP50 in endothelial cells. Int. J. Biochem. Cell Biol. 2017, 82, 10–17. [Google Scholar] [CrossRef]
- Jones, P.A.; May, G.R.; Mcluckie, J.A.; Iwashita, A.; Sharkey, J. Apoptosis is not an invariable component of in vitro models of cortical cerebral ischaemia. Cell Res. 2004, 14, 241–250. [Google Scholar] [CrossRef]
- Li, C.; Zhang, Y.; Wang, Q.; Meng, H.; Zhang, Q.; Wu, Y.; Xiao, W.; Wang, Y.; Tu, P. Dragon’s Blood exerts cardio-protection against myocardial injury through PI3K-AKT-mTOR signaling pathway in acute myocardial infarction mice model. J. Ethnopharmacol. 2018, 227, 279–289. [Google Scholar] [CrossRef]
- Xue, M.; Yao, S.; Hu, M.; Li, W.; Hao, T.; Zhou, F.; Zhu, X.; Lu, H.; Qin, D.; Yan, Q.; et al. HIV-1 Nef and KSHV oncogene K1 synergistically promote angiogenesis by inducing cellular miR-718 to regulate the PTEN/AKT/mTOR signaling pathway. Nucleic Acids Res. 2014, 42, 9862–9879. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Hu, W.; Yi, Y.S.; Hettinghouse, A.; Sun, G.; Bi, Y.; He, W.; Zhang, L.; Gao, G.; Liu, J.; et al. TNFR2/14-3-3ε signaling complex instructs macrophage plasticity in inflammation and autoimmunity. J. Clin. Investig. 2021, 131, e144016. [Google Scholar] [CrossRef]
- He, K.; Yan, L.; Lin, S.Q.; Liu, Y.Y.; Hu, B.H.; Chang, X.; Zhao, X.R.; He, S.Y.; Wei, X.H.; Fan, J.Y.; et al. Implication of IGF1R signaling in the protective effect of Astragaloside IV on ischemia and reperfusion-induced cardiac microvascular endothelial hyperpermeability. Phytomedicine 2022, 100, 154045. [Google Scholar] [CrossRef]
- Mejias, M.; Coch, L.; Berzigotti, A.; Garcia-Pras, E.; Gallego, J.; Bosch, J.; Fernandez, M. Antiangiogenic and antifibrogenic activity of pigment epithelium-derived factor (PEDF) in bile duct-ligated portal hypertensive rats. Gut 2015, 64, 657–666. [Google Scholar] [CrossRef]
- Liakouli, V.; Elies, J.; El-Sherbiny, Y.M.; Scarcia, M.; Grant, G.; Abignano, G.; Derrett-Smith, E.C.; Esteves, F.; Cipriani, P.; Emery, P.; et al. Scleroderma fibroblasts suppress angiogenesis via TGF-β/caveolin-1 dependent secretion of pigment epithelium-derived factor. Ann. Rheum. Dis. 2018, 77, 431–440. [Google Scholar] [CrossRef]
- Becerra, S.P.; Notario, V. NotarioThe effects of PEDF on cancer biology: Mechanisms of action and therapeutic potential. Nat. Rev. Cancer 2013, 13, 258–271. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Zhong, M.; Kawaguchi, R.; Kassai, M.; Al-Ubaidi, M.; Deng, J.; Ter-Stepanian, M.; Sun, H. Identification of PLXDC1 and PLXDC2 as the transmembrane receptors for the multifunctional factor PEDF. Elife 2014, 3, e05401. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Chen, W.; Yan, M.; Liu, J.; Luo, H.; Wang, C.; Yang, P. Rapamycin regulates the balance between cardiomyocyte apoptosis and autophagy in chronic heart failure by inhibiting mTOR signaling. Int. J. Mol. Med. 2020, 45, 195–209. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Wang, Y.; Fan, Y.; Zhu, Y.; Chien, S.; Wang, N. Suppression of c-Cbl tyrosine phosphorylation inhibits neointimal formation in balloon-injured rat arteries. Circulation 2008, 118, 764–772. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Zhang, H.; Zhuang, W.; Yuan, G.; Sun, T.; Jiang, X.; Zhou, Z.; Yuan, H.; Zhang, Z.; Dong, H. PEDF and PEDF-derived peptide 44mer protect cardiomyocytes against hypoxia-induced apoptosis and necroptosis via anti-oxidative effect. Sci. Rep. 2014, 4, 5637. [Google Scholar] [CrossRef]
- Kawaguchi, T.; Yamagishi, S.I.; Sata, M. Structure-function relationships of PEDF. Review 2010, 10, 302–311. [Google Scholar] [CrossRef]
- Kim, J.E.; Park, H.; Jeong, M.J.; Kang, T.C. Epigallocatechin-3-Gallate and PEDF 335 Peptide, 67LR Activators, Attenuate Vasogenic Edema, and Astroglial Degeneration Following Status Epilepticus. Antioxidants 2020, 9, 854. [Google Scholar] [CrossRef]
- Lu, P.; Zhang, Y.Q.; Zhang, H.; Li, Y.F.; Wang, X.Y.; Xu, H.; Liu, Z.W.; Li, L.; Dong, H.Y.; Zhang, Z.M. Pigment Epithelium-Derived Factor (PEDF) Improves Ischemic Cardiac Functional Reserve Through Decreasing Hypoxic Cardiomyocyte Contractility through PEDF Receptor (PEDF-R). J. Am. Heart Assoc. 2016, 5, e003179. [Google Scholar] [CrossRef]
- Yadav, V.; Matsakas, A.; Lorca, S.; Narkar, V.A. PGC1β activates an antiangiogenic program to repress neoangiogenesis in muscle ischemia. Cell Rep. 2014, 8, 783–791. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, J.; Luo, Q.; Shen, N.; Qin, X.; Jia, C.; Chao, Z.; Zhang, L.; Qin, H.; Liu, X.; Quan, X.; et al. PEDF Protects Endothelial Barrier Integrity during Acute Myocardial Infarction via 67LR. Int. J. Mol. Sci. 2023, 24, 2787. https://doi.org/10.3390/ijms24032787
Liang J, Luo Q, Shen N, Qin X, Jia C, Chao Z, Zhang L, Qin H, Liu X, Quan X, et al. PEDF Protects Endothelial Barrier Integrity during Acute Myocardial Infarction via 67LR. International Journal of Molecular Sciences. 2023; 24(3):2787. https://doi.org/10.3390/ijms24032787
Chicago/Turabian StyleLiang, Jingtian, Qifeng Luo, Ningning Shen, Xichun Qin, Caili Jia, Zhixiang Chao, Li Zhang, Hao Qin, Xiucheng Liu, Xiaoyu Quan, and et al. 2023. "PEDF Protects Endothelial Barrier Integrity during Acute Myocardial Infarction via 67LR" International Journal of Molecular Sciences 24, no. 3: 2787. https://doi.org/10.3390/ijms24032787
APA StyleLiang, J., Luo, Q., Shen, N., Qin, X., Jia, C., Chao, Z., Zhang, L., Qin, H., Liu, X., Quan, X., Yuan, Y., & Zhang, H. (2023). PEDF Protects Endothelial Barrier Integrity during Acute Myocardial Infarction via 67LR. International Journal of Molecular Sciences, 24(3), 2787. https://doi.org/10.3390/ijms24032787





