Benefits of the Non-Steroidal Mineralocorticoid Receptor Antagonist Finerenone in Metabolic Syndrome-Related Heart Failure with Preserved Ejection Fraction
Abstract
1. Introduction
2. Results
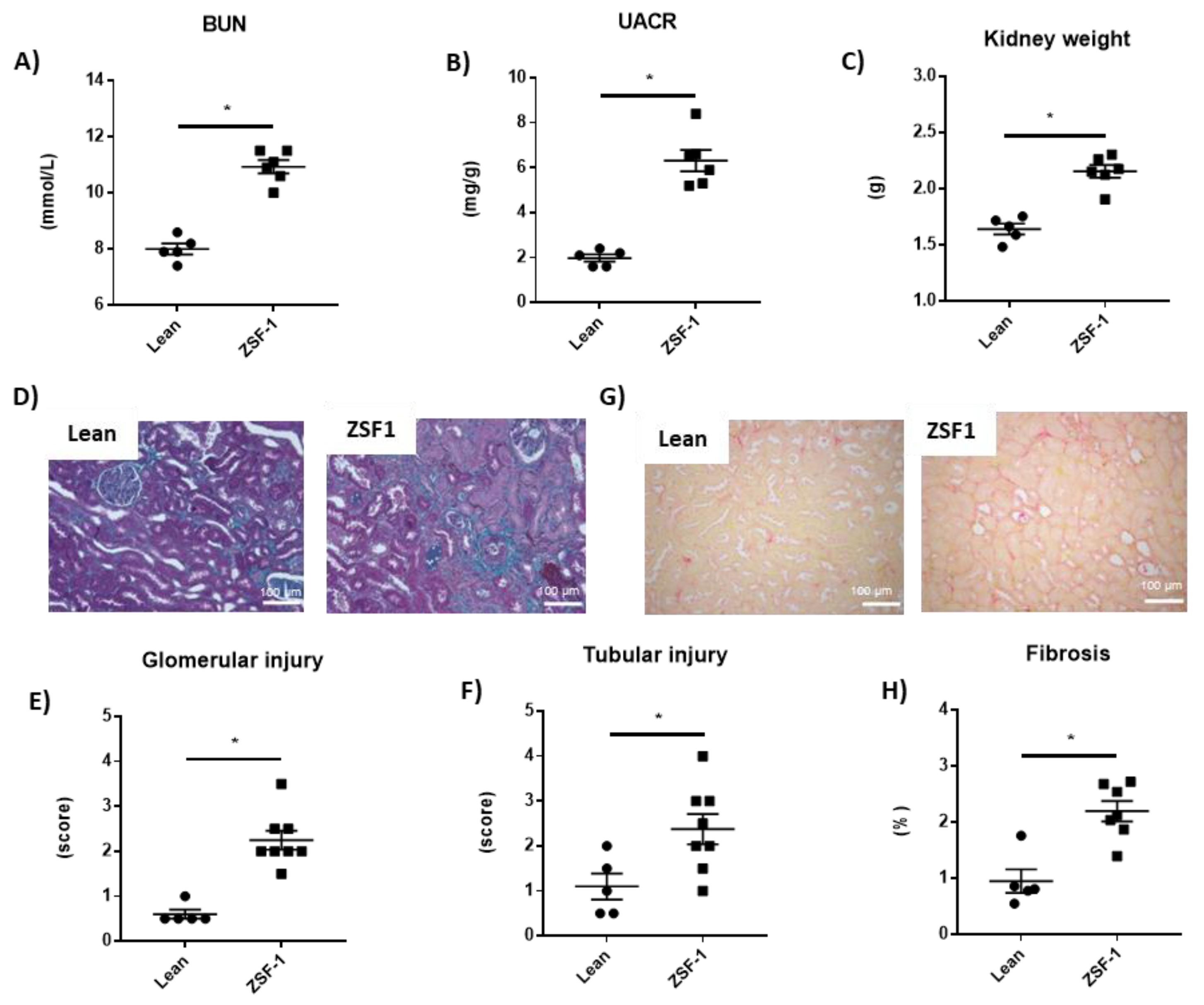
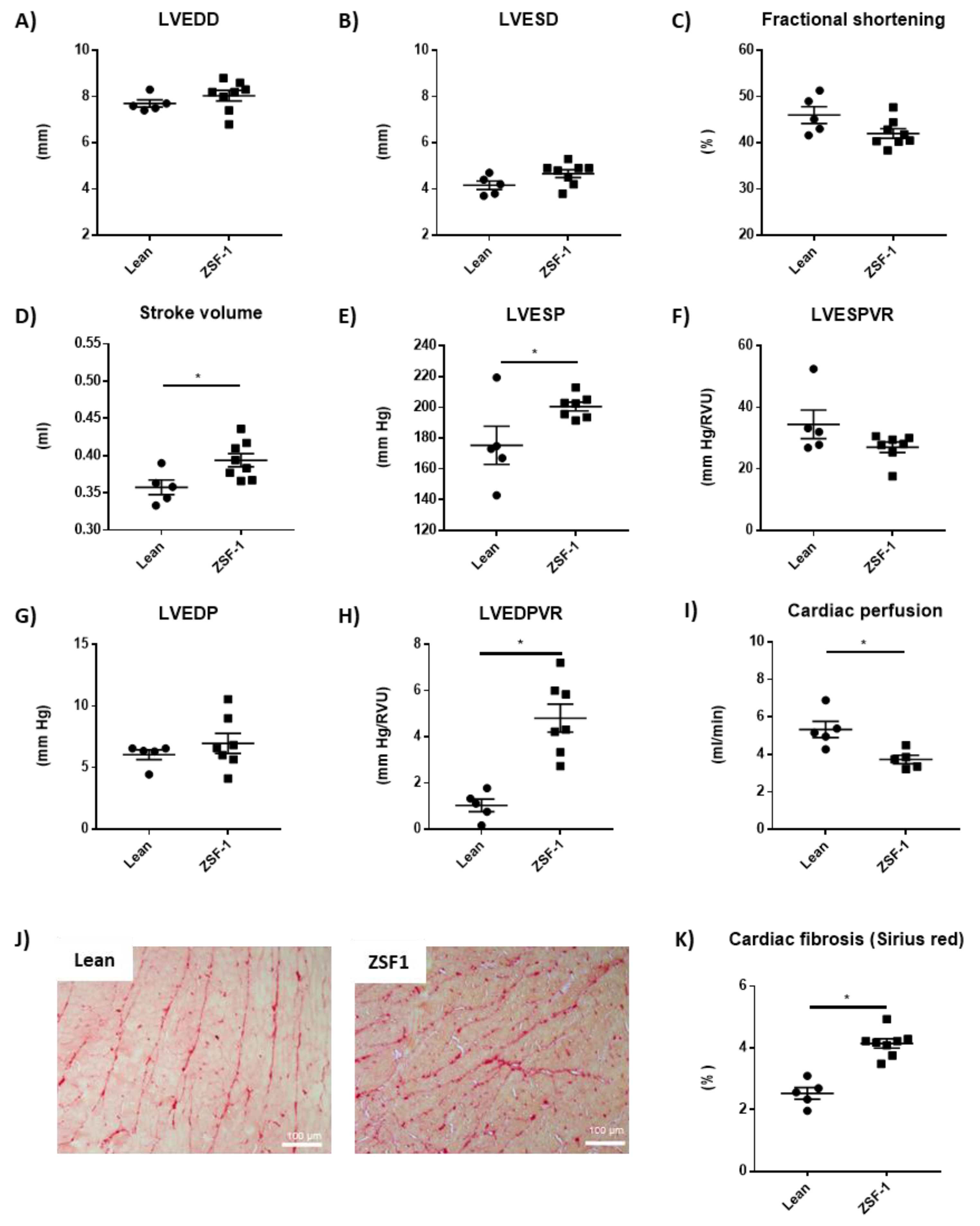
2.1. Renal and Cardiac Characteristics in the ZSF-1 Rats
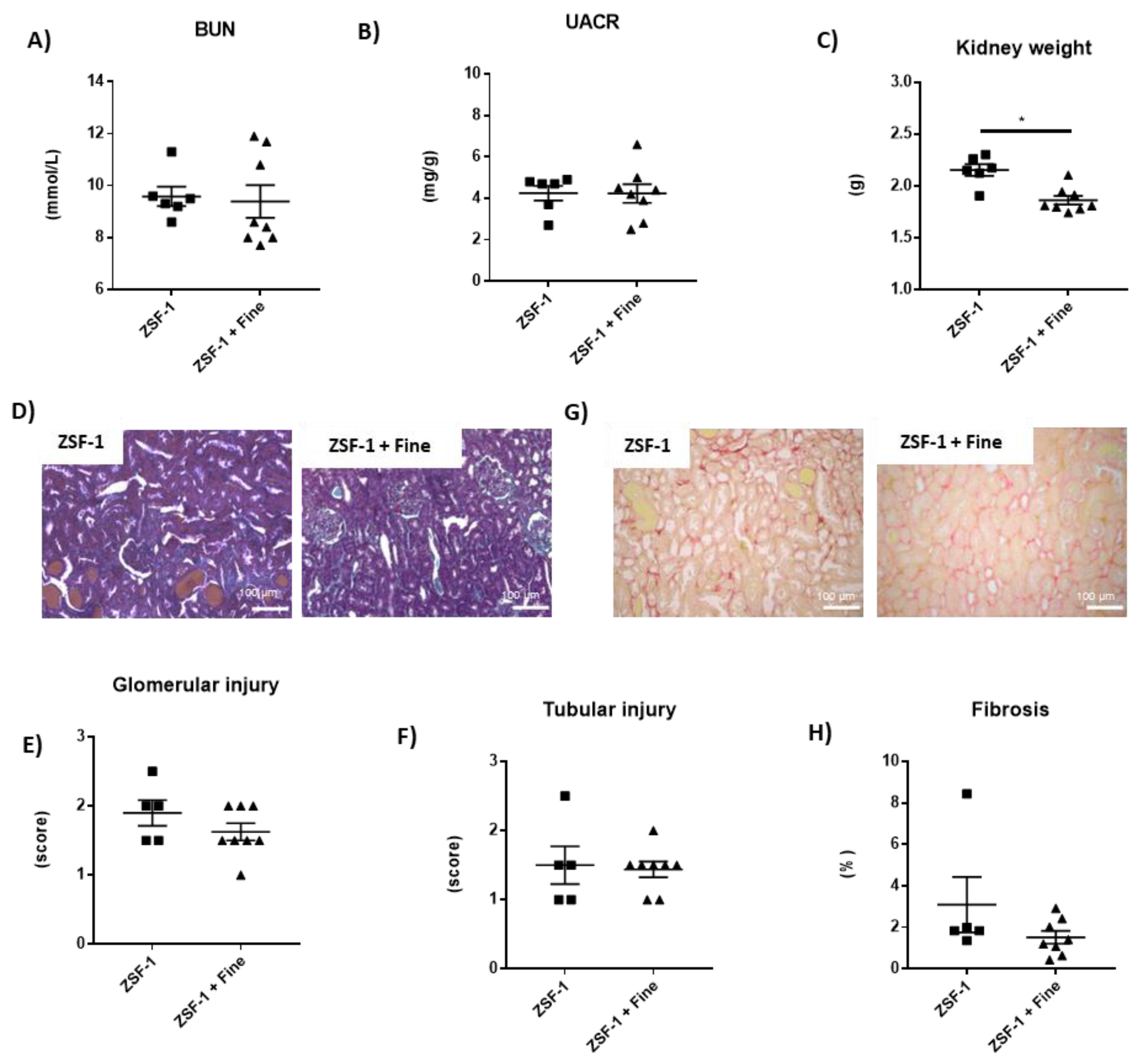
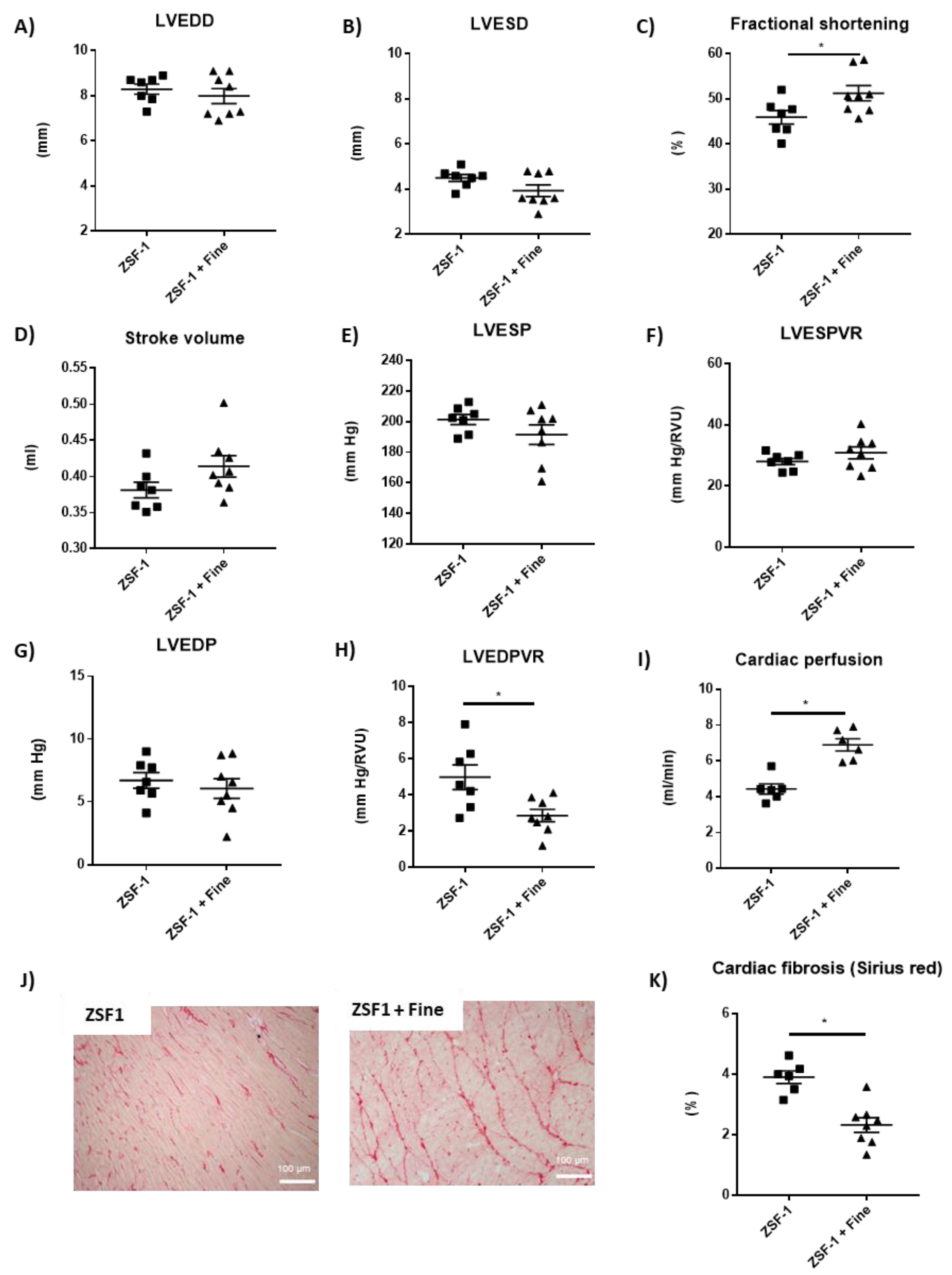
2.2. Impact of Finerenone in Renal and Cardiac Dysfunction in the ZSF-1 Rats
2.3. Involvement of Nitric Oxide (NO) to the Effects of Finerenone on LV Function and LV Tissue Perfusion
3. Discussion
4. Materials and Methods
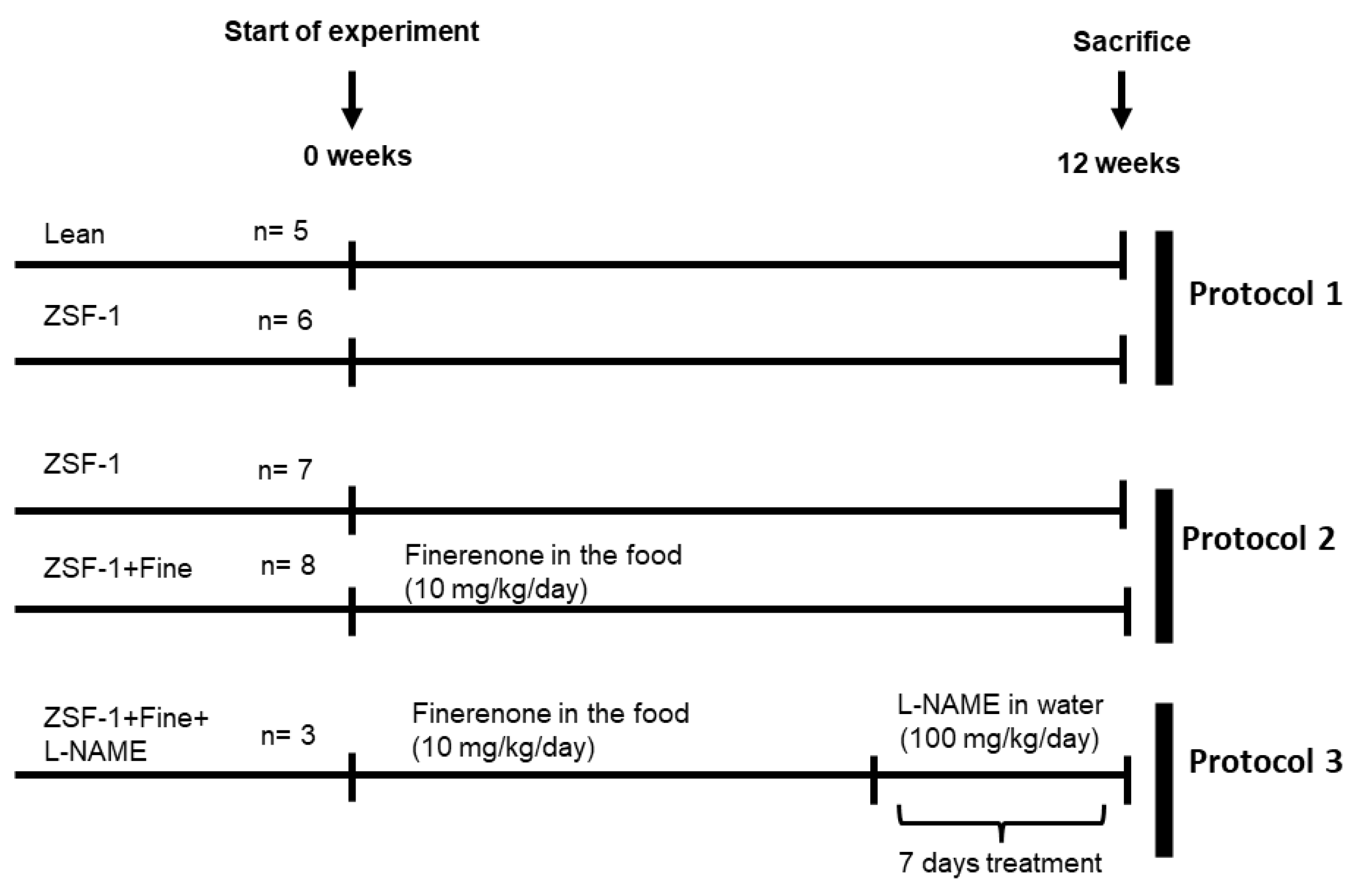
4.1. Experimental Design
4.2. Biochemical Studies
4.3. Echocardiography
4.4. Magnetic Resonance Imaging (MRI)
4.5. Hemodynamic Studies
4.6. Histology
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IDF Diabetes Atlas|Tenth Edition. 2021. Available online: https://diabetesatlas.org/ (accessed on 8 June 2022).
- Anders, H.J.; Huber, T.B.; Isermann, B.; Schiffer, M. CKD in diabetes: Diabetic kidney disease versus nondiabetic kidney disease. Nat. Rev. Nephrol. 2018, 14, 361–377. [Google Scholar] [CrossRef] [PubMed]
- Vijay, K.; Neuen, B.L.; Lerma, E.V. Heart Failure in Patients with Diabetes and Chronic Kidney Disease: Challenges and Opportunities. Cardiorenal Med. 2022, 12, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Lau, E. Sex differences in heart failure with preserved ejection fraction: From traditional risk factors to sex-specific risk factors. Women’s Health 2022, 18, 17455057221140209. [Google Scholar] [CrossRef] [PubMed]
- Paulus, W.J.; Tschöpe, C. A novel paradigm for heart failure with preserved ejection fraction: Comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J. Am. Coll. Cardiol. 2013, 62, 263–271. [Google Scholar] [CrossRef]
- Shenouda, S.M.; Widlansky, M.E.; Chen, K.; Xu, G.; Holbrook, M.; Tabit, C.E.; Hamburg, N.M.; Frame, A.A.; Caiano, T.L.; Kluge, M.A.; et al. Altered mitochondrial dynamics contributes to endothelial dysfunction in diabetes mellitus. Circulation 2011, 124, 444–453. [Google Scholar] [CrossRef]
- Tsai, C.-H.; Pan, C.-T.; Chang, Y.-Y.; Peng, S.-Y.; Lee, P.-C.; Liao, C.-W.; Shun, C.-T.; Li, P.-T.; Wu, V.-C.; Chou, C.-H.; et al. Aldosterone Excess Induced Mitochondria Decrease and Dysfunction via Mineralocorticoid Receptor and Oxidative Stress In Vitro and In Vivo. Biomedicines 2021, 9, 946. [Google Scholar] [CrossRef]
- Gutiérrez-Tenorio, J.; Marín-Royo, G.; Martínez-Martínez, E.; Martín, R.; Miana, M.; López-Andrés, N.; Jurado-López, R.; Gallardo, I.; Luaces, M.; Román, J.A.S.; et al. The role of oxidative stress in the crosstalk between leptin and mineralocorticoid receptor in the cardiac fibrosis associated with obesity. Sci. Rep. 2017, 7, 16802. [Google Scholar] [CrossRef]
- Gueret, A.; Harouki, N.; Favre, J.; Galmiche, G.; Nicol, L.; Henry, J.-P.; Besnier, M.; Thuillez, C.; Richard, V.; Kolkhof, P.; et al. Vascular smooth muscle mineralocorticoid receptor contributes to coronary and left ventricular dysfunction after myocardial infarction. Hypertension 2016, 67, 717–723. [Google Scholar] [CrossRef]
- Kuster, G.M.; Kotlyar, E.; Rude, M.K.; Siwik, D.A.; Liao, R.; Colucci, W.S.; Sam, F. Mineralocorticoid receptor inhibition ameliorates the transition to myocardial failure and decreases oxidative stress and inflammation in mice with chronic pressure overload. Circulation 2005, 111, 420–427. [Google Scholar] [CrossRef]
- Xiong, Y.; Chang, Y.; Hao, J.; Zhang, C.; Yang, F.; Wang, Z.; Liu, Y.; Wang, X.; Mu, S.; Xu, Q. Eplerenone Attenuates Fibrosis in the Contralateral Kidney of UUO Rats by Preventing Macrophage-to-Myofibroblast Transition. Front. Pharmacol. 2021, 12, 620433. [Google Scholar] [CrossRef]
- Barrera-Chimal, J.; Rocha, L.; Amador-Martínez, I.; Pérez-Villalva, R.; González, R.; Cortés-González, C.; Uribe, N.; Ramírez, V.; Berman, N.; Gamba, G.; et al. Delayed spironolactone administration prevents the transition from acute kidney injury to chronic kidney disease through improving renal inflammation. Nephrol. Dial. Transpl. 2019, 34, 794–801. [Google Scholar] [CrossRef]
- Barrera-Chimal, J.; André-Grégoire, G.; Cat, A.N.D.; Lechner, S.M.; Cau, J.; Prince, S.; Kolkhof, P.; Loirand, G.; Sauzeau, V.; Hauet, T.; et al. Benefit of mineralocorticoid receptor antagonism in AKI: Role of vascular smooth muscle Rac1. J. Am. Soc. Nephrol. 2017, 28, 1216–1226. [Google Scholar] [CrossRef] [PubMed]
- Palacios-Ramirez, R.; Lima-Posada, I.; Bonnard, B.; Genty, M.; Fernandez-Celis, A.; Hartleib-Geschwindner, J.; Foufelle, F.; Lopez-Andres, N.; Bamberg, K.; Jaisser, F. Mineralocorticoid Receptor Antagonism Prevents the Synergistic Effect of Metabolic Challenge and Chronic Kidney Disease on Renal Fibrosis and Inflammation in Mice. Front. Physiol. 2022, 13, 656. [Google Scholar] [CrossRef]
- Kolkhof, P.; Nowack, C.; Eitner, F. Nonsteroidal antagonists of the mineralocorticoid receptor. Curr. Opin. Nephrol. Hypertens. 2015, 24, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Kintscher, U.; Bakris, G.L.; Kolkhof, P. Novel non-steroidal mineralocorticoid receptor antagonists in cardiorenal disease. Br. J. Pharmacol. 2022, 179, 3220–3234. [Google Scholar] [CrossRef] [PubMed]
- Bs, M.L.; Barrera-Chimal, J.; Nicol, L.; Rémy-Jouet, I.; Renet, S.; Dumesnil, A.; Wecker, D.; Richard, V.; Kolkhof, P.; Jaisser, F.; et al. Short- and long-term administration of the non-steroidal mineralocorticoid receptor antagonist finerenone opposes metabolic syndrome-related cardio-renal dysfunction. Diabetes Obes. Metab. 2018, 20, 2399–2407. [Google Scholar] [CrossRef]
- Barrera-Chimal, J.; Estrela, G.R.; Lechner, S.M.; Giraud, S.; El Moghrabi, S.; Kaaki, S.; Kolkhof, P.; Hauet, T.; Jaisser, F. The myeloid mineralocorticoid receptor controls inflammatory and fibrotic responses after renal injury via macrophage interleukin-4 receptor signaling. Kidney Int. 2018, 93, 1344–1355. [Google Scholar] [CrossRef]
- Lattenist, L.; Lechner, S.M.; Messaoudi, S.; Le Mercier, A.; El Moghrabi, S.; Prince, S.; Bobadilla, N.A.; Kolkhof, P.; Jaisser, F.; Barrera-Chimal, J. Nonsteroidal Mineralocorticoid Receptor Antagonist Finerenone Protects Against Acute Kidney Injury-Mediated Chronic Kidney Disease: Role of Oxidative Stress. Hypertension 2017, 69, 870–878. [Google Scholar] [CrossRef]
- Pitt, B.; Kober, L.; Ponikowski, P.; Gheorghiade, M.; Filippatos, G.; Krum, H.; Nowack, C.; Kolkhof, P.; Kim, S.-Y.; Zannad, F. Safety and tolerability of the novel non-steroidal mineralocorticoid receptor antagonist BAY 94-8862 in patients with chronic heart failure and mild or moderate chronic kidney disease: A randomized, double-blind trial. Eur. Heart J. 2013, 34, 2453–2463. [Google Scholar] [CrossRef]
- Bakris, G.L.; Agarwal, R.; Anker, S.D.; Pitt, B.; Ruilope, L.M.; Rossing, P.; Kolkhof, P.; Nowack, C.; Schloemer, P.; Joseph, A.; et al. Effect of Finerenone on Chronic Kidney Disease Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2020, 383, 2219–2229. [Google Scholar] [CrossRef]
- Pitt, B.; Filippatos, G.; Agarwal, R.; Anker, S.D.; Bakris, G.L.; Rossing, P.; Joseph, A.; Kolkhof, P.; Nowack, C.; Schloemer, P.; et al. Cardiovascular Events with Finerenone in Kidney Disease and Type 2 Diabetes. N. Engl. J. Med. 2021, 385, 2252–2263. [Google Scholar] [CrossRef] [PubMed]
- Kolkhof, P.; Joseph, A.; Kintscher, U. Nonsteroidal mineralocorticoid receptor antagonism for cardiovascular and renal disorders–New perspectives for combination therapy. Pharmacol. Res. 2021, 172, 105859. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R.; Rossignol, P.; Romero, A.; Garza, D.; Mayo, M.R.; Warren, S.; Ma, J.; White, W.B.; Williams, B. Patiromer versus placebo to enable spironolactone use in patients with resistant hypertension and chronic kidney disease (AMBER): A phase 2, randomised, double-blind, placebo-controlled trial. Lancet 2019, 394, 1540–1550. [Google Scholar] [CrossRef] [PubMed]
- Bonnard, B.; Pieronne-Deperrois, M.; Djerada, Z.; Elmoghrabi, S.; Kolkhof, P.; Ouvrard-Pascaud, A.; Mulder, P.; Jaisser, F.; Messaoudi, S. Mineralocorticoid receptor antagonism improves diastolic dysfunction in chronic kidney disease in mice. J. Mol. Cell. Cardiol. 2018, 121, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Bode, D.; Semmler, L.; Wakula, P.; Hegemann, N.; Primessnig, U.; Beindorff, N.; Powell, D.; Dahmen, R.; Ruetten, H.; Oeing, C.; et al. Dual SGLT-1 and SGLT-2 inhibition improves left atrial dysfunction in HFpEF. Cardiovasc. Diabetol. 2021, 20, 1–14. [Google Scholar] [CrossRef]
- Hohendanner, F.; Bode, D.; Primessnig, U.; Guthof, T.; Doerr, R.; Jeuthe, S.; Reimers, S.; Zhang, K.; Bach, D.; Wakula, P.; et al. Cellular mechanisms of metabolic syndrome-related atrial decompensation in a rat model of HFpEF. J. Mol. Cell. Cardiol. 2018, 115, 10–19. [Google Scholar] [CrossRef]
- Leite, S.; Oliveira-Pinto, J.; Tavares-Silva, M.; Abdellatif, M.; Fontoura, D.; Falcão-Pires, I.; Leite-Moreira, A.F.; Lourenço, A.P. Echocardiography and invasive hemodynamics during stress testing for diagnosis of heart failure with preserved ejection fraction: An experimental study. Am. J. Physiol. Heart Circ. Physiol. 2015, 308, H1556–H1563. [Google Scholar] [CrossRef]
- Bode, D.; Rolim, N.P.; Guthof, T.; Hegemann, N.; Wakula, P.; Primessnig, U.; Berre, A.M.O.; Adams, V.; Wisløff, U.; Pieske, B.M.; et al. Effects of different exercise modalities on cardiac dysfunction in heart failure with preserved ejection fraction. ESC Heart Fail. 2021, 8, 1806–1818. [Google Scholar] [CrossRef]
- Hegemann, N.; Primessnig, U.; Bode, D.; Wakula, P.; Beindorff, N.; Klopfleisch, R.; Michalick, L.; Grune, J.; Hohendanner, F.; Messroghli, D.; et al. Right-ventricular dysfunction in HFpEF is linked to altered cardiomyocyte Ca2+ homeostasis and myofilament sensitivity. ESC Heart Fail. 2021, 8, 3130–3144. [Google Scholar] [CrossRef]
- Schauer, A.; Adams, V.; Augstein, A.; Jannasch, A.; Draskowski, R.; Kirchhoff, V.; Goto, K.; Mittag, J.; Galli, R.; Männel, A.; et al. Sacubitril/Valsartan Improves Diastolic Function but not Skeletal Muscle Function in a Rat Model of HFpEF. Int. J. Mol. Sci. 2021, 22, 3570. [Google Scholar] [CrossRef]
- Lodhi, A.H.; Ahmad, F.-U.; Furwa, K.; Madni, A. Role of Oxidative Stress and Reduced Endogenous Hydrogen Sulfide in Diabetic Nephropathy. Drug Des. Devel. Ther. 2021, 15, 1031–1043. [Google Scholar] [CrossRef] [PubMed]
- Dong, D.; Fan, T.-T.; Ji, Y.-S.; Yu, J.-Y.; Wu, S.; Zhang, L. Spironolactone alleviates diabetic nephropathy through promoting autophagy in podocytes. Int. Urol. Nephrol. 2019, 51, 755–764. [Google Scholar] [CrossRef]
- Koszegi, S.; Molnar, A.; Lenart, L.; Hodrea, J.; Balogh, D.B.; Lakat, T.; Szkibinszkij, E.; Hosszu, A.; Sparding, N.; Genovese, F.; et al. RAAS inhibitors directly reduce diabetes-induced renal fibrosis via growth factor inhibition. J. Physiol. 2019, 597, 193–209. [Google Scholar] [CrossRef] [PubMed]
- Lian, M.; Hewitson, T.D.; Wigg, B.; Samuel, C.S.; Chow, F.; Becker, G.J. Long-term mineralocorticoid receptor blockade ameliorates progression of experimental diabetic renal disease. Nephrol. Dial. Transpl. 2012, 27, 906–912. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Gong, W.; He, M.; Liu, Y.; Yang, Y.; Wang, M.; Wu, M.; Guo, S.; Yu, Y.; Wang, X.; et al. Spironolactone Protects against Diabetic Cardiomyopathy in Streptozotocin-Induced Diabetic Rats. J. Diabetes Res. 2018, 2018, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, U.B.; Patil, P.D.; Chandrayan, G.; Patil, C.R.; Agrawal, Y.O.; Ojha, S.; Goyal, S.N. Eplerenone pretreatment protects the myocardium against ischaemia/reperfusion injury through the phosphatidylinositol 3-kinase/Akt-dependent pathway in diabetic rats. Mol. Cell. Biochem. 2018, 446, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Vranic, A.; Simovic, S.; Ristic, P.; Nikolic, T.; Stojic, I.; Srejovic, I.; Zivkovic, V.; Jakovljevic, V.; Djuric, D. The acute effects of different spironolactone doses on cardiac function in streptozotocin-induced diabetic rats. Can. J. Physiol. Pharmacol. 2017, 95, 1343–1350. [Google Scholar] [CrossRef] [PubMed]
- Bender, S.B.; Demarco, V.G.; Padilla, J.; Jenkins, N.T.; Habibi, J.; Garro, M.; Pulakat, L.; Aroor, A.R.; Jaffe, I.Z.; Sowers, J.R. Mineralocorticoid receptor antagonism treats obesity-associated cardiac diastolic dysfunction. Hypertension 2015, 65, 1082–1088. [Google Scholar] [CrossRef]
- Ramírez, E.; Klett-Mingo, M.; Ares-Carrasco, S.; Picatoste, B.; Ferrarini, A.; Rupérez, F.J.; Caro-Vadillo, A.; Barbas, C.; Egido, J.; Tuñón, J.; et al. Eplerenone attenuated cardiac steatosis, apoptosis and diastolic dysfunction in experimental type-II diabetes. Cardiovasc. Diabetol. 2013, 12, 172. [Google Scholar] [CrossRef]
- Patel, B.M.; Kakadiya, J.; Goyal, R.K.; Mehta, A.A. Effect of spironolactone on cardiovascular complications associated with type-2 diabetes in rats. Exp. Clin. Endocrinol. Diabetes 2013, 121, 441–447. [Google Scholar] [CrossRef]
- Pessôa, B.S.; Peixoto, E.B.; Papadimitriou, A.; de Faria, J.M.L.; de Faria, J.B.L. Spironolactone improves nephropathy by enhancing glucose-6-phosphate dehydrogenase activity and reducing oxidative stress in diabetic hypertensive rat. J. Renin Angiotensin Aldosterone Syst. 2012, 13, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Martinez-Vasquez, D.; Mendez, G.P.; Toniolo, M.F.; Yao, T.M.; Oestreicher, E.M.; Kikuchi, T.; Lapointe, N.; Pojoga, L.; Williams, G.H.; et al. Mineralocorticoid receptor antagonist reduces renal injury in rodent models of types 1 and 2 diabetes mellitus. Endocrinology 2006, 147, 5363–5373. [Google Scholar] [CrossRef] [PubMed]
- Hirohama, D.; Nishimoto, M.; Ayuzawa, N.; Kawarazaki, W.; Fujii, W.; Oba, S.; Shibata, S.; Marumo, T.; Fujita, T. Activation of Rac1-Mineralocorticoid Receptor Pathway Contributes to Renal Injury in Salt-Loaded db/db Mice. Hypertension 2021, 78, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Li, X.; Peng, C.; Gao, R.; Ma, L.; Hu, J.; Luo, T.; Qing, H.; Wang, Y.; Ge, Q.; et al. miR-196b-5p-enriched extracellular vesicles from tubular epithelial cells mediated aldosterone-induced renal fibrosis in mice with diabetes. BMJ Open Diabetes Res. Care 2020, 8, e001101. [Google Scholar] [CrossRef]
- Riboulet, W. ZSF1 rat: A model of chronic cardiac and renal diseases in the context of metabolic syndrome. Characterization with anti-oxidant, mineralocorticoid receptor antagonist and aldosterone synthase inhibitor. Hum. Health Pathol. Univ. Lorraine 2015. Available online: https://hal.univ-lorraine.fr/tel-01751560 (accessed on 1 December 2022).
- Feraco, A.; Marzolla, V.; Scuteri, A.; Armani, A.; Caprio, M. Mineralocorticoid Receptors in Metabolic Syndrome: From Physiology to Disease. Trends Endocrinol. Metab. 2020, 31, 205–217. [Google Scholar] [CrossRef]
- Nagase, M.; Yoshida, S.; Shibata, S.; Nagase, T.; Gotoda, T.; Ando, K.; Fujita, T. Enhanced aldosterone signaling in the early nephropathy of rats with metabolic syndrome: Possible contribution of fat-derived factors. J. Am. Soc. Nephrol. 2006, 17, 3438–3446. [Google Scholar] [CrossRef]
- Cat, N.D.; Friederich-Persson, M.; White, A.; Touyz, R.M. Adipocytes, aldosterone and obesity-related hypertension. J. Mol. Endocrinol. 2016, 57, F7–F21. [Google Scholar] [CrossRef]
- Pereira, D.; Azevedo, I.; Monteiro, R.; Martins, M.J. 11β-Hydroxysteroid dehydrogenase type 1: Relevance of its modulation in the pathophysiology of obesity, the metabolic syndrome and type 2 diabetes mellitus. Diabetes. Obes. Metab. 2012, 14, 869–881. [Google Scholar] [CrossRef]
- Lee, S.H.; Yoo, T.-H.; Nam, B.-Y.; Kim, D.K.; Li, J.J.; Jung, D.-S.; Kwak, S.-J.; Ryu, D.-R.; Han, S.H.; Lee, J.E.; et al. Activation of local aldosterone system within podocytes is involved in apoptosis under diabetic conditions. Am. J. Physiol. Ren. Physiol. 2009, 297, F1381–F1390. [Google Scholar] [CrossRef]
- Jaisser, F.; Farman, N. Emerging roles of the mineralocorticoid receptor in pathology: Toward new paradigms in clinical pharmacology. Pharmacol. Rev. 2016, 68, 49–75. [Google Scholar] [CrossRef] [PubMed]
- Cuijpers, I.; Simmonds, S.; Van Bilsen, M.; Czarnowska, E.; Miqueo, A.G.; Heymans, S.; Kuhn, A.; Mulder, P.; Ratajska, A.; Jones, E.A.V.; et al. Microvascular and lymphatic dysfunction in HFpEF and its associated comorbidities. Basic Res. Cardiol. 2020, 115, 1–15. [Google Scholar] [CrossRef]
- De Santis, V.; Vitale, D.; Santoro, A.; Magliocca, A.; Porto, A.G.; Nencini, C.; Tritapepe, L. Ivabradine: Potential clinical applications in critically ill patients. Clin. Res. Cardiol. 2013, 102, 171–178. [Google Scholar] [CrossRef]
- Prabhakar, S.; Starnes, J.; Shi, S.; Lonis, B.; Tran, R. Diabetic nephropathy is associated with oxidative stress and decreased renal nitric oxide production. J. Am. Soc. Nephrol. 2007, 18, 2945–2952. [Google Scholar] [CrossRef]
- Schmederer, Z.; Rolim, N.; Bowen, T.S.; Linke, A.; Wisloff, U.; Adams, V. Endothelial function is disturbed in a hypertensive diabetic animal model of HFpEF: Moderate continuous vs. high intensity interval training. Int. J. Cardiol. 2018, 273, 147–154. [Google Scholar] [CrossRef] [PubMed]
- RGonzález-Blázquez, R.; Somoza, B.; Gil-Ortega, M.; Ramos, M.M.; Cortijo, D.R.; Vega-Martín, E.; Schulz, A.; Ruilope, L.M.; Kolkhof, P.; Kreutz, R.; et al. Finerenone Attenuates Endothelial Dysfunction and Albuminuria in a Chronic Kidney Disease Model by a Reduction in Oxidative Stress. Front. Pharmacol. 2018, 9, 1131. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-H.; Farooq, M.A.; Gaertner, S.; Bruckert, C.; Qureshi, A.W.; Lee, H.-H.; Benrahla, D.; Pollet, B.; Stephan, D.; Ohlmann, P.; et al. Empagliflozin improved systolic blood pressure, endothelial dysfunction and heart remodeling in the metabolic syndrome ZSF1 rat. Cardiovasc. Diabetol. 2020, 19, 1–14. [Google Scholar] [CrossRef]
- Kolkhof, P.; Hartmann, E.; Freyberger, A.; Pavkovic, M.; Mathar, I.; Sandner, P.; Droebner, K.; Joseph, A.; Hüser, J.; Eitner, F. Effects of Finerenone Combined with Empagliflozin in a Model of Hypertension-Induced End-Organ Damage. Am. J. Nephrol. 2021, 52, 642–652. [Google Scholar] [CrossRef]
- Provenzano, M.; Puchades, M.J.; Garofalo, C.; Jongs, N.; D’Marco, L.; Andreucci, M.; De Nicola, L.; Gorriz, J.L.; Heerspink, H.J. Albuminuria-Lowering Effect of Dapagliflozin, Eplerenone, and Their Combination in Patients with Chronic Kidney Disease: A Randomized Crossover Clinical Trial. J. Am. Soc. Nephrol. 2022, 33, 1569–1580. [Google Scholar] [CrossRef]
- Fang, Y.; Nicol, L.; Harouki, N.; Monteil, C.; Wecker, D.; Debunne, M.; Bauer, F.; Lallemand, F.; Richard, V.; Thuillez, C.; et al. Improvement of left ventricular diastolic function induced by β-blockade: A comparison between nebivolol and metoprolol. J. Mol. Cell. Cardiol. 2011, 51, 168–176. [Google Scholar] [CrossRef]
- Peschanski, N.; Harouki, N.; Soulie, M.; Lachaux, M.; Nicol, L.; Remy-Jouet, I.; Henry, J.; Dumesnil, A.; Renet, S.; Fougerousse, F.; et al. Transient heart rate reduction improves acute decompensated heart failure-induced left ventricular and coronary dysfunction. ESC Heart Fail. 2021, 8, 1085–1095. [Google Scholar] [CrossRef] [PubMed]
| ZSF1 + Fine n = 8 | ZSF1 + Fine + L-NAME n = 3 | |||||
|---|---|---|---|---|---|---|
| SBP | 195 | ± | 5.5 | 202 | ± | 5.8 |
| HR | 327.3 | ± | 6.8 | 291 | ± | 7.0 * |
| LVESP | 191.5 | ± | 6.4 | 205.4 | ± | 5.5 |
| dP/dt max | 11,467.8 | ± | 504.4 | 9757 | ± | 419.0 * |
| LVESPVR | 30.8 | ± | 1.9 | 29.7 | ± | 1.5 |
| LVEDP | 6.05 | ± | 0.7 | 14.6 | ± | 3.0 * |
| dP/dt min | 9979.5 | ± | 641.4 | 4420.6 | ± | 177.4 * |
| Tau | 12.38 | ± | 0.5 | 17.29 | ± | 0.5 * |
| LVEDPVR | 2.86 | ± | 0.3 | 3.76 | ± | 0.1 * |
| LV tissue perfusion | 6.9 | ± | 0.3 | 3.6 | ± | 0.2 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lima-Posada, I.; Stephan, Y.; Soulié, M.; Palacios-Ramirez, R.; Bonnard, B.; Nicol, L.; Kolkhof, P.; Jaisser, F.; Mulder, P. Benefits of the Non-Steroidal Mineralocorticoid Receptor Antagonist Finerenone in Metabolic Syndrome-Related Heart Failure with Preserved Ejection Fraction. Int. J. Mol. Sci. 2023, 24, 2536. https://doi.org/10.3390/ijms24032536
Lima-Posada I, Stephan Y, Soulié M, Palacios-Ramirez R, Bonnard B, Nicol L, Kolkhof P, Jaisser F, Mulder P. Benefits of the Non-Steroidal Mineralocorticoid Receptor Antagonist Finerenone in Metabolic Syndrome-Related Heart Failure with Preserved Ejection Fraction. International Journal of Molecular Sciences. 2023; 24(3):2536. https://doi.org/10.3390/ijms24032536
Chicago/Turabian StyleLima-Posada, Ixchel, Yohan Stephan, Matthieu Soulié, Roberto Palacios-Ramirez, Benjamin Bonnard, Lionel Nicol, Peter Kolkhof, Frederic Jaisser, and Paul Mulder. 2023. "Benefits of the Non-Steroidal Mineralocorticoid Receptor Antagonist Finerenone in Metabolic Syndrome-Related Heart Failure with Preserved Ejection Fraction" International Journal of Molecular Sciences 24, no. 3: 2536. https://doi.org/10.3390/ijms24032536
APA StyleLima-Posada, I., Stephan, Y., Soulié, M., Palacios-Ramirez, R., Bonnard, B., Nicol, L., Kolkhof, P., Jaisser, F., & Mulder, P. (2023). Benefits of the Non-Steroidal Mineralocorticoid Receptor Antagonist Finerenone in Metabolic Syndrome-Related Heart Failure with Preserved Ejection Fraction. International Journal of Molecular Sciences, 24(3), 2536. https://doi.org/10.3390/ijms24032536






