Comparison of Tandem Mass Spectrometry and the Fluorometric Method—Parallel Phenylalanine Measurement on a Large Fresh Sample Series and Implications for Newborn Screening for Phenylketonuria
Abstract
1. Introduction
2. Results
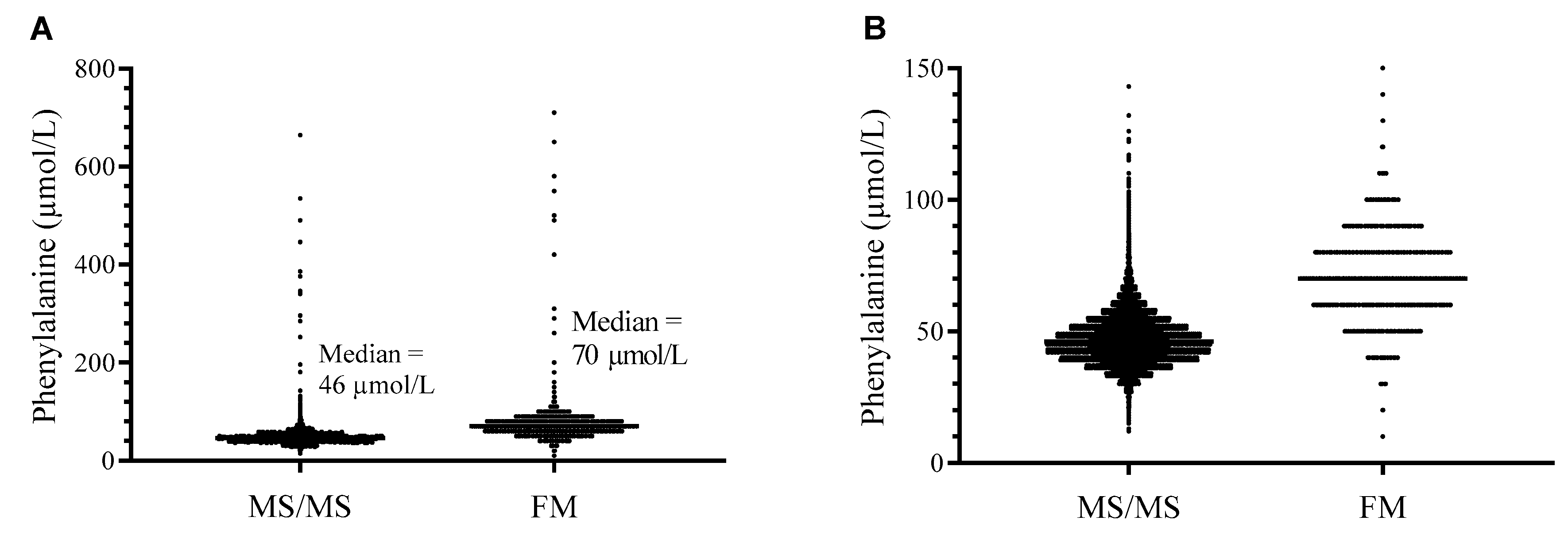
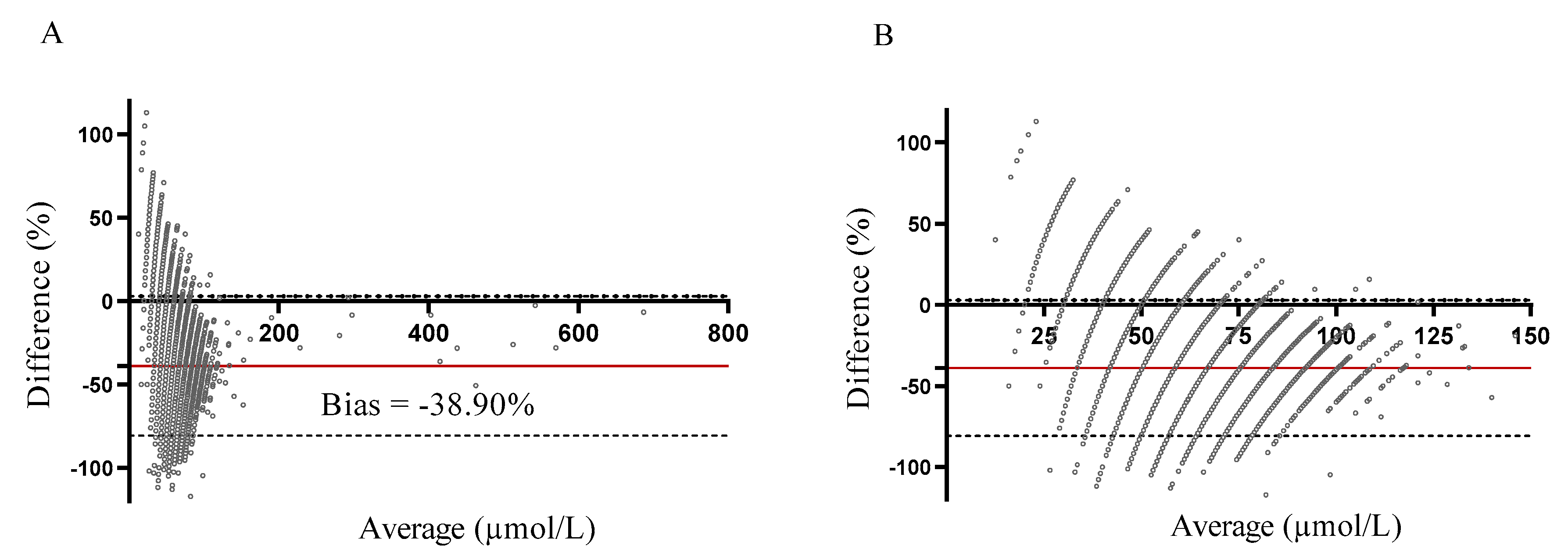
2.1. Methods Comparison
2.2. Methods Comparison—Implication for NBS
Adjustment of the Cut-Off Value for the MS/MS Method
3. Discussion
4. Materials and Methods
4.1. Subjects
4.2. Specimen Collection
4.3. The Fluorometric Method
4.4. MS/MS Method
4.5. Statistical Analysis
4.6. Implications for NBS for PKU
4.6.1. Current Diagnostic Algorithm for NBS
4.6.2. Method Comparison—Implications for NBS
4.6.3. Adjustment of the Cut-Off Value—The MS/MS Method
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Pourfarzam, M.; Zadhoush, F. Newborn screening for inherited metabolic disorders; news and views. J. Res. Med. Sci. 2013, 18, 801–808. [Google Scholar]
- Repič Lampret, B.; Remec, Ž.I.; Drole Torkar, A.; Žerjav Tanšek, M.; Šmon, A.; Koračin, V.; Čuk, V.; Perko, D.; Ulaga, B.; Jelovšek, A.M.; et al. Expanded newborn screening program in Slovenia using tandem mass spectrometry and confirmatory next generation sequencing genetic testing. Zdr. Varst. 2020, 95, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, S. The Phenylalanine Hydroxylating System. Adv. Enzymol. Relat. Areas Mol. Biol. 2006, 67, 77–264. [Google Scholar] [CrossRef]
- Grošelj, U.; Tanšek, M.Ž.; Podkrajšek, K.T.; Battelino, T. Genetske in klinične značilnosti bolnikov s fenilketonurijo v Sloveniji. Zdr. Vestn. 2013, 82, 767–777. [Google Scholar]
- Maxim, L.D.; Niebo, R.; Utell, M.J. Screening tests: A review with examples. Inhal. Toxicol. 2014, 26, 811–828. [Google Scholar] [CrossRef]
- Blau, N.; Van Spronsen, F.J.; Levy, H.L. Phenylketonuria. Lancet 2010, 376, 1417–1427. [Google Scholar] [CrossRef]
- Groselj, U.; Tansek, M.Z.; Kovac, J.; Hovnik, T.; Podkrajsek, K.T.; Battelino, T. Five novel mutations and two large deletions in a population analysis of the phenylalanine hydroxylase gene. Mol. Genet. Metab. 2012, 106, 142–148. [Google Scholar] [CrossRef]
- MacLeod, E.L.; Ney, D.M. Nutritional management of phenylketonuria. Ann. Nestle 2010, 68, 58–69. [Google Scholar] [CrossRef]
- Shoraka, H.R.; Haghdoost, A.A.; Baneshi, M.R.; Bagherinezhad, Z.; Zolala, F. Global prevalence of classic phenylketonuria based on neonatal screening program data: Systematic review and meta-analysis. Korean J. Pediatr. 2020, 63, 34–43. [Google Scholar] [CrossRef]
- Groselj, U.; Tansek, M.Z.; Battelino, T. Fifty years of phenylketonuria newborn screening—A great success for many, but what about the rest? Mol. Genet. Metab. 2014, 113, 8–10. [Google Scholar] [CrossRef]
- Battelino, T.; Kržišnik, C.; Pavlin, K. Early detection and follow up of children with phenylketonuria in Slovenia. Zdr. Vestn 1994, 63, 25–28. [Google Scholar]
- Levy, H.L. Newborn screening by tandem mass spectrometry: A new era. Clin. Chem. 1998, 44, 2401–2402. [Google Scholar] [CrossRef] [PubMed]
- Šmon, A.; Grošelj, U.; Žerjav Tanšek, M.; Biček, A.; Oblak, A.; Zupančič, M.; Kržišnik, C.; Repič Lampret, B.; Murko, S.; Hojker, S.; et al. Newborn screening in Slovenia. Zdr. Varst. 2015, 54, 86–90. [Google Scholar] [CrossRef]
- van Spronsen, F.J.; Blau, N.; Harding, C.; Burlina, A.; Longo, N.; Bosch, A.M. Phenylketonuria. Nat. Rev. Dis. Prim. 2021, 7, 36. [Google Scholar] [CrossRef]
- Smon, A.; Repic Lampret, B.; Groselj, U.; Zerjav Tansek, M.; Kovac, J.; Perko, D.; Bertok, S.; Battelino, T.; Trebusak Podkrajsek, K. Next generation sequencing as a follow-up test in an expanded newborn screening programme. Clin. Biochem. 2018, 52, 48–55. [Google Scholar] [CrossRef]
- Vittozzi, L.; Burgard, P.; Cornel, M.; Hoffmann, G.F.; Lindner, M.; Loeber, J.G.; Rigter, T.; Rupp, K.; Taruscio, D.; Weinreich, S. Executive Report to the European Commission on newborn screening in the European Union Table of Contents. Available online: https://isns-neoscreening.org/wp-content/uploads/2016/06/Executive-Report-to-EC-FINAL.pdf (accessed on 16 November 2021).
- Asano, Y. Screening and development of enzymes for determination and transformation of amino acids. Biosci. Biotechnol. Biochem. 2019, 83, 1402–1416. [Google Scholar] [CrossRef]
- MacReady, R.; Hussey, M. Newborn phenylketonuria detection program in Massachussets. Am. J. Public Health Nations Health 1964, 54, 2075–2091. [Google Scholar] [CrossRef] [PubMed]
- Chace, D.H.; Kalas, T.A.; Naylor, E.W. Use of Tandem Mass Spectrometry for Multianalyte Screening of Dried Blood Specimens from Newborns. Clin. Chem. 2003, 49, 1797–1817. [Google Scholar] [CrossRef] [PubMed]
- Weitzel, K.; Chemie, F.; Rev, M.S.; Introduction, I.; Reference, C. The use of mass spectormetry to analyse dried blood spots. WHO Libr. Cat. Data 2011, 221–235. [Google Scholar] [CrossRef]
- Koracin, V.; Mlinaric, M.; Baric, I.; Brincat, I.; Djordjevic, M.; Drole Torkar, A.; Fumic, K.; Kocova, M.; Milenkovic, T.; Moldovanu, F.; et al. Current Status of Newborn Screening in Southeastern Europe. Front. Pediatr. 2021, 9, 391. [Google Scholar] [CrossRef]
- Sebastião, F.M.; Burin, M.G.; Civallero, G.; Tirelli, K.M.; Sitta, A.; de Coelho, D.M.; Vargas, C.R.; Wajner, M.; Giugliani, R.; de Bitencourt, F.H.; et al. Monitoring of Phenylalanine Levels in Patients with Phenylketonuria Using Dried Blood Spots: A Comparison of Two Methods. J. Inborn Errors Metab. Screen. 2020, 8. [Google Scholar] [CrossRef]
- Allard, P.; Cowell, L.D.; Zytkovicz, T.H.; Korson, M.S.; Ampola, M.G. Determination of phenylalanine and tyrosine in dried blood specimens by ion-exchange chromatography using the Hitachi L-8800 analyzer. Clin. Biochem. 2004, 37, 857–862. [Google Scholar] [CrossRef]
- Carducci, C.; Birarelli, M.; Leuzzi, V.; Santagata, G.; Serafini, P.; Antonozzi, I. Automated method for the measurement of amino acids in urine by high-performance liquid chromatography. J. Chromatogr. A 1996, 729, 173–180. [Google Scholar] [CrossRef]
- Gregory, C.O.; Yu, C.; Singh, R.H. Blood phenylalanine monitoring for dietary compliance among patients with phenylketonuria: Comparison of methods. Genet. Med. 2007, 9, 761–765. [Google Scholar] [CrossRef] [PubMed]
- Kazanasmaz, H.; Karaca, M. Comparison of Fluorometric İmmunoassay and Tandem Mass Spectrometry Methods Used in the Diagnosis of Phenylalanine Metabolism Disorders in Turkish Children. Istanbul Med. J. 2019, 20, 279–284. [Google Scholar] [CrossRef]
- Rivero, A.; Allue, J.A.; Grijalba, A.; Palacios, M.; Garcia Merlo, S. Comparison of two different methods for measurement of phenylalanine in dried blood spots. Clin. Chem. Lab. Med. 2000, 38, 773–776. [Google Scholar] [CrossRef] [PubMed]
- Baieli, S.; Pavone, L.; Meli, C.; Fiumara, A.; Coleman, M. Autism and phenylketonuria. J. Autism Dev. Disord. 2003, 33, 201–204. [Google Scholar] [CrossRef]
- Waisbren, S.E.; Albers, S.; Amato, S.; Ampola, M.; Brewster, T.G.; Demmer, L.; Eaton, R.B.; Greenstein, R.; Korson, M.; Larson, C.; et al. Effect of Expanded Newborn Screening for Biochemical Genetic Disorders on Child Outcomes and Parental Stress. J. Am. Med. Assoc. 2003, 290, 2564–2572. [Google Scholar] [CrossRef]
- Schmidt, J.L.; Castellanos-Brown, K.; Childress, S.; Bonhomme, N.; Oktay, J.S.; Terry, S.F.; Kyler, P.; Davidoff, A.; Greene, C. The impact of false-positive newborn screening results on families: A qualitative study. Genet. Med. 2012, 14, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Lipstein, E.A.; Perrin, J.M.; Waisbren, S.E.; Prosser, L.A. Impact of false-positive newborn metabolic screening results on earlyhealth care utilization. Genet Med. 2009, 1, 716–721. [Google Scholar] [CrossRef]
- Tu, W.J.; He, J.; Chen, H.; Shi, X.D.; Li, Y. Psychological effects of false-positive results in expanded newborn screening in China. PLoS ONE 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Sharman, R.; Sullivan, K.; Young, R.; McGill, J. A preliminary investigation of the role of the phenylalanine:tyrosine ratio in children with early and continuously treated phenylketonuria: Toward identification of “safe” levels. Dev. Neuropsychol. 2010, 35, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Sharman, R.; Sullivan, K.A.; Young, R.M.; McGill, J.J. Tyrosine monitoring in children with early and continuously treated phenylketonuria: Results of an international practice survey. J. Inherit. Metab. Dis. 2010, 33, 417–420. [Google Scholar] [CrossRef]
- Regier, D.S.; Greene, C.L. Phenylalanine Hydroxylase Deficiency Summary Diagnosis Suggestive Findings. 2019:1-23. Available online: https://www.ncbi.nlm.nih.gov/books/NBK1504/pdf/Bookshelf_NBK1504.pdf (accessed on 16 November 2021).
- Spronsen, F.; Rijn, M.; Bekhof, J.; Koch, R.; Peter GA, S. Phenylketonuria: Tyrosine supplementation in phenylalanine-restricted diets. Am. J. Clin. Nutr. 2001, 73, 153–157. [Google Scholar] [CrossRef]
- Groselj, U.; Murko, S.; Zerjav Tansek, M.; Kovac, J.; Trampus Bakija, A.; Repic Lampret, B.; Battelino, T. Comparison of tandem mass spectrometry and amino acid analyzer for phenylalanine and tyrosine monitoring-Implications for clinical management of patients with hyperphenylalaninemia. Clin. Biochem. 2015, 48, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Perko, D.; Repic Lampret, B.; Remec, Z.I.; Zerjav Tansek, M.; Drole Torkar, A.; Krhin, B.; Bicek, A.; Oblak, A.; Battelino, T.; Groselj, U. Optimizing the Phenylalanine Cut-Off Value in a Newborn Screening Program. Genes 2022, 13, 517. [Google Scholar] [CrossRef]

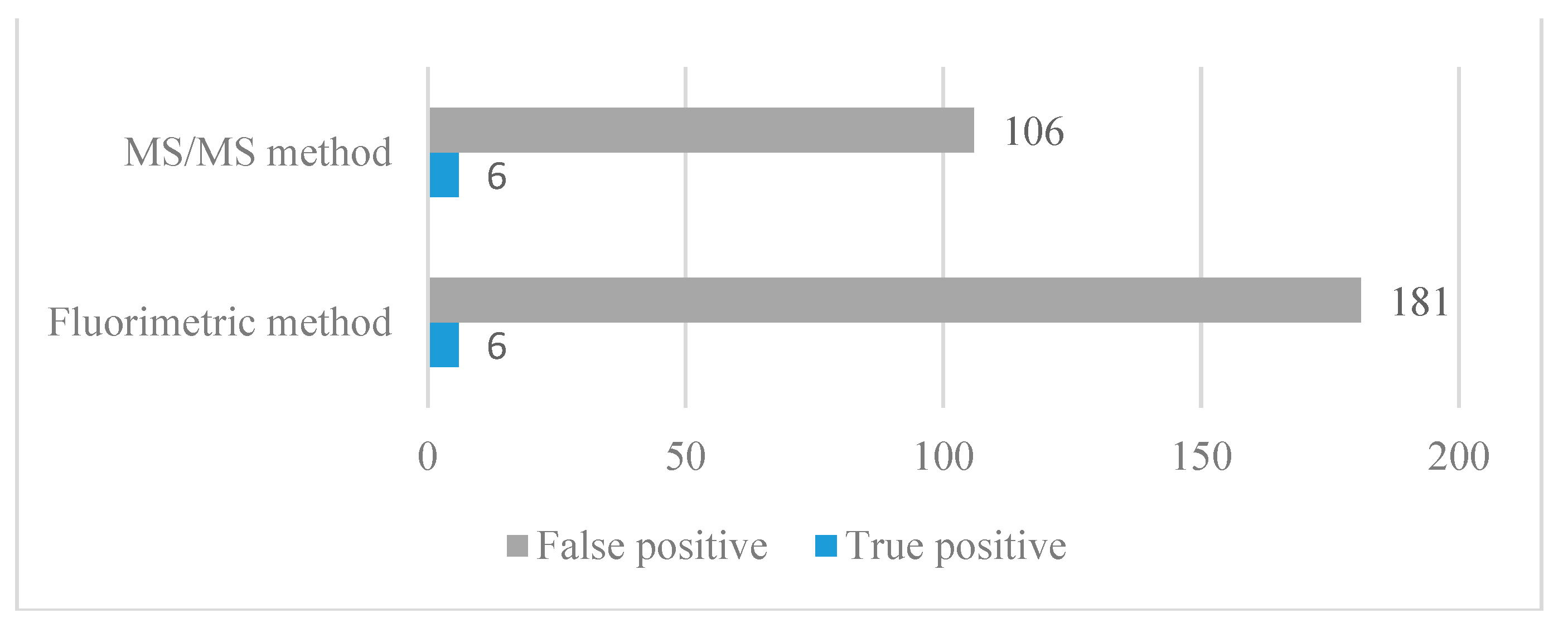

| Patient | DBS Phe (µmol/L)—Fluorometric Method | DBS Phe (µmol/L)—MS/MS Method | Recall DBS Phe (µmol/L)—Fluorometric Method | NBS +/− | Conformation Analyses and Diagnostic Algorithm | TP | FP | Note | ||
|---|---|---|---|---|---|---|---|---|---|---|
| S-Phe (µmol/L) | Genotype (PAH Gene) | Diet | ||||||||
| 1 | 500 | 376 | / | + | 569 | NM_000277.1:c [143T > C(;)913-7A > G] | yes | X | ||
| 2 | 420 | 386 | / | + | 1118 | NM_000277.2:c[143T > C(;)1222C > T] | yes | X | ||
| 3 | 550 | 535 | / | + | 802 | NM_000277.2:c[842C > T(;)1222C > T] | yes | X | ||
| 4 | 490 | 340 | / | + | 751 | NM_000277.3:c[473G > A];[473G > A] | yes | X | ||
| 5 | 580 | 446 | / | + | 983 | NM_000277.3:c[473G > A];[473G > A] | yes | X | ||
| 6 | 290 | 296 | / | + | 593 | NM_000277.1:c[442-5C > G(;)842C > T] | yes | X | ||
| 7 | 310 | 252 | / | + | 241 | NM_000277.2:c[58C > T(;)165T > G] | no | X | ||
| 8 | 260 | 196 | / | + | 250 | NM_000277.3:c[1208C > T(;)1222C > T] | no | X | ||
| 9 | 310 | 285 | / | + | 282 | NM_000277.3:c[473G > A (;)1243G > A] | no | X | ||
| 10 | 180 | 143 | 200 | + | 229 | NM_000277.3:c[473G > A(;)827T > C] | no | X | ||
| 11 | 160 | 132 | 180 | + | 200 | NM_000277.3:c[898G > T(;)1208C > T] | no | X | ||
| 12 | 180 | 126 | 130 | + | 123 | / | no | X | ||
| 13 | 150 | 116 | 120 | + | 247 | NM_000277.3:c[678G > C(;)734T > C] | no | X | ||
| 14 | 150 | 115 | 170 | + | 194 | / | no | X | ||
| 15 | 130 | 86 | 180 | + | 131 | / | no | X | ||
| 16 | 130 | 74 | 120 | + | 92 | / | no | X | ||
| 17 | 130 | 92 | 130 | + | 135 | / | no | X | ||
| 18 | 180 | 100 | 160 | + | 144 | / | no | X | ||
| 19 | 130 | 86 | 140 | + | 149 | / | no | X | ||
| 20 | 650 | 490 | / | + | 67 | / | no | X | PN | |
| 21 | 200 | 105 | 33 | + | / | / | no | X | ↓GS | |
| 22 | 710 | 664 | / | + | 417 | / | no | X | GALD | |
| Recalls (N) | False Positive (N) | True Positive (N) | False Negative (N) | True Negative (N) | Sensitivity (%) | Specificity (%) | Positive Predictive Value (%) | |
|---|---|---|---|---|---|---|---|---|
| Tandem mass spectrometry | 112 | 106 | 6 | 0 | 54,822 | 100 | 99.81 | 5.36 |
| Fluorometric method | 187 | 181 | 6 | 0 | 54,747 | 100 | 99.67 | 3.21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perko, D.; Groselj, U.; Cuk, V.; Iztok Remec, Z.; Zerjav Tansek, M.; Drole Torkar, A.; Krhin, B.; Bicek, A.; Oblak, A.; Battelino, T.; et al. Comparison of Tandem Mass Spectrometry and the Fluorometric Method—Parallel Phenylalanine Measurement on a Large Fresh Sample Series and Implications for Newborn Screening for Phenylketonuria. Int. J. Mol. Sci. 2023, 24, 2487. https://doi.org/10.3390/ijms24032487
Perko D, Groselj U, Cuk V, Iztok Remec Z, Zerjav Tansek M, Drole Torkar A, Krhin B, Bicek A, Oblak A, Battelino T, et al. Comparison of Tandem Mass Spectrometry and the Fluorometric Method—Parallel Phenylalanine Measurement on a Large Fresh Sample Series and Implications for Newborn Screening for Phenylketonuria. International Journal of Molecular Sciences. 2023; 24(3):2487. https://doi.org/10.3390/ijms24032487
Chicago/Turabian StylePerko, Dasa, Urh Groselj, Vanja Cuk, Ziga Iztok Remec, Mojca Zerjav Tansek, Ana Drole Torkar, Blaz Krhin, Ajda Bicek, Adrijana Oblak, Tadej Battelino, and et al. 2023. "Comparison of Tandem Mass Spectrometry and the Fluorometric Method—Parallel Phenylalanine Measurement on a Large Fresh Sample Series and Implications for Newborn Screening for Phenylketonuria" International Journal of Molecular Sciences 24, no. 3: 2487. https://doi.org/10.3390/ijms24032487
APA StylePerko, D., Groselj, U., Cuk, V., Iztok Remec, Z., Zerjav Tansek, M., Drole Torkar, A., Krhin, B., Bicek, A., Oblak, A., Battelino, T., & Repic Lampret, B. (2023). Comparison of Tandem Mass Spectrometry and the Fluorometric Method—Parallel Phenylalanine Measurement on a Large Fresh Sample Series and Implications for Newborn Screening for Phenylketonuria. International Journal of Molecular Sciences, 24(3), 2487. https://doi.org/10.3390/ijms24032487








