Abstract
Airborne fungi are ubiquitous in the environment and are commonly associated with airway inflammatory diseases. The innate immune defense system eliminates most inhaled fungi. However, some influence the development of chronic rhinosinusitis. Fungal CRS is thought of as not a common disease, and its incidence increases over time. Fungi are present in CRS patients and in healthy sinonasal mucosa. Although the immunological mechanisms have not been entirely explained, CRS patients may exhibit different immune responses than healthy people against airborne fungi. Fungi can induce Th1 and Th2 immune responses. In CRS, Th2-related immune responses against fungi are associated with pattern recognition receptors in nasal epithelial cells, the production of inflammatory cytokines and chemokines from nasal epithelial cells, and interaction with innate type 2 cells, lymphocytes, and inflammatory cells. Fungi also interact with neutrophils and eosinophils and induce neutrophil extracellular traps (NETs) and eosinophil extracellular traps (EETs). NETs and EETs are associated with antifungal properties and aggravation of chronic inflammation in CRS by releasing intracellular granule proteins. Fungal and bacterial biofilms are commonly found in CRS and may support chronic and recalcitrant CRS infection. The fungal–bacterial interaction in the sinonasal mucosa could affect the survival and virulence of fungi and bacteria and host immune responses. The interaction between the mycobiome and microbiome may also influence the host immune response, impacting local inflammation and chronicity. Although the exact immunopathologic role of fungi in the pathogenesis of CRS is not completely understood, they contribute to the development of sinonasal inflammatory responses in CRS.
1. Introduction
Airborne fungi are ubiquitous in the environment and continuously inhaled and deposited in the airway mucosa. The number of fungal species exceeds 50,000 with only a few species implicated in human diseases. Alternaria, Aspergillus, Cladosporium, Penicillium, and Candida are commonly associated with airway mucosal diseases [1,2]. Airborne fungal bioparticles such as spores, mycelia, and hyphal fragments act as allergens that induce type 1 hypersensitivity with the production of specific immunoglobulin E (IgE). Alternatively, they can act as an irritant, causing local or systemic infection, especially in an immunocompromised host. In the air, 50–50,000 spores/m3 are present, and they are continuously inhaled into the airway, where they come into contact with the airway mucosa [3]. Fungi and their components or products contribute to the development of airway inflammatory diseases through innate or adaptive immune responses in airway mucosa.
Fungal rhinosinusitis (FRS) used to be an uncommon disease; however, the number of diagnosed cases has increased over time with the improvement of diagnostic technologies [4]. The rise in FRS may be related to the increased usage of antibiotics, longer life expectancy, global warming, increased air pollution, and increase in the amount of time spent indoors [5,6]. Based on histopathologic findings, FRS can be divided into invasive and noninvasive types. Invasive FRS has histologic characteristics of mucosal infiltration with fungal elements that can be divided into acute fulminant invasive, chronic invasive, and chronic granulomatous FRS. Noninvasive FRS includes saprophytic fungal infection, fungus ball, and allergic fungal rhinosinusitis (AFR) [7]. Fungus ball refers to the accumulation of a dense conglomeration of fungal hyphae in the sinus cavity with or without characteristic hyperdense radiologic findings. In early studies, Aspergillus was thought to be the leading cause of fungus ball, similar to bronchopulmonary aspergilloma or allergic bronchopulmonary aspergillosis [8,9]. The definition of AFR is often confused with fungus-related eosinophilic mucin rhinosinusitis [10]. In AFR, Dematiaceous fungi and Aspergillus are common etiologic fungi. Typically, AFS is found in immunocompetent atopic patients with chronic rhinosinusitis (CRS) and nasal polyps who develop an allergic response to fungal organisms that have colonized the sinus mucosa with eosinophilic mucus [9]. In some cases, eosinophilic mucin can be found in CRS patients without type I hypersensitivity against fungi [11].
CRS is one of the most frequently reported chronic diseases. In contrast to acute rhinosinusitis, where the bacterial or viral etiology is well established, the etiology of CRS is not completely understood. Noninfectious inflammation with inflammatory cell infiltration of the sinonasal mucosa is a histologic hallmark of CRS [12,13]. Several studies have attempted to elucidate the mechanisms of CRS and numerous hypotheses concerning its pathogenesis have been proposed, such as chronic bacterial infection, superantigens, biofilms, anatomic abnormalities, and immune cell dysfunction [14,15]. In the late 1990s, the Mayo group reported that CRS patients and healthy volunteers had fungal culture-positive nasal secretions. Additionally, they suggested that CRS patients may show abnormal immune responses against airborne fungi [11]. Subsequently, many researchers attempted to detect fungi in nasal secretions in various ways [16,17]. Most fungal CRS are associated with eosinophilic CRS, which develops early olfactory dysfunction and bilateral nasal polyps with opacification of the posterior ethmoid sinus and the olfactory cleft in early CT images in comparison with noneosinophilic CRS [18]. CRS patients have a larger burden of fungi than healthy controls, and sinus surgery can significantly reduce the number of fungal organisms in the sinonasal mucosa [19]. Although CRS patients show abnormal or inappropriate innate and adaptive immune responses against fungi, the role of fungi in the pathogenesis of CRS remains under debate. Because antifungal therapy is not efficacious in controlling CRS, the hypothesis that fungi play a significant etiologic role in CRS development has been rejected [20]. However, many studies have suggested that fungi affect immune responses in the upper airway mucosa. This paper aims to review the recent studies on the immunologic roles and interaction of fungi with the upper airway mucosa and inflammatory cells and its effect on the development of airway mucosal inflammatory diseases or CRS.
2. Upper Airway Mucosal Immune Responses against Fungi
The human airway mucosa defends against invasion by environmental pathogens through innate and adaptive immune responses [21]. Innate immunity is nonspecific and includes the activation of the complement system, phagocytosis, and physical and chemical barriers against infectious agents. Most pathogenic organisms are detected and destroyed within hours by innate defense mechanisms. Innate immune responses are followed by adaptive immunity with specialized immunological memory to eliminate or prevent the growth of pathogens and provide long-term clearance of the infection. Airway epithelial cells are essential in the innate immune system as the first mucosal defense against allergens and pathogens. The physical barrier function of the nasal mucosa is determined by the integrity of the apical junction complex, which is composed of tight and adherens junctions [22]. This innate barrier function seems to be altered in chronic airway inflammation. In CRS, the impaired epithelial barrier function leads to increased susceptibility to pathogenic agents and diminished transepithelial migration of inflammatory cells [23]. Fungal components may induce airway epithelial barrier dysfunction with intracellular production of reactive oxygens species and increased epithelial permeability associated with structural and functional alteration in the junctional complexes of the epithelial layer [24,25]. Alternaria influences tight junction molecule expression and rarely affects apical junction components in the nasal epithelial cells [25,26]. The imbalance of fungal protease and protease inhibitors within the airway mucosa can cause epithelial barrier dysfunction [27]. Fungal proteases affect the innate immune system of epithelial cells through increased transepithelial access of pathogens and critical immune cells, leading to tissue damage and immune activation [25]. Fungal proteases have immunomodulatory functions by inducing inflammatory cytokines and chemokines in airway epithelial cells, leading to the recruitment, activation, and survival of inflammatory cells [28]. Protease-activated receptors recognize fungal protease in airway epithelial cells. Fungal proteases cleave extracellular ligands, which are freed to initiate the production of inflammatory mediators [29,30]. Alternaria induces the production of granulocyte-macrophage colony-stimulating factor (GM-CSF), interleukin (IL)-6, IL-8, and thymic stromal lymphopoietin (TSLP) via the activation of protease-activated receptor (PAR)2, facilitating the development of airway inflammation. Blocking the protease activity of fungi can decrease airway inflammation and hyperresponsiveness [29]. Fungi can also drive airway inflammation and epithelial cytokine production independent of protease-activated receptors [31]. Fungal proteases impair the innate mucosal defense by protein degradation and fungicidal protein activity or by inactivating the complement system [32]. The reduced production of antimicrobial peptides, such as surfactant protein-D, lactoferrin, and histatins, from airway epithelial cells could impair antifungal immunity [33].
Airway epithelial cells actively participate in inflammatory responses by releasing inflammatory mediators that directly affect the airway mucosa and other inflammatory cells. Nasal epithelial cells express pattern recognition receptors (PRRs), which play a crucial role in the proper function of the mucosal innate immune system [34]. PRRs interact with pathogen-associated molecular patterns of fungi, such as glucans and chitin. Toll-like receptors (TLRs) are expressed on various immune cells and non-immune epithelial and endothelial cells and fibroblasts. TLRs play a role in pathogen recognition and in the induction and regulation of innate and adaptive immune responses [35]. TLR2 and TLR4 mRNA expression are significantly increased in nasal epithelial cells of CRS patients [36]. TLR2 interacts with β-glucan and phospholipomannans from fungal conidia and hyphae, and TLR4 can be activated in conjunction with O-linked mannans and Dectin-1 [37,38]. The interaction of fungal allergens with PRRs induces the production of pro-inflammatory cytokines, the respiratory burst, and Th1 immune responses for the antifungal effector function of respiratory epithelial cells. Aspergillus and Alternaria induce the production of IL-8 and GM-CSF from nasal epithelial cells, which have been implicated in the development of protective immunity against fungi [39].
Mucosal dendritic cells ingest fungal antigens and migrate to the regional lymph nodes, promoting the proliferation and polarization of naïve T-cells. Protective and successful clearance of fungal antigens by Th1 and Th17 immune responses and antifungal responses can be repressed by inhibitory cytokines produced by Th2 cells [40,41]. Th1 responses enhance the functions of phagocytic cells and the promotion of B-cell production of opsonizing antifungal antibodies. Th17 responses activate structural cells, resulting in the production of chemokines that induce the recruitment of phagocytic cells [41,42]. Th2 immunity to fungi is characterized by an inability to clear fungal pathogens with less effective activation of macrophages, and it arises as a consequence of fungal infections [43]. PRRs, such as TLRs and C-type lectin-like receptors, recognize fungal elements and activate epithelial cells to release innate inflammatory cytokines. Fungi-induced epithelial cell derived cytokines, such as IL-25, IL-33, and TSLP, are critical regulators of Th2 immune responses in the nasal mucosa. These cytokines activate group 2 innate lymphoid cells (ILC2) and polarize naïve CD4+ T-cells to Th2 cells at the site of inflammation [44,45]. Alternaria induces the production of epithelial cell-derived cytokines through the nuclear factor (NF)-κB, activator protein (AP)-1, and mitogen-activated protein kinase (MAPK) pathways [45]. Aspergillus fumigatus also activates the innate immune response and induces the production of IL-33 from nasal polyp epithelial cells through PAR interactions [46,47]. ILC2s play a critical role in fungus-induced immunopathology in airway inflammation. ILC2 induces epithelial repair in the allergic immune response to fungi through the production of the epidermal growth factor [48]. Intranasal instillation of Alternaria induces the production of cysteine leukotriene, the activation of ILC2, and Th2 recruitment and proliferation [49]. Several fungal allergens induce type 2 inflammations by enhancing the production of epithelial cell-derived cytokines and potentiating ILC2-associated innate and adaptive immune responses in chronic airway inflammation. The immunopathologic roles of fungi in airway mucosa are summarized in Figure 1.
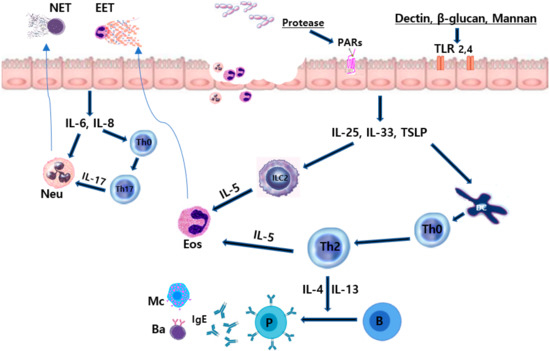
Figure 1.
Immunopathologic effects of fungi on the sinonasal mucosa. Fungal proteases and components interact with epithelial cell receptors and release innate inflammatory cytokines. Fungi can induce eosinophilic and neutrophilic inflammation in sinonasal mucosa. IL, interleukin; TSLP, thymic stromal lymphopoietin; IgE, immunoglobulin E; Th, T helper cell; B, B cells; P, plasma cells, Ba, basophils; Mc, mast cells; TLR, toll-like receptor; PAR, protease-activated receptor; Eos, eosinophils; Neu, neutrophils; EET, eosinophil extracellular trap; NET, neutrophil extracellular trap (Authors own work).
Recurrent exposure of naïve mice to fungal extracts or in combination with other airborne allergens induces CRS with an increase in airway eosinophils, tissue remodeling, and allergen-specific IgE [50,51,52]. Intranasal instillation of Aspergillus-derived proteases or a combined extract of house dust mite, A. fumigatus, Alternaria alternata, and proteases from Staphylococcus aureus successfully induced Th2-type CRS in a murine model with subepithelial infiltration of eosinophils and increased IL-4, IL-5, eotaxin, and other inflammatory mediators in the sinonasal mucosa or nasal lavage fluid [50,51]. The allergic background of the host could influence the immune response against fungi, with allergic predisposed mice showing Th2 and eosinophil dominant immune responses and those without allergic predisposition showing Th2, Th1, and Treg immune responses with eosinophilic and neutrophilic mucosal inflammation [52]. In CRS, fungi induce the innate immune response in airway epithelial cells and ILC2 and the adaptive immune response via B-cells and T-cells, which aggravate type 2 inflammations with severe or intractable chronic airway inflammation. Additional studies are required to determine the effect of host or genetic immunologic backgrounds against fungi on the development of CRS.
3. Fungal Biofilms in CRS
Biofilms are microbial-derived sessile communities with microorganisms and extracellular polymeric substances. In human hosts, fungal biofilms can evade host defenses and demonstrate resistance to antimicrobial host immunity and antifungal resistance as a reservoir for recalcitrant infection [53]. Several hundred conidia enter the airway each day, and persistent contact with the airway mucosa can germinate and produce mycelium. In this manner, a biofilm is created via multicellular community formation and interactions with polysaccharides in the extracellular matrix. In CRS patients, the prevalence of biofilm ranges from 44% to 92%. Fungal elements are commonly found within sinonasal mucosal biofilms, especially in eosinophilic mucus CRS [54]. Fungal biofilm colonization of the respiratory mucosa may be an important factor in chronic inflammatory diseases. Inhaled hyphae spread across the mucosal surface with extracellular matrix secretions and can develop fungal biofilms by gluing the hyphae together. In an in vitro study, A. fumigatus could develop biofilms on primary nasal epithelial cells [55]. However, in an in vivo study, fungal biofilms could not develop in the sinonasal mucosa with intact innate immune defenses. However, fungal biofilms can develop when innate immune systems are impaired, such as impaired mucociliary clearance or epithelial injury [56]. Impaired mucosal innate defense systems and aggravated mucosal inflammatory responses increase the risk of developing fungal biofilms and fungal inflammatory diseases in the sinonasal mucosa [57]. Preoperative or intraoperative biofilm detection is associated with a high risk of recalcitrance, which requires more aggressive postoperative antimicrobial and anti-inflammatory therapy [58]. The major molecular components that play an important role in the transition from a planktonic form to biofilm form may determine the pathologic characteristics of fungal biofilms.
Microbial biofilm formation from a planktonic form requires interactions between different pathogen and host factors. Bacterial and fungal biofilms commonly co-exist in the sinonasal mucosa of CRS patients [59]. S. aureus biofilm is significantly associated with fungal biofilm in CRS. In animal models, inoculation with fungal spores in an obstructed sinus did not develop a fungal biofilm. However, when the fungi were inoculated with S. aureus, there was robust biofilm formation in the sinonasal mucosa [56]. Co-inoculation with S. aureus and fungi can enhance the formation of fungal biofilms in sheep models, and the degree of mucosal inflammation and mucosal injury was more severe than bacterial or fungal inoculation alone [56]. However, producing fungal biofilms in animal models of sinus mucosa with intact innate immune defenses is difficult. Fungal biofilms developed when A. fumigatus was cultured in vitro on nasal or bronchial epithelial cells [57,60]. In CRS, bacterial biofilms in sinonasal mucosa damage the epithelial layer, and then fungal biofilm formation enhanced by aggravated mucosal inflammation and severe recalcitrant disease. Bacterial biofilms may significantly damage mucociliary transport systems that clear inoculated fungal spores. Bacterial and fungal biofilms have a potentially mutualistic relationship. This implies that for the development of sinonasal mucosal inflammation, the interaction between bacteria, especially S. aureus, both with colonizing fungi and inhaled fungal allergens, is pathogenic. However, the relationship between the fungus and the bacterium may be synergistic or antagonistic depending on the secretion of bacterial quorum-sensing molecules and host immune factors [61]. The initial interaction between the fungus and bacteria is synergistic. However, during the formation of biofilms, it may become competitive or antagonistic depending on the species of microbe and host immune factors. Biofilms may affect the chronicity and recalcitrant nature of CRS, but further studies are needed to determine the pathophysiologic role of biofilms on the development of CRS.
4. Neutrophil and Eosinophil Extracellular Traps against Fungi
Neutrophils and eosinophils act as effector immune cells that mediate antifungal immunity. Extracellular traps are a type of rapid cell death characterized by the release of intact cytoplasmic organelles via nuclear and plasma membrane breakdown [62]. Extracellular traps remove pathogens by capturing and immobilizing them by releasing a mixture of DNA, histones, and granule proteins. Immunologic characteristics of NETs and EETs were summarized in Table 1. Neutrophil extracellular traps (NETs) and eosinophil extracellular traps (EETs) are commonly found in CRS [63,64]. Tissue eosinophilia and EETs or tissue neutrophilia and NETs may affect the prognosis and refractory nature of CRS. Neutrophils are abundant in humans and quickly respond to kill harmful agents by trapping and releasing antimicrobial molecules [65]. NETs provide protection against bacterial infections; for instance, lipopolysaccharide and flagella trigger NET formation with bacteriostatic or bactericidal effects. NETs play a significant role in clearing infections, such as with mycobacteria, fungi, viruses, and parasites [65,66,67,68]. NETs are characterized by morphological changes in nuclear shape, chromatin condensation, and leakage of the nuclear envelope with phorbol-12-myristate-13-acetate-induced cell death. However, NETs can also develop independent of cell death by granulocyte/macrophage colony-stimulating factor, lipopolysaccharide or complement factor 5a, which induce the release of mitochondria-derived DNA with an intact nuclear membrane and neutrophil viability [69]. NETs significantly decrease the extracellular microbial burden to inhibit infection and increase host survival. Bacteria, fungi, viruses, and immune complexes induce NETs through nicotinamide adenine dinucleotide phosphate (NADPH)-oxidase and the reactive oxygen species (ROS)-dependent response of neutrophils with activation of intracellular granular proteases [66]. Neutrophils and their NETs are key players in innate defense against fungi. Neutrophils and other immune cells can remove small fungal structures, such as conidia and yeast through phagocytosis. Fungal hyphal filaments are too large to be removed by phagocytosis, but these may be the main targets of NETs [70]. The size of fungal components and their morphology also influence NETs through various signaling pathways. β-glucan particles or molecules trigger NETs through the Syk kinase-dependent pathway [71]. A. fumigatus induce NET formation within 3–4 h after exposure to neutrophils without prior stimulation. Fungal hydrophobin RodA on the surface of conidia with β-glucan inhibit NET production, but the fungal pigment DHN-melanin was not involved in the evasion of neutrophil killing [70]. During the formation of fungal biofilms, the expression of RodA increased compared with the planktonic condition. Fungal biofilms show inhibition of NETs with immune evasion [72]. The number of NETs-forming neutrophils was significantly higher in acute exacerbated CRS patients, meaning that microorganisms including bacteria and viruses trigger NETs to kill microbes or prevent microbial dissemination [63]. However, NETs show antifungal properties and can be pathogenic by being cytotoxic to airway epithelial cells and structural cells, aggravating chronic inflammatory diseases. However, until now, drawing conclusions has been difficult regarding the interactions between fungi and NETs in sinonasal mucosa as playing an important role in development of CRS.

Table 1.
Immunopatholoic characteristics of neutrophil extracellular trap (NET) and eosinophil extracellular trap (EET).
Eosinophils protect the host against nonpagocytable pathogenic fungi by releasing cytotoxic granules [73]. Eosinophil cationic proteins, major basic protein, eosinophil-derived neurotoxin, and eosinophil peroxidase are four major intracellular crystalloid-bearing granule proteins. Lilly et al. demonstrated that eosinophils are essential for fungal clearance and fugal growth suppression through the production of pro-inflammatory cytokines and chemokines [74]. Eosinophils and their degranulation products are abundant in the eosinophilic mucin of CRS patients [75]. EETs can occur either independent of cell death based on the activation of NADPH oxidase and β2-integrin or dependent on cytolytic cell death with extrusion of histone-enriched nuclear DNA associated with clustering of intact granules [76,77]. Direct contact with fungal components, such as fungal cell wall protease, chitin, and β-glucans, initiates eosinophil activation, resulting in antifungal responses [78]. Eosinophils also have fungicidal activity against A. fumigatus without direct cell contact [74]. Fungal conidia and eosinophils provide an adhesive surface for microorganism entrapment and EET formation. The eosinophilic mucin of CRS patients contains the extracellular deposition of cytotoxic granule proteins that destroy fungal elements [79]. The increased viscosity of nasal secretions in CRS is not only associated with the overproduction of mucin glycoproteins from airway epithelial cells but also EETs, which contain debris of inflammatory cells and large polymers including DNA [80]. Abundant filamentous DNA fragments in eosinophilic mucin form a sticky scaffold for clustered eosinophils and free eosinophilic granules. EETs provide an adhesive surface for fungal entrapment. Eosinophils form EETs in response to A. fumigatus conidia through a cytolytic process with intact granules [81]. Phorbol myristate acetate (PMA) induces EET in an oxidative burst and in a NADPH oxidase-dependent manner. Compared with NETs, which are associated with NADPH oxidase or mitochondrial ROS, EET development by fungi occurs independently of ROS production in leukocytes. These differences between eosinophils and neutrophils in the A. fumigatus-induced release of extracellular DNA traps may be related to the difference in how they recognize or respond to fungi. The interaction of an eosinophil β2-integrin molecule, CD11b, and fungal cell wall β-glucan play an important role in the release of EETs [81]. A. fumigatus induces EET formation by a cytolytic process via a Mac-1 and Syk tyrosine kinase pathway-dependent mechanism [81]. However, the role of PPRs and the interaction with other structural cells in EET release remain to be studied. EETs can eradicate fungi, although how they interact with the immune system under normal physiological conditions has yet to be determined, with either a beneficial or harmful effect on the development of CRS. EETs are an innate defense system against extracellular pathogens, but they induce barrier dysfunction and contribute to the stability and high viscosity of mucus, which in turn impairs pathogen clearance with inflammatory cell infiltration, biofilm growth, and the potential for chronic diseases [82].
5. Mycobiome
Inhaled microbial colonization may play a role in the initiation and maintenance of the inflammatory process of CRS. In the field of mucosal immunity, various studies have elucidated the role of the microbiome for the initiation, adaptation, and maintenance of the mucosal immune responses. Dysbiosis of the sinonasal mucosa microbiota may contribute to the development and exacerbation of chronic inflammatory diseases [83,84]. However, most microbiome or microbiological studies are focused on bacteria, and the characteristics or composition of fungal species in human body are not commonly studied. Like the microbiome, the mycobiome may also play an important immunologic role in host innate and adaptive immunity. Mycobiota are the fungal component of a given microbial community, whereas the mycobiome is their corresponding genomes [85]. Fungi are ubiquitous and normal commensals of sinonasal mucosa in healthy and CRS subjects, but the interactions with the immune system under normal or pathologic conditions has yet to be determined. Fungal culture has been difficult using traditional culture methods. However, the development of next-generation sequencing (NGS) methods and state-of-the art molecular biologic techniques are enabling the detection of fungi by culture-free, fast, and sensitive single-molecule sequencing [86]. Based on traditional methods, Aspergillus, Alternaria, Cladosporium, and Penicillium are commonly isolated from sinonasal mucosa [11,87]. With NGS, Aspergillus sp., Schizophyllum sp., Curvularia sp., and Malassezia sp. are most frequently detected fungi in the nose [88]. When using nanopore sequencing, Malassezia sp., M restricta and M. sympodialis, are predominant in the sinonasal mucosa of healthy and CRS subjects, followed by Aspergillus sp. and Candida albicans [89]. Because Malassezia is lipid-dependent and is typically found in sebum-rich areas of the body, they require special culture media to grow. The presence of Malassezia in the sinonasal mucosa may come from the nasal vestibule. An opportunistic infection by Malassezia can damage nasal epithelial cells and induce Th2 immune responses in the sinonasal mucosa [90]. However, the role of Malassezia in the development of the CRS is not completely understood. The immunopathologic role of the development of CRS based on this organism requires further study. A. fumigatus is often involved in the development of allergic fungal rhinosinusitis and fungus ball. Fungal components are commonly found in the eosinophilic mucin of CRS patients [79]. Alterations in the mycobiome are associated with the development of airway diseases [91]. Fungal dysbiosis is related to the altered fungal composition or changes in total fungal content or abundance. Although fungal diversity should be higher in more severe disease, fungal dysbiosis may be implicated in the development or exacerbation of airway inflammatory diseases through interactions with hose immune responses.
Fungal and bacterial interactions and their relationship with hosts has been studied [92,93]. Bacterial and fungi co-exist in the human body, and microbial interactions influence human health and disease. Bacteria can directly affect fungal morphology, survival, growth, virulence, and attachment [92,94]. Fungal–bacterial interactions could affect the survival and virulence of fungi, bacteria, and both their own and host immune responses. In an in vitro study, S. aureus enhanced the tissue invasion of fungi, and Pseudomonas aeruginosa showed an antifungal effect [93,95]. Pyocyanin and phenazine, metabolites from P. aeruginosa, inhibited the growth of A. fumigatus, and A. fumigatus suppressed the growth of P. aeruginosa [96]. Co-infection of Malassezia sympodialis and S. aureus demonstrated synergistic interactions in growth and virulence with aggravated type2 and type17 inflammation in the sinonasal mucosa [97]. Interactions between fungi and the host can occur directly or through metabolites produced during colonization and infection. The interactions between fungi and bacteria show species-specific immune responses in the sinonasal mucosa [97]. The downregulation of bacterial loading by antibiotics could influence fungal composition or growth, which may aggravate or suppress mucosal inflammation However, dysbiosis of the mycobiome is primary or secondary to an immune response as an imbalance of the bacterial microbiome. The interaction between the mycobiome and the microbiome may show specific immune responses and influence the host immune response, which may affect local inflammation and disease progression. Therefore, understanding microbial interactions and host-microbiota interactions will be crucial in establishing the function of microbial communities in CRS and implementing new therapeutic strategies.
6. Summary and Conclusions
Fungi are ubiquitous in the environment and co-exist with hosts as saprophytes or commensals. Nonetheless, some airborne fungi cause airway inflammatory diseases, and some are associated with the pathogenesis of CRS. Although fungi play a pathogenic role in certain types of CRS, the immunopathologic role of fungi in pathogenesis of CRS is not completely understood. Airborne fungi interact with the sinonasal mucosa by innate and adaptive immune responses. The Th1 immune response commonly associated with antifungal immunity and the Th2 immune response is related to pathologic immunity. However, the host genetic background, airway mucosal fungal loading, fungal species inhaled from the air, susceptibility to fungal allergens, and environmental factors influence the type of mucosal immune responses. Although Th2 inflammation is commonly associated with the pathologic role of fungi in CRS development, Th1 inflammation is also found in the sinonasal mucosa [43,45]. Bacterial–fungal interactions, such as the development of biofilms and the composition of mycobiomes, may also play an important role in determining the inflammatory immune responses in the sinonasal mucosa. Furthermore, their interactions with the host need to be elucidated. In recent years, knowledge has been accumulated through many studies on the immunologic characteristics of CRS, and various immunological mechanisms and hypotheses have been proposed for the role of fungi in CRS development. Although fungi are present not only in CRS or chronic inflammatory airway disease mucosa but also in normal healthy mucosa, they interact with innate and adaptive immune responses to remove pathologic fungi from the sinonasal mucosa or contribute to the development of airway inflammatory diseases. Fungi contribute to the development of CRS, and additional studies are required to reveal a more detailed understanding of the immunologic interactions between fungi, bacteria, and the host sinonasal mucosa.
Author Contributions
Conceptualization, S.-H.S. and M.-K.Y.; data acquisition: D.-W.L. and S.-Y.G.; writing—original draft preparation, D.-W.L. and S.-Y.G.; writing—review and editing, S.-H.S.; project administration, M.-K.Y.; Funding acquisition, S.-H.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Research Foundation of Korea (NRF) grant funded by the Korea government (Ministry of Science and ICT) (2019R1F1A1047757).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kauffman, H.F.; Van Der Heide, S. Exposure, sensitization, and mechanisms of fungus-induced asthma. Curr. Allergy Asthma Rep. 2003, 3, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Babiceanu, M.; Howard, B.A.; Rumore, A.; Kita, H.; Lawrence, C. Analysis of global gene expression changes in human bronchial epithelial cells exposed to spores of the allergenic fungus, Alternaria alternata. Front. Microbiol. 2013, 4, 196. [Google Scholar] [CrossRef] [PubMed]
- Pashley, C.H.; Fairs, A.; Free, R.C.; Wardlaw, A.J. DNA analysis of outdoor air reveals a high degree of fungal diversity, temporal variability, and genera not seen by spore morphology. Fungal Biol. 2012, 116, 214–224. [Google Scholar] [CrossRef] [PubMed]
- Wani, M.K.; Ruckenstein, M.J.; Parikh, S. Magnetic Resonance Imaging of the Paranasal Sinuses: Incidental Abnormalities and Their Relationship to Patient Symptoms. J. Otolaryngol. 2001, 30, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Solache, M.A.; Casadevall, A. Global Warming Will Bring New Fungal Diseases for Mammals. mBio 2010, 1, e00061-10. [Google Scholar]
- Pacheco, S.E.; Guidos-Fogelbach, G.; Annesi-Maesano, I.; Pawankar, R.; Amato, G.D.; Latour-Staffeld, P.; Urrutia-Pereira, M.; Kesic, M.J.; Hernandez, M.L.; American Academy of Allergy; et al. Climate change and global issues in allergy and immunology. J. Allergy Clin. Immunol. 2021, 148, 1366–1377. [Google Scholar] [CrossRef]
- Chakrabarti, A.; Denning, D.; Ferguson, B.J.; Ponikau, J.; Buzina, W.; Kita, H.; Marple, B.; Panda, N.; Vlaminck, S.; Kauffmann-Lacroix, C.; et al. Fungal rhinosinusitis: A categorization and definitional schema addressing current controversies. Laryngoscope 2009, 119, 1809–1818. [Google Scholar] [CrossRef]
- Hora, J.F. Primary aspergillosis of the paranasal sinuses and associated areas. Laryngoscope 1965, 75, 768–773. [Google Scholar] [CrossRef]
- Katzenstein, A.L.; Sale, S.R.; Greenberger, P.A. Allergic Aspergillus sinusitis: A newly recognized form of sinusitis. J. Allergy Clin. Immunol. 1983, 72, 89–93. [Google Scholar] [CrossRef]
- Ferguson, B.J. Eosinophilic Mucin Rhinosinusitis: A Distinct Clinicopathological Entity. Laryngoscope 2000, 110, 799–813. [Google Scholar] [CrossRef]
- Ponikau, J.U.; Sherris, D.A.; Kern, E.B.; Homburger, H.A.; Frigas, E.; Gaffey, T.A.; Roberts, G.D. The Diagnosis and Incidence of Allergic Fungal Sinusitis. Mayo Clin. Proc. 1999, 74, 877–884. [Google Scholar] [CrossRef]
- Ikeda, K.; Shiozawa, A.; Ono, N.; Kusunoki, T.; Hirotsu, M.; Homma, H.; Saitoh, T.; Murata, J. Subclassification of chronic rhinosinusitis with nasal polyp based on eosinophil and neutrophil. Laryngoscope 2013, 123, E1–E9. [Google Scholar] [CrossRef]
- Fokkens, W.J.; Lund, V.J.; Hopkins, C.; Hellings, P.W.; Kern, R.; Reitsma, S.; Toppila-Salmi, S.; Bernal-Sprekelsen, M.; Mullol, J.; Alobid, I.; et al. European position paper on rhinosinusitis and nasal polyps 2020. Rhinology 2020, 58, 1–464. [Google Scholar] [CrossRef]
- Rha, M.-S.; Kim, S.-W.; Chang, D.-Y.; Lee, J.-K.; Kim, J.; Park, S.-H.; Khalmuratova, R.; Lim, H.-S.; Eun, K.M.; Hong, S.-N.; et al. Superantigen-related TH2 CD4+ T cells in nonasthmatic chronic rhinosinusitis with nasal polyps. J. Allergy Clin. Immunol. 2020, 145, 1378–1388.e10. [Google Scholar] [CrossRef]
- Mbbs, B.S.P.; Cooksley, C.M.; Ramezanpour, M.; Vediappan, R.S.; Bassiouni, A.; Wormald, P.J.; Psaltis, A.J.; Vreugde, S. Staphylococcus aureus biofilm exoproteins are cytotoxic to human nasal epithelial barrier in chronic rhinosinusitis. Int. Forum Allergy Rhinol. 2020, 10, 871–883. [Google Scholar] [CrossRef]
- Kim, S.T.; Choi, J.H.; Jeon, H.G.; Cha, H.E.; Hwang, Y.J.; Chung, Y.-S. Comparison between polymerase chain reaction and fungal culture for the detection of fungi in patients with chronic sinusitis and normal controls. Acta Oto-Laryngol. 2005, 125, 72–75. [Google Scholar] [CrossRef]
- Polzehl, D.; Weschta, M.; Podbielski, A.; Riechelmann, H.; Rimek, D. Fungus culture and PCR in nasal lavage samples of patients with chronic rhinosinusitis. J. Med. Microbiol. 2005, 54, 31–37. [Google Scholar] [CrossRef]
- Cho, S.-W.; Kim, D.W.; Kim, J.-W.; Lee, C.H.; Rhee, C.-S. Classification of chronic rhinosinusitis according to a nasal polyp and tissue eosinophilia: Limitation of current classification system for Asian population. Asia Pac. Allergy 2017, 7, 121–130. [Google Scholar] [CrossRef]
- Murr, A.H.; Goldberg, A.N.; Pletcher, S.D.; Bs, K.D.; Ms, L.J.W.; Vesper, S.J. Some chronic rhinosinusitis patients have elevated populations of fungi in their sinuses. Laryngoscope 2012, 122, 1438–1445. [Google Scholar] [CrossRef]
- Fokkens, W.J.; van Drunen, C.; Georgalas, C.; Ebbens, F. Role of fungi in pathogenesis of chronic rhinosinusitis: The hypothesis rejected. Curr. Opin. Otolaryngol. Head Neck Surg. 2012, 20, 19–23. [Google Scholar] [CrossRef]
- Schleimer, R.P.; Kato, A.; Kern, R.; Kuperman, D.; Avila, P.C. Epithelium: At the interface of innate and adaptive immune responses. J. Allergy Clin. Immunol. 2007, 120, 1279–1284. [Google Scholar] [CrossRef] [PubMed]
- Georas, S.N.; Rezaee, F. Epithelial barrier function: At the front line of asthma immunology and allergic airway inflammation. J. Allergy Clin. Immunol. 2014, 134, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Tieu, D.D.; Kern, R.C.; Schleimer, R.P. Alterations in epithelial barrier function and host defense responses in chronic rhinosinusitis. J. Allergy Clin. Immunol. 2009, 124, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Leino, M.S.; Loxham, M.; Blume, C.; Swindle, E.J.; Jayasekera, N.P.; Dennison, P.W.; Shamji, B.W.H.; Edwards, M.J.; Holgate, S.T.; Howarth, P.H.; et al. Barrier Disrupting Effects of Alternaria Alternata Extract on Bronchial Epithelium from Asthmatic Donors. PLoS ONE 2013, 8, e71278. [Google Scholar] [CrossRef]
- Shin, S.; Ye, M.; Lee, D.; Che, M. Alternaria-induced barrier dysfunction of nasal epithelial cells: Role of serine protease and reactive oxygen species. Int. Forum Allergy Rhinol. 2019, 9, 514–521. [Google Scholar] [CrossRef]
- Koizumi, J.-I.; Kojima, T.; Ogasawara, N.; Kamekura, R.; Kurose, M.; Go, M.; Harimaya, A.; Murata, M.; Osanai, M.; Chiba, H.; et al. Protein Kinase C Enhances Tight Junction Barrier Function of Human Nasal Epithelial Cells in Primary Culture by Transcriptional Regulation. Mol. Pharmacol. 2008, 74, 432–442. [Google Scholar] [CrossRef]
- Kouzaki, H.; Matsumoto, K.; Kikuoka, H.; Kato, T.; Tojima, I.; Shimizu, S.; Kita, H.; Shimizu, T. Endogenous Protease Inhibitors in Airway Epithelial Cells Contribute to Eosinophilic Chronic Rhinosinusitis. Am. J. Respir. Crit. Care Med. 2017, 195, 737–747. [Google Scholar] [CrossRef]
- Jang, Y.J.; Lee, Y.-H.; Shin, S.-H. Rhinovirus-infected nasal polyp epithelial cells: Effect on the activation and migration of eosinophils by airborne fungi. Ann. Allergy Asthma Immunol. 2010, 104, 434–439. [Google Scholar] [CrossRef]
- Matsuwaki, Y.; Wada, K.; White, T.; Moriyama, H.; Kita, H. Alternaria Fungus Induces the Production of GM-CSF, Interleukin-6 and Interleukin-8 and Calcium Signaling in Human Airway Epithelium through Protease-Activated Receptor 2. Int. Arch. Allergy Immunol. 2012, 158 (Suppl. S1), 19–29. [Google Scholar] [CrossRef]
- Rivas, C.M.; Schiff, H.V.; Moutal, A.; Khanna, R.; Kiela, P.R.; Dussor, G.; Price, T.J.; Vagner, J.; DeFea, K.A.; Boitano, S. Alternaria alternata-induced airway epithelial signaling and inflammatory responses via protease-activated receptor-2 expression. Biochem. Biophys. Res. Commun. 2022, 591, 13–19. [Google Scholar] [CrossRef]
- Daines, M.; Zhu, L.; Pereira, R.; Zhou, X.; Bondy, C.; Pryor, B.M.; Zhou, J.; Chen, Y. Alternaria induces airway epithelial cytokine expression independent of protease-activated receptor. Respirology 2020, 25, 502–510. [Google Scholar] [CrossRef]
- Chotirmall, S.H.; Mirkovic, B.; Lavelle, G.M.; McElvaney, N.G. Immunoevasive Aspergillus Virulence Factors. Mycopathologia 2014, 178, 363–370. [Google Scholar] [CrossRef]
- Tyler, M.A.; Dietz, C.J.P.; Russell, C.B.; Citardi, M.J.; Assassi, S.; Ying, J.; Luong, A.U. Distinguishing Molecular Features of Allergic Fungal Rhinosinusitis. Otolaryngol. Neck Surg. 2018, 159, 185–193. [Google Scholar] [CrossRef]
- Bartemes, K.R.; Kita, H. Innate and adaptive immune responses to fungi in the airway. J. Allergy Clin. Immunol. 2018, 142, 353–363. [Google Scholar] [CrossRef]
- Delneste, Y.; Beauvillain, C.; Jeannin, P. Innate immunity: Structure and function of TLRs. Med. Sci. 2007, 23, 67–73. [Google Scholar] [CrossRef]
- Dong, Z.; Yang, Z.; Wang, C. Expression of TLR2 and TLR4 Messenger RNA in the Epithelial Cells of the Nasal Airway. Am. J. Rhinol. 2005, 19, 236–239. [Google Scholar] [CrossRef]
- Williams, P.B.; Barnes, C.S.; Portnoy, J.M.; Baxi, S.; Grimes, C.; Horner, W.E.; Kennedy, K.; Larenas-Linnemann, D.; Levetin, E.; Miller, J.D.; et al. Innate and Adaptive Immune Response to Fungal Products and Allergens. J. Allergy Clin. Immunol. Pract. 2016, 4, 386–395. [Google Scholar] [CrossRef]
- Loures, F.; Araújo, E.F.; Feriotti, C.; Bazan, S.B.; Calich, V.L.G. TLR-4 cooperates with Dectin-1 and mannose receptor to expand Th17 and Tc17 cells induced by Paracoccidioides brasiliensis stimulated dendritic cells. Front. Microbiol. 2015, 6, 261. [Google Scholar] [CrossRef]
- Shin, S.-H.; Lee, Y.-H. Airborne Fungi Induce Nasal Polyp Epithelial Cell Activation and Toll-Like Receptor Expression. Int. Arch. Allergy Immunol. 2010, 153, 46–52. [Google Scholar] [CrossRef]
- Jolink, H.; de Boer, R.; Hombrink, P.; Jonkers, R.E.; van Dissel, J.T.; Falkenburg, J.F.; Heemskerk, M.H. Pulmonary immune responses against Aspergillus fumigatus are characterized by high frequencies of IL-17 producing T-cells. J. Infect. 2016, 74, 81–88. [Google Scholar] [CrossRef]
- Zhang, Z.; Biagini Myers, J.M.; Brandt, E.B.; Ryan, P.H.; Lindsey, M.; Mintz-Cole, R.A.; Reponen, T.; Vesper, S.J.; Forde, F.; Ruff, B.; et al. β-Glucan exacerbates allergic asthma independent of fungal sensitization and promotes steroid-resistant TH2/TH17 responses. J. Allergy Clin. Immunol. 2017, 139, 54–65.e8. [Google Scholar] [CrossRef] [PubMed]
- Romani, L. Immunity to fungal infections. Nat. Rev. Immunol. 2011, 11, 275–288. [Google Scholar] [CrossRef] [PubMed]
- Iijima, K.; Kobayashi, T.; Hara, K.; Kephart, G.M.; Ziegler, S.F.; McKenzie, A.N.; Kita, H. IL-33 and Thymic Stromal Lymphopoietin Mediate Immune Pathology in Response to Chronic Airborne Allergen Exposure. J. Immunol. 2014, 193, 1549–1559. [Google Scholar] [CrossRef]
- Kouzaki, H.; Iijima, K.; Kobayashi, T.; O’Grady, S.M.; Kita, H. The Danger Signal, Extracellular ATP, Is a Sensor for an Airborne Allergen and Triggers IL-33 Release and Innate Th2-Type Responses. J. Immunol. 2011, 186, 4375–4387. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.-H.; Ye, M.-K.; Lee, D.-W.; Chae, M.-H.; Han, B.-D. Nasal Epithelial Cells Activated with Alternaria and House Dust Mite Induce Not Only Th2 but Also Th1 Immune Responses. Int. J. Mol. Sci. 2020, 21, 2693. [Google Scholar] [CrossRef]
- Millien, V.O.; Lu, W.; Shaw, J.; Yuan, X.; Mak, G.; Roberts, L.; Song, L.-Z.; Knight, J.M.; Creighton, C.J.; Luong, A.; et al. Cleavage of Fibrinogen by Proteinases Elicits Allergic Responses Through Toll-Like Receptor 4. Science 2013, 341, 792–796. [Google Scholar] [CrossRef]
- Dietz, C.J.; Sun, H.; Yao, W.C.; Citardi, M.J.; Corry, D.B.; Luong, A.U. Aspergillus fumigatus induction of IL-33 expression in chronic rhinosinusitis is PAR2-dependent. Laryngoscope 2019, 129, 2230–2235. [Google Scholar] [CrossRef]
- Strohm, A.N.; Doherty, T.A. Detection, Isolation, and Functional Studies of Mouse Pulmonary Group 2 Innate Lymphoid Cells. Methods Mol. Biol. 2022, 2506, 167–186. [Google Scholar] [CrossRef]
- Doherty, T.A.; Khorram, N.; Lund, S.; Mehta, A.K.; Croft, M.; Broide, D.H. Lung type 2 innate lymphoid cells express cysteinyl leukotriene receptor 1, which regulates TH2 cytokine production. J. Allergy Clin. Immunol. 2013, 132, 205–213. [Google Scholar] [CrossRef]
- Kim, H.C.; Lim, J.Y.; Kim, S.; Kim, J.H.; Jang, Y.J. Development of a mouse model of eosinophilic chronic rhinosinusitis with nasal polyp by nasal instillation of an Aspergillus protease and ovalbumin. Eur. Arch. Oto-Rhino-Laryngol. 2017, 274, 3899–3906. [Google Scholar] [CrossRef]
- Park, S.C.; Kim, S.I.; Hwang, C.S.; Cho, H.-J.; Yoon, J.-H.; Kim, C.-H. Multiple airborne allergen-induced eosinophilic chronic rhinosinusitis murine model. Eur. Arch. Oto-Rhino-Laryngol. 2019, 276, 2273–2282. [Google Scholar] [CrossRef]
- Shin, S.-H.; Ye, M.-K.; Lee, D.-W.; Chae, M.-H.; Choi, S.-Y. Development and immunopathological characteristics of an Alternaria-induced chronic rhinosinusitis mouse model. PLoS ONE 2020, 15, e0234731. [Google Scholar] [CrossRef]
- Jain, R.; Douglas, R. When and how should we treat biofilms in chronic sinusitis? Curr. Opin. Otolaryngol. Head Neck Surg. 2014, 22, 16–21. [Google Scholar] [CrossRef]
- Healy, D.Y.; Leid, J.G.; Sanderson, A.R.; Hunsaker, D.H. Biofilms with fungi in chronic rhinosinusitis. Otolaryngol. Neck Surg. 2008, 138, 641–647. [Google Scholar] [CrossRef]
- Singhal, D.; Baker, L.; Wormald, P.-J.; Tan, L. Aspergillus FumigatusBiofilm on Primary Human Sinonasal Epithelial Culture. Am. J. Rhinol. Allergy 2011, 25, 219–225. [Google Scholar] [CrossRef]
- Boase, S.; Jervis-Bardy, J.; Cleland, E.; Pant, H.; Tan, L.; Wormald, P.-J. Bacterial-induced epithelial damage promotes fungal biofilm formation in a sheep model of sinusitis. Int. Forum Allergy Rhinol. 2013, 3, 341–348. [Google Scholar] [CrossRef]
- Shin, S.-H.; Ye, M.-K.; Lee, D.-W.; Chae, M.-H. Asian Sand Dust Particles Enhance the Development of Aspergillus fumigatus Biofilm on Nasal Epithelial Cells. Int. J. Mol. Sci. 2022, 23, 3030. [Google Scholar] [CrossRef]
- Foreman, A.; Boase, S.; Psaltis, A.; Wormald, P.-J. Role of Bacterial and Fungal Biofilms in Chronic Rhinosinusitis. Curr. Allergy Asthma Rep. 2012, 12, 127–135. [Google Scholar] [CrossRef]
- Hochstim, C.J.; Choi, J.Y.; Lowe, D.; Masood, R.; Rice, D.H. Biofilm Detection With Hematoxylin-Eosin Staining. Arch. Otolaryngol. Neck Surg. 2010, 136, 453–456. [Google Scholar] [CrossRef]
- Seidler, M.J.; Salvenmoser, S.; Muüller, F.-M.C. Aspergillus fumigatus Forms Biofilms with Reduced Antifungal Drug Susceptibility on Bronchial Epithelial Cells. Antimicrob. Agents Chemother. 2008, 52, 4130–4136. [Google Scholar] [CrossRef]
- Kaur, S.; Singh, S. Biofilm formation by Aspergillus fumigatus. Med. Mycol. 2014, 52, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Von Köckritz-Blickwede, M.; Nizet, V. Innate immunity turned inside-out: Antimicrobial defense by phagocyte extracellular traps. J. Mol. Med. 2009, 87, 775–783. [Google Scholar] [CrossRef] [PubMed]
- Hwang, C.S.; Park, S.C.; Cho, H.-J.; Park, D.-J.; Yoon, J.-H.; Kim, C.-H. Eosinophil extracellular trap formation is closely associated with disease severity in chronic rhinosinusitis regardless of nasal polyp status. Sci. Rep. 2019, 9, 8061. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.W.; Kim, J.H.; Kim, H.J.; Choi, I.H.; Han, H.M.; Lee, K.J.; Kim, T.H.; Lee, S.H. Neutrophil extracellular traps in nasal secretions of patients with stable and exacerbated chronic rhinosinusitis and their contribution to induce chemokine secretion and strengthen the epithelial barrier. Clin. Exp. Allergy 2019, 49, 1306–1320. [Google Scholar] [CrossRef]
- Azzouz, L.; Cherry, A.; Riedl, M.; Khan, M.; Pluthero, F.G.; Kahr, W.H.; Palaniyar, N.; Licht, C. Relative antibacterial functions of complement and NETs: NETs trap and complement effectively kills bacteria. Mol. Immunol. 2018, 97, 71–81. [Google Scholar] [CrossRef]
- Röhm, M.; Grimm, M.J.; D’Auria, A.C.; Almyroudis, N.G.; Segal, B.H.; Urban, C.F. NADPH Oxidase Promotes Neutrophil Extracellular Trap Formation in Pulmonary Aspergillosis. Infect. Immun. 2014, 82, 1766–1777. [Google Scholar] [CrossRef]
- Schönrich, G.; Raftery, M.J. Neutrophil Extracellular Traps Go Viral. Front. Immunol. 2016, 7, 366. [Google Scholar] [CrossRef]
- Abi Abdallah, D.S.; Lin, C.; Ball, C.J.; King, M.R.; Duhamel, G.E.; Denkers, E.Y. Toxoplasma gondii Triggers Release of Human and Mouse Neutrophil Extracellular Traps. Infect. Immun. 2012, 80, 768–777. [Google Scholar] [CrossRef]
- Yipp, B.G.; Petri, B.; Salina, D.; Jenne, C.N.; Scott, B.N.V.; Zbytnuik, L.D.; Pittman, K.; Asaduzzaman, M.; Wu, K.; Meijndert, H.C.; et al. Infection-induced NETosis is a dynamic process involving neutrophil multitasking in vivo. Nat. Med. 2012, 18, 1386–1393. [Google Scholar] [CrossRef]
- Bruns, S.; Kniemeyer, O.; Hasenberg, M.; Aimanianda, V.; Nietzsche, S.; Thywien, A.; Jeron, A.; Latgé, J.P.; Brakhage, A.A.; Gunzer, M. Production of Extracellular Traps against Aspergillus fumigatus In Vitro and in Infected Lung Tissue Is Dependent on Invading Neutrophils and Influenced by Hydrophobin RodA. PLoS Pathog. 2010, 6, e1000873. [Google Scholar] [CrossRef]
- Nanì, S.; Fumagalli, L.; Sinha, U.; Kamen, L.; Scapini, P.; Berton, G. Src Family Kinases and Syk Are Required for Neutrophil Extracellular Trap Formation in Response to β-Glucan Particles. J. Innate Immun. 2015, 7, 59–73. [Google Scholar] [CrossRef]
- Kernien, J.F.; Snarr, B.D.; Sheppard, D.C.; Nett, J.E. The Interface between Fungal Biofilms and Innate Immunity. Front. Immunol. 2017, 8, 1968. [Google Scholar] [CrossRef]
- Figueiredo, R.T.; Neves, J.S. Eosinophils in fungal diseases: An overview. J. Leukoc. Biol. 2018, 104, 49–60. [Google Scholar] [CrossRef]
- Lilly, L.M.; Scopel, M.; Nelson, M.P.; Burg, A.R.; Dunaway, C.W.; Steele, C. Eosinophil Deficiency Compromises Lung Defense against Aspergillus fumigatus. Infect. Immun. 2014, 82, 1315–1325. [Google Scholar] [CrossRef]
- Lam, K.; Schleimer, R.P.; Kern, R.C. The Etiology and Pathogenesis of Chronic Rhinosinusitis: A Review of Current Hypotheses. Curr. Allergy Asthma Rep. 2015, 15, 41. [Google Scholar] [CrossRef]
- Yousefi, S.; Gold, J.; Andina, N.; Lee, J.J.; Kelly, A.M.; Kozlowski, E.; Schmid, I.; Straumann, A.; Reichenbach, J.; Gleich, G.J.; et al. Catapult-like release of mitochondrial DNA by eosinophils contributes to antibacterial defense. Nat. Med. 2008, 14, 949–953. [Google Scholar] [CrossRef]
- Ueki, S.; Melo, R.; Ghiran, I.; Spencer, L.A.; Dvorak, A.M.; Weller, P.F. Eosinophil extracellular DNA trap cell death mediates lytic release of free secretion-competent eosinophil granules in humans. Blood 2013, 121, 2074–2083. [Google Scholar] [CrossRef]
- Gaur, P.; Zaffran, I.; George, T.; Alekberli, F.R.; Ben-Zimra, M.; Levi-Schaffer, F. The regulatory role of eosinophils in viral, bacterial, and fungal infections. Clin. Exp. Immunol. 2022, 209, 72–82. [Google Scholar] [CrossRef]
- Ponikau, J.U.; Sherris, D.A.; Kephart, G.M.; Kern, E.B.; Congdon, D.J.; Adolphson, C.R.; Springett, M.J.; Gleich, G.J.; Kita, H. Striking deposition of toxic eosinophil major basic protein in mucus: Implications for chronic rhinosinusitis. J. Allergy Clin. Immunol. 2005, 116, 362–369. [Google Scholar] [CrossRef]
- Lillehoj, E.P.; Kato, K.; Lu, W.; Kim, K.C. Cellular and Molecular Biology of Airway Mucins. Int. Rev. Cell Mol. Biol. 2013, 303, 139–202. [Google Scholar] [CrossRef]
- Muniz, V.S.; Silva, J.C.; Braga, Y.A.; Melo, R.C.; Ueki, S.; Takeda, M.; Hebisawa, A.; Asano, K.; Figueiredo, R.T.; Neves, J.S. Eosinophils release extracellular DNA traps in response to Aspergillus fumigatus. J. Allergy Clin. Immunol. 2018, 141, 571–585.e7. [Google Scholar] [CrossRef] [PubMed]
- Ueki, S.; Konno, Y.; Takeda, M.; Moritoki, Y.; Hirokawa, M.; Matsuwaki, Y.; Honda, K.; Ohta, N.; Yamamoto, S.; Takagi, Y.; et al. Eosinophil extracellular trap cell death–derived DNA traps: Their presence in secretions and functional attributes. J. Allergy Clin. Immunol. 2016, 137, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Chalermwatanachai, T.; Vilchez-Vargas, R.; Holtappels, G.; Lacoere, T.; Jáuregui, R.; Kerckhof, F.-M.; Pieper, D.H.; Van de Wiele, T.; Vaneechoutte, M.; Van Zele, T.; et al. Chronic rhinosinusitis with nasal polyps is characterized by dysbacteriosis of the nasal microbiota. Sci. Rep. 2018, 8, 7926. [Google Scholar] [CrossRef] [PubMed]
- Cho, D.-Y.; Hunter, R.C.; Ramakrishnan, V.R. The Microbiome and Chronic Rhinosinusitis. Immunol. Allergy Clin. North Am. 2020, 40, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Whiteside, S.A.; McGinniss, J.E.; Collman, R.G. The lung microbiome: Progress and promise. J. Clin. Investig. 2021, 131, e150473. [Google Scholar] [CrossRef]
- Thompson, J.F.; Steinmann, K.E. Single Molecule Sequencing with a HeliScope Genetic Analysis System. Curr. Protoc. Mol. Biol. 2010, 92, 7.10.1–7.10.14. [Google Scholar] [CrossRef]
- Shin, S.-H.; Ye, M.-K.; Lee, Y.-H. Fungus Culture of the Nasal Secretion of Chronic Rhinosinusitis Patients: Seasonal Variations in Daegu, Korea. Am. J. Rhinol. 2007, 21, 556–559. [Google Scholar] [CrossRef]
- Zhao, Y.C.; Bassiouni, A.; Tanjararak, K.; Vreugde, S.; Wormald, P.-J.; Psaltis, A.J. Role of fungi in chronic rhinosinusitis through ITS sequencing. Laryngoscope 2018, 128, 16–22. [Google Scholar] [CrossRef]
- Jiang, R.-S.; Shih, C.-H.; Jiang, Y.-H.; Hsieh, H.-H.; Chiang, Y.-F.; Chuang, H.-N.; Hsiao, T.-H. Nasal Mycology of Chronic Rhinosinusitis Revealed by Nanopore Sequencing. Diagnostics 2022, 12, 2735. [Google Scholar] [CrossRef]
- Blanco, J.L.; Garcia, M.E. Immune response to fungal infections. Veter-Immunol. Immunopathol. 2008, 125, 47–70. [Google Scholar] [CrossRef]
- Caballero, J.D.D.; Cantón, R.; Ponce-Alonso, M.; García-Clemente, M.M.; de la Pedrosa, E.G.G.; López-Campos, J.L.; Máiz, L.; del Campo, R.; Martínez-García, M. The Human Mycobiome in Chronic Respiratory Diseases: Current Situation and Future Perspectives. Microorganisms 2022, 10, 810. [Google Scholar] [CrossRef]
- Schlecht, L.M.; Peters, B.M.; Krom, B.P.; Freiberg, J.A.; Hänsch, G.M.; Filler, S.G.; Jabra-Rizk, M.A.; Shirtliff, M.E. Systemic Staphylococcus aureus infection mediated by Candida albicans hyphal invasion of mucosal tissue. Microbiology 2015, 161, 168–181. [Google Scholar] [CrossRef]
- Harriott, M.M.; Noverr, M.C. Candida albicans and Staphylococcus aureus Form Polymicrobial Biofilms: Effects on Antimicrobial Resistance. Antimicrob. Agents Chemother. 2009, 53, 3914–3922. [Google Scholar] [CrossRef]
- Peters, B.M.; Ovchinnikova, E.S.; Krom, B.P.; Schlecht, L.M.; Zhou, H.; Hoyer, L.L.; Busscher, H.J.; van der Mei, H.C.; Jabra-Rizk, M.A.; Shirtliff, M.E. Staphylococcus aureus adherence to Candida albicans hyphae is mediated by the hyphal adhesin Als3p. Microbiology 2012, 158, 2975–2986. [Google Scholar] [CrossRef]
- Hogan, D.A.; Vik, Å.; Kolter, R. A Pseudomonas aeruginosa quorum-sensing molecule influences Candida albicans morphology. Mol. Microbiol. 2004, 54, 1212–1223. [Google Scholar] [CrossRef]
- Reece, E.; Doyle, S.; Greally, P.; Renwick, J.; McClean, S. Aspergillus fumigatus Inhibits Pseudomonas aeruginosa in Co-culture: Implications of a Mutually Antagonistic Relationship on Virulence and Inflammation in the CF Airway. Front. Microbiol. 2018, 9, 1205. [Google Scholar] [CrossRef]
- Lee, K.; Zhang, I.; Kyman, S.; Kask, O.; Cope, E.K. Co-infection of Malassezia sympodialis with Bacterial Pathobionts Pseudomonas aeruginosa or Staphylococcus aureus Leads to Distinct Sinonasal Inflammatory Responses in a Murine Acute Sinusitis Model. Front. Cell. Infect. Microbiol. 2020, 10, 472. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).