Abstract
4-methylumbelliferone (4MU) is a well-known hyaluronic acid synthesis inhibitor and an approved drug for the treatment of cholestasis. In animal models, 4MU decreases inflammation, reduces fibrosis, and lowers body weight, serum cholesterol, and insulin resistance. It also inhibits tumor progression and metastasis. The broad spectrum of effects suggests multiple and yet unknown targets of 4MU. Aiming at 4MU target deconvolution, we have analyzed publicly available data bases, including: 1. Small molecule library Bio Assay screening (PubChemBioAssay); 2. GO pathway databases screening; 3. Protein Atlas Database. We also performed comparative liver transcriptome analysis of mice on normal diet and mice fed with 4MU for two weeks. Potential targets of 4MU public data base analysis fall into two big groups, enzymes and transcription factors (TFs), including 13 members of the nuclear receptor superfamily regulating lipid and carbohydrate metabolism. Transcriptome analysis revealed changes in the expression of genes involved in bile acid metabolism, gluconeogenesis, and immune response. It was found that 4MU feeding decreased the accumulation of the glycogen granules in the liver. Thus, 4MU has multiple targets and can regulate cell metabolism by modulating signaling via nuclear receptors.
1. Introduction
In cell culture, 4-methylumbelliferone (4MU) is a coumarin derivative well-known for inhibiting the synthesis of hyaluronic acid (HA). Moreover, 4MU, under the trade name “Hymecromone”, is a prescribed drug for the treatment of cholestasis. Studies in different animal models clearly show that 4MU treatment leads to a wide range of biological effects summarized in reviews [1,2]. Further, 4MU administration inhibits tumor growth and metastasis [3,4,5], fibrosis development [6,7], and inflammation [1,8,9]. A broad spectrum of 4MU biological effects can be explained by inhibiting hyaluronic acid production, but pharmacological targets of 4MU are still under debate. Direct interaction of 4MU and hyaluronan synthase enzyme was not demonstrated. Moreover, 4MU does not inhibit bacterial HAS activity in membrane preparations after the disintegration with ultrasound [10]. Cell-free hyaluronic acid synthesis by membrane preparations from human fibroblasts was not affected by 4MU, too [11]. These experimental facts point towards the possible indirect effect of 4MU on HA synthesis.
It was suggested that 4MU suppresses HA production by depleting the intracellular pool of UDP-glucuronic acid, a precursor of HA [12]. 4MU is a competitive substrate for UGT-glycosyltransferase (UGT), an enzyme which generates precursors for HA synthesis, UDP-glucuronic acid (UDP-GlcA) and UDP-N-acetylglucosamine (UDP-GlcNAc). Recently published data suggested that this interpretation may be insufficient. Nagy et al. demonstrated that in vivo administration of 4MU significantly decreased the level of UDP-GlcA but did not reduce the total concentration of HA in mouse pancreas, muscle, and liver [13]. The fact that 4MU does not inhibit chondroitin sulfate and heparan sulfate synthesis, where both HA precursors are involved, contradicts the UDP-GlcA depletion hypothesis [14,15]. According to published studies and the data from our laboratory, 4MU exerts a dual action on mammalian cells: at low micromolar concentration, it inhibits the HA synthesis, but in concentrations higher than 200 μm, it strongly affects multiple gene expressions, including HAS2 [6,15,16]. Our transcriptomic analysis demonstrated strong effects of 4MU on gene expression in hepatocytes, the cells which do not express HAS enzyme [6]. All these data point to multiple biological effects of 4MU.
As mentioned in many papers, 4MU treatment has a significant influence on the immune response [17,18]. Moreover, 4MU treatment decreases inflammation in mouse models of type 1 and 2 diabetes [19,20,21], multiple sclerosis [22], autoimmune arthritis [23], acute lung injury [18], liver fibrosis [6], and many others. Further, 4MU inhibits antigen presentation by dendritic cells [24] and blocks T cell proliferation activation and Th1 polarization [17,25]. In addition, 4MU primes Th2 polarization and induction of Foxp3+ regulatory T cells [17]. Treatment with 4MU also reduces macrophage accumulation in the fibrotic area [6,7], switches hepatic macrophages to proinflammatory M1 phenotype, and reduces the aggressiveness of hepatocellular carcinoma [26].
Recently published data also indicates that 4MU administration induces a metabolic switch in different cell types, and this effect is not directly linked to HA inhibition. It was shown that 4MU treatment inhibits glycolysis in chondrocytes [8,9] and melanoma cell lines [27]. At present direct biological targets and mechanistic explanations of the biological effects of 4MU are missing. This hampers the development of new, more specific, and more potent drugs than hymecromone.
Here, we present an analysis of publicly available data bases: 1. Summary of 4MU targets identivied by small molecule libraries screening, depositted in PubChemBioAssay; 2. GO pathway analysis of identified 4MU target proteins; 3. Analysis of 4MU target protein expression across various human cell types (Single Cell and HPA datasets from Protein Atlas). Database analysis and our experimental data reveal that 4MU has multiple targets and can regulate cell metabolism through the modulation of nuclear receptor signaling.
2. Results
2.1. Analysis of 4 MU Targets and Open Access Data Bases
2.1.1. Potential 4MU Biological Targets Revealed by PubChem BioAssay Data
As of 30 May 2022, there were 1,465,985 bioassays deposited on the PubChem BioAssay database (https://pubchemdocs.ncbi.nlm.nih.gov/bioassays; accessed on 30 May 2022) including 111,398,703 compounds, encompassing 103,628 genes and 185,202 proteins [28]. The dataset for analysis was collected manually from the following queries: 4-methylumbelliferone (4MU, 1676 records), 4-methylumbelliferyl-beta-D-glucuronide (4MUG, 71 records) and 4-methylumbelliferyl sulfate (4MUS, 15 records), all henceforth jointly referred to as 4MU targets. Each record describes the effect of 4MU observed in small molecule libraries screening experiments targeting a particular protein or process activity. We excluded entries where 4MU did not have any activity, partially curated data records and experiments dedicated to the antibacterial properties of 4MU. After parsing the initial targets through the exclusion criteria, the 51 records obtained were sorted into either Active or Inconclusive sets based on PubChem BioAssay activity score. The final result after deduplication of 4MU and 4MUG common targets (HSD17B4, HSD17B10 for “Active” and GBA, HPGD for “Inconclusive”) contained 22 “Active” targets and 39 “Inconclusive” targets 10 of which were common in both sets (Figure 1B). Using the Gene Ontology database, we clustered the target proteins by function into three main groups namely: Enzymes (23 records), Transcription factors (TF; 20 records), and Others (nine records), mainly transport and scaffold proteins, as shown in Figure 1C,D and Table 1.
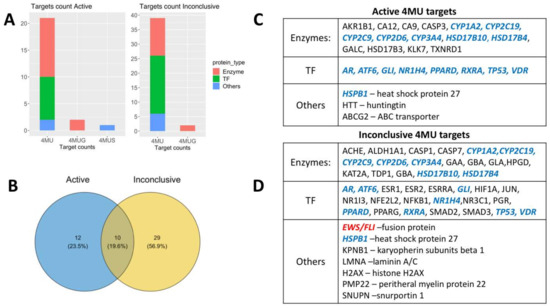
Figure 1.
4MU, 4MUG and 4MUS targets retrieved from PubChem BioAssay database. (A)—target counts in Active and Inconclusive data sets with respect to target function and 4MU derivative. (B)—Venn diagram with Active and Inconclusive sets intersection. (C)—List of Active targets, (D)—list of Inconclusive targets. Common targets for Active and Inconclusive sets are shown in blue, and EWS/FLI protein, which was excluded from further analysis, is shown in red.

Table 1.
Function of the acquired 4MU target proteins.
2.1.2. 4MU Target Proteins Revealed by Analysis of Affected Molecular Pathways
For the investigation of the involvement of 4MU target proteins in biological processes and pathways, we used the GO Database terms. TFs were mainly identified using DNA-templated RNA transcription through cis-regulatory regions. Among identified TFs, 13 belong to the superfamily of nuclear receptors binding lipophilic ligands. The Enzymes group included enzymes primarily involved in steroid hormone metabolism, monoterpenoid metabolic processes, exogenous drug metabolism, long-chain fatty acid biosynthetic processes, and apoptosis. These enzymes possess hydroxylase, oxidoreductase, hydrolase and caspase activities “Others” group included proteins involved in nuclear membrane reassembly, organisation, and nuclear import with transmembrane transporter activity, RNA cap, and dynactin binding (Figure 2A,B).
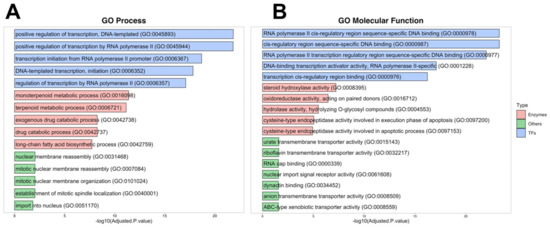
Figure 2.
Main GO ontologies and pathways of 4MU targets. (A)—GO Process ontologies, (B)—GO Molecular Function.
The type of quantitative High throughput screens performed (qHTS) are highlighted and were accessed in PubChem BioAssay using the AID numbers shown in Table 2.

Table 2.
4MU active concentration and effect on target protein function.
Treatment with 4MU activates cellular stress response pathways mediated by TP53, HIF1A, NFE2L2, and ATF6 and initiates NFkB-dependent transcription (Table 2). It also activates RXRA, the main dimerization partner for type I nuclear receptors, and mediates the biological effects of retinoic acid. Such pattern of TFs indicates activation of the defence program against different stress stimuli followed by a shift in fatty acid and glucose metabolism. 4MU has a dual effect (activation and inhibition) on 12 TFs (Table 2), mainly nuclear receptors. Furthermore, 4MU elicits different responses depending on the cell line and experimental conditions as is the case with VDR and NR3C1 (glucocorticoid receptor) which are well-known modulators of the immune response.
In addition, 4MU stimulated the activity of enzymes, such as CASP3, GALC, KLK7, GAA, and GBA, which modulate protein processing, cell motility, membrane glycolipid composition and glycogen breakdown. In this Enzymes group, KLK7 peptidase was activated by the lowest concentration of 4MU (7 µM). KLK7 peptidase is involved in skin shedding, cancer and Alzheimer’s disease progression [90,91,92].
It was found that 4MU inhibited 18 out of 24 enzymes (Table 2). These enzymes are involved in lipid and drug metabolism, oxidative stress response, cytokine activation, transcription and DNA repair processes. CA9 was inhibited by the lowest concentration of 4MU (0.56 μM). CA9 is abundantly expressed in many cancer types where it maintains the normal pH level in tumor cells in the hypoxic environment [93].
Transcription activity of GLI and NR1I3 and nuclear imports mediated by SNUPN and KPNB1 from the other groups was also inhibited by 4MU. Interestingly, we found three proteins in this group specifically involved in the development of inherited neurological disorders:
- (i)
- Huntingtin protein (HTT) is involved in axonal transport. The mutation of the Huntingtin gene causes Huntington’s disease. Treatment with 4MU leads to cytoprotection in the cell model of Huntington’s disease (PubChem bioassay AID 1471).
- (ii)
- PMP22 is integrated into the myelin sheath of Schwann cells and is involved in Charcot–Marie–Tooth disease. Treatment with 4MU inhibits the PMP22 expression in Swann cells.
- (iii)
- Nuclear Lamin A (LMNA), a nuclear envelope scaffold protein involved in chromatin organisation, remodelling, and double stranded break DNA repair [94]. Mutations in LMNA cause the development of Hutchinson-Gilford syndrome, characterised by early premature ageing. Moreover, 4MU modulates the expression of Lamin A (PubChem Bioassay 1487).
In vitro, 4MU activated the phosphorylation of H2AX on Ser-139, a sensitive marker of DNA double strand break [95], and stimulated the expression of HSPB1, indicating the activation of the heat shock response pathway. These data correlate with ATF6α activation, which is the main sensor of unfolded protein stress in ER [58]. All these proteins from the “Others” group (HSPB1, LMNA, HTT, PMP22 and H2AX) can be considered indirect targets of 4MU.
2.1.3. 4MU Target Proteins Revealed by Analysis of Gene Expression in Various Human Cell Types
The target gene expression of 4MU was evaluated in 51 human cell types from the Single Cell data of the Protein Atlas Database. The target genes 4MU fell into three clusters. Cluster 1 comprised of 11 genes; NR2H4, CYP1A2, KLK7, CA9, ESR2, PGR, HSD17B3, AR, CLI3, NR1I3, and ESR1. Expression of these genes varied significantly across cell types. Cluster 2 consisted of 26 genes, all highly expressed in almost all cell types. Cluster 3, composed of eight genes, namely ACHE, CA12, ABCG2, CASP1, VDR, TDP1, HPGD, and PPARG, showed a moderately high expression in some cell types. All gene clusters were detected in the cardiomyocytes in contrast to erythroid cells in which gene expression was undetectable (Figure 3). These data indicate that 4MU alters metabolic pathways in a vast majority of cell types.
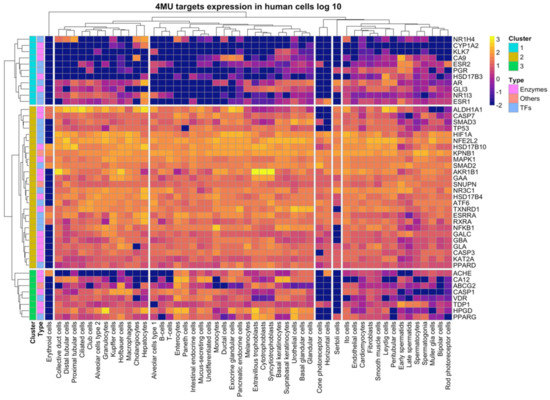
Figure 3.
4MU targets expression across human cell types. The expression levels of individual genes were retrieved manually from the Single Cell RNA-Seq dataset of the Human protein atlas database (www.proteinatlas.org). Data presented in log 10 scale, −2 values correspond to the undetectable expression level.
We also analysed 4MU target gene expression in immune cells using the data from the HPA dataset deposited in the Protein atlas database. Immune cells express 43 out of 45 genes which form three clusters. PGR and NR1H4 expression was undetectable (Figure 4).
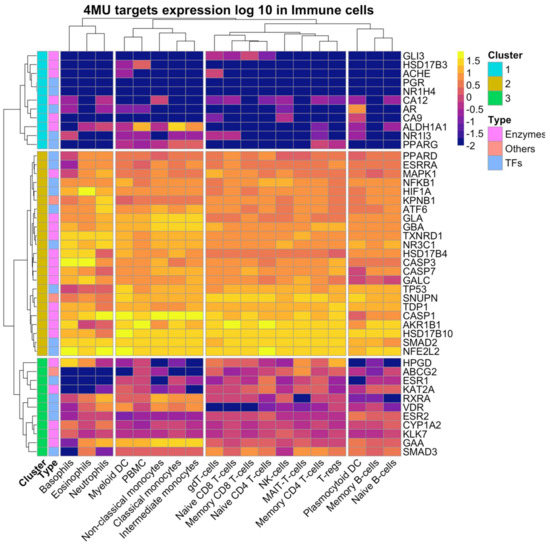
Figure 4.
Expression of 4MU targets in the immune cells. Data were retrieved manually from the HPA RNA expression dataset from the Human Protein Atlas database. Data present in log 10 scale, −2 value represent the absence of expression.
Cluster 1 includes eight genes whose expression is generally low and is specific to peripheral blood mononuclear cell (PBMC), non-classical, classical, and intermediate monocytes. The co-expression of ALDH1A1, NR1I3, and PPARG is characteristic of PMBC, myeloid dendritic cells (DC), macrophages, and memory CD4+ T cells. CA12, CA9, AR, ALDH1A1, and NR1I3 co expression is unique to plasmacytoid DC.
Cluster 2 contained 26 genes with ubiquitous and high expression across all analysed cell types. The group of TFs was comprised of ATF6, ESRRA, HIF1A, SMAD2, NFE2L2, NFKB1, PPARD, TP53, and NR3C1 and the enzymes group consisted of AKR1B1, HSD17B4, HSD17B10, CASP1, CASP3, CASP7, CYP1A2, GALC, GBA, GLA, TXNRD1, and TDP1.
Cluster 3 contained 11 genes expressed in most analysed cell types (Figure 4). This cluster included HPGD, ABCG2, ESR1, KAT2A, RXRA, VDR, ESR2, SMAD3, CYP1A2, GAA, and KLK7. The expression levels of cluster 3 genes were lower than those of cluster 2 genes (Figure 4).
Based on the target annotation above, we postulate that 4MU influences lipid metabolism, cytokine production, antioxidant activity and apoptosis in immune cells. NFE2L2 is a 4MU target, which is highly expressed in lymphocytes. This TF upregulates the transcription of many antioxidant and cytoprotective proteins, including TXNRD1 [96]. NFE2L2 signalling pathway antagonises NFkB-pathway regulating expression of the genes involved in inflammatory, immune and acute-phase responses [97,98]. Furthermore, NFE2L2 inhibits TGF-β signalling and HIF1-mediated immune response [99,100].
Earlier on, we highlighted that the type of response to 4MU elicited is dependant type of cell line and experimental conditions as is the case with VDR and NR3C1. NR3C1 is highly expressed in all immune cells and regulates innate and adaptive immunity. Generally, NR3C1 suppresses proinflammatory activation of macrophages, inhibits the production of cytokines (IL-1, IL-6, IL-8) and chemokines (Ccl2, Ccl3, Ccl4, Cxcl9, and Cxcl11) which promotes the acquisition of inflammation resolving phenotype [70]. Functional activity of NR3C1 is essential for T-cell polyclonal activation and maturation of either Th1- and Th2- cell types [101]. It is also involved in the modulation of B-cell apoptosis [102].
VDR is a potent immune stimulator, upon activation boosts the innate immune cells’ chemotactic and phagocytic capabilities. Moreover, VDR induces the transcription of cathelicidin and defensin β2 [78]. It was shown that VDR activation prevents nuclear translocation of p65/p50 subunits of NFkB and degrades IκBα protein, thereby inhibiting the inflammatory response [103]. Its activation suppresses autoimmunity driven by Th1 and Th17 cells [78]. The mild immunosuppressive properties of 4MU can be explained by the synergistic action of both VDR and NR3C1, which occurred in the 30–50 μM range according to PubChem BioAssay data analysis (Table 2).
2.2. Experimental Analysis of 4MU Effect on Liver Transcriptome and Glycogene Storage
2.2.1. Effect of 4MU Treatment on the Gene Expression Profile of Normal Mouse Liver
According to the previously published data, the liver is one of the most prominent target organs of 4MU action [13]. Our previously published results demonstrated a profound effect of 4MU on gene expression after two weeks of treatment and its cessation after four weeks in CCL4 induced murine model of hepatic fibrosis [6].
In this study, we present a detailed transcriptomic analysis of 4MU-dependent changes in a healthy liver. Bulk RNA-Seq data analysis showed a high degree of similarity between control and 4MU treated liver samples, as reflected by Spearman correlation coefficients (Figure 5A). Principal component analysis (PCA) showed that 40.6% of sample variance originated from the first two components, which clearly separated the experimental groups into two clusters (Figure 5A). Oral 4MU treatment of healthy animals has very mild effects on gene expression. We found 62 genes which were significantly up- (26 genes) and downregulated by 4MU (36 genes), (Figure 5B). The complete list of DEGs with their relative expression levels is presented in Figure 5C,D and Table 3. Affected genes were annotated according to GO Biological Pathways database terms (Figure 5E,F). Most of the downregulated genes (29 out of 36) are involved in different aspects of immune response (Figure 5E). These genes encode the surface markers of different immune cells: Ly6d is a marker of B and dendritic cells, Ly6c1, Themis2, and Ms4a4b, are markers of T and NK cells; whilst Fcgr1 is characteristic of liver macrophages. Treatment with 4MU significantly decreases the expression level of 14 genes involved in interferon signalling and response (Tgtp1, Mx1, Epsti1, Ifit1, Ifit3, Ifit3b, Gbp3, Isg15, Il18bp, Usp18, Irf7, Oasl2, Ifi27l2a, Oas1a). The expression level of Naip1 was also significantly diminished by 4MU. Naip 1, whose role is to recognise bacterial proteins, is abundantly expressed in dendritic cells and macrophages.
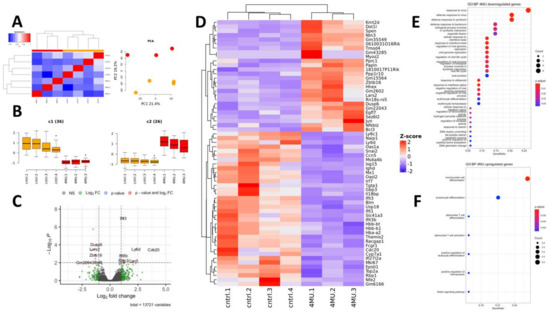
Figure 5.
Characterisation of 4MU differentially expressed genes. (A)—Correlation between control and 4MU-treated samples. (B)—Mean expression of down- and upregulated genes across the samples; (C)—Volcano plot of differentially expressed genes; (D)—Heatmap of DEGs. (E)—Pathway enrichment analysis of down-regulated genes; (F)—Pathway enrichment analysis of upregulated genes; Gene ratio—the number of DEG which falls into the specific pathway to the total number of genes in this pathway. Symbol meaning 6B: c1(36) the number of down regulated genes (n = 36) in 4MU group (4MU.1, 4MU.2, 4MU.3) in comparison with control group (cntrl.1, cntrl.2, cntrl.3, cntrl.4′); c2(26) the number of up regulated genes (n = 26) in 4MU treated group.

Table 3.
Top 52 genes differentially expressed in the liver of mice fed with 4MU.
In the 4-MU treated mice, we observed a decreased expression of proteins (Cdc20, Mki67, Ccn5, Racgap1) involved in cycle progression, and maintenance of DNA integrity and stability (Top2a, Blm). Among other downregulated genes, we identified two transcriptional factors (Nfe2, Snai2), haemoglobin’s chains genes (Hba-a2, Hbb-b1, Hbb-bt), retinol-binding protein 1 (Rbp1), Ig delta chain C region (Ighd) and mitochondrial Mg2+ transporter (Slc41a3). Surprisingly, Cyp7a1 one of the critical enzymes of bile acid production was downregulated four-fold [104].
The upregulated genes are involved in the modulation of transcriptional activity (Bcl3, Spen), histone acetylation (Dot1l, Kmt2d), protein phosphatase activity (Dusp6, Ppp1r10), mitochondrial biogenesis and function (Lars2, Pprc1), extracellular matrix remodelling (Egfl7, Ntn3, Papln), muscle cells differentiation (Myoz2, Tmod4), and cancer progression (Sez6l2). Moreover, 4MU induced a nearly two-fold increase in the expression levels of transcriptional factors Hhex, Jun, Nfkbiz, and Zbtb16 (Figure 5C,D; Table 3). Hhex is an essential transcription factor for hepatoblast differentiation and intrahepatic bile duct morphogenesis [105]. Embryonic knockout of Hhex is lethal because it causes abnormal liver development. In adult mice, transcriptional activation of Hhex expression depends on Farnesoid X receptor (NR1H4) activation by bile acid. Overstimulation of this signalling pathway leads to liver hypertrophy [106]. Jun is involved in hepatocyte proliferation and liver regeneration [107]. It has been demonstrated that c-Jun is involved in the bile-acid-induced downregulation of Cyp7a1 expression [108]. Nfkbiz is a transcriptional regulator that augments the inflammatory responses from Toll-like receptors or interleukin signalling. The hepatocyte-specific knockout of this gene significantly accelerates the progression of non-alcoholic fatty liver disease in mice, while its overexpression protects the liver from steatosis. Microarray analysis revealed that the overexpression of Nfkbiz downregulates the expression of genes involved in the triglyceride metabolism pathway [109]. Zbtb16, also known as promyelocytic leukaemia zinc finger protein (PLZF), is a transcription repressor involved in energy metabolism maintenance and pathogenesis of metabolic diseases. In the adult liver, Zbtb16 is an essential regulator of gluconeogenesis. The activation of Zbtb16 expression induces the expression of vital gluconeogenic genes in vitro and in vivo [110]. Zbtb16 expression is activated by the glucocorticoid receptor (NR3C1) in many cell types, including hepatocytes [110], breast cancer cell line [111], endometrial stromal cells, and myometrial smooth muscle cells [112].
This finding is in concert with the observed depletion of glycogen granules in mice after treatment with 4MU, as shown in Figure 6.
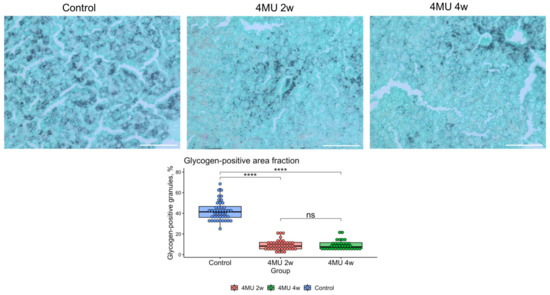
Figure 6.
Glycogen granules in mouse hepatocytes after 4MU treatment. Gomori-Grocott methenamine silver staining of glycogen granules in mice hepatocytes after 2 (2w) and 4 weeks(4w) of 4MU treatment. In the figure, hepatocytes are light green and dark grey while glycogen granules are black. Scale bar—100 μm. The plot represents the percentage of glycogen-positive area to total tissue area. Statistical difference was estimated by one-way ANOVA with Tukey post-hoc test (****—p.val < 0.0001; ns—not significant).
2.2.2. Effect of 4MU on Glycogen Storage in the Liver
Public data analysis (Pub Chem Bio Assay, Table 2 assay id 2112) shows 4MU activation of acid alpha-glucosidase (GAA), a key enzyme involved in lysosomal glycogen metabolism. The half-maximal efficacy concentration of 4MU for GAA was 25.1 μM in vitro (Table 1 and Table 2). We performed glycogen granules staining with Gomori-Grocott methenamine silver stain in healthy liver at two or four weeks after 4MU treatment n = 3 controls, n = 4 in 4 MU treated group. As anticipated, GAA activation by 4MU depleted hepatocyte glycogen granules after two weeks (Figure 6). However, the glycogen granules increased after four weeks.
3. Discussion
There are two major processes of drug discovery, namely target-based drug discovery (TBDD) and phenotype-based drug discovery (PBD). TBDD is a predominant route taken by pharmaceutical companies developing drugs interacting with well-characterised targets. In contrast to TBDB, PBD evaluates a compound’s ability to modulate a certain trait of a given biological system, the effect of a biologically active compound is based on the analysis of cell culture and whole-body phenotypes. Initially, the biological target and molecular mechanism of action are unknown. Nonetheless, effective drug development demands we identify the drug targets of the biologically active compounds.
In this study we identified 4MU targets by analysing data from publicly available databases coupled with our RNA-Seq transcriptomic profiling of bulk liver. We show that 4MU modulates the functional activity of at least 45 proteins, mainly TFs and enzymes. These proteins are expressed in 51 different human cell types, including immune cells, which explains the wide range of biological effects upon 4MU treatment. According to the data, 4MU modulates the activity of at least 13 nuclear receptors, namely the transcription factors which regulate various metabolic pathways and immune responses. Our analysis of publicly available data indicates that 4MU treatment leads to activation of cell stress response pathways via HIF1A, NFkB, NFE2L2 and ATF6 transcription factors. In addition, 4MU exposure activates several lysosomal enzymes involved in the degradation of glycolipids and glycogen (GAA, GBA, GALC and GLA).
In our previously published work [6], we showed that 4MU inhibits HAS2 expression and hyaluronan deposition in liver parenchyma during the development of liver fibrosis in mice. In the current study, we evaluate the effect of 4MU treatment in normal liver, which expresses very low levels of HAS2 [113]. Our data indicate that 4MU influences energy metabolism in a healthy liver by activating glycogen utilisation. Exposure of mice to 4MU for two weeks led to the depletion of glycogen granules in the liver. These findings are consistent with publicly available data of high content screening experiments targeting GAA, the main glycogen catabolic enzyme. The exhaustion of glycogen indicates an alteration in the liver’s energy and carbohydrate metabolic signature. This finding is in correlation with the conclusion of the recently published extensive research on the effects of 4MU on energy metabolism in mice [20].
Liver transcriptome analysis of mice on normal and 4MU diet confirms this finding: 4MU strongly affected the expression of Zbtb16, one of the key regulators of hepatic gluconeogenesis [110]. Concurrently, 4MU treatment significantly affected the expression of genes involved in bile acid metabolism. Even though 4MU was introduced in some countries as a drug for cholestasis treatment more than 30 years ago, the precise mechanism of its action remains unknown. Our findings reveal an upregulation of Hhex and Jun, the transcription factors regulating the conversion of cholesterol into bile acids and a downregulation of Cyp7a1 which is the rate-limiting enzyme in bile acid synthesis via the classical pathway [104,106,108]. The expression of Cyp7a1 is tightly regulated; it is induced by feeding and suppressed by high levels of bile acid in the serum through activation of NR1H4 (Farnesoid X receptor). Such an autoregulatory loop controls the enterohepatic circulation of bile acids and maintains a constant circulating level of bile acid [114]. It has been shown that bile acid regulates the expression of c-Jun via nuclear receptor SHP [115], and c-Jun is involved in NR1H4-dependent downregulation of Cyp7a1 [108]. Our study revealed that 4MU treatment significantly modified the expression of two NR1H4-dependent genes, namely Cyp7a1 and Hhex. This finding is consistent with the PubChem BioAssay data, which clearly states that 4MU is a non-specific ligand for nuclear receptors, including NR1H4. We also observed an indubitable downregulation of immune-associated genes involved in interferon signalling.
PubChem BioAssay database revealed high-affinity specific inhibition of HSD17b3, HSD17b4, and HSD17b10 belonging to the HSD17B family of NAD(P)H/NAD(P)+-dependent short-chain oxidoreductases. HSD17B1, HSD17B2, HSD17B3, HSD17B5, and HSD17B6 catalyse the interconversion between less potent 17-ketosteroids and more potent 17-hydroxysteroids to maintain the balance between 17-keto/17β-hydroxy forms of estrogens and androgens [116]. Other members of this family are involved in fatty acid metabolism, cholesterol biosynthesis, and bile acid production [117]. One member of this family, HSD17B13, is of paramount interest as a potential target for drug development. HSD17B13 was first cloned from the human liver cDNA library [118]. It was later shown that the HSD17B13 genetic polymorphism rs72613567, causing splicing alteration and loss of HSD17B13 activity, is associated with a substantial decrease in serum alanine aminotransferase and aspartate aminotransferase levels in a European population study. This polymorphism was also associated with a lower risk of NAFLD and non-alcoholic cirrhosis [119]. These findings were later confirmed in an independent study by Pirola, C.J. et al. [120]. Although data on 4MU inhibition of HSD17B13 are missing from the PubChem BioAssay database, the high homology of HSD17 family members and inhibition of b3, b4 and b10 by low micromolar concentration of 4MU allow us to infer the possible inhibition of HSD17b13 activity by 4MU, which may contribute to its protective effect in liver fibrosis [6].
Inhibition of KAT2A by 4MU in low micromolar concentrations (Table 2) underpins the recent discovery of its involvement in biliary fibrosis in mice. KAT2A, also known as GCN5, is a histone acetyltransferase (HAT) that functions primarily as a transcription activator. It also represses NFkB activity by promoting the ubiquitination of the NFkB subunit RelA (p65) in a HAT-independent manner [121]. Both pharmacologic inhibition of KAT2A lysine acetyltransferase activity and cholangiocyte-specific deletion of KAT2A were protective in mouse models of biliary fibrosis [122].
TGF-β ligands that activate the SMAD-2/3 intracellular pathway are considered a major driver of human fibrotic pathologies [123,124,125]. The involvement of the TGF-β/SMAD3 pathway is well documented in hepatic stellate cell activation and collagen production [124]. SMAD3 signalling was mechanistically linked to the liver fibrosis development in mice. Specifically, the anti-fibrotic action of Umbelliferon, a close analogue of 4MU, was associated with inhibition of TGF-β SMAD3 signalling [126]. SMAD3 inhibition by 4MU was reported in four independent bioassays (Table 2). Together, these data justify the validity of our bioinformatic approach, which independently identified SMAD3 as a potential 4MU target.
By its nature the big data analysis presented here only suggested potential targets of 4MU which have to be individually validated in our ongoing research using in vitro and in vivo techniques, including gene knockout and knockdown.
4. Materials and Methods
Data collection. Publicly available data were collected from the PubChem BioAssay (U.S. National Library of Medicine, Bethesda, MD, USA) [28] database for the next CIDs: 5280567 (Hymecromone, 4MU), 84843 (4-methylumbelliferone sulfate, MUS), 91553 (4-methylumbelliferone glucuronide, MUG). Data were downloaded manually, and all subsequent data manipulations were performed with a custom-made R script in RStudio version 1.4.1106, R version 3.6.3 (Posid, Boston, MA, USA). Briefly, the collected dataset containing 1762 rows was filtered according to substance activity. Individual observations without target names (calibration controls) were excluded from further analysis. In the final step, we identified common 4MU targets and its derivatives based on protein name. Expression profiles of 4MU target proteins in different cell types were manually downloaded from the Human protein atlas database [127] (https://www.proteinatlas.org/; accessed on 3 June 2022) from Single Cell and HPA datasets.
Mice experiments. All animal procedures were conducted following the Russian Academy of Science Guidelines for Animal Experimentation and were approved by the Institute of Developmental Biology RAS Ethics committee. 8-week-old female Balb/c mice (18–20 g.) were obtained from “Scientific Centre for Biomedical Technologies” of the Federal Medical and Biological Agency, Moscow, Russia. Mice were fed a diet of natural ingredients and housed in a 12-h light/dark cycle with an ad libitum access to food and water at a room temperature of 22 °C and relative humidity of 55–65%. Mice were allowed to acclimatise to the conditions for 2–3 days prior to the experiment. The 4MU was purchased from Sigma-Aldrich (Cat # M1381, St. Louis, MO, USA), mixed with 0.5% methylcellulose, and administered per os through gavage at a concentration of 600 mg/kg daily for 2 or 4 weeks in total. To the control mice, 5% methylcellulose slurry was administered via the same route. At the end of the experiment, animals were terminally anaesthetised with 5% isoflurane and humanely sacrificed by rapid exsanguination followed by cervical dislocation. Liver samples were collected for RNA isolation and histological examination.
Liver histology and staining. Freshly isolated liver samples were cut into 3 × 3 mm pieces and fixed in 10% buffered formalin for 24 h. After an extensive wash in diH2O, samples were dehydrated in isopropanol solutions with rising concentrations from 70% to 100%, followed by two immersions in xylene and then embedded in Histomix (Biovitrum, Russia) at 56 °C. Embedded tissue samples were sectioned by microtome at 5 µm slices and mounted onto SuperFrost glass slides. We used commercially available kits for Pass-Shiff and Gomori-Grocott staining (Biovitrum, Russia). The images of histological specimens were taken with microscope Keyence BZ9000 BioRevo (Yokogawa Electric Corporation, Tokyo, Japan).
RNA isolation and library preparation. TotRNA was isolated from liver tissue samples (60–100 mg) using TRI Reagent (Sigma-Aldrich, Merck KGaA, Darmstadt, Germany) according to the manufacture protocol. 3–5 μg of totRNA was used for isolated mRNA by NEBNext Poly(A) mRNA Magnetic Isolation Module (New England BioLabs, MA, USA) according to the manufacture protocol. RNA concentration was measured by Nanodrop (Thermo Fisher Scientific, MA, USA), and RNA samples were analysed by capillary electrophoresis using an Agilent capillary electrophoresis system (Agilent, CA, USA). cDNA libraries were constructed using NEBNext Ultra II Directional RNA Library Prep Kit for Illumina (New England BioLabs, MA, USA) following the manufacturer’s protocol. cDNA libraries were sequenced using the NextSeq500 (Illumina, San Diego, CA USA) instrument. Hence, 33–41 million raw reads were obtained for each sample with a 75-bp read length.
RNASeq data analysis. Raw reads were preprocessed as follows: trimmed with Trimmomatic (v0.39) [124] to remove the adapters. The processed reads were aligned to the Mus musculus genome (assembly GRCm39.105) using the HISAT2 algorithm [128]. Follow analysis was performed in R version 3.4.2. Gene read counts were calculated using Rsubread package, function feature Counts (with parameters countMultiMappingReads = F, isPairedEnd = TRUE) [129]. Genes with less than 10 reads on average for each sample were filtered out. To check the data for self-consistency, the following analysis was carried out: correlation analysis and MDS using the Spearman correlation and PCA using normalised gene counts as CPM (count per million). DEGs were identified using edgeR package version 3.20.9 [130] as follows. First, the read counts were normalised using the calcNormFactors function (RLE algorithm). Genes differentially expressed between each group of samples and appropriate control were identified using the estimateDisp, glmFit, and glmLRT functions with a 0.05 FDR significance threshold [131]. Hierarchical clustering was carried out to identify the modules of genes using hclust and cutree functions. Gene ontology analysis of DEGs modules was performed using the ClusterProfiler package with all significant genes as background [132]. Pathway enrichment analyses were performed across Bioplanet 2019, GO Molecular function [133,134], MSigDB_Hellmark_2020, and KEGG_Pathways_2019 CHEA 2016 databases to annotate differences in molecular functions, biological processes, and signalling pathways between experimental groups with Enrichr (http://amp.pharm.mssm.edu/Enrichr; New York, NY, USA).
5. Conclusions
Our study presents multiple biological targets of 4MU, including enzymes, nuclear receptors, and transcription factors. Detailed investigation of unravelled 4MU targets presented here will be pivotal in the discovery of new drugs for the efficient treatment of pathological processes associated with energy metabolism, inflammation, carcinogenesis, and tissue repair. Our transcriptomic data support the public data indicating nuclear receptors as the main targets of 4MU action. Future work will allow us to validate these targets using in vivo gene knockdown techniques in experimental models of fibrosis as well as immunological and neurodegenerative disorders.
Author Contributions
Conceptualisation, A.A.T., I.N.A. and Y.K.; data curation, A.A.T., M.S. and R.R.; formal analysis, A.A.T., M.S. and R.R.; investigation, N.H., I.N.A., N.O.D. and M.N.; writing—original draft, A.A.T. and Y.K.; writing—review and editing, Y.K., N.H., A.M. and N.H.; resources, E.V.B.; supervision and project administration, Y.K. and A.M.; funding acquisition, Y.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was conducted under the IDB RAS Government basic research program in 2022 No. 0088-2021-0017 and funded by Russian Foundation for Basic Research (grant number: 19-29-04123). The work was supported by the Ministry of Higher Education and Science of the Russian Federation (Project No. 075-15-2021-1075).
Institutional Review Board Statement
The animal study protocol was approved by the Ethics Committee of the Koltzov Institute of Developmental Biology of the Russian Academy of Sciences (protocol number: 49; 22 July 2021).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data analysed in the study are publicly available in PubChem BioAssay database with the following CIDs: 5280567 (Hymecromone, 4MU), 84843 (4-methylumbelliferone sulfate, MUS), 91553 (4-methylumbelliferone glucuronide, MUG). The Rna-Seq dataset is available from corresponding author upon request.
Acknowledgments
The research was performed using equipment from the Core Centrum of the Institute of Developmental Biology RAS. cDNA library preparation and RNseq were conducted by Skoltech Genomics Centre for Collective under the students’ core facility grant.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Nagy, N.; Kuipers, H.F.; Frymoyer, A.R.; Ishak, H.D.; Bollyky, J.B.; Wight, T.N.; Bollyky, P.L. 4-Methylumbelliferone Treatment and Hyaluronan Inhibition as a Therapeutic Strategy in Inflammation, Autoimmunity, and Cancer. Front. Immunol. 2015, 6, 123. [Google Scholar] [CrossRef] [PubMed]
- Vitale, D.L.; Icardi, A.; Rosales, P.; Spinelli, F.M.; Sevic, I.; Alaniz, L.D. Targeting the Tumor Extracellular Matrix by the Natural Molecule 4-Methylumbelliferone: A Complementary and Alternative Cancer Therapeutic Strategy. Front. Oncol. 2021, 11, 3938. [Google Scholar] [CrossRef] [PubMed]
- Piccioni, F.; Malvicini, M.; Garcia, M.G.; Rodriguez, A.; Atorrasagasti, C.; Kippes, N.; Piedra Buena, I.T.; Rizzo, M.M.; Bayo, J.; Aquino, J.; et al. Antitumor Effects of Hyaluronic Acid Inhibitor 4-Methylumbelliferone in an Orthotopic Hepatocellular Carcinoma Model in Mice. Glycobiology 2012, 22, 400–410. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Tamura, D.; Nakamura, T.; Makita, Y.; Ariyama, H.; Komiyama, K.; Yoshihara, T.; Asano, R. 4-Methylumbelliferone Leads to Growth Arrest and Apoptosis in Canine Mammary Tumor Cells. Oncol. Rep. 2013, 29, 335–342. [Google Scholar] [CrossRef]
- Saito, T.; Dai, T.; Asano, R. The Hyaluronan Synthesis Inhibitor 4-Methylumbelliferone Exhibits Antitumor Effects against Mesenchymal-like Canine Mammary Tumor Cells. Oncol. Lett. 2013, 5, 1068–1074. [Google Scholar] [CrossRef]
- Andreichenko, I.N.; Tsitrina, A.A.; Fokin, A.V.; Gabdulkhakova, A.I.; Maltsev, D.I.; Perelman, G.S.; Bulgakova, E.V.; Kulikov, A.M.; Mikaelyan, A.S.; Kotelevtsev, Y.V. 4-Methylumbelliferone Prevents Liver Fibrosis by Affecting Hyaluronan Deposition, FSTL1 Expression and Cell Localization. Int. J. Mol. Sci. 2019, 20, 6301. [Google Scholar] [CrossRef]
- Collum, S.D.; Molina, J.G.; Hanmandlu, A.; Bi, W.; Pedroza, M.; Mertens, T.C.J.; Wareing, N.; Wei, W.; Wilson, C.; Sun, W.; et al. Adenosine and Hyaluronan Promote Lung Fibrosis and Pulmonary Hypertension in Combined Pulmonary Fibrosis and Emphysema. Dis. Model. Mech. 2019, 12, dmm038711. [Google Scholar] [CrossRef]
- Terabe, K.; Ohashi, Y.; Tsuchiya, S.; Ishizuka, S.; Knudson, C.B.; Knudson, W. Chondroprotective Effects of 4-Methylumbelliferone and Hyaluronan Synthase-2 Overexpression Involve Changes in Chondrocyte Energy Metabolism. J. Biol. Chem. 2019, 294, 17799–17817. [Google Scholar] [CrossRef]
- Idota, M.; Ishizuka, S.; Hiraiwa, H.; Yamashita, S.; Oba, H.; Kawamura, Y.; Sakaguchi, T.; Haga, T.; Mizuno, T.; Kawashima, I.; et al. 4-Methylumbelliferone Suppresses Catabolic Activation in Anterior Cruciate Ligament-Derived Cells via a Mechanism Independent of Hyaluronan Inhibition. J. Orthop. Surg. Res. 2021, 16, 507. [Google Scholar] [CrossRef]
- Kakizaki, I.; Takagaki, K.; Endo, Y.; Kudo, D.; Ikeya, H.; Miyoshi, T.; Baggenstoss, B.A.; Tlapak-Simmons, V.L.; Kumari, K.; Nakane, A.; et al. Inhibition of Hyaluronan Synthesis in Streptococcus Equi FM100 by 4-Methylumbelliferone. Eur. J. Biochem. 2002, 269, 5066–5075. [Google Scholar] [CrossRef]
- Nakamura, T.; Funahashi, M.; Takagaki, K.; Munakata, H.; Endo, M.; Tanaka, K.; Saito, Y. Effect of 4-Methylumbelliferone on Cell-Free Synthesis of Hyaluronic Acid. IUBMB Life 1997, 43, 263–268. [Google Scholar] [CrossRef]
- Kultti, A.; Pasonen-Seppänen, S.; Jauhiainen, M.; Rilla, K.J.; Kärnä, R.; Pyöriä, E.; Tammi, R.H.; Tammi, M.I. 4-Methylumbelliferone Inhibits Hyaluronan Synthesis by Depletion of Cellular UDP-Glucuronic Acid and Downregulation of Hyaluronan Synthase 2 and 3. Exp. Cell Res. 2009, 315, 1914–1923. [Google Scholar] [CrossRef]
- Nagy, N.; Gurevich, I.; Kuipers, H.F.; Ruppert, S.M.; Marshall, P.L.; Xie, B.J.; Sun, W.; Malkovskiy, A.V.; Rajadas, J.; Grandoch, M.; et al. 4-Methylumbelliferyl Glucuronide Contributes to Hyaluronan Synthesis Inhibition. J. Biol. Chem. 2019, 294, 7864–7877. [Google Scholar] [CrossRef]
- Gandhi, N.S.; Mancera, R.L. The Structure of Glycosaminoglycans and Their Interactions with Proteins. Chem. Biol. Drug Des. 2008, 72, 455–482. [Google Scholar] [CrossRef]
- Rilla, K.; Pasonen-Seppänen, S.; Rieppo, J.; Tammi, M.; Tammi, R. The Hyaluronan Synthesis Inhibitor 4-Methylumbelliferone Prevents Keratinocyte Activation and Epidermal Hyperproliferation Induced by Epidermal Growth Factor. J. Investig. Dermatol. 2004, 123, 708–714. [Google Scholar] [CrossRef]
- Tsitrina, A.A.; Krasylov, I.V.; Maltsev, D.I.; Andreichenko, I.N.; Moskvina, V.S.; Ivankov, D.N.; Bulgakova, E.V.; Nesterchuk, M.; Shashkovskaya, V.; Dashenkova, N.O.; et al. Inhibition of Hyaluronan Secretion by Novel Coumarin Compounds and Chitin Synthesis Inhibitors. Glycobiology 2021, 31, 959–974. [Google Scholar] [CrossRef]
- Kuipers, H.F.; Rieck, M.; Gurevich, I.; Nagy, N.; Butte, M.J.; Negrin, R.S.; Wight, T.N.; Steinman, L.; Bollyky, P.L. Hyaluronan Synthesis Is Necessary for Autoreactive T-Cell Trafficking, Activation, and Th1 Polarization. Proc. Natl. Acad. Sci. USA 2016, 113, 1339–1344. [Google Scholar] [CrossRef]
- McKallip, R.J.; Ban, H.; Uchakina, O.N. Treatment with the Hyaluronic Acid Synthesis Inhibitor 4-Methylumbelliferone Suppresses LPS-Induced Lung Inflammation. Inflammation 2015, 38, 1250–1259. [Google Scholar] [CrossRef]
- Sim, M.-O.; Ham, J.R.; Lee, H.-I.; Seo, K.-I.; Lee, M.-K. Long-Term Supplementation of Umbelliferone and 4-Methylumbelliferone Alleviates High-Fat Diet Induced Hypertriglyceridemia and Hyperglycemia in Mice. Chem.-Biol. Interact. 2014, 216, 9–16. [Google Scholar] [CrossRef]
- Grandoch, M.; Flögel, U.; Virtue, S.; Maier, J.K.; Jelenik, T.; Kohlmorgen, C.; Feldmann, K.; Ostendorf, Y.; Castañeda, T.R.; Zhou, Z.; et al. 4-Methylumbelliferone Improves the Thermogenic Capacity of Brown Adipose Tissue. Nat. Metab. 2019, 1, 546–559. [Google Scholar] [CrossRef]
- Nagy, N.; Kaber, G.; Johnson, P.Y.; Gebe, J.A.; Preisinger, A.; Falk, B.A.; Sunkari, V.G.; Gooden, M.D.; Vernon, R.B.; Bogdani, M.; et al. Inhibition of Hyaluronan Synthesis Restores Immune Tolerance during Autoimmune Insulitis. J. Clin. Investig. 2015, 125, 3928–3940. [Google Scholar] [CrossRef] [PubMed]
- Mueller, A.M.; Yoon, B.H.; Sadiq, S.A. Inhibition of Hyaluronan Synthesis Protects against Central Nervous System (CNS) Autoimmunity and Increases CXCL12 Expression in the Inflamed CNS. J. Biol. Chem. 2014, 289, 22888–22899. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, Y.; Kozawa, E.; Urakawa, H.; Arai, E.; Futamura, N.; Zhuo, L.; Kimata, K.; Ishiguro, N.; Nishida, Y. Suppression of Hyaluronan Synthesis Alleviates Inflammatory Responses in Murine Arthritis and in Human Rheumatoid Synovial Fibroblasts. Arthritis Rheum. 2013, 65, 1160–1170. [Google Scholar] [CrossRef] [PubMed]
- Bollyky, P.L.; Evanko, S.P.; Wu, R.P.; Potter-Perigo, S.; Long, S.A.; Kinsella, B.; Reijonen, H.; Guebtner, K.; Teng, B.; Chan, C.K.; et al. Th1 Cytokines Promote T-Cell Binding to Antigen-Presenting Cells via Enhanced Hyaluronan Production and Accumulation at the Immune Synapse. Cell. Mol. Immunol. 2010, 7, 211–220. [Google Scholar] [CrossRef]
- Mahaffey, C.L.; Mummert, M.E. Hyaluronan Synthesis Is Required for IL-2-Mediated T Cell Proliferation. J. Immunol. 2007, 179, 8191–8199. [Google Scholar] [CrossRef]
- Rodríguez, M.M.; Onorato, A.; Cantero, M.J.; Domínguez, L.; Bayo, J.; Fiore, E.; García, M.; Atorrasagasti, C.; Canbay, A.; Malvicini, M.; et al. 4-Methylumbelliferone-Mediated Polarization of M1 Macrophages Correlate with Decreased Hepatocellular Carcinoma Aggressiveness in Mice. Sci. Rep. 2021, 11, 6310. [Google Scholar] [CrossRef]
- Abildgaard, C.; Rizza, S.; Christiansen, H.; Schmidt, S.; Dahl, C.; Abdul-Al, A.; Christensen, A.; Filomeni, G.; Guldberg, P. Screening of Metabolic Modulators Identifies New Strategies to Target Metabolic Reprogramming in Melanoma. Sci. Rep. 2021, 11, 4390. [Google Scholar] [CrossRef]
- PubChem Statistics. Available online: https://pubchemdocs.ncbi.nlm.nih.gov/statistics (accessed on 1 June 2022).
- Soreq, H.; Seidman, S. Acetylcholinesterase—New Roles for an Old Actor. Nat. Rev. Neurosci. 2001, 2, 294–302. [Google Scholar] [CrossRef]
- Khayami, R.; Hashemi, S.R.; Kerachian, M.A. Role of Aldo-Keto Reductase Family 1 Member B1 (AKR1B1) in the Cancer Process and Its Therapeutic Potential. J. Cell. Mol. Med. 2020, 24, 8890–8902. [Google Scholar] [CrossRef]
- Petrosino, J.M.; DiSilvestro, D.; Ziouzenkova, O. Aldehyde Dehydrogenase 1A1: Friend or Foe to Female Metabolism? Nutrients 2014, 6, 950–973. [Google Scholar] [CrossRef]
- Doyen, J.; Parks, S.; Marcié, S.; Pouysségur, J.; Chiche, J. Knock-down of Hypoxia-Induced Carbonic Anhydrases IX and XII Radiosensitizes Tumor Cells by Increasing Intracellular Acidosis. Front. Oncol. 2013, 2, 199. [Google Scholar] [CrossRef]
- Pastorekova, S.; Gillies, R.J. The Role of Carbonic Anhydrase IX in Cancer Development: Links to Hypoxia, Acidosis, and Beyond. Cancer Metastasis Rev. 2019, 38, 65–77. [Google Scholar] [CrossRef]
- Supuran, C.T. Carbonic Anhydrases: Novel Therapeutic Applications for Inhibitors and Activators. Nat. Rev. Drug Discov. 2008, 7, 168–181. [Google Scholar] [CrossRef]
- Thornberry, N.A.; Bull, H.G.; Calaycay, J.R.; Chapman, K.T.; Howard, A.D.; Kostura, M.J.; Miller, D.K.; Molineaux, S.M.; Weidner, J.R.; Aunins, J.; et al. A Novel Heterodimeric Cysteine Protease Is Required for Interleukin-1βprocessing in Monocytes. Nature 1992, 356, 768–774. [Google Scholar] [CrossRef]
- Gaggero, A.; Ambrosis, A.D.; Mezzanzanica, D.; Piazza, T.; Rubartelli, A.; Figini, M.; Canevari, S.; Ferrini, S. A Novel Isoform of Pro-Interleukin-18 Expressed in Ovarian Tumors Is Resistant to Caspase-1 and -4 Processing. Oncogene 2004, 23, 7552–7560. [Google Scholar] [CrossRef]
- Ball, D.P.; Taabazuing, C.Y.; Griswold, A.R.; Orth, E.L.; Rao, S.D.; Kotliar, I.B.; Vostal, L.E.; Johnson, D.C.; Bachovchin, D.A. Caspase-1 Interdomain Linker Cleavage Is Required for Pyroptosis. Life Sci. Alliance 2020, 3, 1–11. [Google Scholar] [CrossRef]
- Nicholson, D.W.; Ali, A.; Thornberry, N.A.; Vaillancourt, J.P.; Ding, C.K.; Gallant, M.; Gareau, Y.; Griffin, P.R.; Labelle, M.; Lazebnik, Y.A. Identification and Inhibition of the ICE/CED-3 Protease Necessary for Mammalian Apoptosis. Nature 1995, 376, 37–43. [Google Scholar] [CrossRef]
- Thorn, C.F.; Aklillu, E.; Klein, T.E.; Altman, R.B. PharmGKB Summary: Very Important Pharmacogene Information for CYP1A2. Pharm. Genom. 2012, 22, 73–77. [Google Scholar] [CrossRef]
- Zhou, S.-F.; Yang, L.-P.; Zhou, Z.-W.; Liu, Y.-H.; Chan, E. Insights into the Substrate Specificity, Inhibitors, Regulation, and Polymorphisms and the Clinical Impact of Human Cytochrome P450 1A2. AAPS J. 2009, 11, 481–494. [Google Scholar] [CrossRef]
- Roig-Zamboni, V.; Cobucci-Ponzano, B.; Iacono, R.; Ferrara, M.C.; Germany, S.; Bourne, Y.; Parenti, G.; Moracci, M.; Sulzenbacher, G. Structure of Human Lysosomal Acid α-Glucosidase–a Guide for the Treatment of Pompe Disease. Nat. Commun. 2017, 8, 1111. [Google Scholar] [CrossRef]
- Paciotti, S.; Albi, E.; Parnetti, L.; Beccari, T. Lysosomal Ceramide Metabolism Disorders: Implications in Parkinson’s Disease. J. Clin. Med. 2020, 9, 594. [Google Scholar] [CrossRef] [PubMed]
- Wendell, S.G.; Golin-Bisello, F.; Wenzel, S.; Sobol, R.W.; Holguin, F.; Freeman, B.A. 15-Hydroxyprostaglandin Dehydrogenase Generation of Electrophilic Lipid Signaling Mediators from Hydroxy ω-3 Fatty Acids. J. Biol. Chem. 2015, 290, 5868–5880. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-Y.; He, X.-Y.; Miller, D. HSD17B10: A Gene Involved in Cognitive Function through Metabolism of Isoleucine and Neuroactive Steroids. Mol. Genet. Metab. 2007, 92, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Zschocke, J.; Ruiter, J.P.; Brand, J.; Lindner, M.; Hoffmann, G.F.; Wanders, R.J.; Mayatepek, E. Progressive Infantile Neurodegeneration Caused by 2-Methyl-3-Hydroxybutyryl-CoA Dehydrogenase Deficiency: A Novel Inborn Error of Branched-Chain Fatty Acid and Isoleucine Metabolism. Pediatr. Res. 2000, 48, 852–855. [Google Scholar] [CrossRef]
- Mendonca, B.B.; Gomes, N.L.; Costa, E.M.F.; Inacio, M.; Martin, R.M.; Nishi, M.Y.; Carvalho, F.M.; Tibor, F.D.; Domenice, S. 46,XY Disorder of Sex Development (DSD) Due to 17β-Hydroxysteroid Dehydrogenase Type 3 Deficiency. J. Steroid Biochem. Mol. Biol. 2017, 165, 79–85. [Google Scholar] [CrossRef]
- Rebourcet, D.; Mackay, R.; Darbey, A.; Curley, M.K.; Jørgensen, A.; Frederiksen, H.; Mitchell, R.T.; O’Shaughnessy, P.J.; Nef, S.; Smith, L.B. Ablation of the Canonical Testosterone Production Pathway via Knockout of the Steroidogenic Enzyme HSD17B3, Reveals a Novel Mechanism of Testicular Testosterone Production. FASEB J. 2020, 34, 10373–10386. [Google Scholar] [CrossRef]
- Violante, S.; Achetib, N.; van Roermund, C.W.T.; Hagen, J.; Dodatko, T.; Vaz, F.M.; Waterham, H.R.; Chen, H.; Baes, M.; Yu, C.; et al. Peroxisomes Can Oxidize Medium- and Long-Chain Fatty Acids through a Pathway Involving ABCD3 and HSD17B4. FASEB J. 2019, 33, 4355–4364. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, Y.R.; Liu, K.; Yin, Z.; Liu, R.; Xia, Y.; Tan, L.; Yang, P.; Lee, J.-H.; Li, X.-J.; et al. KAT2A Coupled with the α-KGDH Complex Acts as a Histone H3 Succinyltransferase. Nature 2017, 552, 273–277. [Google Scholar] [CrossRef]
- Zieger, K.; Weiner, J.; Kunath, A.; Gericke, M.; Krause, K.; Kern, M.; Stumvoll, M.; Klöting, N.; Blüher, M.; Heiker, J.T. Ablation of Kallikrein 7 (KLK7) in Adipose Tissue Ameliorates Metabolic Consequences of High Fat Diet-Induced Obesity by Counteracting Adipose Tissue Inflammation in Vivo. Cell. Mol. Life Sci. 2018, 75, 727–742. [Google Scholar] [CrossRef]
- Kunath, A.; Weiner, J.; Krause, K.; Rehders, M.; Pejkovska, A.; Gericke, M.; Biniossek, M.L.; Dommel, S.; Kern, M.; Ribas-Latre, A.; et al. Role of Kallikrein 7 in Body Weight and Fat Mass Regulation. Biomedicines 2021, 9, 131. [Google Scholar] [CrossRef]
- Cargnello, M.; Roux, P.P. Activation and Function of the MAPKs and Their Substrates, the MAPK-Activated Protein Kinases. Microbiol. Mol. Biol. Rev. 2011, 75, 50–83. [Google Scholar] [CrossRef]
- Interthal, H.; Chen, H.J.; Champoux, J.J. Human Tdp1 Cleaves a Broad Spectrum of Substrates, Including Phosphoamide Linkages. J. Biol. Chem. 2005, 280, 36518–36528. [Google Scholar] [CrossRef]
- Hofman, E.R.; Boyanapalli, M.; Lindner, D.J.; Weihua, X.; Hassel, B.A.; Jagus, R.; Gutierrez, P.L.; Kalvakolanu, D.V. Thioredoxin Reductase Mediates Cell Death Effects of the Combination of Beta Interferon and Retinoic Acid. Mol. Cell. Biol. 1998, 18, 6493–6504. [Google Scholar] [CrossRef]
- Yu, I.-C.; Lin, H.-Y.; Sparks, J.D.; Yeh, S.; Chang, C. Androgen Receptor Roles in Insulin Resistance and Obesity in Males: The Linkage of Androgen-Deprivation Therapy to Metabolic Syndrome. Diabetes 2014, 63, 3180–3188. [Google Scholar] [CrossRef]
- Kovats, S. Estrogen Receptors Regulate Innate Immune Cells and Signaling Pathways. Cell. Immunol. 2015, 294, 63–69. [Google Scholar] [CrossRef]
- Masuyama, H.; Hiramatsu, Y. Potential Role of Estradiol and Progesterone in Insulin Resistance through Constitutive Androstane Receptor. J. Mol. Endocrinol. 2011, 47, 229–239. [Google Scholar] [CrossRef]
- DeZwaan-McCabe, D.; Sheldon, R.D.; Gorecki, M.C.; Guo, D.-F.; Gansemer, E.R.; Kaufman, R.J.; Rahmouni, K.; Gillum, M.P.; Taylor, E.B.; Teesch, L.M.; et al. ER Stress Inhibits Liver Fatty Acid Oxidation While Unmitigated Stress Leads to Anorexia-Induced Lipolysis and Both Liver and Kidney Steatosis. Cell Rep. 2017, 19, 1794–1806. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, F.; Gong, Q.; Cui, A.; Zhuo, S.; Hu, Z.; Han, Y.; Gao, J.; Sun, Y.; Liu, Z.; et al. Hepatic ATF6 Increases Fatty Acid Oxidation to Attenuate Hepatic Steatosis in Mice Through Peroxisome Proliferator–Activated Receptor α. Diabetes 2016, 65, 1904–1915. [Google Scholar] [CrossRef]
- Wang, Y.; Vera, L.; Fischer, W.H.; Montminy, M. The CREB Coactivator CRTC2 Links Hepatic ER Stress and Fasting Gluconeogenesis. Nature 2009, 460, 534–537. [Google Scholar] [CrossRef]
- Matissek, S.J.; Elsawa, S.F. GLI3: A Mediator of Genetic Diseases, Development and Cancer. Cell Commun. Signal. 2020, 18, 54. [Google Scholar] [CrossRef]
- Matz-Soja, M.; Rennert, C.; Schönefeld, K.; Aleithe, S.; Boettger, J.; Schmidt-Heck, W.; Weiss, T.S.; Hovhannisyan, A.; Zellmer, S.; Klöting, N.; et al. Hedgehog Signaling Is a Potent Regulator of Liver Lipid Metabolism and Reveals a GLI-Code Associated with Steatosis. eLife 2016, 5, e13308. [Google Scholar] [CrossRef] [PubMed]
- Masoud, G.N.; Li, W. HIF-1α Pathway: Role, Regulation and Intervention for Cancer Therapy. Acta Pharm. Sin. B 2015, 5, 378–389. [Google Scholar] [CrossRef]
- Huppke, P.; Weissbach, S.; Church, J.A.; Schnur, R.; Krusen, M.; Dreha-Kulaczewski, S.; Kühn-Velten, W.N.; Wolf, A.; Huppke, B.; Millan, F.; et al. Activating de Novo Mutations in NFE2L2 Encoding NRF2 Cause a Multisystem Disorder. Nat. Commun. 2017, 8, 818. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-ΚB Signaling in Inflammation. Signal Transduct. Target. Ther. 2017, 2, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Saha, P.K.; Chan, L.; Moore, D.D. Farnesoid X Receptor Is Essential for Normal Glucose Homeostasis. J. Clin. Investig. 2006, 116, 1102–1109. [Google Scholar] [CrossRef]
- Shen, L.-L.; Liu, H.; Peng, J.; Gan, L.; Lu, L.; Zhang, Q.; Li, L.; He, F.; Jiang, Y. Effects of Farnesoid X Receptor on the Expression of the Fatty Acid Synthetase and Hepatic Lipase. Mol. Biol. Rep. 2011, 38, 553–559. [Google Scholar] [CrossRef]
- Stayrook, K.R.; Bramlett, K.S.; Savkur, R.S.; Ficorilli, J.; Cook, T.; Christe, M.E.; Michael, L.F.; Burris, T.P. Regulation of Carbohydrate Metabolism by the Farnesoid X Receptor. Endocrinology 2005, 146, 984–991. [Google Scholar] [CrossRef]
- Pustylnyak, Y.A.; Gulyaeva, L.F.; Pustylnyak, V.O. Noncanonical Constitutive Androstane Receptor Signaling in Gene Regulation. Int. J. Mol. Sci. 2020, 21, 6735. [Google Scholar] [CrossRef]
- Escoter-Torres, L.; Caratti, G.; Mechtidou, A.; Tuckermann, J.; Uhlenhaut, N.H.; Vettorazzi, S. Fighting the Fire: Mechanisms of Inflammatory Gene Regulation by the Glucocorticoid Receptor. Front. Immunol. 2019, 10, 1859. [Google Scholar] [CrossRef]
- Peters, J.M.; Shah, Y.M.; Gonzalez, F.J. The Role of Peroxisome Proliferator-Activated Receptors in Carcinogenesis and Chemoprevention. Nat. Rev. Cancer 2012, 12, 181–195. [Google Scholar] [CrossRef]
- Ma, F.; Liu, S.-Y.; Razani, B.; Arora, N.; Li, B.; Kagechika, H.; Tontonoz, P.; Núñez, V.; Ricote, M.; Cheng, G. Retinoid X Receptor α Attenuates Host Antiviral Response by Suppressing Type I Interferon. Nat. Commun. 2014, 5, 5494. [Google Scholar] [CrossRef]
- Ma, X.; Warnier, M.; Raynard, C.; Ferrand, M.; Kirsh, O.; Defossez, P.-A.; Martin, N.; Bernard, D. The Nuclear Receptor RXRA Controls Cellular Senescence by Regulating Calcium Signaling. Aging Cell 2018, 17, e12831. [Google Scholar] [CrossRef]
- Massagué, J.; Wotton, D. Transcriptional Control by the TGF-Beta/Smad Signaling System. EMBO J. 2000, 19, 1745–1754. [Google Scholar] [CrossRef]
- Blank, U.; Karlsson, S. The Role of Smad Signaling in Hematopoiesis and Translational Hematology. Leukemia 2011, 25, 1379–1388. [Google Scholar] [CrossRef]
- Zou, M.-L.; Chen, Z.-H.; Teng, Y.-Y.; Liu, S.-Y.; Jia, Y.; Zhang, K.-W.; Sun, Z.-L.; Wu, J.-J.; Yuan, Z.-D.; Feng, Y.; et al. The Smad Dependent TGF-β and BMP Signaling Pathway in Bone Remodeling and Therapies. Front. Mol. Biosci. 2021, 8, 593310. [Google Scholar] [CrossRef]
- Aubrey, B.J.; Strasser, A.; Kelly, G.L. Tumor-Suppressor Functions of the TP53 Pathway. Cold Spring Harb. Perspect. Med. 2016, 6, a026062. [Google Scholar] [CrossRef]
- Lin, R. Crosstalk between Vitamin D Metabolism, VDR Signalling, and Innate Immunity. Biomed. Res. Int. 2016, 2016, 1375858. [Google Scholar] [CrossRef]
- Kongsbak, M.; Levring, T.B.; Geisler, C.; von Essen, M.R. The Vitamin D Receptor and T Cell Function. Front. Immunol. 2013, 4, 148. [Google Scholar] [CrossRef]
- Taylor, N.M.I.; Manolaridis, I.; Jackson, S.M.; Kowal, J.; Stahlberg, H.; Locher, K.P. Structure of the Human Multidrug Transporter ABCG2. Nature 2017, 546, 504–509. [Google Scholar] [CrossRef]
- Dickey, J.S.; Redon, C.E.; Nakamura, A.J.; Baird, B.J.; Sedelnikova, O.A.; Bonner, W.M. H2AX: Functional Roles and Potential Applications. Chromosoma 2009, 118, 683–692. [Google Scholar] [CrossRef]
- Arrigo, A.-P. Mammalian HspB1 (Hsp27) Is a Molecular Sensor Linked to the Physiology and Environment of the Cell. Cell Stress Chaperones 2017, 22, 517–529. [Google Scholar] [CrossRef] [PubMed]
- Leavitt, B.R.; Kordasiewicz, H.B.; Schobel, S.A. Huntingtin-Lowering Therapies for Huntington Disease: A Review of the Evidence of Potential Benefits and Risks. JAMA Neurol. 2020, 77, 764–772. [Google Scholar] [CrossRef] [PubMed]
- Stelma, T.; Leaner, V.D. KPNB1-Mediated Nuclear Import Is Required for Motility and Inflammatory Transcription Factor Activity in Cervical Cancer Cells. Oncotarget 2017, 8, 32833–32847. [Google Scholar] [CrossRef] [PubMed]
- Andrés, V.; González, J.M. Role of A-Type Lamins in Signaling, Transcription, and Chromatin Organization. J. Cell Biol. 2009, 187, 945–957. [Google Scholar] [CrossRef]
- Burke, B.; Stewart, C.L. The Nuclear Lamins: Flexibility in Function. Nat. Rev. Mol. Cell Biol. 2013, 14, 13–24. [Google Scholar] [CrossRef]
- Mittendorf, K.F.; Marinko, J.T.; Hampton, C.M.; Ke, Z.; Hadziselimovic, A.; Schlebach, J.P.; Law, C.L.; Li, J.; Wright, E.R.; Sanders, C.R.; et al. Peripheral Myelin Protein 22 Alters Membrane Architecture. Sci. Adv. 2017, 3, e1700220. [Google Scholar] [CrossRef]
- Serfecz, J.; Bazick, H.; Al Salihi, M.O.; Turner, P.; Fields, C.; Cruz, P.; Renne, R.; Notterpek, L. Downregulation of the Human Peripheral Myelin Protein 22 Gene by MiR-29a in Cellular Models of Charcot–Marie–Tooth Disease. Gene 2019, 26, 455–464. [Google Scholar] [CrossRef]
- Strasser, A.; Dickmanns, A.; Lührmann, R.; Ficner, R. Structural Basis for M3G-Cap-Mediated Nuclear Import of Spliceosomal UsnRNPs by Snurportin1. EMBO J. 2005, 24, 2235–2243. [Google Scholar] [CrossRef]
- Kidana, K.; Tatebe, T.; Ito, K.; Hara, N.; Kakita, A.; Saito, T.; Takatori, S.; Ouchi, Y.; Ikeuchi, T.; Makino, M.; et al. Loss of Kallikrein-related Peptidase 7 Exacerbates Amyloid Pathology in Alzheimer’s Disease Model Mice. EMBO Mol. Med. 2018, 10, e8184. [Google Scholar] [CrossRef]
- Talieri, M.; Mathioudaki, K.; Prezas, P.; Alexopoulou, D.K.; Diamandis, E.P.; Xynopoulos, D.; Ardavanis, A.; Arnogiannaki, N.; Scorilas, A. Clinical Significance of Kallikrein-Related Peptidase 7 (KLK7) in Colorectal Cancer. Thromb. Haemost. 2009, 101, 741–747. [Google Scholar] [CrossRef]
- Chen, J.-Q.; Liang, B.-H.; Li, H.-P.; Mo, Z.-Y.; Zhu, H.-L. Roles of Kallikrein-Related Peptidase in Epidermal Barrier Function and Related Skin Diseases. Int. J. Dermatol. Venereol. 2019, 2, 150–155. [Google Scholar] [CrossRef]
- Ivanov, S.V.; Kuzmin, I.; Wei, M.-H.; Pack, S.; Geil, L.; Johnson, B.E.; Stanbridge, E.J.; Lerman, M.I. Down-Regulation of Transmembrane Carbonic Anhydrases in Renal Cell Carcinoma Cell Lines by Wild-Type von Hippel-Lindau Transgenes. Proc. Natl. Acad. Sci. USA 1998, 95, 12596–12601. [Google Scholar] [CrossRef]
- Gonzalo, S.; Kreienkamp, R.; Askjaer, P. Hutchinson-Gilford Progeria Syndrome: A Premature Aging Disease Caused by LMNA Gene Mutations. Ageing Res. Rev. 2017, 33, 18–29. [Google Scholar] [CrossRef]
- Zhao, H.; Huang, X.; Halicka, H.D.; Darzynkiewicz, Z. Detection of Histone H2AX Phosphorylation on Ser-139 as an Indicator of DNA Damage. Curr. Protoc. Cytom. 2019, 89, e55. [Google Scholar] [CrossRef]
- Malhotra, D.; Portales-Casamar, E.; Singh, A.; Srivastava, S.; Arenillas, D.; Happel, C.; Shyr, C.; Wakabayashi, N.; Kensler, T.W.; Wasserman, W.W.; et al. Global Mapping of Binding Sites for Nrf2 Identifies Novel Targets in Cell Survival Response through ChIP-Seq Profiling and Network Analysis. Nucleic Acids Res. 2010, 38, 5718–5734. [Google Scholar] [CrossRef]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative Stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef]
- Cuadrado, A.; Martín-Moldes, Z.; Ye, J.; Lastres-Becker, I. Transcription Factors NRF2 and NF-ΚB Are Coordinated Effectors of the Rho Family, GTP-Binding Protein RAC1 during Inflammation. J. Biol. Chem. 2014, 289, 15244–15258. [Google Scholar] [CrossRef]
- Song, M.-K.; Ryoo, I.-G.; Ryu, D.; Lee, S.-H.; Kwak, M.-K. NFE2L2 (Nrf2) Attenuates Tgf-Β1 Signaling by Elevation of Smad7 in Mouse Mesangial MES-13 Cells. FASEB J. 2018, 32, 562.12. [Google Scholar] [CrossRef]
- Harris, A.J.; Thompson, A.R.; Whyte, M.K.; Walmsley, S.R. HIF-Mediated Innate Immune Responses: Cell Signaling and Therapeutic Implications. HP 2014, 2, 47–58. [Google Scholar] [CrossRef]
- Brewer, J.A.; Khor, B.; Vogt, S.K.; Muglia, L.M.; Fujiwara, H.; Haegele, K.E.; Sleckman, B.P.; Muglia, L.J. T-Cell Glucocorticoid Receptor Is Required to Suppress COX-2-Mediated Lethal Immune Activation. Nat. Med. 2003, 9, 1318–1322. [Google Scholar] [CrossRef]
- Gruver-Yates, A.L.; Quinn, M.A.; Cidlowski, J.A. Analysis of Glucocorticoid Receptors and Their Apoptotic Response to Dexamethasone in Male Murine B Cells During Development. Endocrinology 2014, 155, 463–474. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, J.; Ge, X.; Du, J.; Deb, D.K.; Li, Y.C. Vitamin D Receptor Inhibits Nuclear Factor ΚB Activation by Interacting with IκB Kinase β Protein. J. Biol. Chem. 2013, 288, 19450–19458. [Google Scholar] [CrossRef]
- Li, T.; Francl, J.M.; Boehme, S.; Chiang, J.Y.L. Regulation of Cholesterol and Bile Acid Homeostasis by the CYP7A1/SREBP2/MiR-33a Axis. Hepatology 2013, 58, 1111–1121. [Google Scholar] [CrossRef] [PubMed]
- Hunter, M.P.; Wilson, C.M.; Jiang, X.; Cong, R.; Vasavada, H.; Kaestner, K.H.; Bogue, C.W. The Homeobox Gene Hhex Is Essential for Proper Hepatoblast Differentiation and Bile Duct Morphogenesis. Dev. Biol. 2007, 308, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Xing, X.; Burgermeister, E.; Geisler, F.; Einwächter, H.; Fan, L.; Hiber, M.; Rauser, S.; Walch, A.; Röcken, C.; Ebeling, M.; et al. Hematopoietically Expressed Homeobox Is a Target Gene of Farnesoid X Receptor in Chenodeoxycholic Acid-Induced Liver Hypertrophy. Hepatology 2009, 49, 979–988. [Google Scholar] [CrossRef] [PubMed]
- Behrens, A.; Sibilia, M.; David, J.-P.; Möhle-Steinlein, U.; Tronche, F.; Schütz, G.; Wagner, E.F. Impaired Postnatal Hepatocyte Proliferation and Liver Regeneration in Mice Lacking C-Jun in the Liver. EMBO J. 2002, 21, 1782–1790. [Google Scholar] [CrossRef]
- Gupta, S.; Stravitz, R.T.; Dent, P.; Hylemon, P.B. Down-Regulation of Cholesterol 7α-Hydroxylase (CYP7A1) Gene Expression by Bile Acids in Primary Rat Hepatocytes Is Mediated by the c-Jun N-Terminal Kinase Pathway. J. Biol. Chem. 2001, 276, 15816–15822. [Google Scholar] [CrossRef]
- Ishikawa, H.; Hayakawa, M.; Baatartsogt, N.; Kakizawa, N.; Ohto-Ozaki, H.; Maruyama, T.; Miura, K.; Suzuki, K.; Rikiyama, T.; Ohmori, T. IκBζ Regulates the Development of Nonalcoholic Fatty Liver Disease through the Attenuation of Hepatic Steatosis in Mice. Sci. Rep. 2022, 12, 11634. [Google Scholar] [CrossRef]
- Chen, S.; Qian, J.; Shi, X.; Gao, T.; Liang, T.; Liu, C. Control of Hepatic Gluconeogenesis by the Promyelocytic Leukemia Zinc Finger Protein. Mol. Endocrinol. 2014, 28, 1987–1998. [Google Scholar] [CrossRef]
- Wan, Y.; Nordeen, S.K. Overlapping but Distinct Gene Regulation Profiles by Glucocorticoids and Progestins in Human Breast Cancer Cells. Mol. Endocrinol. 2002, 16, 1204–1214. [Google Scholar] [CrossRef]
- Fahnenstich, J.; Nandy, A.; Milde-Langosch, K.; Schneider-Merck, T.; Walther, N.; Gellersen, B. Promyelocytic Leukaemia Zinc Finger Protein (PLZF) Is a Glucocorticoid- and Progesterone-induced Transcription Factor in Human Endometrial Stromal Cells and Myometrial Smooth Muscle Cells. Mol. Hum. Reprod. 2003, 9, 611–623. [Google Scholar] [CrossRef]
- Yang, Y.M.; Noureddin, M.; Liu, C.; Ohashi, K.; Kim, S.Y.; Ramnath, D.; Powell, E.E.; Sweet, M.J.; Roh, Y.S.; Hsin, I.-F.; et al. Hyaluronan Synthase 2–Mediated Hyaluronan Production Mediates Notch1 Activation and Liver Fibrosis. Sci. Transl. Med. 2019, 11, eaat9284. [Google Scholar] [CrossRef]
- Chiang, J.Y.L.; Ferrell, J.M. Up to Date on Cholesterol 7 Alpha-Hydroxylase (CYP7A1) in Bile Acid Synthesis. Liver Res. 2020, 4, 47–63. [Google Scholar] [CrossRef]
- Park, W.I.; Park, M.J.; An, J.K.; Choi, Y.H.; Kim, H.Y.; Cheong, J.; Yang, U.S. Bile Acid Regulates C-Jun Expression through the Orphan Nuclear Receptor SHP Induction in Gastric Cells. Biochem. Biophys. Res. Commun. 2008, 369, 437–443. [Google Scholar] [CrossRef]
- Poutanen, M.; Penning, T.M. Biology and Clinical Relevance of Hydroxysteroid (17beta) Dehydrogenase Enzymes. Mol. Cell. Endocrinol. 2019, 489, 1–2. [Google Scholar] [CrossRef]
- Saloniemi, T.; Jokela, H.; Strauss, L.; Pakarinen, P.; Poutanen, M. The Diversity of Sex Steroid Action: Novel Functions of Hydroxysteroid (17β) Dehydrogenases as Revealed by Genetically Modified Mouse Models. J. Endocrinol. 2012, 212, 27–40. [Google Scholar] [CrossRef]
- Liu, S.; Huang, C.; Li, D.; Ren, W.; Zhang, H.; Qi, M.; Li, X.; Yu, L. Molecular Cloning and Expression Analysis of a New Gene for Short-Chain Dehydrogenase/Reductase 9. Acta Biochim. Pol. 2007, 54, 213–218. [Google Scholar] [CrossRef]
- Abul-Husn, N.S.; Cheng, X.; Li, A.H.; Xin, Y.; Schurmann, C.; Stevis, P.; Liu, Y.; Kozlitina, J.; Stender, S.; Wood, G.C.; et al. A Protein-Truncating HSD17B13 Variant and Protection from Chronic Liver Disease. N. Engl. J. Med. 2018, 378, 1096–1106. [Google Scholar] [CrossRef]
- Pirola, C.J.; Garaycoechea, M.; Flichman, D.; Arrese, M.; San Martino, J.; Gazzi, C.; Castaño, G.O.; Sookoian, S. Splice Variant Rs72613567 Prevents Worst Histologic Outcomes in Patients with Nonalcoholic Fatty Liver Disease [S]. J. Lipid Res. 2019, 60, 176–185. [Google Scholar] [CrossRef]
- Mao, X.; Gluck, N.; Li, D.; Maine, G.N.; Li, H.; Zaidi, I.W.; Repaka, A.; Mayo, M.W.; Burstein, E. GCN5 Is a Required Cofactor for a Ubiquitin Ligase That Targets NF-KappaB/RelA. Genes Dev. 2009, 23, 849–861. [Google Scholar] [CrossRef]
- Aseem, S.O.; Jalan-Sakrikar, N.; Chi, C.; Navarro-Corcuera, A.; De Assuncao, T.M.; Hamdan, F.H.; Chowdhury, S.; Banales, J.M.; Johnsen, S.A.; Shah, V.H.; et al. Epigenomic Evaluation of Cholangiocyte Transforming Growth Factor-β Signaling Identifies a Selective Role for Histone 3 Lysine 9 Acetylation in Biliary Fibrosis. Gastroenterology 2021, 160, 889–905.e10. [Google Scholar] [CrossRef]
- Walton, K.L.; Johnson, K.E.; Harrison, C.A. Targeting TGF-β Mediated SMAD Signaling for the Prevention of Fibrosis. Front. Pharmacol. 2017, 8, 461. [Google Scholar] [CrossRef] [PubMed]
- Zisser, A.; Ipsen, D.H.; Tveden-Nyborg, P. Hepatic Stellate Cell Activation and Inactivation in NASH-Fibrosis—Roles as Putative Treatment Targets? Biomedicines 2021, 9, 365. [Google Scholar] [CrossRef]
- Shang, H.; Liu, X.; Guo, H. Knockdown of Fstl1 Attenuates Hepatic Stellate Cell Activation through the TGF-β1/Smad3 Signaling Pathway. Mol. Med. Rep. 2017, 16, 7119–7123. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.M.; Hozayen, W.G.; Hasan, I.H.; Shaban, E.; Bin-Jumah, M. Umbelliferone Ameliorates CCl4-Induced Liver Fibrosis in Rats by Upregulating PPARγ and Attenuating Oxidative Stress, Inflammation, and TGF-Β1/Smad3 Signaling. Inflammation 2019, 42, 1103–1116. [Google Scholar] [CrossRef] [PubMed]
- Human Protein Atlas Database. Available online: https://www.proteinatlas.org/humanproteome/single+cell+type (accessed on 3 June 2022).
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A Flexible Trimmer for Illumina Sequence Data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Kim, D.; Paggi, J.M.; Park, C.; Bennett, C.; Salzberg, S.L. Graph-Based Genome Alignment and Genotyping with HISAT2 and HISAT-Genotype. Nat. Biotechnol. 2019, 37, 907–915. [Google Scholar] [CrossRef]
- Liao, Y.; Smyth, G.K.; Shi, W. The R Package Rsubread Is Easier, Faster, Cheaper and Better for Alignment and Quantification of RNA Sequencing Reads. Nucleic Acids Res. 2019, 47, e47. [Google Scholar] [CrossRef]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. EdgeR: A Bioconductor Package for Differential Expression Analysis of Digital Gene Expression Data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef]
- ClusterProfiler: An R Package for Comparing Biological Themes Among Gene Clusters|OMICS: A Journal of Integrative Biology. Available online: https://www.liebertpub.com/doi/10.1089/omi.2011.0118 (accessed on 3 June 2022).
- BioPlanet. Available online: https://tripod.nih.gov/bioplanet/ (accessed on 3 June 2022).
- Gene Ontology. Available online: http://geneontology.org/ (accessed on 3 June 2022).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).