ER Stress-Perturbed Intracellular Protein O-GlcNAcylation Aggravates Podocyte Injury in Diabetes Nephropathy
Abstract
:1. Introductions
2. Results
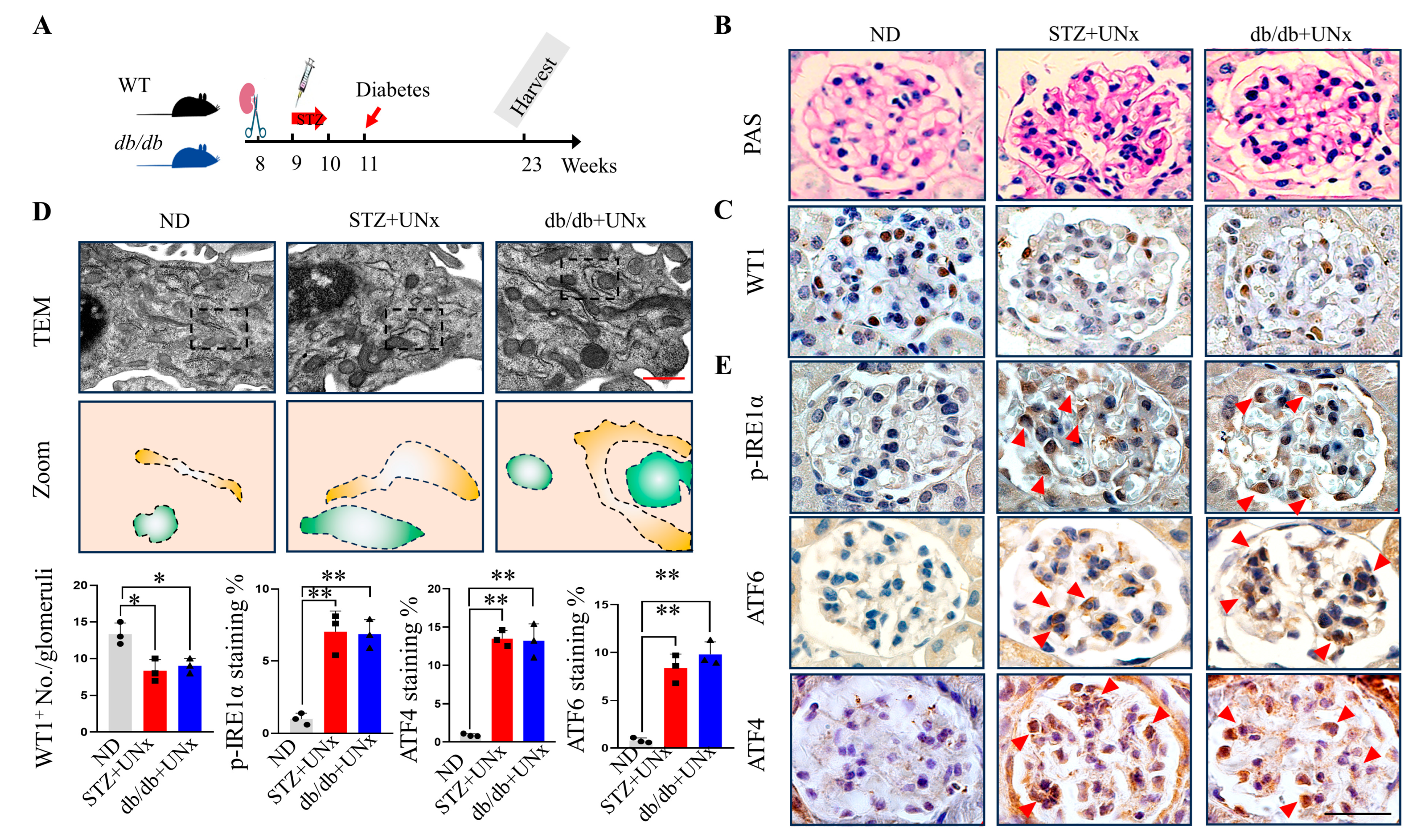
2.1. ER Stress Is Induced in Both Type 1 and Type 2 DN
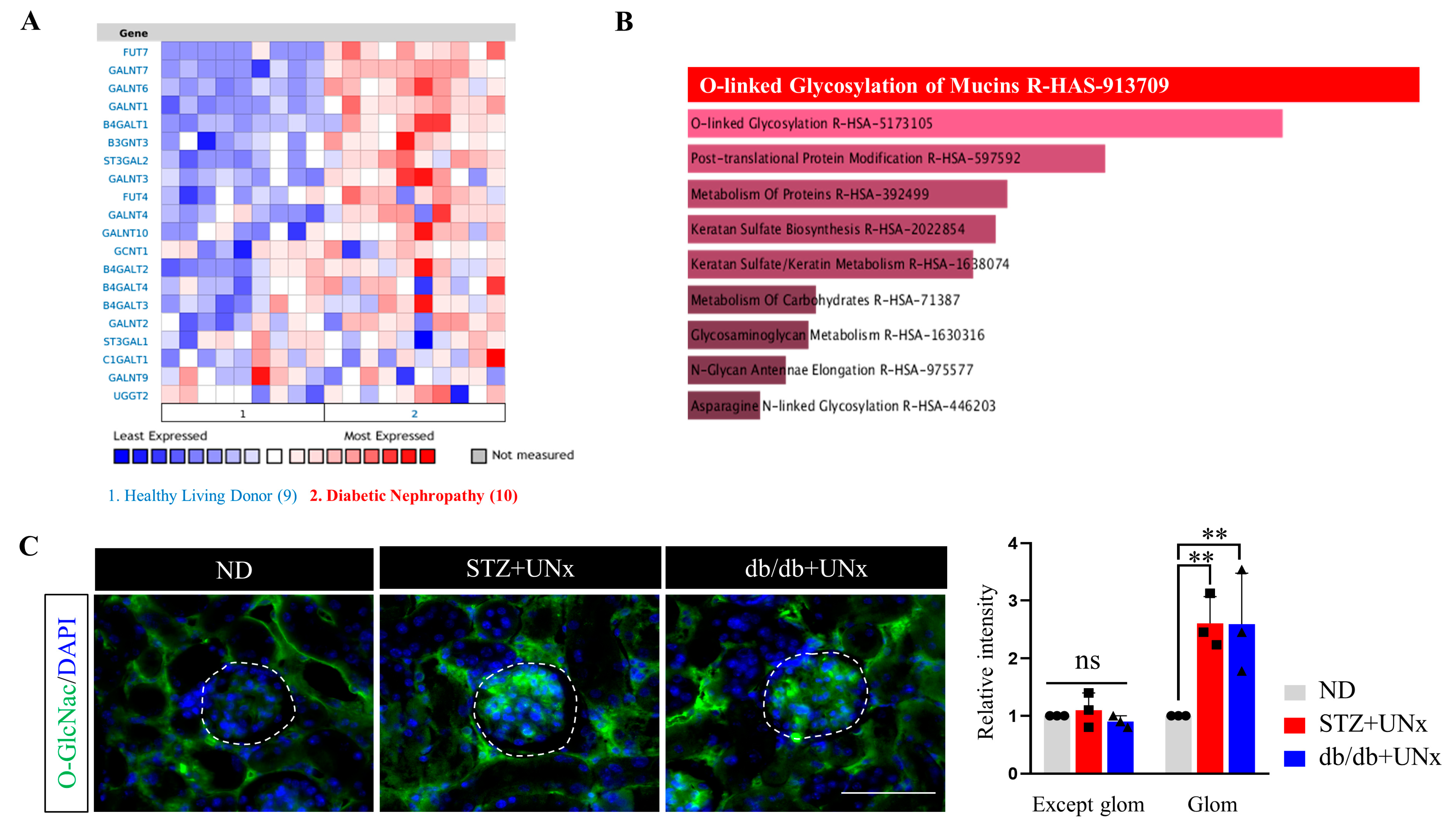
2.2. Increased Protein O-GlcNAcylation Is Identified in the Glomeruli of the DN Mice
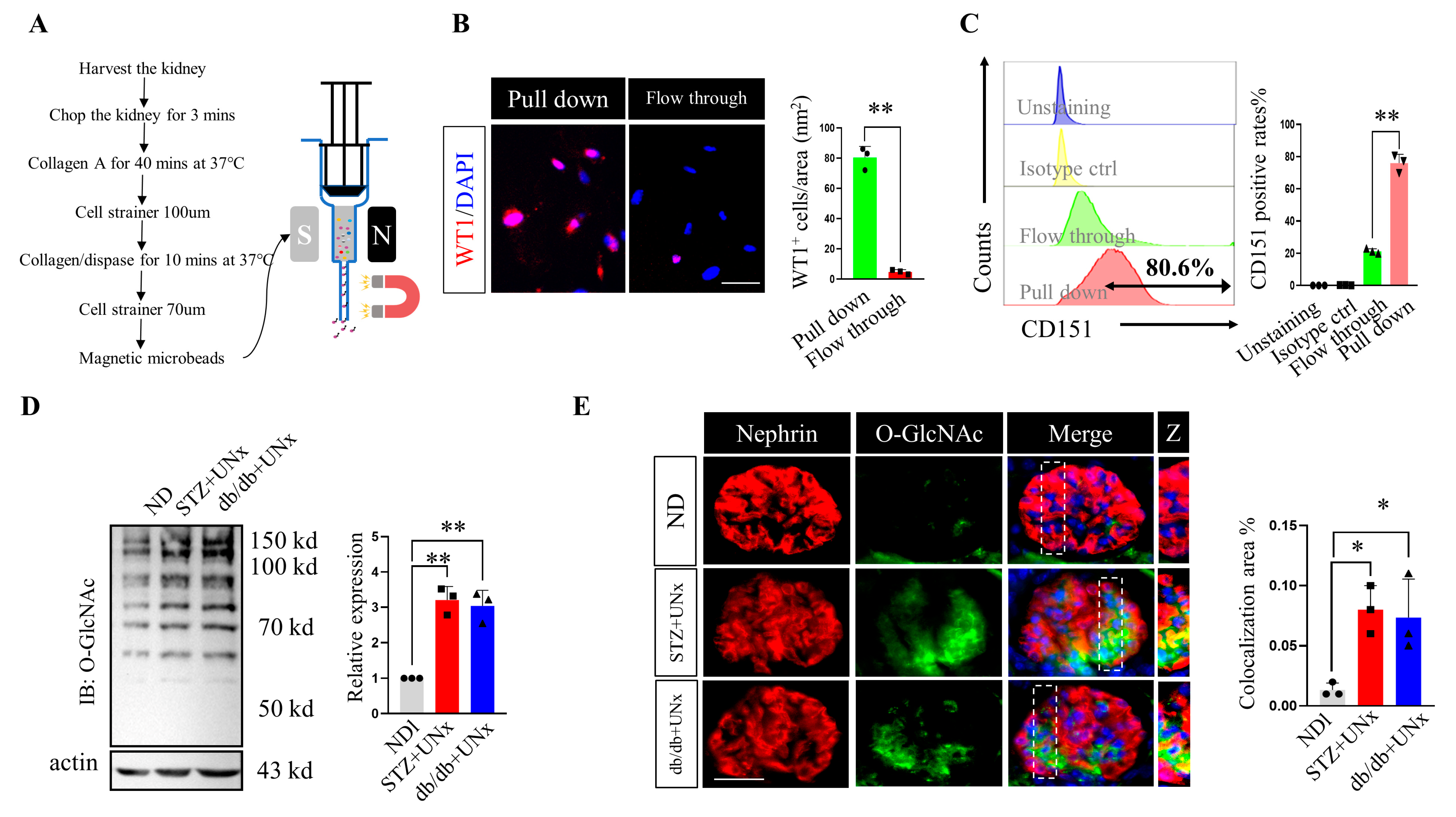
2.3. O-GlcNAc Protein Modification Is Increased in Podocytes
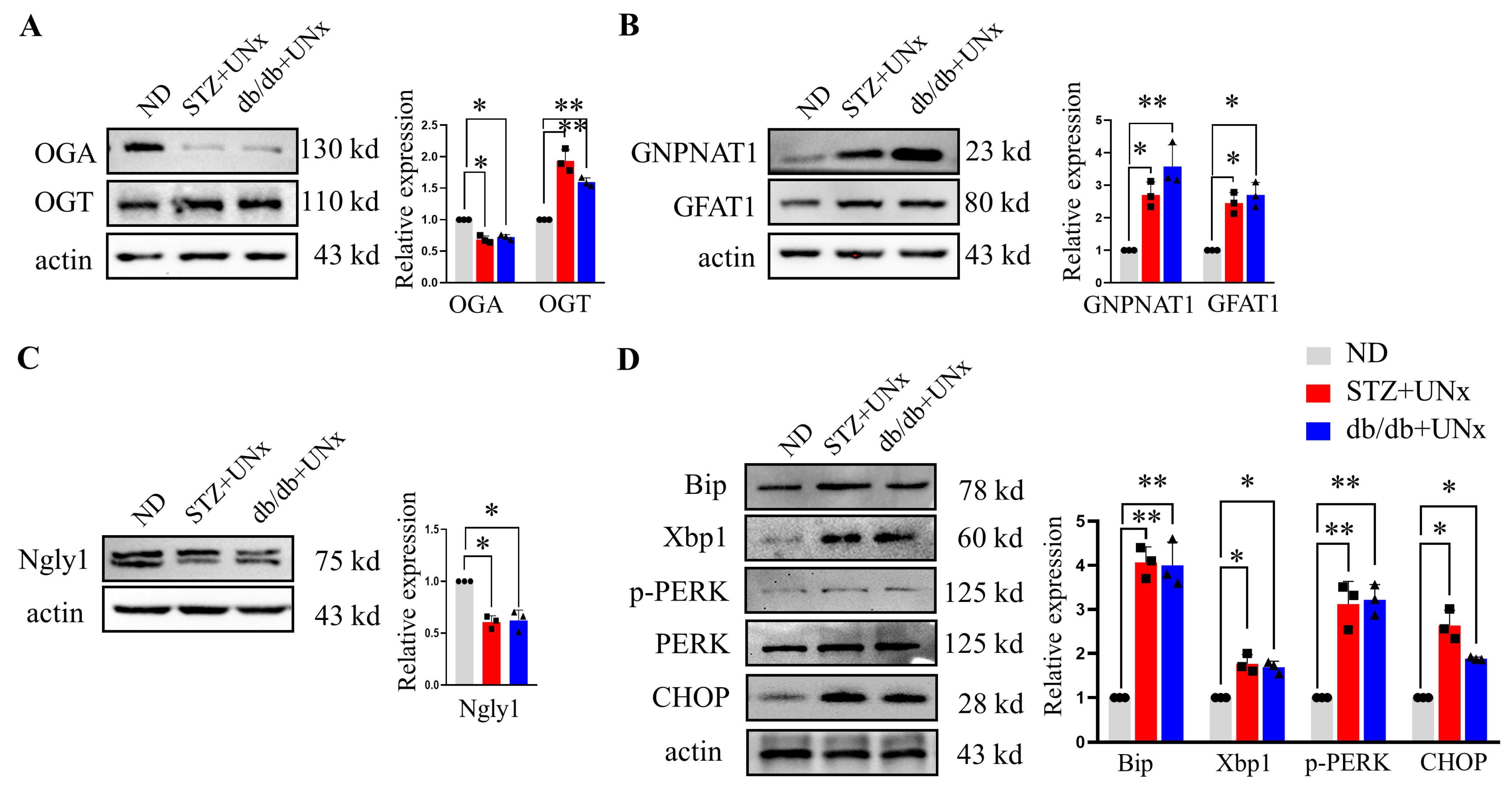
2.4. An Increase in O-GlcNAc Modification Is Correlated with ER Stress
2.5. Pharmacological Mediation of Protein O-GlcNAcylation in Podocyte Injuries
2.6. ER Stress Drives O-GlcNAc Protein Modification In Vitro
3. Discussion
4. Materials and Methods
4.1. Animal Study
4.2. STZ Plus Unilateral Nephrectomy-Induced Diabetic Kidney Nephropathy Model
4.3. Unilateral Nephrectomy-Induced Diabetic Kidney Nephropathy in db/db Mice
4.4. Immunofluorescence Staining
4.5. Immunohistochemistry Staining
4.6. Immunoblotting
4.7. Cell Culture and Treatments
4.8. Flow Cytometry Analysis
4.9. Nephroseq V5 Data Acquisition
4.10. Transmission Electron Microscopy
4.11. Magnetic Microbeads Podocyte Pull-down Assay
4.12. Plasma Analysis
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gross, J.L.; De Azevedo, M.J.; Silveiro, S.P.; Canani, L.H.; Caramori, M.L.; Zelmanovitz, T. Diabetic nephropathy: Diagnosis, prevention, and treatment. Diabetes Care 2005, 28, 164–176. [Google Scholar] [CrossRef]
- Koye, D.N.; Magliano, D.J.; Nelson, R.G.; Pavkov, M.E. The Global Epidemiology of Diabetes and Kidney Disease. Adv. Chronic Kidney Dis. 2018, 25, 121–132. [Google Scholar] [CrossRef]
- Madhusudhan, T.; Wang, H.; Dong, W.; Ghosh, S.; Bock, F.; Thangapandi, V.R.; Ranjan, S.; Wolter, J.; Kohli, S.; Shahzad, K.; et al. Defective podocyte insulin signalling through p85-XBP1 promotes ATF6-dependent maladaptive ER-stress response in diabetic nephropathy. Nat. Commun. 2015, 6, 6496. [Google Scholar] [CrossRef]
- Fan, Y.; Xiao, W.; Li, Z.; Li, X.; Chuang, P.Y.; Jim, B.; Zhang, W.; Wei, C.; Wang, N.; Jia, W.; et al. RTN1 mediates progression of kidney disease by inducing ER stress. Nat. Commun. 2015, 6, 7841. [Google Scholar] [CrossRef]
- Qi, W.; Mu, J.; Luo, Z.F.; Zeng, W.; Guo, Y.H.; Pang, Q.; Ye, Z.L.; Liu, L.; Yuan, F.H.; Feng, B. Attenuation of diabetic nephropathy in diabetes rats induced by streptozotocin by regulating the endoplasmic reticulum stress inflammatory response. Metab. Clin. Exp. 2011, 60, 594–603. [Google Scholar] [CrossRef]
- Li, C.; Krothapalli, S.; Chen, Y.M. Targeting Endoplasmic Reticulum for Novel Therapeutics and Monitoring in Acute Kidney Injury. Nephron 2023, 147, 21–24. [Google Scholar] [CrossRef]
- Chen, Y.M.; Zhou, Y.; Go, G.; Marmerstein, J.T.; Kikkawa, Y.; Miner, J.H. Laminin β2 gene missense mutation produces endoplasmic reticulum stress in podocytes. J. Am. Soc. Nephrol. JASN 2013, 24, 1223–1233. [Google Scholar] [CrossRef]
- Shu, S.; Zhu, J.; Liu, Z.; Tang, C.; Cai, J.; Dong, Z. Endoplasmic reticulum stress is activated in post-ischemic kidneys to promote chronic kidney disease. EBioMedicine 2018, 37, 269–280. [Google Scholar] [CrossRef]
- Inagi, R.; Nangaku, M.; Onogi, H.; Ueyama, H.; Kitao, Y.; Nakazato, K.; Ogawa, S.; Kurokawa, K.; Couser, W.G.; Miyata, T. Involvement of endoplasmic reticulum (ER) stress in podocyte injury induced by excessive protein accumulation. Kidney Int. 2005, 68, 2639–2650. [Google Scholar] [CrossRef]
- Park, S.J.; Kim, Y.; Yang, S.M.; Henderson, M.J.; Yang, W.; Lindahl, M.; Urano, F.; Chen, Y.M. Discovery of endoplasmic reticulum calcium stabilizers to rescue ER-stressed podocytes in nephrotic syndrome. Proc. Natl. Acad. Sci. USA 2019, 116, 14154–14163. [Google Scholar] [CrossRef]
- Cao, Y.; Hao, Y.; Li, H.; Liu, Q.; Gao, F.; Liu, W.; Duan, H. Role of endoplasmic reticulum stress in apoptosis of differentiated mouse podocytes induced by high glucose. Int. J. Mol. Med. 2014, 33, 809–816. [Google Scholar] [CrossRef] [PubMed]
- Cybulsky, A.V. Endoplasmic reticulum stress, the unfolded protein response and autophagy in kidney diseases. Nat. Rev. Nephrol. 2017, 13, 681–696. [Google Scholar] [CrossRef]
- Hassan, H.; Tian, X.; Inoue, K.; Chai, N.; Liu, C.; Soda, K.; Moeckel, G.; Tufro, A.; Lee, A.H.; Somlo, S.; et al. Essential Role of X-Box Binding Protein-1 during Endoplasmic Reticulum Stress in Podocytes. J. Am. Soc. Nephrol. JASN 2016, 27, 1055–1065. [Google Scholar] [CrossRef] [PubMed]
- Zachara, N.E.; O’Donnell, N.; Cheung, W.D.; Mercer, J.J.; Marth, J.D.; Hart, G.W. Dynamic O-GlcNAc modification of nucleocytoplasmic proteins in response to stress. A survival response of mammalian cells. J. Biol. Chem. 2004, 279, 30133–30142. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.S.; Lee, J.; Lee, P.; Jeon, B.C.; Song, M.Y.; Kwak, S.; Lee, J.; Kim, J.S.; Kim, D.J.; Kim, J.H.; et al. Inhibition of O-GlcNAcylation protects from Shiga toxin-mediated cell injury and lethality in host. EMBO Mol. Med. 2022, 14, e14678. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.V.; Deng, Y.; Gao, N.; Pedrozo, Z.; Li, D.L.; Morales, C.R.; Criollo, A.; Luo, X.; Tan, W.; Jiang, N.; et al. Spliced X-box binding protein 1 couples the unfolded protein response to hexosamine biosynthetic pathway. Cell 2014, 156, 1179–1192. [Google Scholar] [CrossRef] [PubMed]
- Wells, L.; Vosseller, K.; Hart, G.W. Glycosylation of nucleocytoplasmic proteins: Signal transduction and O-GlcNAc. Science 2001, 291, 2376–2378. [Google Scholar] [CrossRef]
- Ruan, H.-B.; Singh, J.P.; Li, M.-D.; Wu, J.; Yang, X. Cracking the O-GlcNAc code in metabolism. Trends Endocrinol. Metab. 2013, 24, 301–309. [Google Scholar] [CrossRef]
- Ruan, H.-B.; Ma, Y.; Torres, S.; Zhang, B.; Feriod, C.; Heck, R.M.; Qian, K.; Fu, M.; Li, X.; Nathanson, M.H. Calcium-dependent O-GlcNAc signaling drives liver autophagy in adaptation to starvation. Genes Dev. 2017, 31, 1655–1665. [Google Scholar] [CrossRef]
- Ruan, H.-B.; Dietrich, M.O.; Liu, Z.-W.; Zimmer, M.R.; Li, M.-D.; Singh, J.P.; Zhang, K.; Yin, R.; Wu, J.; Horvath, T.L.; et al. O-GlcNAc Transferase Enables AgRP Neurons to Suppress Browning of White Fat. Cell 2014, 159, 306–317. [Google Scholar] [CrossRef]
- Yang, X.; Qian, K. Protein O-GlcNAcylation: Emerging mechanisms and functions. Nat. Rev. Mol. Cell Biol. 2017, 18, 452–465. [Google Scholar] [CrossRef]
- Liu, B.; Salgado, O.C.; Singh, S.; Hippen, K.L.; Maynard, J.C.; Burlingame, A.L.; Ball, L.E.; Blazar, B.R.; Farrar, M.A.; Hogquist, K.A.; et al. The lineage stability and suppressive program of regulatory T cells require protein O-GlcNAcylation. Nat. Commun. 2019, 10, 354. [Google Scholar] [CrossRef]
- Gellai, R.; Hodrea, J.; Lenart, L.; Hosszu, A.; Koszegi, S.; Balogh, D.; Ver, A.; Banki, N.F.; Fulop, N.; Molnar, A.; et al. Role of O-linked N-acetylglucosamine modification in diabetic nephropathy. Am. J. Physiol. Ren. Physiol. 2016, 311, F1172–F1181. [Google Scholar] [CrossRef]
- Kim, G.; Cao, L.; Reece, E.A.; Zhao, Z. Impact of protein O-GlcNAcylation on neural tube malformation in diabetic embryopathy. Sci. Rep. 2017, 7, 11107. [Google Scholar] [CrossRef]
- Brosius, F.C., 3rd; Alpers, C.E.; Bottinger, E.P.; Breyer, M.D.; Coffman, T.M.; Gurley, S.B.; Harris, R.C.; Kakoki, M.; Kretzler, M.; Leiter, E.H.; et al. Mouse models of diabetic nephropathy. J. Am. Soc. Nephrol. JASN 2009, 20, 2503–2512. [Google Scholar] [CrossRef]
- Fu, Y.; Sun, Y.; Wang, M.; Hou, Y.; Huang, W.; Zhou, D.; Wang, Z.; Yang, S.; Tang, W.; Zhen, J.; et al. Elevation of JAML Promotes Diabetic Kidney Disease by Modulating Podocyte Lipid Metabolism. Cell Metab. 2020, 32, 1052–1062.e8. [Google Scholar] [CrossRef]
- Uil, M.; Scantlebery, A.M.L.; Butter, L.M.; Larsen, P.W.B.; de Boer, O.J.; Leemans, J.C.; Florquin, S.; Roelofs, J. Combining streptozotocin and unilateral nephrectomy is an effective method for inducing experimental diabetic nephropathy in the ‘resistant’ C57Bl/6J mouse strain. Sci. Rep. 2018, 8, 5542. [Google Scholar] [CrossRef]
- Viggiano, D. Mechanisms of Diabetic Nephropathy Not Mediated by Hyperglycemia. J. Clin. Med. 2023, 12, 6848. [Google Scholar] [CrossRef]
- Mandel, N.; Büttner, M.; Poschet, G.; Kuner, R.; Agarwal, N. SUMOylation Modulates Reactive Oxygen Species (ROS) Levels and Acts as a Protective Mechanism in the Type 2 Model of Diabetic Peripheral Neuropathy. Cells 2023, 12, 2511. [Google Scholar] [CrossRef]
- Shen, W.B.; Wang, B.; Yao, R.; Goetzinger, K.R.; Wu, S.; Gao, H.; Yang, P. Obesity impacts placental function through activation of p-IRE1a-XBP1s signaling. Front. Cell Dev. Biol. 2023, 11, 1023327. [Google Scholar] [CrossRef]
- Vallon, V. Glucose transporters in the kidney in health and disease. Pflug. Arch. Eur. J. Physiol. 2020, 472, 1345–1370. [Google Scholar] [CrossRef]
- Pozzi, A.; Zent, R. Hold tight or you’ll fall off: CD151 helps podocytes stick in high-pressure situations. J. Clin. Investig. 2012, 122, 13–16. [Google Scholar] [CrossRef]
- Allison, S.J. CD151—Role in strengthening podocyte–GBM binding. Nat. Rev. Nephrol. 2012, 8, 132. [Google Scholar] [CrossRef]
- Masson, E.; Wiernsperger, N.; Lagarde, M.; El Bawab, S. Glucosamine induces cell-cycle arrest and hypertrophy of mesangial cells: Implication of gangliosides. Biochem. J. 2005, 388, 537–544. [Google Scholar] [CrossRef]
- Boehmelt, G.; Wakeham, A.; Elia, A.; Sasaki, T.; Plyte, S.; Potter, J.; Yang, Y.; Tsang, E.; Ruland, J.; Iscove, N.N.; et al. Decreased UDP-GlcNAc levels abrogate proliferation control in EMeg32-deficient cells. EMBO J. 2000, 19, 5092–5104. [Google Scholar] [CrossRef]
- Huang, C.; Harada, Y.; Hosomi, A.; Masahara-Negishi, Y.; Seino, J.; Fujihira, H.; Funakoshi, Y.; Suzuki, T.; Dohmae, N.; Suzuki, T. Endo-β-N-acetylglucosaminidase forms N-GlcNAc protein aggregates during ER-associated degradation in Ngly1-defective cells. Proc. Natl. Acad. Sci. USA 2015, 112, 1398–1403. [Google Scholar] [CrossRef]
- Fujihira, H.; Masahara-Negishi, Y.; Tamura, M.; Huang, C.; Harada, Y.; Wakana, S.; Takakura, D.; Kawasaki, N.; Taniguchi, N.; Kondoh, G.; et al. Lethality of mice bearing a knockout of the Ngly1-gene is partially rescued by the additional deletion of the Engase gene. PLoS Genet. 2017, 13, e1006696. [Google Scholar] [CrossRef]
- Peterson, S.B.; Hart, G.W. New insights: A role for O-GlcNAcylation in diabetic complications. Crit. Rev. Biochem. Mol. Biol. 2016, 51, 150–161. [Google Scholar] [CrossRef]
- Macauley, M.S.; Shan, X.; Yuzwa, S.A.; Gloster, T.M.; Vocadlo, D.J. Elevation of Global O-GlcNAc in rodents using a selective O-GlcNAcase inhibitor does not cause insulin resistance or perturb glucohomeostasis. Chem. Biol. 2010, 17, 949–958. [Google Scholar] [CrossRef]
- Hofmann, C.; Blackwood, E.A.; Jakobi, T.; Sandmann, C.; Groß, J.; Herzog, N.; Kaufman, R.J.; Katus, H.A.; Völkers, M.; Glembotski, C.C. Age-related decline of the unfolded protein response in the heart promotes protein misfolding and cardiac pathology. bioRxiv 2021. [Google Scholar] [CrossRef]
- Akimoto, Y.; Miura, Y.; Toda, T.; Wolfert, M.A.; Wells, L.; Boons, G.J.; Hart, G.W.; Endo, T.; Kawakami, H. Morphological changes in diabetic kidney are associated with increased O-GlcNAcylation of cytoskeletal proteins including α-actinin 4. Clin. Proteom. 2011, 8, 15. [Google Scholar] [CrossRef] [PubMed]
- Na, J.; Sweetwyne, M.T.; Park, A.S.; Susztak, K.; Cagan, R.L. Diet-Induced Podocyte Dysfunction in Drosophila and Mammals. Cell Rep. 2015, 12, 636–647. [Google Scholar] [CrossRef]
- Li, X.; Yang, Q.; Liu, S.; Song, S.; Wang, C. Mitochondria-associated endoplasmic reticulum membranes promote mitochondrial fission through AKAP1-Drp1 pathway in podocytes under high glucose conditions. Exp. Cell Res. 2023, 424, 113512. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Li, X.; Wen, R.; Chen, L.; Yang, Q.; Song, S.; Xiao, G.; Su, Z.; Wang, C. Increased thromboxane/prostaglandin receptors contribute to high glucose-induced podocyte injury and mitochondrial fission through ROCK1-Drp1 signaling. Int. J. Biochem. Cell Biol. 2022, 151, 106281. [Google Scholar] [CrossRef]
- Deng, Q.; Wen, R.; Liu, S.; Chen, X.; Song, S.; Li, X.; Su, Z.; Wang, C. Increased long noncoding RNA maternally expressed gene 3 contributes to podocyte injury induced by high glucose through regulation of mitochondrial fission. Cell Death Dis. 2020, 11, 814. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.A.; O’Hare, M.J.; Reiser, J.; Coward, R.J.; Inward, C.D.; Farren, T.; Xing, C.Y.; Ni, L.; Mathieson, P.W.; Mundel, P. A conditionally immortalized human podocyte cell line demonstrating nephrin and podocin expression. J. Am. Soc. Nephrol. JASN 2002, 13, 630–638. [Google Scholar] [CrossRef]


| ND | db/m | STZ plus UNx | db/db plus UNx | |
|---|---|---|---|---|
| Body weight, baseline (g) | 24.4 ± 2.71 | 23.67 ± 3.66 | 24.8 ± 3.21 | 29.5 ± 3.72 |
| Body weight, endpoint (g) | 34.7 ± 2.44 | 36.72 ± 4.23 | 25.9 ± 2.62 * | 42.3 ± 3.87 # |
| Glucose, baseline (mmol/L) | 5.3 ± 0.65 | 6.11 ± 0.35 | 4.7 ± 0.67 | 14.3 ± 3.41 |
| Glucose, endpoint (mmol/L) | 4.9 ± 0.52 | 5.54 ± 0.46 | 26.7 ± 4.46 * | 28.3 ± 4.84 # |
| Plasma creatinine (mg/dL) | 0.22 ± 0.07 | 0.16 ± 0.09 | 0.53 ± 0.06 * | 0.62 ± 0.10 # |
| Plasma BUN (mg/dL) | 19.62 ± 4.66 | 17.34 ± 5.72 | 28.55 ± 6.86 * | 35.69 ± 5.12 # |
| Urinary albumin excretion (mg/d) | 8.67 ± 2.88 | 9.32 ± 3.31 | 233.72 ± 65.43 * | 271.26 ± 73.41 # |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, S.; Hu, T.; Shi, X.; Jin, Y.; Liu, S.; Li, X.; Zou, W.; Wang, C. ER Stress-Perturbed Intracellular Protein O-GlcNAcylation Aggravates Podocyte Injury in Diabetes Nephropathy. Int. J. Mol. Sci. 2023, 24, 17603. https://doi.org/10.3390/ijms242417603
Song S, Hu T, Shi X, Jin Y, Liu S, Li X, Zou W, Wang C. ER Stress-Perturbed Intracellular Protein O-GlcNAcylation Aggravates Podocyte Injury in Diabetes Nephropathy. International Journal of Molecular Sciences. 2023; 24(24):17603. https://doi.org/10.3390/ijms242417603
Chicago/Turabian StyleSong, Shicong, Tiantian Hu, Xu Shi, Yongjie Jin, Sirui Liu, Xuehong Li, Wei Zou, and Cheng Wang. 2023. "ER Stress-Perturbed Intracellular Protein O-GlcNAcylation Aggravates Podocyte Injury in Diabetes Nephropathy" International Journal of Molecular Sciences 24, no. 24: 17603. https://doi.org/10.3390/ijms242417603
APA StyleSong, S., Hu, T., Shi, X., Jin, Y., Liu, S., Li, X., Zou, W., & Wang, C. (2023). ER Stress-Perturbed Intracellular Protein O-GlcNAcylation Aggravates Podocyte Injury in Diabetes Nephropathy. International Journal of Molecular Sciences, 24(24), 17603. https://doi.org/10.3390/ijms242417603







