Caffeine and Its Interactions with Antiseizure Medications—Is There a Correlation between Preclinical and Clinical Data?
Abstract
:1. Introduction
2. Search Strategy and Selection Criteria
3. Caffeine and the Anticonvulsant Activity of ASMs
3.1. Data from Electroconvulsive Tests in Rodents—Maximal Electroshock Test (MES) or Maximal Electroshock Threshold Test (MEST)
3.2. Data from Pentylenetetrazol-Induced Clonic Seizure Activity
4. Do Other Methylxanthines Share Caffeine’s Propensity to Affect the Anticonvulsant Activity of ASMs?
5. Do the Experimental Results Translate into Clinical Studies?
5.1. Studies Pointing to a Relationship between Caffeine Intake and Seizure Activity
5.2. Studies Showing Mainly No Association between Dietary Caffeine and Seizure Precipitation
6. Discussion
7. Final Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Schwabe, U.; Ukena, D.; Lohse, M.J. Xanthine derivatives as antagonists at A1 and A2 adenosine receptors. Naunyn Schmiedebergs Arch. Pharmacol. 1985, 330, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Svenningsson, P.; Hall, M.; Sedval, G.; Fredholm, B.B. Distribution of adenosine receptors in the postmortem human brain: An extended autoradiographic study. Synapse 1997, 27, 322–335. [Google Scholar] [CrossRef]
- Londos, C.; Cooper, D.; Wolff, J. Subclasses of external adenosine receptors. Proc. Natl. Acad. Sci. USA 1980, 77, 2551–2554. [Google Scholar] [CrossRef] [PubMed]
- Daly, J.W.; Butts-Lamb, P.; Padgett, W. Subclasses of adenosine receptors in the central nervous system: Interactions with caffeine and related methylxanthines. Cell Mol. Neurobiol. 1983, 3, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Sebastiao, A.M.; Ribeiro, J.A. Adenosine A2 receptor mediated excitatory action on the nervous system. Prog. Neurobiol. 1996, 48, 167–189. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Sharma, A. Adenosine: An endogenous modulator of innate immune system with therapeutic potential. Eur. J. Pharmacol. 2009, 616, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Fredholm, B.B.; IJzerman, A.P.; Jacobson, K.A.; Klotz, K.N.; Linden, J. International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol. Rev. 2001, 51, 527–552. [Google Scholar]
- Dingledine, R.; McBain, C.; McNamara, J.O. Excitatory amino acid receptors in epilepsy. Trends Pharmacol. Sci. 1990, 11, 334–338. [Google Scholar] [CrossRef]
- Zeraati, M.; Mirmajafi-Zadeh, J.; Fathollahi, Y.; Namvar, S.; Rezvani, M.E. Adenosine A1 and A2A receptors of hippocampal CA1 region have opposite effects on piriform cortex kindled seizures in rats. Seizure 2006, 15, 41–48. [Google Scholar] [CrossRef]
- Von Lubitz, D.K.; Carter, M.F.; Deutsch, S.I.; Lin, R.C.; Mastropaolo, J.; Meshulam, Y.; Jacobson, K.A. The effects of adenosine A3 receptor stimulation on seizures in mice. Eur. J. Pharmacol. 1995, 275, 23–29. [Google Scholar] [CrossRef]
- Bernstein, G.A.; Carrol, M.E.; Thuras, P.D.; Cosgrove, K.P.; Roth, M.E. Caffeine dependence in teenagers. Drug Alcohol Depend. 2002, 66, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Derossi, A.; Ricci, I.; Caporizzi, R.; Fiore, A.; Severini, C. How grinding level and brewing method (Espresso, American, Turkish) could affect the antioxidant activity and bioactive compounds in a coffee cup. J. Sci. Food Agric. 2018, 98, 3198–3207. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.-N.; Ho, S.C.; Zhou, C.; Ling, W.-H.; Chen, W.-Q.; Wang, C.-L.; Chen, Y.-M. Coffee consumption and risk of coronanry heart diseases: A meta-analysis of 21 prospective cohort studies. Int. J. Cardiol. 2009, 137, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Chrościńska-Krawczyk, M.; Radzik, I.; Miziak, B.; Czuczwar, S.J. Safety considerations for patients with epilepsy taking antiepileptic drugs alongside caffeine or other methylxanthine derivatives. Expert Opin. Drug Metab. Toxicol. 2014, 10, 981–989. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, J.E.C.; da Silva, A.L.M.; Veira, L.R.; Nascimento, P.C.; Pereira, R.G.; Rodriguez, S.F.; Hamoy, A.O.; de Mello, V.J.; de Araújo, D.B.; Barbas, L.A.L.; et al. Caffeine intoxication: Behavioral and electrocorticographic patterns in Wistar rats. Food Chem. Toxicol. 2022, 170, 113452. [Google Scholar] [CrossRef] [PubMed]
- Jailani, M.; Mubarak, M.; Sarkhouh, M.; Al Mahrezi, A.; Abdulnabi, H.; Naiser, M.; Alaradi, H.; Alabbad, A.; Hassan, M.; Kamal, A. The effect of low-doses of caffeine and taurine on convulsive seizure parameters in rats. Behav. Sci. 2020, 10, 43. [Google Scholar] [CrossRef] [PubMed]
- Bankstahl, M.; Bankstahl, J.P.; Bloms-Funke, P.; Löscher, W. Striking differences in proconvulsant-induced alterations of seizure threshold in two rat models. Neurotoxicology 2012, 33, 127–137. [Google Scholar] [CrossRef]
- Cutrufo, C.; Bortot, L.; Giachetti, A.; Manzini, S. Different effects of various methylxanthines on pentylenetetrazole-induced seizures in rats: An EEG and behavioural study. Eur. J. Pharmacol. 1992, 222, 1–6. [Google Scholar] [CrossRef]
- Asadi-Pooya, A.A.; Zeraatpisheh, Z.; Rostaminejad, M.; Damabi, N.M. Caffeinated drinks, fruit juices, and epilepsy. A systematic review. Acta Neurol. Scand. 2022, 145, 127–138. [Google Scholar] [CrossRef]
- Löscher, W.; Fassbender, C.P.; Nolting, B. The role of technical, biological and pharmacological factors in the laboratory evaluation of anticonvulsant drugs. II. Maximal electroshock seizure models. Epilepsy Res. 1991, 8, 79–94. [Google Scholar] [CrossRef]
- Kulkarni, C.; Joseph, T.; David, J. Influence of adenosine receptor antagonists, aminophylline and caffeine, on seizure protective ability of antiepileptic drugs in rats. Indian J. Exp. Biol. 1991, 29, 751–754. [Google Scholar] [CrossRef] [PubMed]
- Jargiełło-Baszak, M.; Chrościńska-Krawczyk, M.; Andres-Mach, M.; Łuszczki, J.J.; Czuczwar, S.J. Influence of caffeine on the protective activity of gabapentin and topiramate in a mouse model of generalized tonic-clonic seizures. Pharmacol. Rep. 2016, 68, 680–685. [Google Scholar] [PubMed]
- Chwedorowicz, R.; Łukawski, K.; Raszewski, G.; Czuczwar, S.J. Effect of caffeine on the anticonvulsant action of pregabalin against electroconvulsions in mice. Pharmacol. Rep. 2022, 74, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Chwedorowicz, R.; Łukawski, K.; Raszewski, G.; Czuczwar, S.J. Caffeine impairs anticonvulsant effects of levetiracetam in the maximal electroshock seizure threshold test in mice. J. Basic Clin. Physiol. Pharmacol. 2022, 34, 57–364. [Google Scholar] [CrossRef]
- Löscher, W.; Hönack, D.; Fassbender, C.P.; Nolting, B. The role of technical, biological and pharmacological factors in the laboratory evaluation of anticonvulsant drugs. III. Pentylenetetrazole seizure models. Epilepsy Res. 1991, 8, 171–189. [Google Scholar] [CrossRef] [PubMed]
- Goto, M.; Mirishita, S.; Fukuda, F. Anticonvulsant action of diazepam in mice pretreated with caffeine. J. Pharmacobiodyn. 1983, 6, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Mahemuti, G.; Zhang, H.; Li, J.; Tieliwaerdi, N.; Ren, L. Efficacy and side effects of intravenous theophylline in acute asthma: A systematic review and meta-analysis. Drug Res. Devel. Ther. 2018, 12, 99–120. [Google Scholar] [CrossRef]
- Czuczwar, S.J.; Janusz, W.; Wamil, A.; Kleinrok, Z. Inhibition of aminophylline -induced convulsions in mice by antiepileptic drugs and other agents. Eur. J. Pharmacol. 1987, 144, 309–315. [Google Scholar] [CrossRef]
- Kaufman, K.; Sachdeo, R. Caffeinated beverages and decreased seizure control. Seizure 2003, 12, 519–521. [Google Scholar] [CrossRef]
- Bonilha, L.; Li, M. Heavy coffee drinking and epilepsy. Seizure 2004, 13, 284–285. [Google Scholar] [CrossRef]
- Mackow, M.J.; Krishnan, B.; Bingaman, W.E.; Najm, I.M.; Alexopoulos, A.V.; Nair, D.R. Increased caffeine intake leads to worsening of electrocorticographic epileptiform discharges as recorded with a responsive neurostimulation device. Clin. Neurophysiol. 2016, 127, 2341–2342. [Google Scholar] [CrossRef] [PubMed]
- Błaszczyk, B. Caffeine and management of epilepsy—A clinical evidence. Pharmacol. Rep. 2007, 59, 116. [Google Scholar]
- Samsonsen, C.; Brathen, G.; Reimers, A.; Helde, G.; Brodtkorp, E. Is dietary caffeine involved in seizure precipitation? Epilepsy Behav. 2013, 28, 147–150. [Google Scholar] [CrossRef] [PubMed]
- Dvoretzky, B.A.; Bromfield, E.B.; Townsend, M.K.; Kang, J.H. A prospective study of smoking, caffeine, and alcohol as risk factors for seizures in young adult women; data from the Nurses’ Health Study II. Epilepsia 2010, 51, 198–205. [Google Scholar]
- Bourgeois-Vionnet, J.; Rywlin, P.; Elsensohn, M.-H.; Michel, V.; Valton, L.; Derambure, P.; Frazzini, V.; Hirsch, E.; Maillard, L.; Bartolomei, F.; et al. Coffee consumption and seizure frequency in patients with drug-resistant focal epilepsy. Epilepsy Behav. 2022, 126, 108486. [Google Scholar] [CrossRef]
- Baltos, J.-A.; Casillas-Espinosa, P.M.; Rollo, B.; Gregory, K.J.; White, P.J.; Christopoulos, A.; Kwan, P.; O’Brien, T.J.; May, L.T. The role of the adenosine system in epilepsy and its comorbidities. Br. J. Pharmacol. 2023. online ahead of print. [Google Scholar] [CrossRef]
- Booker, S.A.; Pires, N.; Cobb, S.; Soares-da-Silva, P.; Vida, I. Carbamazepine and oxcarbazepine, but not eslicarbazepine, enhance excitatory synaptic transmission onto hippocampal CA1 pyramidal cells through an antagonist action at adenosine A1 receptors. Neuropharmacology 2015, 93, 103–115. [Google Scholar] [CrossRef]
- Nehlig, A.; Daval, J.L.; Debry, G. Caffeine and the central nervous system: Mechanism of action, biochemical, metabolic and psychostimulant effects. Brain Res. Rev. 1992, 17, 139–170. [Google Scholar] [CrossRef]
- Neering, I.R.; McBurney, R.N. Role for microsomal Ca storage in mammalian neurones? Nature 1984, 309, 158–160. [Google Scholar] [CrossRef]
- Margineau, D.G.; Klitgaard, H. Caffeine-induced epileptiform field potentials in rat hippocampal slices: A pharmacological characterization. Neuropharmacology 2004, 47, 926–934. [Google Scholar] [CrossRef]
- Lasoń, W.; Dudra-Jastrzębska, M.; Rejdak, K.; Czuczwar, S.J. Basic mechanisms of antiepileptic drugs and their pharmacokinetic/pharmacodynamic interactions: An update. Pharmacol. Rep. 2011, 63, 271–292. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, D.; Elger, C.E. What is the evidence that oxcarbazepine and carbamazepine are distinctly different antiepileptic drugs? Epilepsy Behav. 2004, 5, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Hainsworth, A.H.; McNaughton, N.C.L.; Pereverzev, A.; Schneider, T.; Randall, A.D. Actions of sipatrigine, 202W92 and lamotrigine on R-type and T-type Ca2+ channel currents. Eur. J. Pharmacol. 2003, 467, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Dibue-Adjei, M.; Kamp, M.A.; Alpdogan, S.; Tevoufouet, E.E.; Neiss, W.F.; Heschler, J.; Scheider, T. Cav2.3 (R-type) calcium channels are critical for mediating anticonvulsive and neuroprotective properties of lamotrigine in vivo. Cell Physiol Biochem. 2017, 44, 935–947. [Google Scholar] [CrossRef] [PubMed]
- Calabresi, P.; De Murtas, M.; Stefani, A.; Pisani, A.; Sancesario, G.; Mercuri, N.B.; Bernardi, G. Action of GP 47779, the active metabolite of oxcarbazepine, on the corticostriatal system. I. Modulation of corticostriatal synaptic transmission. Epilepsia 1995, 36, 990–996. [Google Scholar] [CrossRef] [PubMed]
- Stefani, A.; Pisani, A.; De Murtas, M.; Mercuri, N.B.; Marciani, M.G.; Calabresi, P. Action of GP 47779, the active metabolite of oxcarbazepine, on the corticostriatal system. II. Modulation of high-voltage-activated calcium currents. Epilepsia 1995, 36, 997–1002. [Google Scholar] [CrossRef] [PubMed]
- Kuzmiski, J.B.; Barr, W.; Zamponi, G.W.; MacVicar, B.A. Topiramate inhibits the initiation of plateau potentials in CA1 neurons by depressing R-type calcium channels. Epilepsia 2005, 46, 481–489. [Google Scholar] [CrossRef]
- van Koert, R.R.; Bauer, P.R.; Schuitema, I.; Sander, J.W.; Visser, G.H. Caffeine and seizures: A systematic review and quantitative analysis. Epilepsy Behav. 2018, 80, 37–47. [Google Scholar] [CrossRef]
- Nehlig, A. Interindividual differences in caffeine metabolism and factors driving caffeine consumption. Pharmacol. Rev. 2018, 70, 384–411. [Google Scholar] [CrossRef]
- Burg, A.W. Physiological disposition of caffeine. Drug Metab. Rev. 1975, 4, 199–228. [Google Scholar] [CrossRef]
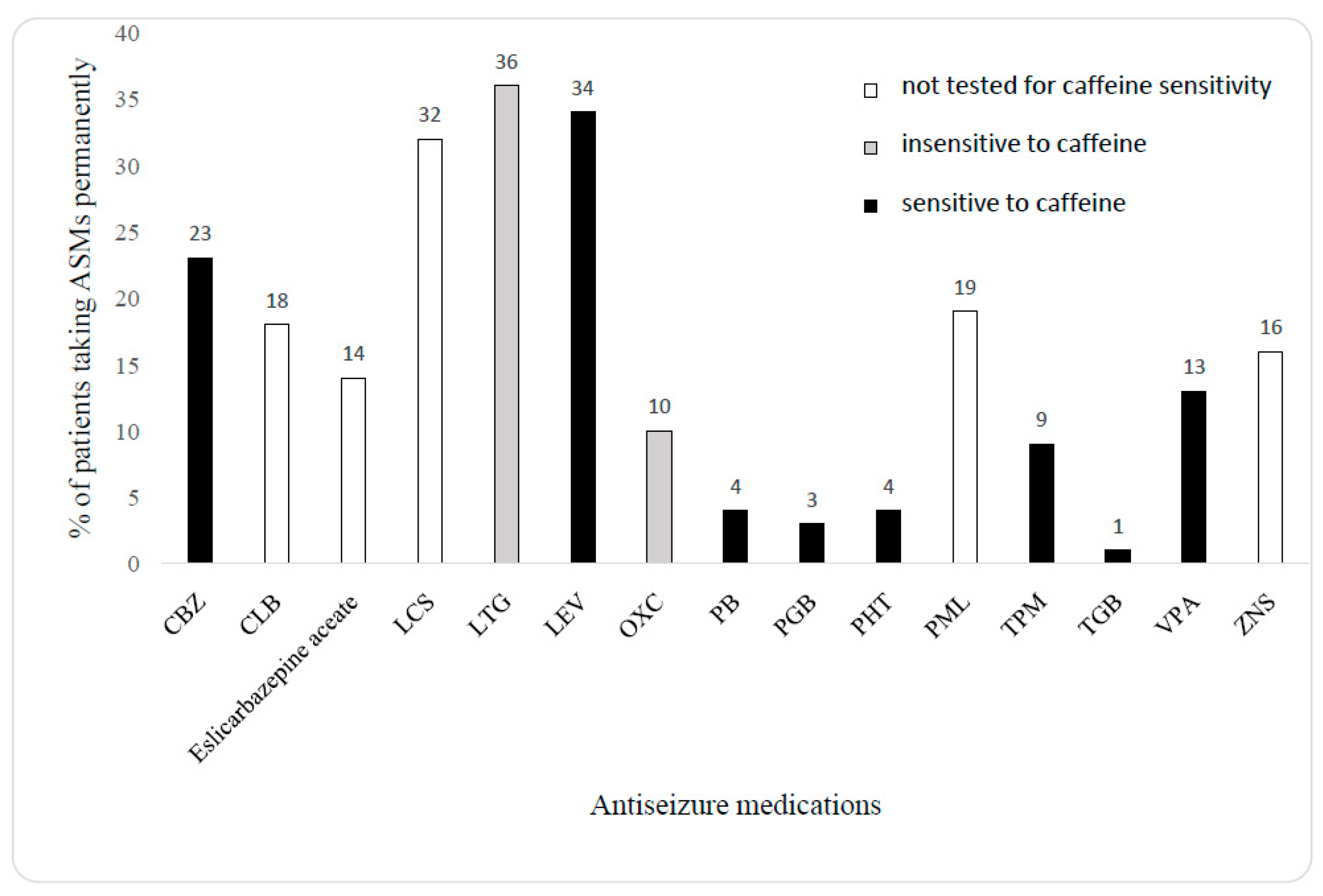
| ASM | Initial Doses of ASMs (mg/kg) | Caffeine | Seizure Model | Animal Model | ASM Efficacy | Bibliography | ||
|---|---|---|---|---|---|---|---|---|
| Dose of Caffeine (mg/kg) | Acute | Chronic | ||||||
| Carbamazepine | 13 | 46.2 | + | NT | MES | mouse | ↓ | [14] |
| 13 | 92.4 | + | NT | MES | mouse | ↓ | [14] | |
| 15 | 11.55 | NT | + | MES | mouse | ↓ | [14] | |
| 15 | 11.55 | + | NT | MES | mouse | 0 | [14] | |
| 15 | 23.1 | + | NT | MES | mouse | ↓ | [14] | |
| 15 | 23.1 | NT | + | MES | mouse | ↓ | [14] | |
| Diazepam | 0.5 | 200 | + | NT | PTZ | mouse | ↓ | [26] |
| 1 | 200 | + | NT | PTZ | rat | ↓ | [21] | |
| 10 | 200 | + | NT | MES | rat | ↓ | [21] | |
| Ethosuximide | 127.7 | 69.3 | + | NT | PTZ | mouse | ↓ | [14] |
| 127.7 | 92.4 | + | NT | PTZ | mouse | ↓ | [14] | |
| 200 | 200 | + | NT | PTZ | mouse | 0 | [21] | |
| Felbamate | 110 | 161.7 | + | NT | MES | mouse | ↓ | [14] |
| Clonazepam | 0.026 | 92.4 | + | NT | PTZ | mouse | 0 | [14] |
| Lamotrigine | 7.5 | 46.2 | + | + | MES | mouse | 0 | [14] |
| 7.5 | 23.1 | + | + | MES | mouse | 0 | [14] | |
| Oxcarbazepine | 13 | 23.1 | + | + | MES | mouse | 0 | [14] |
| 13 | 46.2 | + | + | MES | mouse | 0 | [14] | |
| Phenobarbital | 10 | 200 | + | NT | MES | rat | ↓ | [21] |
| 19.5 | 92.4 | + | NT | MES | mouse | ↓ | [14] | |
| 17.8 | 23.1 | + | + | MES | mouse | ↓ | [14] | |
| 11.4 | 92.4 | + | NT | PTZ | mouse | 0 | [14] | |
| 10 | 200 | + | NT | PTZ | rat | ↓ | [14] | |
| Phenytoin | 12 | 92.4 | + | NT | MES | mouse | ↓ | [14] |
| 12 | 46.2 | + | NT | MES | mouse | ↓ | [14] | |
| 20 | 200 | + | NT | MES | rat | ↓ | [21] | |
| Pregabalin | 379.3 | 23.1 | + | NT | MES | mouse | ↓ | [23] |
| Topiramate | 44.8 | 23.1 | + | + | MES | mouse | ↓ | [22] |
| 46.2 | + | + | MES | mouse | ↓ | [22] | ||
| Valproate | 300 | 200 | + | NT | MES | rat | 0 | [21] |
| 270 | 46.2 | + | NT | MES | mouse | ↓ | [14] | |
| 273 | 23.1 | NT | + | MES | mouse | ↓ | [14] | |
| 270 | 92.4 | + | NT | MES | mouse | ↓ | [14] | |
| 130.7 | 92.4 | + | NT | PTZ | mouse | 0 | [14] | |
| 300 | 200 | + | NT | PTZ | mouse | 0 | [21] | |
| ASM | Dosage of ASMs (mg/kg) | Caffeine | Seizure Model | Animal Model | Convuslive Threshold | Bibliography | ||
|---|---|---|---|---|---|---|---|---|
| Dose of Caffeine (mg/kg) | Acute | Chronic | ||||||
| NT | NT | 50 | + | NT | PTZ | rat | ↓ | [16] |
| 60 | + | NT | PTZ | rat | 0 | [17] | ||
| 92.4 | + | NT | PTZ | mouse | ↓ | [14] | ||
| 92.4 | + | NT | PTZ | rat | ↓ | [14] | ||
| 80 | + | NT | MEST | rat | ↓ | [17] | ||
| 80 | NT | + | PTZ | rat | ↓ | [16] | ||
| Gabapentin | 200 | 23.1 | NT | + | MEST | mouse | ↓ | [22] |
| 200 | 46.2 | + | + | MEST | mouse | ↓ | [22] | |
| Levetiracetam | 500 | 46.2 | + | NT | MEST | mouse | ↓ | [24] |
| 500 | 69.3 | + | NT | MEST | mouse | ↓ | [24] | |
| Tiagabine | 4.9 | 23.1 | + | + | MEST | mouse | 0 | [14] |
| 4.9 | 46.2 | + | + | MEST | mouse | 0 | [14] | |
| Study | Age of Patients (Years) | Gender | Type of Seizures | Exposure to Increased Doses of Caffeine | Seizure Frequency | Seizure Frequency after Withdrawal of Caffeine Source | Bibliography | |
|---|---|---|---|---|---|---|---|---|
| Case Reports | Clinical Study | |||||||
| + | 49 | M | absence, atonic, and myoclonic seizures | + | ↑ | ↓ | [29] | |
| + | 40 | M | simple and complex focal seizures | + | ↑ | ↓ | [30] | |
| + | 33 | F | temporal lobe epilepsy recurrent episodes of status epilepticus | + | ↑ | ↓ | [31] | |
| + | 42.6 (mean of years) | M—57% F—43% | focal seizure onset (89 patients) and genetic generalized epilepsies (22) and absences (5) | + | 0 | 0 | [33] | |
| + | 25–42 | F | self-reported seizure or epilepsy | + | 0 | 0 | [34] | |
| + | 18–77 | M, F | drug-resistant focal epilepsy, focal bilateral clonic-tonic seizures | moderate caffeine | ↓ | No data | [35] | |
| M, F | drug-resistant focal epilepsy, focal bilateral clonic-tonic seizures | high caffeine consumption | ↑ | ↓ | [35] | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miziak, B.; Błaszczyk, B.; Chrościńska-Krawczyk, M.; Czuczwar, S.J. Caffeine and Its Interactions with Antiseizure Medications—Is There a Correlation between Preclinical and Clinical Data? Int. J. Mol. Sci. 2023, 24, 17569. https://doi.org/10.3390/ijms242417569
Miziak B, Błaszczyk B, Chrościńska-Krawczyk M, Czuczwar SJ. Caffeine and Its Interactions with Antiseizure Medications—Is There a Correlation between Preclinical and Clinical Data? International Journal of Molecular Sciences. 2023; 24(24):17569. https://doi.org/10.3390/ijms242417569
Chicago/Turabian StyleMiziak, Barbara, Barbara Błaszczyk, Magdalena Chrościńska-Krawczyk, and Stanisław J. Czuczwar. 2023. "Caffeine and Its Interactions with Antiseizure Medications—Is There a Correlation between Preclinical and Clinical Data?" International Journal of Molecular Sciences 24, no. 24: 17569. https://doi.org/10.3390/ijms242417569
APA StyleMiziak, B., Błaszczyk, B., Chrościńska-Krawczyk, M., & Czuczwar, S. J. (2023). Caffeine and Its Interactions with Antiseizure Medications—Is There a Correlation between Preclinical and Clinical Data? International Journal of Molecular Sciences, 24(24), 17569. https://doi.org/10.3390/ijms242417569







