Development of Magnetizable, Nickel–Ferrite-Decorated Carbon Nanocomposites as Hydrogenation Catalyst for Aniline Synthesis
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of the CNL and N-BCNT Nanomaterials as Catalyst Supports
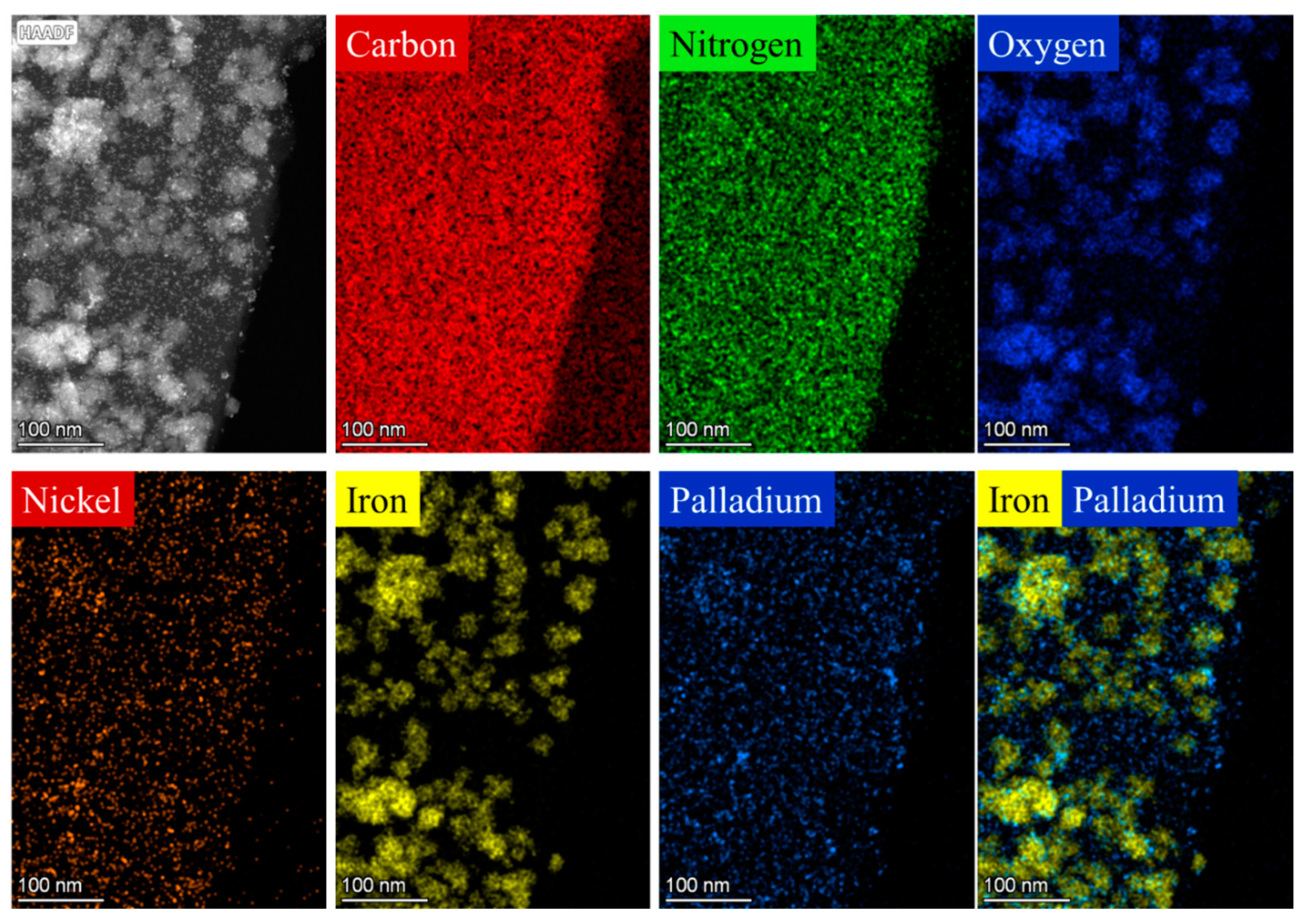
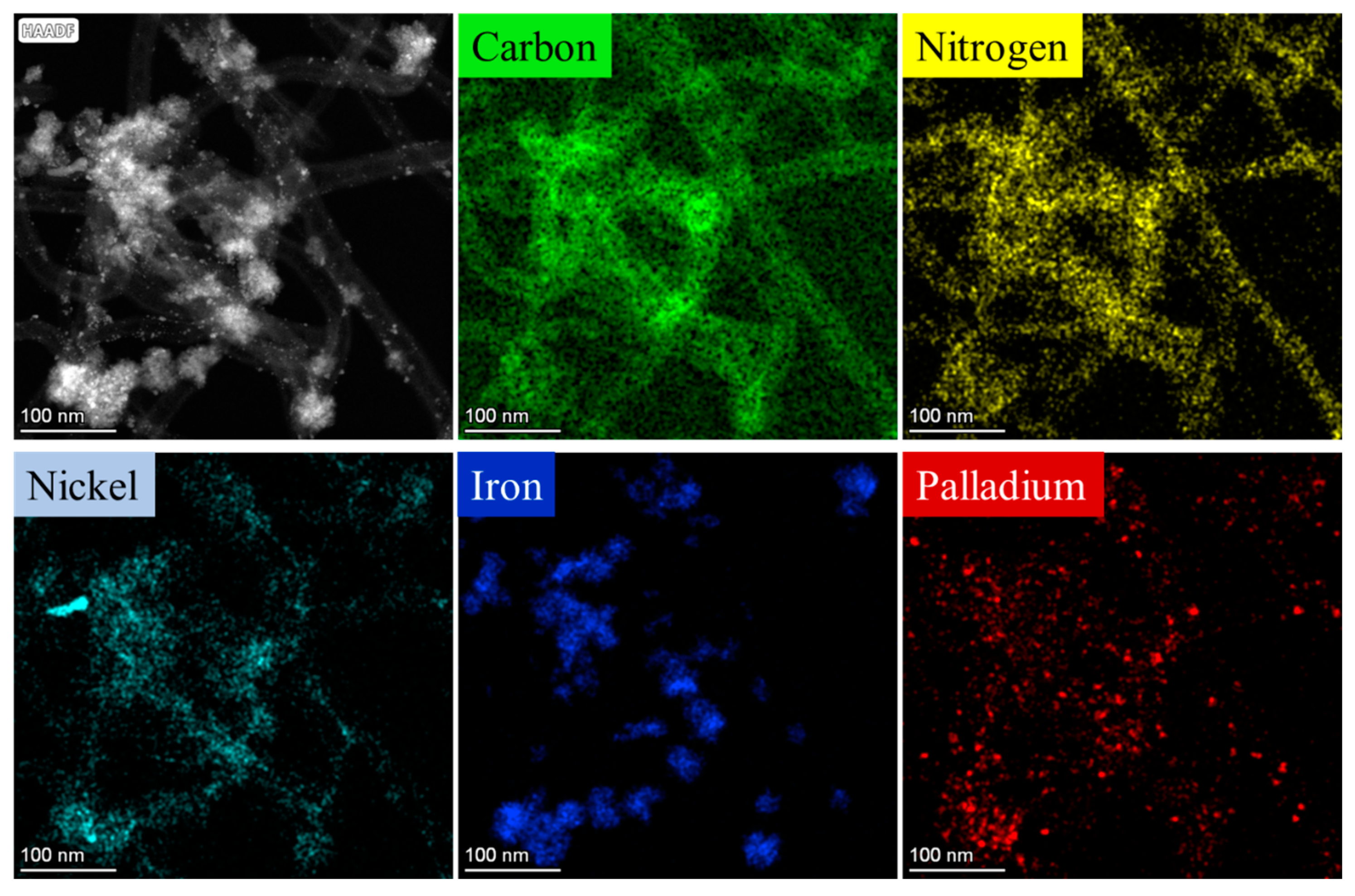
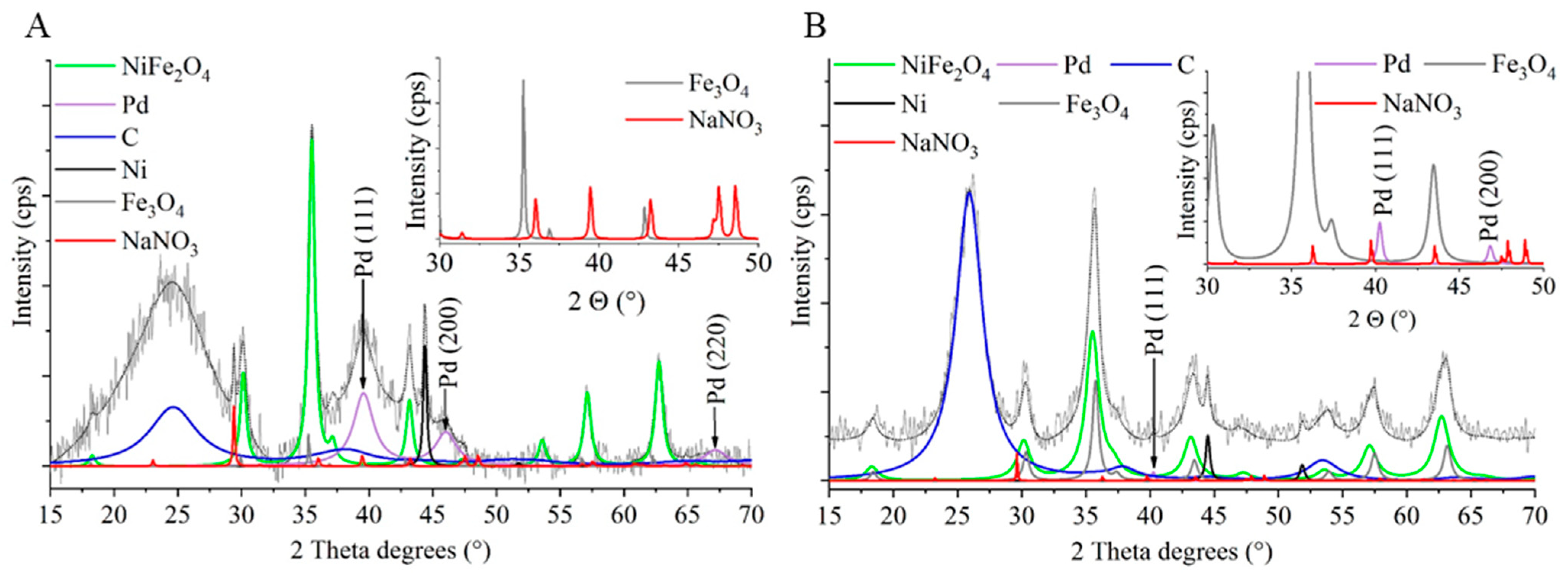
2.2. Characterization of the Magnetizable, Nickel-Ferrite Decorated Carbon Nanomaterials and the Palladium Catalyst
2.3. Catalytic Tests of the Magnetic Carbon-Supported Palladium Catalysts in Nitrobenzene Hydrogenation
3. Materials and Methods
3.1. Materials
3.2. Characterization Techniques
3.3. Preparation of the Carbon Nanolayer (CNL) Sample
3.4. Preparation of the N-BCNT
3.5. Deposition of the Nickel–Ferrite Particles onto the Surfaces of the Carbon Supports
3.6. Deposition of the Palladium Nanoparticles onto the Surfaces of the Ferrite-Decorated Carbon Supports
3.7. Catalytic Tests of the Prepared Palladium Catalysts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wegener, G.; Brandt, M.; Duda, L.; Hofmann, J.; Klesczewski, B.; Koch, D.; Kumpf, R.J.; Orzesek, H.; Pirkl, H.G.; Six, C.; et al. Trends in Industrial Catalysis in the Polyurethane Industry. Appl. Catal. A Gen. 2001, 221, 303–335. [Google Scholar] [CrossRef]
- Serp, P.; Figueiredo, J.L. Carbon Materials for Catalysis; John Wiley & Sons: Hoboken, NJ, USA, 2009; ISBN 9780470403693. [Google Scholar]
- Pham-Huu, C.; Ledoux, M.J. Carbon Nanomaterials with Controlled Macroscopic Shapes as New Catalytic Materials. Top. Catal. 2006, 40, 49–63. [Google Scholar] [CrossRef]
- Macías Pérez, M.C.; Salinas Martínez De Lecea, C.; Linares Solano, A. Platinum Supported on Activated Carbon Cloths as Catalyst for Nitrobenzene Hydrogenation. Appl. Catal. A Gen. 1997, 151, 461–475. [Google Scholar] [CrossRef]
- Hashemi, M.; Khodaei, M.M.; Teymouri, M.; Rashidi, A.; Mohammadi, H. Preparation of NiO Nanocatalyst Supported on MWCNTs and Its Application in Reduction of Nitrobenzene to Aniline in Liquid Phase. Synth. React. Inorganic Met. Nano-Metal Chem. 2016, 46, 959–967. [Google Scholar] [CrossRef]
- Huang, L.; Lv, Y.; Wu, S.; Liu, P.; Xiong, W.; Hao, F.; Luo, H. Activated Carbon Supported Bimetallic Catalysts with Combined Catalytic Effects for Aromatic Nitro Compounds Hydrogenation under Mild Conditions. Appl. Catal. A Gen. 2019, 577, 76–85. [Google Scholar] [CrossRef]
- Ren, G.; Gao, L.; Teng, C.; Li, Y.; Yang, H.; Shui, J.; Lu, X.; Zhu, Y.; Dai, L. Ancient Chemistry “Pharaoh’s Snakes” for Efficient Fe-/N-Doped Carbon Electrocatalysts. ACS Appl. Mater. Interfaces 2018, 10, 10778–10785. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Zhou, M.; Wang, X.; Wang, H.; Yin, Z.; Tan, X.; Li, Y. Preparing Co/N-Doped Carbon as Electrocatalyst toward Oxygen Reduction Reaction via the Ancient “Pharaoh’s Snakes” Reaction. Batteries 2022, 8, 150. [Google Scholar] [CrossRef]
- Gao, Z.; Zhu, H.; Li, Y.; Fan, S.; Luo, H.; Xue, J.; Zhang, J. Preparation and Electrochemical Properties of Sucrose-Based Porous Carbon Materials by Combustion Expansion-Chemical Activation Method. J. Appl. Electrochem. 2020, 50, 549–558. [Google Scholar] [CrossRef]
- Sireesha, M.; Jagadeesh Babu, V.; Kranthi Kiran, A.S.; Ramakrishna, S. A Review on Carbon Nanotubes in Biosensor Devices and Their Applications in Medicine. Nanocomposites 2018, 4, 36–57. [Google Scholar] [CrossRef]
- Das, H.T.; Dutta, S.; Balaji, T.E.; Das, N.; Das, P.; Dheer, N.; Kanojia, R.; Ahuja, P.; Ujjain, S.K. Recent Trends in Carbon Nanotube Electrodes for Flexible Supercapacitors: A Review of Smart Energy Storage Device Assembly and Performance. Chemosensors 2022, 10, 223. [Google Scholar] [CrossRef]
- Yu, Z.; Lau, D. Evaluation on Mechanical Enhancement and Fire Resistance of Carbon Nanotube (CNT) Reinforced Concrete. Coupled Syst. Mech. 2017, 6, 335–349. [Google Scholar] [CrossRef]
- Esteves, L.M.; Oliveira, H.A.; Passos, F.B. Carbon Nanotubes as Catalyst Support in Chemical Vapor Deposition Reaction: A Review. J. Ind. Eng. Chem. 2018, 65, 1–12. [Google Scholar] [CrossRef]
- Yang, D.J.; Zhang, Q.; Chen, G.; Yoon, S.F.; Ahn, J.; Wang, S.G.; Zhou, Q.; Wang, Q.; Li, J.Q. Thermal Conductivity of Multiwalled Carbon Nanotubes. Phys. Rev. B 2002, 66, 165440. [Google Scholar] [CrossRef]
- Esawi, A.M.K.; Morsi, K.; Sayed, A.; Taher, M.; Lanka, S. Effect of Carbon Nanotube (CNT) Content on the Mechanical Properties of CNT-Reinforced Aluminium Composites. Compos. Sci. Technol. 2010, 70, 2237–2241. [Google Scholar] [CrossRef]
- Guo, D.J.; Li, H.L. Highly Dispersed Ag Nanoparticles on Functional MWNT Surfaces for Methanol Oxidation in Alkaline Solution. Carbon 2005, 43, 1259–1264. [Google Scholar] [CrossRef]
- Kim, B.; Sigmund, W.M. Functionalized Multiwall Carbon Nanotube/Gold Nanoparticle Composites. Langmuir 2004, 20, 8239–8242. [Google Scholar] [CrossRef]
- Ma, P.C.; Tang, B.Z.; Kim, J.K. Effect of CNT Decoration with Silver Nanoparticles on Electrical Conductivity of CNT-Polymer Composites. Carbon 2008, 46, 1497–1505. [Google Scholar] [CrossRef]
- Wang, C.; Yang, F.; Yang, W.; Ren, L.; Zhang, Y.; Jia, X.; Zhang, L.; Li, Y. PdO Nanoparticles Enhancing the Catalytic Activity of Pd/Carbon Nanotubes for 4-Nitrophenol Reduction. RSC Adv. 2015, 5, 27526–27532. [Google Scholar] [CrossRef]
- Li, C.-H.; Yu, Z.-X.; Yao, K.-F.; Ji, S.; Liang, J. Nitrobenzene Hydrogenation with Carbon Nanotube-Supported Platinum Catalyst under Mild Conditions. J. Mol. Catal. A Chem. 2005, 226, 101–105. [Google Scholar] [CrossRef]
- Liang, X.-L.; Dong, X.; Lin, G.-D.; Zhang, H.-B. Carbon Nanotube-Supported Pd–ZnO Catalyst for Hydrogenation of CO2 to Methanol. Appl. Catal. B Environ. 2009, 88, 315–322. [Google Scholar] [CrossRef]
- Matus, E.V.; Suboch, A.N.; Lisitsyn, A.S.; Svinsitskiy, D.A.; Modin, E.; Chuvilin, A.; Ismagilov, Z.R.; Podyacheva, O.Y. Beneficial Role of the Nitrogen-Doped Carbon Nanotubes in the Synthesis of the Active Palladium Supported Catalyst. Diam. Relat. Mater. 2019, 98, 107484. [Google Scholar] [CrossRef]
- Chen, P.; Khetan, A.; Yang, F.; Migunov, V.; Weide, P.; Stürmer, S.P.; Guo, P.; Kähler, K.; Xia, W.; Mayer, J.; et al. Experimental and Theoretical Understanding of Nitrogen-Doping-Induced Strong Metal-Support Interactions in Pd/TiO2 Catalysts for Nitrobenzene Hydrogenation. ACS Catal. 2017, 7, 1197–1206. [Google Scholar] [CrossRef]
- Paraknowitsch, J.P.; Thomas, A. Doping Carbons beyond Nitrogen: An Overview of Advanced Heteroatom Doped Carbons with Boron, Sulphur and Phosphorus for Energy Applications. Energy Environ. Sci. 2013, 6, 2839. [Google Scholar] [CrossRef]
- Carlier, S.; Gripekoven, J.; Philippo, M.; Hermans, S. Ru on N-Doped Carbon Supports for the Direct Hydrogenation of Cellobiose into Sorbitol. Appl. Catal. B Environ. 2020, 282, 119515. [Google Scholar] [CrossRef]
- Wang, C.; Ye, B.; Zhou, R.; Jiang, Y.; Hou, Z. Structure-Activity Relationship for the Catalytic Hydrogenation of Nitrobenzene by Single Platinum Atoms Supported on Nitrogen-Doped Carbon. ACS Appl. Nano Mater. 2022, 5, 13601–13611. [Google Scholar] [CrossRef]
- Xia, J.; Zhang, L.; Fu, Y.; He, G.; Sun, X.; Wang, X. Nitrogen-Doped Carbon Black Supported NiCo2S4 Catalyst for Hydrogenation of Nitrophenols under Mild Conditions. J. Mater. Sci. 2018, 53, 4467–4481. [Google Scholar] [CrossRef]
- Niu, J.; Ai, P.; Guo, Q.; Jin, H.; Gao, Z.; Huang, W. The Effect of Nitrogen Doping on Hydrogenation Capability and Stability of Cu-Based Catalyst in Ester Hydrogenation to Methyl Glycolate. Fuel 2023, 351, 128866. [Google Scholar] [CrossRef]
- He, L.; Wang, Y.; Wang, C.; Liu, Z.; Xie, Z. Pyridinic Nitrogen Dominated Doping on Pd/Carbon Catalysts for Enhanced Hydrogenation Performance. Front. Chem. 2022, 10, 1046058. [Google Scholar] [CrossRef]
- Akbarzadeh, P.; Koukabi, N. Magnetic Carbon Nanotube as a Highly Stable Support for the Heterogenization of InCl3 and Its Application in the Synthesis of Isochromeno[4,3-c]Pyrazole-5(1H)-One Derivatives. Appl. Organomet. Chem. 2020, 34, e5746. [Google Scholar] [CrossRef]
- Rao, H.; Fu, H.; Jiang, Y.; Zhao, Y. Easy Copper-Catalyzed Synthesis of Primary Aromatic Amines by Couplings Aromatic Boronic Acids with Aqueous Ammonia at Room Temperature. Angew. Chemie 2009, 121, 1134–1136. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, P.; Wu, J.; Shang, Z. Metal-Free C-N Bond-Forming Reaction: Straightforward Synthesis of Anilines, through Cleavage of Aryl C-O Bond and Amide C-N Bond. Tetrahedron Lett. 2013, 54, 3167–3170. [Google Scholar] [CrossRef]
- He, J.; Yang, S.; Riisager, A. Magnetic Nickel Ferrite Nanoparticles as Highly Durable Catalysts for Catalytic Transfer Hydrogenation of Bio-Based Aldehydes. Catal. Sci. Technol. 2018, 8, 790–797. [Google Scholar] [CrossRef]
- Kaur, M.; Moskvin, A.S.; Gómez García, C.J.; Maji, N.; Dosanjh, H.S. Ferrite Nanoparticles as Catalysts in Organic Reactions: A Mini Review. Magnetochemistry 2023, 9, 156. [Google Scholar] [CrossRef]
- Hajdu, V.; Varga, M.; Muránszky, G.; Karacs, G.; Kristály, F.; Fiser, B.; Viskolcz, B.; Vanyorek, L. Development of Magnetic, Ferrite Supported Palladium Catalysts for 2,4-Dinitrotoluene Hydrogenation. Mater. Today Chem. 2021, 20, 100470. [Google Scholar] [CrossRef]
- Hajdu, V.; Prekob, Á.; Muránszky, G.; Kristály, F.; Daróczi, L.; Harasztosi, L.; Kaleta, Z.; Viskolcz, B.; Nagy, M.; Vanyorek, L. Amine Functionalization Leads to Enhanced Performance for Nickel- and Cobalt-Ferrite-Supported Palladium Catalysts in Nitrobenzene Hydrogenation. Int. J. Mol. Sci. 2023, 24, 13347. [Google Scholar] [CrossRef] [PubMed]
- Toebes, M.L.; Van Dillen, J.A.; De Jong, K.P. Synthesis of Supported Palladium Catalysts. J. Mol. Catal. A Chem. 2001, 173, 75–98. [Google Scholar] [CrossRef]
- Huang, Z.; Wang, C.; Zhao, J.; Wang, S.; Zhou, Y.; Zhang, L. Adsorption Behavior of Pd(II) Ions from Aqueous Solution onto Pyromellitic Acid Modified-UiO-66-NH2. Arab. J. Chem. 2020, 13, 7007–7019. [Google Scholar] [CrossRef]
- Xing, Z.; Ju, Z.; Zhao, Y.; Wan, J.; Zhu, Y.; Qiang, Y.; Qian, Y. One-Pot Hydrothermal Synthesis of Nitrogen-Doped Graphene as High-Performance Anode Materials for Lithium Ion Batteries. Sci. Rep. 2016, 6, 1–10. [Google Scholar] [CrossRef]
- Chen, P.; Chew, L.M.; Kostka, A.; Muhler, M.; Xia, W. The Structural and Electronic Promoting Effect of Nitrogen-Doped Carbon Nanotubes on Supported Pd Nanoparticles for Selective Olefin Hydrogenation. Catal. Sci. Technol. 2013, 3, 1964–1971. [Google Scholar] [CrossRef]
- Feng, Y.; Shao, Q.; Ji, Y.; Cui, X.; Li, Y.; Zhu, X.; Huang, X. Surface-Modulated Palladium-Nickel Icosahedra as High-Performance Non-Platinum Oxygen Reduction Electrocatalysts. Sci. Adv. 2018, 4, 8817–8830. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, Q.; Li, X.; Wang, X.; Dong, S.; Li, J.; Hou, L.; Jiao, T.; Wang, Y.; Gao, F. Three-Dimensional Network Pd-Ni/γ-Al2O3Catalysts for Highly Active Catalytic Hydrogenation of Nitrobenzene to Aniline under Mild Conditions. ACS Omega 2021, 6, 9780–9790. [Google Scholar] [CrossRef] [PubMed]
- Ombaka, L.M.; Ndungu, P.G.; Nyamori, V.O. Pyrrolic Nitrogen-Doped Carbon Nanotubes: Physicochemical Properties, Interactions with Pd and Their Role in the Selective Hydrogenation of Nitrobenzophenone. RSC Adv. 2014, 5, 109–122. [Google Scholar] [CrossRef]

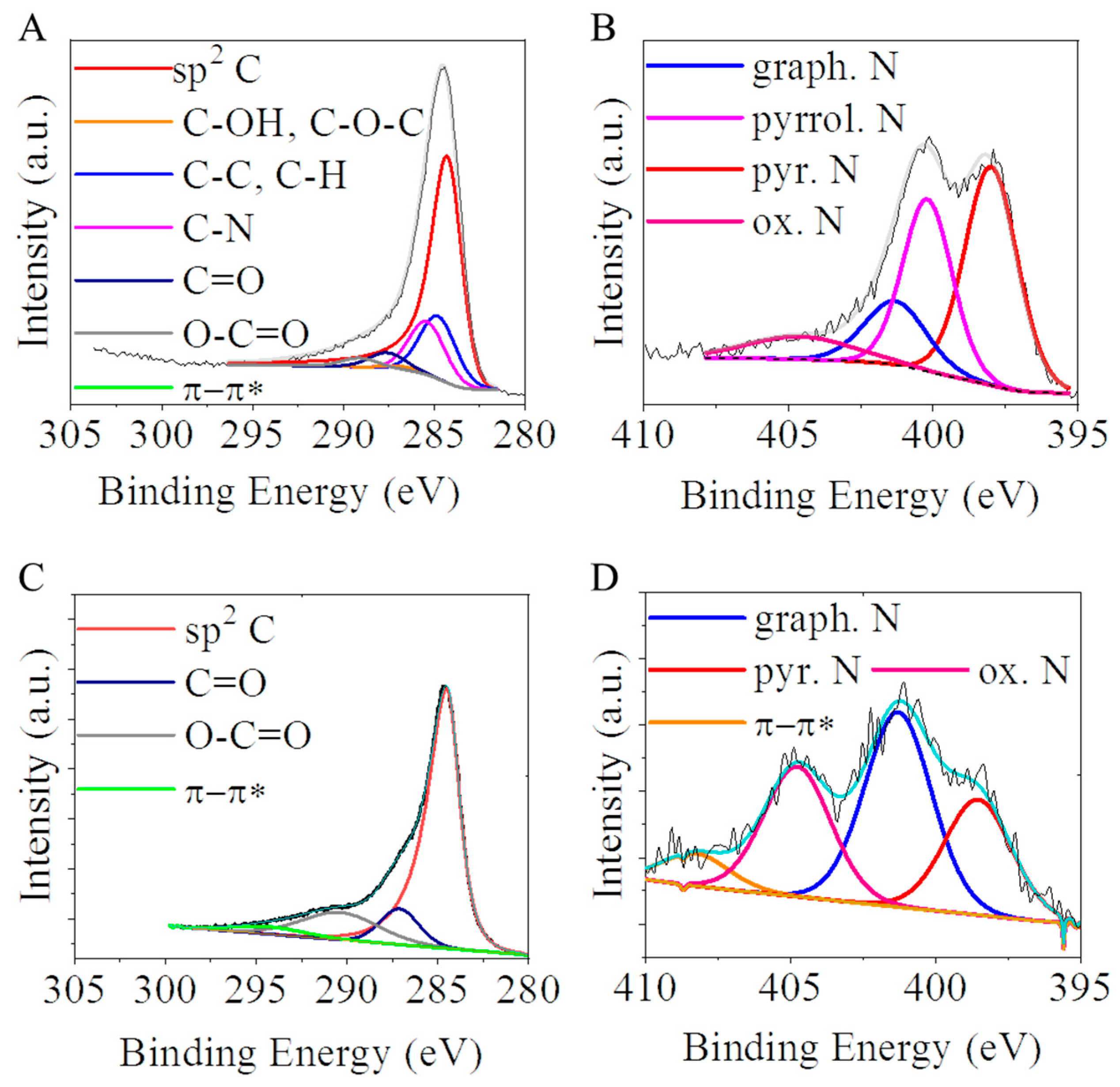

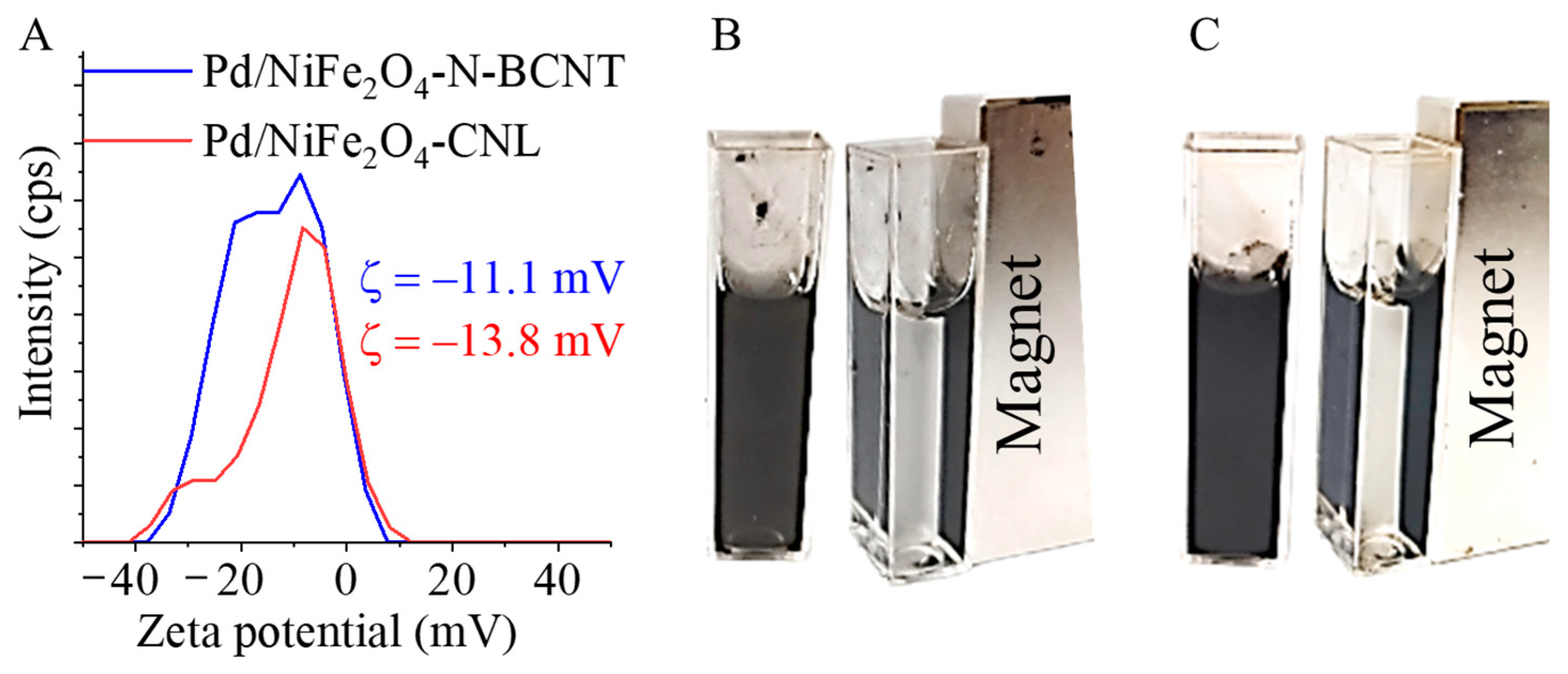
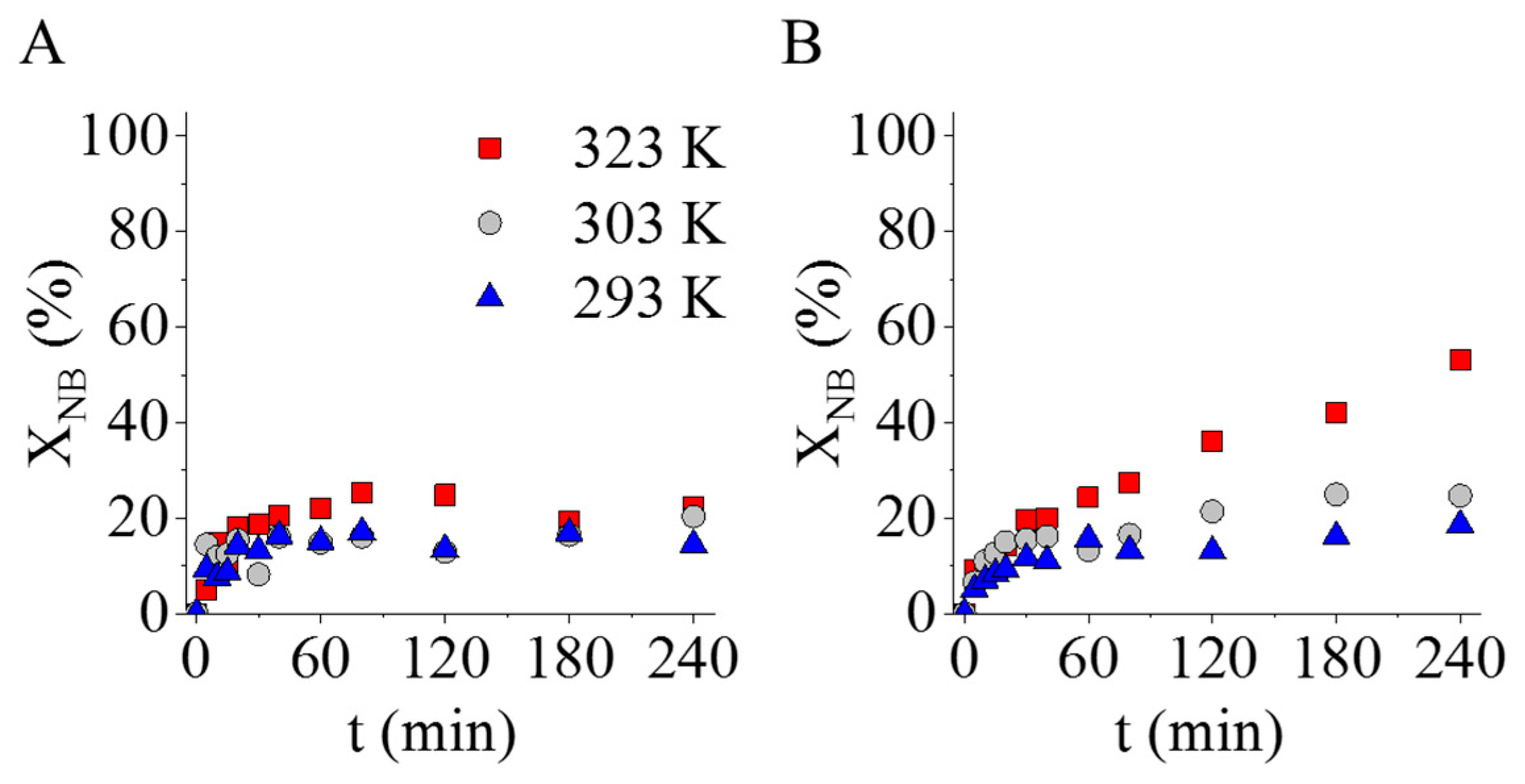


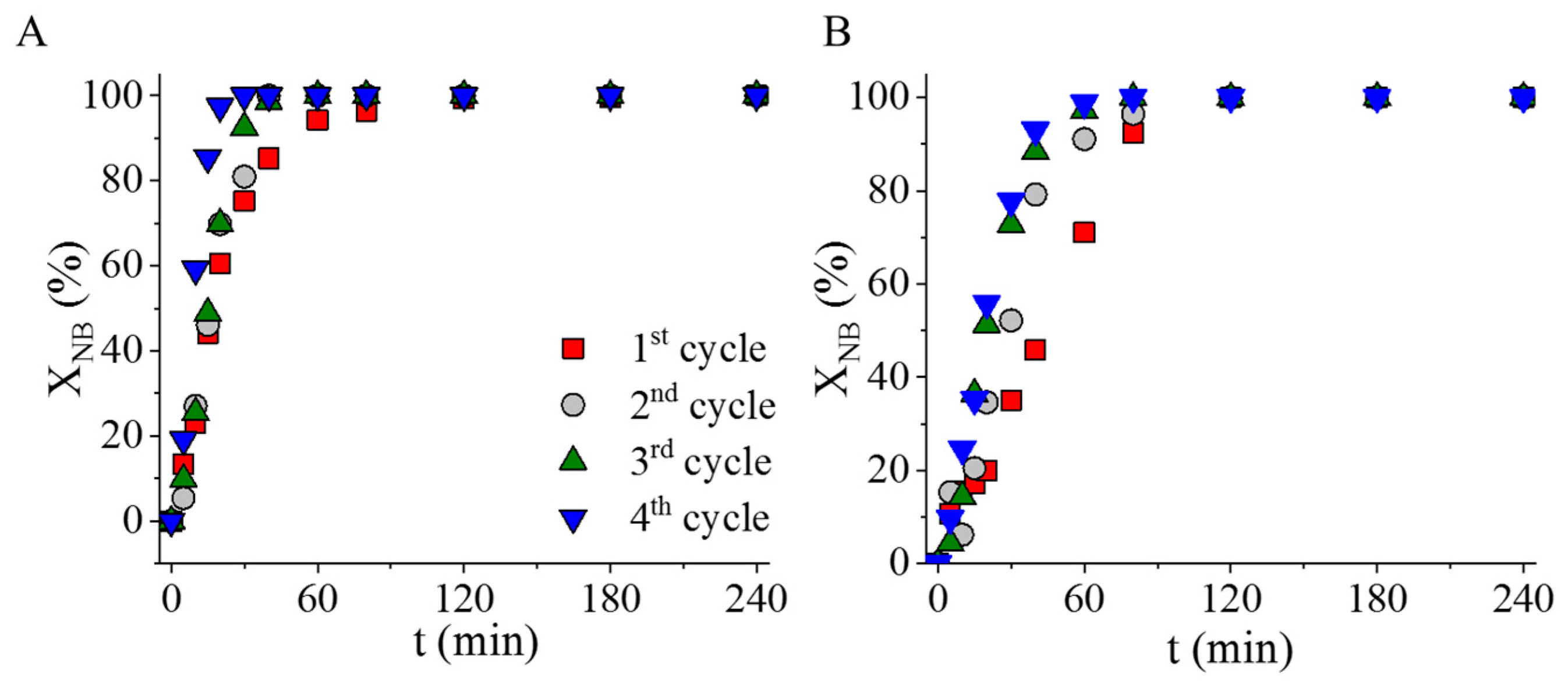
| Sample | Atomic Percentage of Nitrogen (%) | |||
|---|---|---|---|---|
| Pyridinic N | Graphitic N | Pyrrolic N | Oxidized N | |
| CNL | 43.6 | 15 | 32.2 | 9.2 |
| N-BCNT | 31.2 | 35.7 | - | 33.1 |
| wt% | NiFe2O4 | Fe3O4 | Ni | Pd |
|---|---|---|---|---|
| Pd/NiFe2O4-CNL | 13.9 | 0.3 | 0.3 | 3.98 |
| Pd/NiFe2O4-N-BCNT | 19.6 | 5.3 | 1.0 | 4.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prekob, Á.; Szegedi, M.P.; Muránszky, G.; Kristály, F.; Nagy, M.; Halasi, G.; Szamosvölgyi, Á.; Fiser, B.; Viskolcz, B.; Vanyorek, L. Development of Magnetizable, Nickel–Ferrite-Decorated Carbon Nanocomposites as Hydrogenation Catalyst for Aniline Synthesis. Int. J. Mol. Sci. 2023, 24, 17547. https://doi.org/10.3390/ijms242417547
Prekob Á, Szegedi MP, Muránszky G, Kristály F, Nagy M, Halasi G, Szamosvölgyi Á, Fiser B, Viskolcz B, Vanyorek L. Development of Magnetizable, Nickel–Ferrite-Decorated Carbon Nanocomposites as Hydrogenation Catalyst for Aniline Synthesis. International Journal of Molecular Sciences. 2023; 24(24):17547. https://doi.org/10.3390/ijms242417547
Chicago/Turabian StylePrekob, Ádám, Máté Péter Szegedi, Gábor Muránszky, Ferenc Kristály, Miklós Nagy, Gyula Halasi, Ákos Szamosvölgyi, Béla Fiser, Béla Viskolcz, and László Vanyorek. 2023. "Development of Magnetizable, Nickel–Ferrite-Decorated Carbon Nanocomposites as Hydrogenation Catalyst for Aniline Synthesis" International Journal of Molecular Sciences 24, no. 24: 17547. https://doi.org/10.3390/ijms242417547
APA StylePrekob, Á., Szegedi, M. P., Muránszky, G., Kristály, F., Nagy, M., Halasi, G., Szamosvölgyi, Á., Fiser, B., Viskolcz, B., & Vanyorek, L. (2023). Development of Magnetizable, Nickel–Ferrite-Decorated Carbon Nanocomposites as Hydrogenation Catalyst for Aniline Synthesis. International Journal of Molecular Sciences, 24(24), 17547. https://doi.org/10.3390/ijms242417547







