Role of the CB2 Cannabinoid Receptor in the Regulation of Food Intake: A Systematic Review
Abstract
:1. Introduction
1.1. The Endocannabinoid System
1.2. Cellular Mechanisms of the CB2 Receptor
2. Methods
2.1. Information Sources and Search Strategy
2.2. Data Extraction and Assessment of Risk of Bias
3. Results
3.1. Search Results
3.2. Risk of Bias Assessment
| Study | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Q10 |
|---|---|---|---|---|---|---|---|---|---|---|
| Werner 2003 [62] |  |  |  |  |  |  |  |  |  |  |
| Onaivi 2008 [63] |  |  |  |  |  |  |  |  |  |  |
| Agudo 2010 [64] |  |  |  |  |  |  |  |  |  |  |
| Ishiguro 2010 [65] |  |  |  |  |  |  |  |  |  |  |
| Ting 2015 [66] |  |  |  |  |  |  |  |  |  |  |
| Verty 2015 [58] |  |  |  |  |  |  |  |  |  |  |
| Schmitz 2016 [67] |  |  |  |  |  |  |  |  |  |  |
| Diaz-Rocha 2018 [68] |  |  |  |  |  |  |  |  |  |  |
| Alshaarawy 2019 [69] |  |  |  |  |  |  |  |  |  |  |
| Bi 2019 [70] |  |  |  |  |  |  |  |  |  |  |
| Bourdy 2021 [71] |  |  |  |  |  |  |  |  |  |  |
| De Ceglia 2023 [17] |  |  |  |  |  |  |  |  |  |  |
| Rorato 2023 [72] |  |  |  |  |  |  |  |  |  |  |
 ); high risk of bias (
); high risk of bias ( ); unclear risk of bias (
); unclear risk of bias ( ).
).3.3. The Role of the CB2 Cannabinoid Receptor in Food Intake
3.3.1. Administration of CB2 Agonists and Antagonists
3.3.2. Depletion and Expression of the CB2 Receptor
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Belanger, M.J.; Hill, M.A.; Angelidi, A.M.; Dalamaga, M.; Sowers, J.R.; Mantzoros, C.S. COVID-19 and Disparities in Nutrition and Obesity. N. Engl. J. Med. 2020, 383, e69. [Google Scholar] [CrossRef] [PubMed]
- Silventoinen, K.; Konttinen, H. Obesity and Eating Behavior from the Perspective of Twin and Genetic Research. Neurosci. Biobehav. Rev. 2020, 109, 150–165. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.A.; Stuber, G.D. Overlapping Brain Circuits for Homeostatic and Hedonic Feeding. Cell Metab. 2018, 27, 42–56. [Google Scholar] [CrossRef] [PubMed]
- Berthoud, H.-R. The Neurobiology of Food Intake in an Obesogenic Environment. Proc. Nutr. Soc. 2012, 71, 478–487. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.Y.; Gao, Z.; Liang, N.-C. Sex-Dependent Wheel Running Effects on High Fat Diet Preference, Metabolic Outcomes, and Performance on the Barnes Maze in Rats. Nutrients 2020, 12, 2721. [Google Scholar] [CrossRef]
- Guegan, T.; Cutando, L.; Gangarossa, G.; Santini, E.; Fisone, G.; Martinez, A.; Valjent, E.; Maldonado, R.; Martin, M. Operant Behavior to Obtain Palatable Food Modifies ERK Activity in the Brain Reward Circuit. Eur. Neuropsychopharmacol. 2013, 23, 240–252. [Google Scholar] [CrossRef] [PubMed]
- Sallam, N.A.; Borgland, S.L. Insulin and Endocannabinoids in the Mesolimbic System. J. Neuroendocrinol. 2021, 33, e12965. [Google Scholar] [CrossRef]
- Woodward, O.R.M.; Gribble, F.M.; Reimann, F.; Lewis, J.E. Gut Peptide Regulation of Food Intake—Evidence for the Modulation of Hedonic Feeding. J. Physiol. 2022, 600, 1053–1078. [Google Scholar] [CrossRef]
- Bongers, P.; van de Giessen, E.; Roefs, A.; Nederkoorn, C.; Booij, J.; van den Brink, W.; Jansen, A. Being Impulsive and Obese Increases Susceptibility to Speeded Detection of High-Calorie Foods. Health Psychol. 2015, 34, 677–685. [Google Scholar] [CrossRef]
- Satta, V.; Scherma, M.; Giunti, E.; Collu, R.; Fattore, L.; Fratta, W.; Fadda, P. Emotional Profile of Female Rats Showing Binge Eating Behavior. Physiol. Behav. 2016, 163, 136–143. [Google Scholar] [CrossRef]
- Camacho, A.; Montalvo-Martinez, L.; Cardenas-Perez, R.E.; Fuentes-Mera, L.; Garza-Ocañas, L. Obesogenic Diet Intake during Pregnancy Programs Aberrant Synaptic Plasticity and Addiction-like Behavior to a Palatable Food in Offspring. Behav. Brain Res. 2017, 330, 46–55. [Google Scholar] [CrossRef]
- Kantonen, T.; Karjalainen, T.; Pekkarinen, L.; Isojärvi, J.; Kalliokoski, K.; Kaasinen, V.; Hirvonen, J.; Nuutila, P.; Nummenmaa, L. Cerebral μ-Opioid and CB1 Receptor Systems Have Distinct Roles in Human Feeding Behavior. Transl. Psychiatry 2021, 11, 442. [Google Scholar] [CrossRef] [PubMed]
- Calcaterra, V.; Cena, H.; Rossi, V.; Santero, S.; Bianchi, A.; Zuccotti, G. Ultra-Processed Food, Reward System and Childhood Obesity. Children 2023, 10, 804. [Google Scholar] [CrossRef] [PubMed]
- Geisler, C.S.; Hayes, M.R. Metabolic Hormone Action in the VTA: Reward-Directed Behavior and Mechanistic Insights. Physiol. Behav. 2023, 268, 114236. [Google Scholar] [CrossRef]
- Volkow, N.D.; Wise, R.A. How Can Drug Addiction Help Us Understand Obesity? Nat. Neurosci. 2005, 8, 555–560. [Google Scholar] [CrossRef]
- Kringelbach, M.L.; Stein, A.; van Hartevelt, T.J. The Functional Human Neuroanatomy of Food Pleasure Cycles. Physiol. Behav. 2012, 106, 307–316. [Google Scholar] [CrossRef] [PubMed]
- de Ceglia, M.; Micioni Di Bonaventura, M.V.; Romano, A.; Friuli, M.; Micioni Di Bonaventura, E.; Gavito, A.L.; Botticelli, L.; Gaetani, S.; de Fonseca, F.R.; Cifani, C. Anxiety Associated with Palatable Food Withdrawal Is Reversed by the Selective FAAH Inhibitor PF-3845: A Regional Analysis of the Contribution of Endocannabinoid Signaling Machinery. Int. J. Eat. Disord. 2023, 56, 1098–1113. [Google Scholar] [CrossRef]
- Lau, B.K.; Cota, D.; Cristino, L.; Borgland, S.L. Endocannabinoid Modulation of Homeostatic and Non-Homeostatic Feeding Circuits. Neuropharmacology 2017, 124, 38–51. [Google Scholar] [CrossRef]
- de Sa Nogueira, D.; Bourdy, R.; Filliol, D.; Awad, G.; Andry, V.; Goumon, Y.; Olmstead, M.C.; Befort, K. Binge Sucrose-Induced Neuroadaptations: A Focus on the Endocannabinoid System. Appetite 2021, 164, 105258. [Google Scholar] [CrossRef]
- Ceccarini, J.; Weltens, N.; Ly, H.G.; Tack, J.; Van Oudenhove, L.; Van Laere, K. Association between Cerebral Cannabinoid 1 Receptor Availability and Body Mass Index in Patients with Food Intake Disorders and Healthy Subjects: A [(18)F]MK-9470 PET Study. Transl. Psychiatry 2016, 6, e853. [Google Scholar] [CrossRef]
- Bermudez-Silva, F.J.; Viveros, M.P.; McPartland, J.M.; Rodriguez de Fonseca, F. The Endocannabinoid System, Eating Behavior and Energy Homeostasis: The End or a New Beginning? Pharmacol. Biochem. Behav. 2010, 95, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Escartín Pérez, R.E.; Mancilla Díaz, J.M.; Cortés Salazar, F.; López Alonso, V.E.; Florán Garduño, B. CB1/5-HT/GABA Interactions and Food Intake Regulation. Prog. Brain Res. 2021, 259, 177–196. [Google Scholar] [CrossRef] [PubMed]
- Bello, N.T.; Coughlin, J.W.; Redgrave, G.W.; Ladenheim, E.E.; Moran, T.H.; Guarda, A.S. Dietary Conditions and Highly Palatable Food Access Alter Rat Cannabinoid Receptor Expression and Binding Density. Physiol. Behav. 2012, 105, 720–726. [Google Scholar] [CrossRef] [PubMed]
- Cristino, L.; Bisogno, T.; Di Marzo, V. Cannabinoids and the Expanded Endocannabinoid System in Neurological Disorders. Nat. Rev. Neurol. 2020, 16, 9–29. [Google Scholar] [CrossRef] [PubMed]
- Netzahualcoyotzi, C.; Rodríguez-Serrano, L.M.; Chávez-Hernández, M.E.; Buenrostro-Jáuregui, M.H. Early Consumption of Cannabinoids: From Adult Neurogenesis to Behavior. Int. J. Mol. Sci. 2021, 22, 7450. [Google Scholar] [CrossRef] [PubMed]
- Devane, W.A.; Dysarz, F.A.; Johnson, M.R.; Melvin, L.S.; Howlett, A.C. Determination and Characterization of a Cannabinoid Receptor in Rat Brain. Mol. Pharmacol. 1988, 34, 605–613. [Google Scholar]
- Sugiura, T.; Kondo, S.; Sukagawa, A.; Nakane, S.; Shinoda, A.; Itoh, K.; Yamashita, A.; Waku, K. 2-Arachidonoylglycerol: A Possible Endogenous Cannabinoid Receptor Ligand in Brain. Biochem. Biophys. Res. Commun. 1995, 215, 89–97. [Google Scholar] [CrossRef]
- Rasooli-Nejad, S.; Palygin, O.; Lalo, U.; Pankratov, Y. Cannabinoid Receptors Contribute to Astroglial Ca2+-Signalling and Control of Synaptic Plasticity in the Neocortex. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014, 369, 20140077. [Google Scholar] [CrossRef]
- Gandhi, K.; Montoya-Uribe, V.; Martinez, S.; David, S.; Jain, B.; Shim, G.; Li, C.; Jenkins, S.; Nathanielsz, P.; Schlabritz-Loutsevitch, N. Ontogeny and Programming of the Fetal Temporal Cortical Endocannabinoid System by Moderate Maternal Nutrient Reduction in Baboons (Papio Spp.). Physiol. Rep. 2019, 7, e14024. [Google Scholar] [CrossRef]
- Baggelaar, M.P.; Maccarrone, M.; van der Stelt, M. 2-Arachidonoylglycerol: A Signaling Lipid with Manifold Actions in the Brain. Prog. Lipid Res. 2018, 71, 1–17. [Google Scholar] [CrossRef]
- Munro, S.; Thomas, K.L.; Abu-Shaar, M. Molecular Characterization of a Peripheral Receptor for Cannabinoids. Nature 1993, 365, 61–65. [Google Scholar] [CrossRef]
- Onaivi, E.S.; Ishiguro, H.; Gong, J.-P.; Patel, S.; Perchuk, A.; Meozzi, P.A.; Myers, L.; Mora, Z.; Tagliaferro, P.; Gardner, E.; et al. Discovery of the Presence and Functional Expression of Cannabinoid CB2 Receptors in Brain. Ann. N. Y. Acad. Sci. 2006, 1074, 514–536. [Google Scholar] [CrossRef] [PubMed]
- Mechoulam, R.; Parker, L.A. The Endocannabinoid System and the Brain. Annu. Rev. Psychol. 2013, 64, 21–47. [Google Scholar] [CrossRef]
- Gong, J.-P.; Onaivi, E.S.; Ishiguro, H.; Liu, Q.-R.; Tagliaferro, P.A.; Brusco, A.; Uhl, G.R. Cannabinoid CB2 Receptors: Immunohistochemical Localization in Rat Brain. Brain Res. 2006, 1071, 10–23. [Google Scholar] [CrossRef]
- Li, Y.; Kim, J. Neuronal Expression of CB2 Cannabinoid Receptor mRNAs in the Mouse Hippocampus. Neuroscience 2015, 311, 253–267. [Google Scholar] [CrossRef] [PubMed]
- Van Sickle, M.D.; Duncan, M.; Kingsley, P.J.; Mouihate, A.; Urbani, P.; Mackie, K.; Stella, N.; Makriyannis, A.; Piomelli, D.; Davison, J.S.; et al. Identification and Functional Characterization of Brainstem Cannabinoid CB2 Receptors. Science 2005, 310, 329–332. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-Y.; Gao, M.; Liu, Q.-R.; Bi, G.-H.; Li, X.; Yang, H.-J.; Gardner, E.L.; Wu, J.; Xi, Z.-X. Cannabinoid CB2 Receptors Modulate Midbrain Dopamine Neuronal Activity and Dopamine-Related Behavior in Mice. Proc. Natl. Acad. Sci. USA 2014, 111, E5007–E5015. [Google Scholar] [CrossRef] [PubMed]
- Cabral, G.A.; Raborn, E.S.; Griffin, L.; Dennis, J.; Marciano-Cabral, F. CB2 Receptors in the Brain: Role in Central Immune Function. Br. J. Pharmacol. 2008, 153, 240–251. [Google Scholar] [CrossRef]
- Navarrete, F.; García-Gutiérrez, M.S.; Aracil-Fernández, A.; Lanciego, J.L.; Manzanares, J. Cannabinoid CB1 and CB2 Receptors, and Monoacylglycerol Lipase Gene Expression Alterations in the Basal Ganglia of Patients with Parkinson’s Disease. Neurotherapeutics 2018, 15, 459–469. [Google Scholar] [CrossRef]
- Basavarajappa, B.S. Chapter 2—Cannabinoid Receptors and Their Signaling Mechanisms. In The Endocannabinoid System; Murillo-Rodríguez, E., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 25–62. ISBN 978-0-12-809666-6. [Google Scholar]
- Pertwee, R.G.; Howlett, A.C.; Abood, M.E.; Alexander, S.P.H.; Di Marzo, V.; Elphick, M.R.; Greasley, P.J.; Hansen, H.S.; Kunos, G.; Mackie, K.; et al. International Union of Basic and Clinical Pharmacology. LXXIX. Cannabinoid Receptors and Their Ligands: Beyond CB1 and CB2. Pharmacol. Rev. 2010, 62, 588–631. [Google Scholar] [CrossRef]
- Compagnucci, C.; Di Siena, S.; Bustamante, M.B.; Di Giacomo, D.; Di Tommaso, M.; Maccarrone, M.; Grimaldi, P.; Sette, C. Type-1 (CB1) Cannabinoid Receptor Promotes Neuronal Differentiation and Maturation of Neural Stem Cells. PLoS ONE 2013, 8, e54271. [Google Scholar] [CrossRef]
- Brusco, A.; Tagliaferro, P.; Saez, T.; Onaivi, E.S. Postsynaptic Localization of CB2 Cannabinoid Receptors in the Rat Hippocampus. Synapse 2008, 62, 944–949. [Google Scholar] [CrossRef]
- Wu, Q.; Ma, Y.; Liu, Y.; Wang, N.; Zhao, X.; Wen, D. CB2R Agonist JWH-133 Attenuates Chronic Inflammation by Restraining M1 Macrophage Polarization via Nrf2/HO-1 Pathway in Diet-Induced Obese Mice. Life Sci. 2020, 260, 118424. [Google Scholar] [CrossRef]
- Jordan, C.J.; Xi, Z.-X. Progress in Brain Cannabinoid CB2 Receptor Research: From Genes to Behavior. Neurosci. Biobehav. Rev. 2019, 98, 208–220. [Google Scholar] [CrossRef] [PubMed]
- Scherma, M.; Masia, P.; Satta, V.; Fratta, W.; Fadda, P.; Tanda, G. Brain Activity of Anandamide: A Rewarding Bliss? Acta Pharmacol. Sin. 2019, 40, 309–323. [Google Scholar] [CrossRef] [PubMed]
- Ferrisi, R.; Ceni, C.; Bertini, S.; Macchia, M.; Manera, C.; Gado, F. Medicinal Chemistry Approach, Pharmacology and Neuroprotective Benefits of CB2R Modulators in Neurodegenerative Diseases. Pharmacol. Res. 2021, 170, 105607. [Google Scholar] [CrossRef]
- Horn, H.; Böhme, B.; Dietrich, L.; Koch, M. Endocannabinoids in Body Weight Control. Pharmaceuticals 2018, 11, 55. [Google Scholar] [CrossRef] [PubMed]
- Jager, G.; Witkamp, R.F. The Endocannabinoid System and Appetite: Relevance for Food Reward. Nutr. Res. Rev. 2014, 27, 172–185. [Google Scholar] [CrossRef]
- Atwood, B.K.; Mackie, K. CB2: A Cannabinoid Receptor with an Identity Crisis. Br. J. Pharmacol. 2010, 160, 467–479. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.-R.; Canseco-Alba, A.; Zhang, H.-Y.; Tagliaferro, P.; Chung, M.; Dennis, E.; Sanabria, B.; Schanz, N.; Escosteguy-Neto, J.C.; Ishiguro, H.; et al. Cannabinoid Type 2 Receptors in Dopamine Neurons Inhibits Psychomotor Behaviors, Alters Anxiety, Depression and Alcohol Preference. Sci. Rep. 2017, 7, 17410. [Google Scholar] [CrossRef]
- Zhang, H.-Y.; Bi, G.-H.; Li, X.; Li, J.; Qu, H.; Zhang, S.-J.; Li, C.-Y.; Onaivi, E.S.; Gardner, E.L.; Xi, Z.-X.; et al. Species Differences in Cannabinoid Receptor 2 and Receptor Responses to Cocaine Self-Administration in Mice and Rats. Neuropsychopharmacology 2015, 40, 1037–1051. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-Y.; Gao, M.; Shen, H.; Bi, G.-H.; Yang, H.-J.; Liu, Q.-R.; Wu, J.; Gardner, E.L.; Bonci, A.; Xi, Z.-X. Expression of Functional Cannabinoid CB2 Receptor in VTA Dopamine Neurons in Rats. Addict. Biol. 2017, 22, 752–765. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Gao, F.; Larsen, B.; Gao, M.; Luo, Z.; Chen, D.; Ma, X.; Qiu, S.; Zhou, Y.; Xie, J.; et al. Mechanisms of Cannabinoid CB2 Receptor-Mediated Reduction of Dopamine Neuronal Excitability in Mouse Ventral Tegmental Area. EBioMedicine 2019, 42, 225–237. [Google Scholar] [CrossRef]
- Franklin, J.M.; Broseguini de Souza, R.K.; Carrasco, G.A. Cannabinoid 2 Receptors Regulate Dopamine 2 Receptor Expression by a Beta-Arrestin 2 and GRK5-Dependent Mechanism in Neuronal Cells. Neurosci. Lett. 2021, 753, 135883. [Google Scholar] [CrossRef]
- López-Ramírez, G.; Sánchez-Zavaleta, R.; Ávalos-Fuentes, A.; José Sierra, J.; Paz-Bermúdez, F.; Leyva-Gómez, G.; Segovia Vila, J.; Cortés, H.; Florán, B. D2 Autoreceptor Switches CB2 Receptor Effects on [3 H]-Dopamine Release in the Striatum. Synapse 2020, 74, e22139. [Google Scholar] [CrossRef] [PubMed]
- Stempel, A.V.; Stumpf, A.; Zhang, H.-Y.; Özdoğan, T.; Pannasch, U.; Theis, A.-K.; Otte, D.-M.; Wojtalla, A.; Rácz, I.; Ponomarenko, A.; et al. Cannabinoid Type 2 Receptors Mediate a Cell Type-Specific Plasticity in the Hippocampus. Neuron 2016, 90, 795–809. [Google Scholar] [CrossRef] [PubMed]
- Verty, A.N.A.; Stefanidis, A.; McAinch, A.J.; Hryciw, D.H.; Oldfield, B. Anti-Obesity Effect of the CB2 Receptor Agonist JWH-015 in Diet-Induced Obese Mice. PLoS ONE 2015, 10, e0140592. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Urrútia, G.; Bonfill, X. [PRISMA declaration: A proposal to improve the publication of systematic reviews and meta-analyses]. Med. Clin. 2010, 135, 507–511. [Google Scholar] [CrossRef]
- Hooijmans, C.R.; Rovers, M.M.; de Vries, R.B.M.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s Risk of Bias Tool for Animal Studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef]
- Werner, N.A.; Koch, J.E. Effects of the Cannabinoid Antagonists AM281 and AM630 on Deprivation-Induced Intake in Lewis Rats. Brain Res. 2003, 967, 290–292. [Google Scholar] [CrossRef]
- Onaivi, E.S.; Carpio, O.; Ishiguro, H.; Schanz, N.; Uhl, G.R.; Benno, R. Behavioral Effects of CB2 Cannabinoid Receptor Activation and Its Influence on Food and Alcohol Consumption. Ann. N. Y. Acad. Sci. 2008, 1139, 426–433. [Google Scholar] [CrossRef]
- Agudo, J.; Martin, M.; Roca, C.; Molas, M.; Bura, A.S.; Zimmer, A.; Bosch, F.; Maldonado, R. Deficiency of CB2 Cannabinoid Receptor in Mice Improves Insulin Sensitivity but Increases Food Intake and Obesity with Age. Diabetologia 2010, 53, 2629–2640. [Google Scholar] [CrossRef]
- Ishiguro, H.; Carpio, O.; Horiuchi, Y.; Shu, A.; Higuchi, S.; Schanz, N.; Benno, R.; Arinami, T.; Onaivi, E.S. A Nonsynonymous Polymorphism in Cannabinoid CB2 Receptor Gene Is Associated with Eating Disorders in Humans and Food Intake Is Modified in Mice by Its Ligands. Synapse 2010, 64, 92–96. [Google Scholar] [CrossRef]
- Ting, C.-H.; Chi, C.-W.; Li, C.-P.; Chen, C.-Y. Differential Modulation of Endogenous Cannabinoid CB1 and CB2 Receptors in Spontaneous and Splice Variants of Ghrelin-Induced Food Intake in Conscious Rats. Nutrition 2015, 31, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, K.; Mangels, N.; Häussler, A.; Ferreirós, N.; Fleming, I.; Tegeder, I. Pro-Inflammatory Obesity in Aged Cannabinoid-2 Receptor-Deficient Mice. Int. J. Obes. 2016, 40, 366–379. [Google Scholar] [CrossRef] [PubMed]
- Dias-Rocha, C.P.; Almeida, M.M.; Santana, E.M.; Costa, J.C.B.; Franco, J.G.; Pazos-Moura, C.C.; Trevenzoli, I.H. Maternal High-Fat Diet Induces Sex-Specific Endocannabinoid System Changes in Newborn Rats and Programs Adiposity, Energy Expenditure and Food Preference in Adulthood. J. Nutr. Biochem. 2018, 51, 56–68. [Google Scholar] [CrossRef] [PubMed]
- Alshaarawy, O.; Kurjan, E.; Truong, N.; Olson, L.K. Diet-Induced Obesity in Cannabinoid-2 Receptor Knockout Mice and Cannabinoid Receptor 1/2 Double-Knockout Mice. Obesity 2019, 27, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Bi, G.-H.; Galaj, E.; He, Y.; Xi, Z.-X. Cannabidiol Inhibits Sucrose Self-Administration by CB1 and CB2 Receptor Mechanisms in Rodents. Addict. Biol. 2020, 25, e12783. [Google Scholar] [CrossRef] [PubMed]
- Bourdy, R.; Hertz, A.; Filliol, D.; Andry, V.; Goumon, Y.; Mendoza, J.; Olmstead, M.C.; Befort, K. The Endocannabinoid System Is Modulated in Reward and Homeostatic Brain Regions Following Diet-Induced Obesity in Rats: A Cluster Analysis Approach. Eur. J. Nutr. 2021, 60, 4621–4633. [Google Scholar] [CrossRef] [PubMed]
- Rorato, R.; Ferreira, N.L.; Oliveira, F.P.; Fideles, H.J.; Camilo, T.A.; Antunes-Rodrigues, J.; Mecawi, A.S.; Elias, L.L.K. Prolonged Activation of Brain CB2 Signaling Modulates Hypothalamic Microgliosis and Astrogliosis in High Fat Diet-Fed Mice. Int. J. Mol. Sci. 2022, 23, 5527. [Google Scholar] [CrossRef]
- Soethoudt, M.; Grether, U.; Fingerle, J.; Grim, T.W.; Fezza, F.; de Petrocellis, L.; Ullmer, C.; Rothenhäusler, B.; Perret, C.; van Gils, N.; et al. Cannabinoid CB2 Receptor Ligand Profiling Reveals Biased Signalling and Off-Target Activity. Nat. Commun. 2017, 8, 13958. [Google Scholar] [CrossRef]
- Carruthers, E.R.; Grimsey, N.L. Cannabinoid CB2 Receptor Orthologues; in Vitro Function and Perspectives for Preclinical to Clinical Translation. Br. J. Pharmacol. 2023. [Google Scholar] [CrossRef]
- Miljuš, T.; Heydenreich, F.M.; Gazzi, T.; Kimbara, A.; Rogers-Evans, M.; Nettekoven, M.; Zirwes, E.; Osterwald, A.; Rufer, A.C.; Ullmer, C.; et al. Diverse Chemotypes Drive Biased Signaling by Cannabinoid Receptors. bioRxiv 2020. bioRxiv:2020.11.09.375162. [Google Scholar]
- Navarrete, F.; García-Gutiérrez, M.S.; Gasparyan, A.; Navarro, D.; Manzanares, J. CB2 Receptor Involvement in the Treatment of Substance Use Disorders. Biomolecules 2021, 11, 1556. [Google Scholar] [CrossRef]
- Satta, V.; Scherma, M.; Piscitelli, F.; Usai, P.; Castelli, M.P.; Bisogno, T.; Fratta, W.; Fadda, P. Limited Access to a High Fat Diet Alters Endocannabinoid Tone in Female Rats. Front. Neurosci. 2018, 12, 40. [Google Scholar] [CrossRef]
- Flake, N.M.; Zweifel, L.S. Behavioral Effects of Pulp Exposure in Mice Lacking Cannabinoid Receptor 2. J. Endod. 2012, 38, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Romero-Zerbo, S.Y.; Garcia-Gutierrez, M.S.; Suárez, J.; Rivera, P.; Ruz-Maldonado, I.; Vida, M.; Rodriguez de Fonseca, F.; Manzanares, J.; Bermúdez-Silva, F.J. Overexpression of Cannabinoid CB2 Receptor in the Brain Induces Hyperglycaemia and a Lean Phenotype in Adult Mice. J. Neuroendocrinol. 2012, 24, 1106–1119. [Google Scholar] [CrossRef] [PubMed]
- Lillo, J.; Lillo, A.; Zafra, D.A.; Miralpeix, C.; Rivas-Santisteban, R.; Casals, N.; Navarro, G.; Franco, R. Identification of the Ghrelin and Cannabinoid CB2 Receptor Heteromer Functionality and Marked Upregulation in Striatal Neurons from Offspring of Mice under a High-Fat Diet. Int. J. Mol. Sci. 2021, 22, 8928. [Google Scholar] [CrossRef] [PubMed]
- García-Gutiérrez, M.S.; Navarrete, F.; Gasparyan, A.; Navarro, D.; Morcuende, Á.; Femenía, T.; Manzanares, J. Role of Cannabinoid CB2 Receptor in Alcohol Use Disorders: From Animal to Human Studies. Int. J. Mol. Sci. 2022, 23, 5908. [Google Scholar] [CrossRef] [PubMed]
- Hashiesh, H.M.; Sharma, C.; Goyal, S.N.; Jha, N.K.; Ojha, S. Pharmacological Properties, Therapeutic Potential and Molecular Mechanisms of JWH133, a CB2 Receptor-Selective Agonist. Front. Pharmacol. 2021, 12, 702675. [Google Scholar] [CrossRef] [PubMed]
- Bermudez-Silva, F.J.; Sanchez-Vera, I.; Suárez, J.; Serrano, A.; Fuentes, E.; Juan-Pico, P.; Nadal, A.; Rodríguez de Fonseca, F. Role of Cannabinoid CB2 Receptors in Glucose Homeostasis in Rats. Eur. J. Pharmacol. 2007, 565, 207–211. [Google Scholar] [CrossRef] [PubMed]
- O’Keefe, L.; Vu, T.; Simcocks, A.C.; Jenkin, K.A.; Mathai, M.L.; McAinch, A.J.; Hutchinson, D.S.; Hryciw, D.H. Treatment of Diet-Induced Obese Rats with CB2 Agonist AM1241 or CB2 Antagonist AM630 Reduces Leptin and Alters Thermogenic mRNA in Adipose Tissue. Int. J. Mol. Sci. 2023, 24, 7601. [Google Scholar] [CrossRef]
- García-Blanco, A.; Ramírez-López, Á.; Navarrete, F.; García-Gutiérrez, M.S.; Manzanares, J.; Martín-García, E.; Maldonado, R. Role of CB2 Cannabinoid Receptor in the Development of Food Addiction in Male Mice. Neurobiol. Dis. 2023, 179, 106034. [Google Scholar] [CrossRef]
- Rossi, F.; Bellini, G.; Luongo, L.; Manzo, I.; Tolone, S.; Tortora, C.; Bernardo, M.E.; Grandone, A.; Conforti, A.; Docimo, L.; et al. Cannabinoid Receptor 2 as Antiobesity Target: Inflammation, Fat Storage, and Browning Modulation. J. Clin. Endocrinol. Metab. 2016, 101, 3469–3478. [Google Scholar] [CrossRef]
- Marchalant, Y.; Brownjohn, P.W.; Bonnet, A.; Kleffmann, T.; Ashton, J.C. Validating Antibodies to the Cannabinoid CB2 Receptor: Antibody Sensitivity Is Not Evidence of Antibody Specificity. J. Histochem. Cytochem. 2014, 62, 395–404. [Google Scholar] [CrossRef] [PubMed]

| Study | Animal Model Characteristics | Treatment | Results |
|---|---|---|---|
| Werner 2003 [62] | Male Lewis rats (220–240 g) | Administration (i.c.v.) of antagonist (AM630) at different doses: 2.5, 5, 10, and 25 µg | ↑ Food intake (chow) |
| Onaivi 2008 [63] | Male mice: C57Bl/6J, Balb/c, and DBA/2 strains | Acute administration (i.p.) of antagonist (AM630) at 10 mg/kg | The C57BL/6 mice and DBA/s mice ↓ food intake (chow) The Balb/c mice = food intake (chow) |
| Agudo 2010 [64] | Male knockout mice (Cb2−/−) and wild-type littermates (Cb2+/+); ages: 2, 6, and 12 months | Wild-type received chronic administration (i.p.) of antagonist (SR 144528) at 3 mg/kg | CB2R knockout mice ↑ food intake (chow) Wild-type mice = food intake (chow) |
| Ishiguro 2010 [65] | C57Bl/6J male and female mice | Acute administration (i.p.) of antagonist (AM630) at 10 mg/kg | ↑ Food intake (chow) |
| Ting 2015 [66] | Male Sprague Dawley rats (240 to 310 g) | Acute administration (i.p.) of antagonist (AM630) at different doses: 0.3, 1, and 3 mg/kg | ↑ Food intake (chow) |
| Verty 2015 [58] | Male C57BL/6 mice (8 weeks old) | Acute administration (i.p.) of agonist (JWH-015) at different doses: 1.0, 5.0, or 10.0 mg/kg. Co-administered of agonist (JWH-015, 10 mg/kg) with antagonist (AM630, 5 mg/kg) | ↓ Food intake (chow) with administration of JWH-015 (10 mg/kg) = food intake (chow) when co-administered agonist and antagonist |
| Schmitz 2016 [67] | Male and female knockout mice (Cb2−/−) (1.2–1.8 years old) | CB2 receptor deficiency to study age-associated obesity | ↓ Food intake (chow) CB2−/− mice became obese. |
| Dias-Rocha 2018 [68] | Male and female Wistar rats (170 days old) | Maternal high-fat (HF) diet to study effects in rat offspring | ↑ Food preference (high-fat diet) in males and females Females ↑ expression of CB2 receptor |
| Alshaarawy 2019 [69] | Male knockout mice (Cb2−/−) (8 weeks old) | 12 weeks on low-fat or high-fat diet | ↑ Weight gain on high-fat diet, but not different from wild-type mice = Food intake (high-fat diet) |
| Bi 2019 [70] | Male knockout mice (Cb2−/−) (8 to 14 weeks old) | Acute administration (i.p.) of agonist (JWH-015) at different doses: 10 and 20 mg/ kg | ↓ Sucrose self-administration in wild-type mice, but not knockout mice, with doses of 10 and 20 mg/ kg |
| Bourdy 2021 [71] | Male Wistar rats (200 ± 10 g) | 6 weeks of free-choice regimen of high-fat or sugar diet | ↑ Expression of CB2 receptor in NAC due to high sucrose |
| De Ceglia 2023 [17] | Adult male Wistar rats (280–300 g) | 40 days of exposure to palatable cafeteria diet | ↑ Expression of CB2 receptor in prefrontal cortex |
| Rorato 2023 [72] | Male C57BL mice (7–8 weeks old) | 56 days of exposure to high-fat diet. Chronic administration (i.c.v.) of agonist (HU308) at different doses: 1.0, 5.0, or 10.0 mg/kg | = Food intake (high-fat diet) |
| Ligand | Structure | Action Mechanism | pKi on CB2R | CB2R Selectivity * |
|---|---|---|---|---|
| HU 308 | 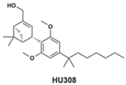 | CB2-selective agonist | 7.15 [73] 7.0 [74] | 12 [73] |
| JWH-015 |  | Moderately CB2-selective agonist | 6.63 [73] 6.5 [74] | 5 [73] |
| AM 630 | 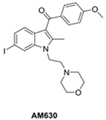 | CB2-selective inverse agonist | 7.66 [73] 7.7 [74] | 115 [73] |
| SR144528 | 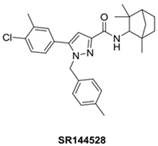 | CB2-selective inverse agonist | 10.7 [73,75] 10.5 [74] | 6026 [73] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Serrano, L.M.; Chávez-Hernández, M.E. Role of the CB2 Cannabinoid Receptor in the Regulation of Food Intake: A Systematic Review. Int. J. Mol. Sci. 2023, 24, 17516. https://doi.org/10.3390/ijms242417516
Rodríguez-Serrano LM, Chávez-Hernández ME. Role of the CB2 Cannabinoid Receptor in the Regulation of Food Intake: A Systematic Review. International Journal of Molecular Sciences. 2023; 24(24):17516. https://doi.org/10.3390/ijms242417516
Chicago/Turabian StyleRodríguez-Serrano, Luis Miguel, and María Elena Chávez-Hernández. 2023. "Role of the CB2 Cannabinoid Receptor in the Regulation of Food Intake: A Systematic Review" International Journal of Molecular Sciences 24, no. 24: 17516. https://doi.org/10.3390/ijms242417516
APA StyleRodríguez-Serrano, L. M., & Chávez-Hernández, M. E. (2023). Role of the CB2 Cannabinoid Receptor in the Regulation of Food Intake: A Systematic Review. International Journal of Molecular Sciences, 24(24), 17516. https://doi.org/10.3390/ijms242417516






