Tonabersat Significantly Reduces Disease Progression in an Experimental Mouse Model of Multiple Sclerosis
Abstract
:1. Introduction
2. Results
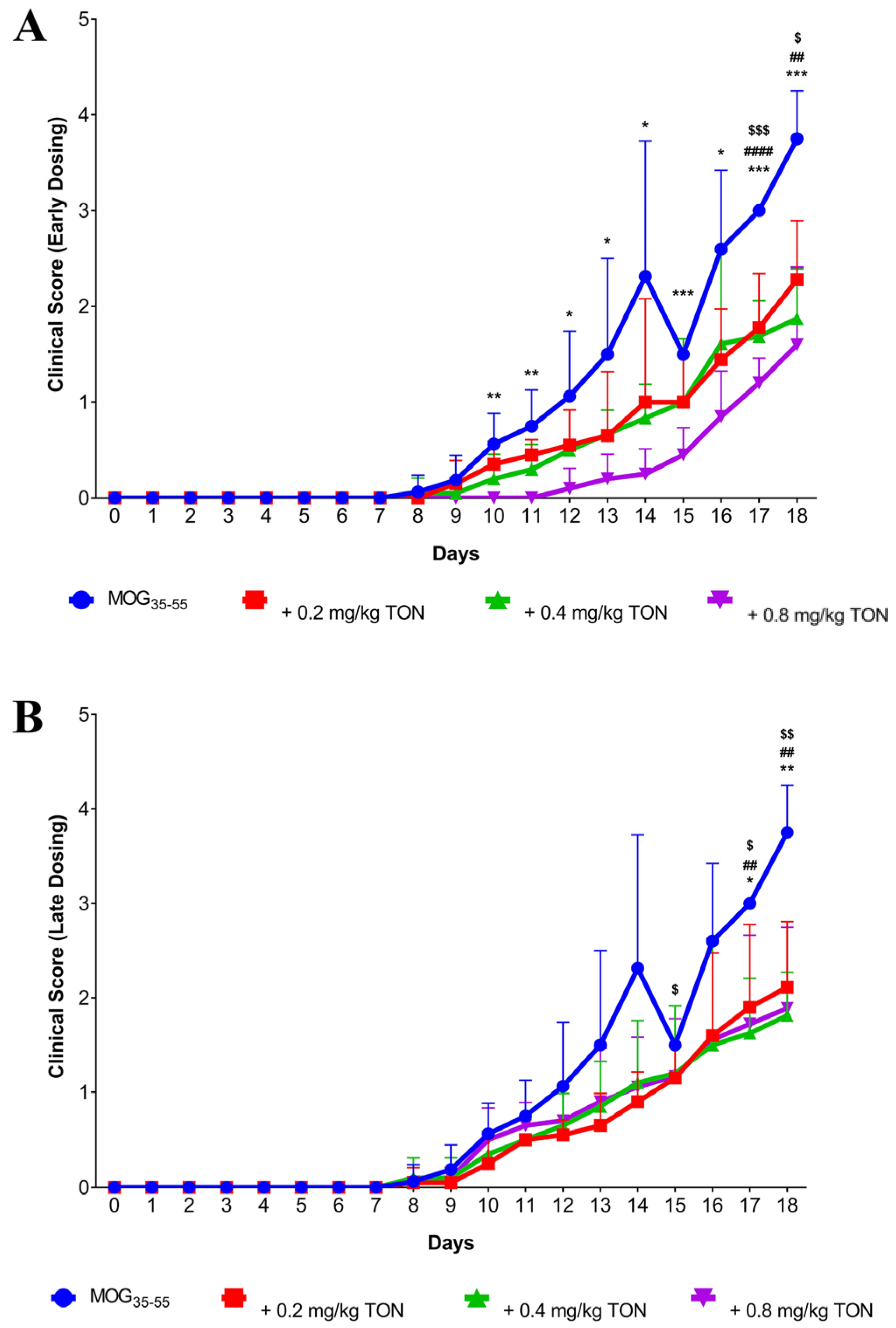
2.1. Behavioural Changes following MOG35–55 EAE and Tonabersat Early Dosing Administration
2.2. Behavioural Changes following MOG35–55 EAE Onset and Tonabersat Late Dosing Administration
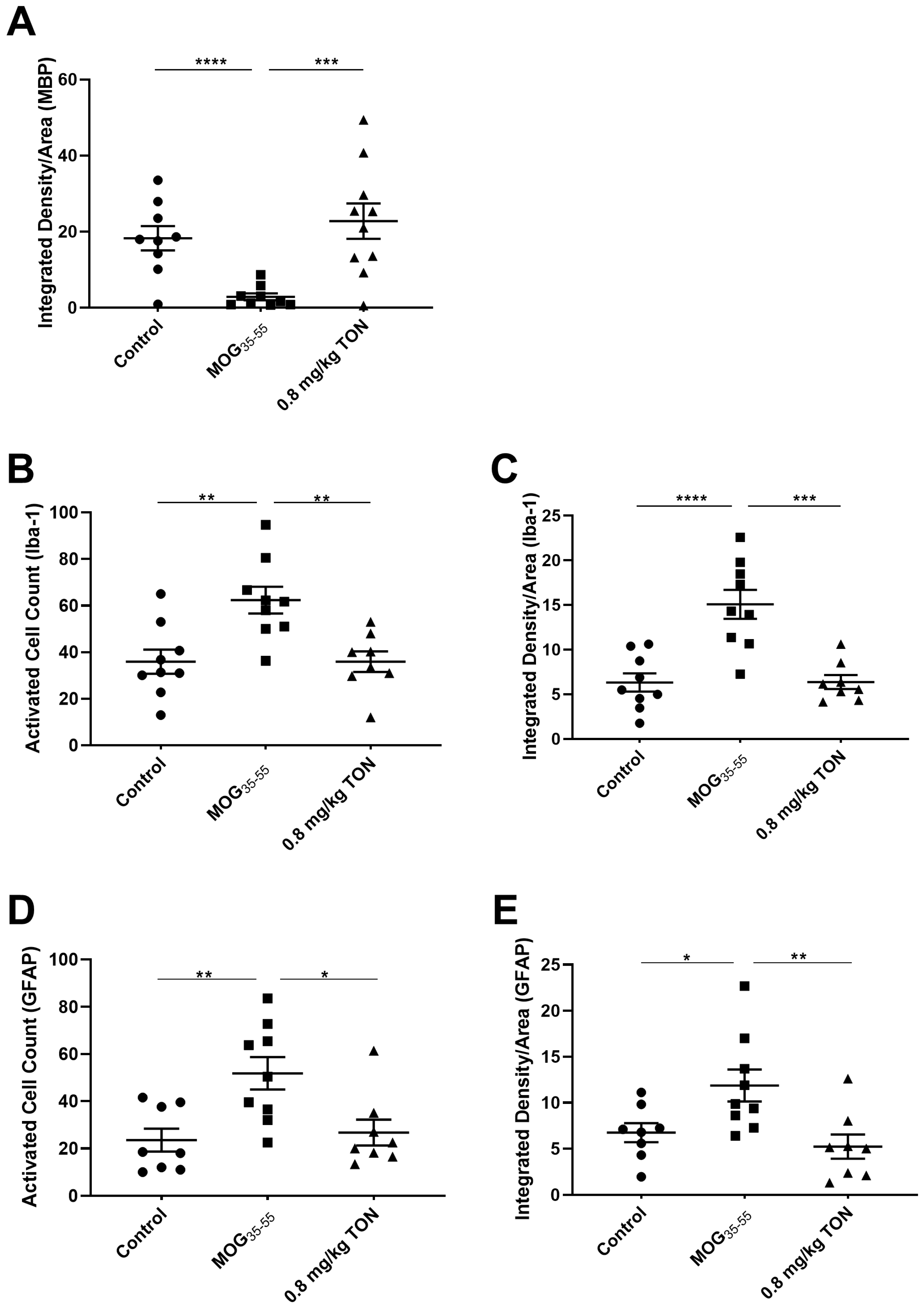
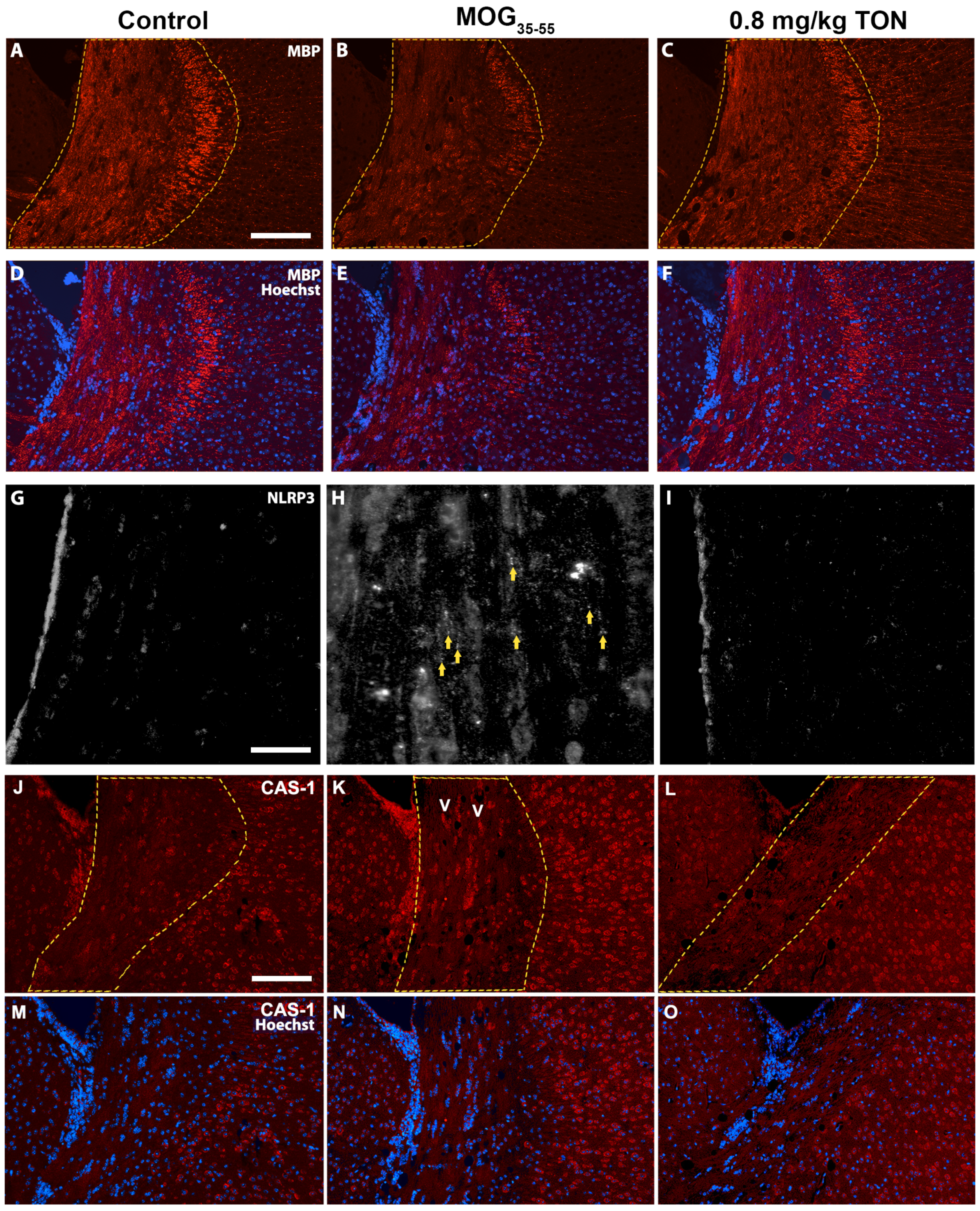
2.3. Immunohistochemical Analysis of MBP, NLRP3, Caspase-1, Iba-1 and GFAP in the Corpus Callosum of Control, MOG35–55 EAE, and MOG35–55 EAE Mice with 0.8 mg/kg Tonabersat Treatment
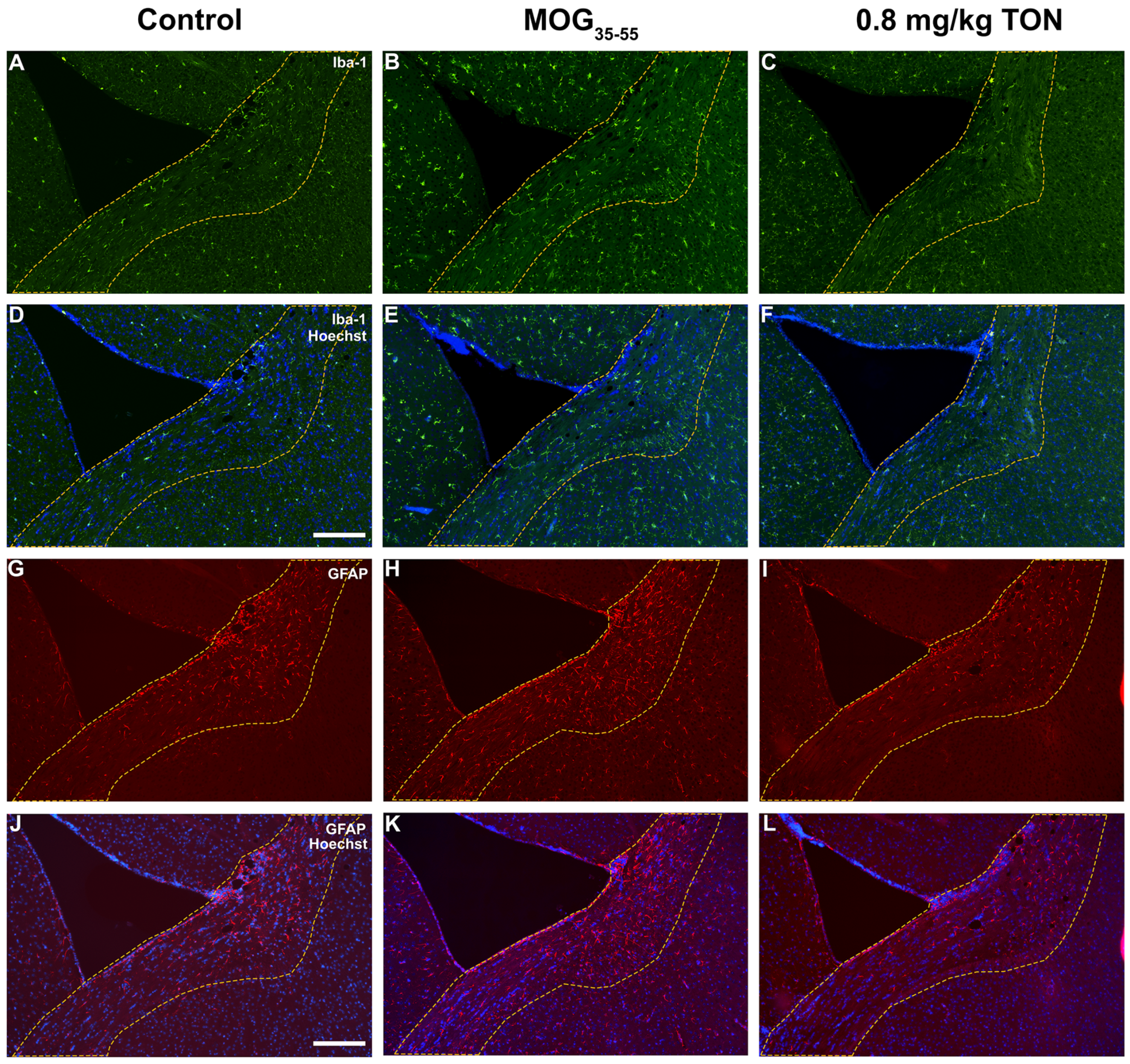
2.4. Immunohistochemical Analysis of Iba-1 and GFAP in the Motor Cortex of Control, MOG35–55 EAE and MOG35–55 EAE Mice with 0.8 mg/kg Tonabersat Treatment
2.5. Immunohistochemical Analysis of Iba-1 and GFAP in the Striatum of Control, MOG35–55 EAE and MOG35–55 EAE Mice with 0.8 mg/kg Tonabersat Treatment
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. EAE Model Preparation
4.3. Tissue Processing
4.4. Immunohistochemistry
4.5. Imaging and Analysis
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Williams, S.K.; Fairless, R.; Maier, O.; Liermann, P.C.; Pichi, K.; Fischer, R.; Eisel, U.L.M.; Kontermann, R.; Herrmann, A.; Weksler, B.; et al. Anti-TNFR1 targeting in humanized mice ameliorates disease in a model of multiple sclerosis. Sci. Rep. 2018, 8, 13628. [Google Scholar] [CrossRef] [PubMed]
- Barclay, W.; Shinohara, M.L. Inflammasome activation in multiple sclerosis and experimental autoimmune encephalomyelitis (EAE). Brain Pathol. 2017, 27, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Lahooti, B.; Chhibber, T.; Bagchi, S.; Varahachalam, S.P.; Jayant, R.D. Therapeutic role of inflammasome inhibitors in neurodegenerative disorders. Brain Behav. Immun. 2021, 91, 771–783. [Google Scholar] [CrossRef] [PubMed]
- Govindarajan, V.; de Rivero Vaccari, J.P.; Keane, R.W. Role of inflammasomes in multiple sclerosis and their potential as therapeutic targets. J. Neuroinflamm. 2020, 17, 260. [Google Scholar] [CrossRef] [PubMed]
- Piancone, F.; La Rosa, F.; Marventano, I.; Saresella, M.; Clerici, M. The Role of the Inflammasome in Neurodegenerative Diseases. Molecules 2021, 26, 953. [Google Scholar] [CrossRef] [PubMed]
- Ke, Q.; Greenawalt, A.N.; Manukonda, V.; Ji, X.; Tisch, R.M. The regulation of self-tolerance and the role of inflammasome molecules. Front. Immunol. 2023, 14, 1154552. [Google Scholar] [CrossRef]
- Vanaja, S.K.; Rathinam, V.A.; Fitzgerald, K.A. Mechanisms of inflammasome activation: Recent advances and novel insights. Trends Cell Biol. 2015, 25, 308–315. [Google Scholar] [CrossRef]
- Koca-Unsal, R.B.; Sehirli, A.O.; Sayiner, S.; Aksoy, U. Relationship of NLRP3 inflammasome with periodontal, endodontic and related systemic diseases. Mol. Biol. Rep. 2022, 49, 11123–11132. [Google Scholar] [CrossRef]
- Cui, Y.; Yu, H.; Bu, Z.; Wen, L.; Yan, L.; Feng, J. Focus on the Role of the NLRP3 Inflammasome in Multiple Sclerosis: Pathogenesis, Diagnosis, and Therapeutics. Front. Mol. Neurosci. 2022, 15, 894298. [Google Scholar] [CrossRef]
- Bakhshi, S.; Shamsi, S. MCC950 in the treatment of NLRP3-mediated inflammatory diseases: Latest evidence and therapeutic outcomes. Int. Immunopharmacol. 2022, 106, 108595. [Google Scholar] [CrossRef]
- Shao, S.; Chen, C.; Shi, G.; Zhou, Y.; Wei, Y.; Fan, N.; Yang, Y.; Wu, L.; Zhang, T. Therapeutic potential of the target on NLRP3 inflammasome in multiple sclerosis. Pharmacol. Ther. 2021, 227, 107880. [Google Scholar] [CrossRef] [PubMed]
- Di Stadio, A.; Romani, L.; Bernitsas, E. Could SARS-CoV-2 affect MS progression? Mult. Scler. Relat. Disord. 2020, 46, 102540. [Google Scholar] [CrossRef] [PubMed]
- Fusco, R.; Siracusa, R.; Genovese, T.; Cuzzocrea, S.; Di Paola, R. Focus on the Role of NLRP3 Inflammasome in Diseases. Int. J. Mol. Sci. 2020, 21, 4223. [Google Scholar] [CrossRef] [PubMed]
- Beynon, V.; Quintana, F.J.; Weiner, H.L. Activated human CD4+CD45RO+ memory T-cells indirectly inhibit NLRP3 inflammasome activation through downregulation of P2X7R signalling. PLoS ONE 2012, 7, e39576. [Google Scholar] [CrossRef]
- Groslambert, M.; Py, B.F. Spotlight on the NLRP3 inflammasome pathway. J. Inflamm. Res. 2018, 11, 359–374. [Google Scholar] [CrossRef] [PubMed]
- Mugisho, O.O.; Green, C.R.; Kho, D.T.; Zhang, J.; Graham, E.S.; Acosta, M.L.; Rupenthal, I.D. The inflammasome pathway is amplified and perpetuated in an autocrine manner through connexin43 hemichannel mediated ATP release. Biochim. Biophys. Acta Gen. Subj. 2018, 1862, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, S.; Costa, C.; Eixarch, H.; Keller, C.W.; Amman, L.; Martinez-Banaclocha, H.; Midaglia, L.; Sarro, E.; Machin-Diaz, I.; Villar, L.M.; et al. NLRP3 inflammasome as prognostic factor and therapeutic target in primary progressive multiple sclerosis patients. Brain 2020, 143, 1414–1430. [Google Scholar] [CrossRef] [PubMed]
- Yi, C.; Mei, X.; Ezan, P.; Mato, S.; Matias, I.; Giaume, C.; Koulakoff, A. Astroglial connexin43 contributes to neuronal suffering in a mouse model of Alzheimer’s disease. Cell Death Differ. 2016, 23, 1691–1701. [Google Scholar] [CrossRef]
- Lyon, H.; Shome, A.; Rupenthal, I.D.; Green, C.R.; Mugisho, O.O. Tonabersat Inhibits Connexin43 Hemichannel Opening and Inflammasome Activation in an In Vitro Retinal Epithelial Cell Model of Diabetic Retinopathy. Int. J. Mol. Sci. 2020, 22, 298. [Google Scholar] [CrossRef]
- Kim, Y.; Davidson, J.O.; Gunn, K.C.; Phillips, A.R.; Green, C.R.; Gunn, A.J. Role of hemichannels in CNS inflammation and the inflammasome pathway. Adv. Protein Chem. Struct. Biol. 2016, 104, 1–37. [Google Scholar]
- Orellana, J.A.; Saez, P.J.; Cortes-Campos, C.; Elizondo, R.J.; Shoji, K.F.; Contreras-Duarte, S.; Figueroa, V.; Velarde, V.; Jiang, J.X.; Nualart, F.; et al. Glucose increases intracellular free Ca2+ in tanycytes via ATP released through connexin 43 hemichannels. Glia 2012, 60, 53–68. [Google Scholar] [CrossRef] [PubMed]
- Maatouk, L.; Yi, C.; Carrillo-de Sauvage, M.A.; Compagnion, A.C.; Hunot, S.; Ezan, P.; Hirsch, E.C.; Koulakoff, A.; Pfrieger, F.W.; Tronche, F.; et al. Glucocorticoid receptor in astrocytes regulates midbrain dopamine neurodegeneration through connexin hemichannel activity. Cell Death Differ. 2019, 26, 580–596. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Griffin, J.M.; Nor, M.N.M.; Zhang, J.; Freestone, P.S.; Danesh-Meyer, H.V.; Rupenthal, I.D.; Acosta, M.; Nicholson, L.F.B.; O’Carroll, S.J. Tonabersat prevents inflammatory damage in the central nervous system by blocking connexin43 hemichannels. Neurotherapeutics 2017, 14, 1148–1165. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Li, Z.; He, X.; Yu, H.; Feng, J. Ghrelin Attenuates Neuroinflammation and Demyelination in Experimental Autoimmune Encephalomyelitis Involving NLRP3 Inflammasome Signaling Pathway and Pyroptosis. Front. Pharmacol. 2019, 10, 1320. [Google Scholar] [CrossRef] [PubMed]
- Hasselmann, J.P.C.; Karim, H.; Khalaj, A.J.; Ghosh, S.; Tiwari-Woodruff, S.K. Consistent induction of chronic experimental autoimmune encephalomyelitis in C57BL/6 mice for the longitudinal study of pathology and repair. J. Neurosci. Methods 2017, 284, 71–84. [Google Scholar] [CrossRef] [PubMed]
- Brambilla, R. The contribution of astrocytes to the neuroinflammatory response in multiple sclerosis and experimental autoimmune encephalomyelitis. Acta Neuropathol. 2019, 137, 757–783. [Google Scholar] [CrossRef] [PubMed]
- Van der Valk, P.; De Groot, C.J.A. Staging of multiple sclerosis (MS) lesions: Pathology of the time frame of MS. Neuropathol. Appl. Neurobiol. 2000, 26, 2–10. [Google Scholar] [CrossRef]
- Pons, V.; Rivest, S. Beneficial Roles of Microglia and Growth Factors in MS, a Brief Review. Front. Cell. Neurosci. 2020, 14, 284. [Google Scholar] [CrossRef]
- Sobue, A.; Komine, O.; Hara, Y.; Endo, F.; Mizoguchi, H.; Watanabe, S.; Murayama, S.; Saito, T.; Saido, T.C.; Sahara, N. Microglial gene signature reveals loss of homeostatic microglia associated with neurodegeneration of Alzheimer’s disease. Acta Neuropathol. Commun. 2021, 9, 1–17. [Google Scholar] [CrossRef]
- Sofroniew, M.V.; Vinters, H.V. Astrocytes: Biology and pathology. Acta Neuropathol. 2010, 119, 7–35. [Google Scholar] [CrossRef]
- Palpagama, T.; Mills, A.R.; Ferguson, M.W.; Vikas Ankeal, P.; Turner, C.; Tippett, L.; van der Werf, B.; Waldvogel, H.J.; Faull, R.L.M.; Kwakowsky, A. Microglial and Astrocytic Responses in the Human Midcingulate Cortex in Huntington’s Disease. Ann. Neurol. 2023, 94, 895–910. [Google Scholar] [CrossRef] [PubMed]
- Freeman, L.C.; Ting, J.P. The pathogenic role of the inflammasome in neurodegenerative diseases. J. Neurochem. 2016, 136 (Suppl. 1), 29–38. [Google Scholar] [CrossRef] [PubMed]
- Jha, S.; Srivastava, S.Y.; Brickey, W.J.; Iocca, H.; Toews, A.; Morrison, J.P.; Chen, V.S.; Gris, D.; Matsushima, G.K.; Ting, J.P.Y. The inflammasome sensor, NLRP3, regulates CNS inflammation and demyelination via caspase-1 and interleukin-18. J. Neurosci. 2010, 30, 15811–15820. [Google Scholar] [CrossRef] [PubMed]
- Chou, Y.K.; Jones, R.E.; Bourdette, D.; Whitham, R.; Hashim, G.; Atherton, J.; Offner, H.; Vandenbark, A.A. Human myelin basic protein (MBP) epitopes recognized by mouse MBP-selected T cell lines from multiple sclerosis patients. J. Neuroimmunol. 1994, 49, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, M.K.; McPherson, T.A.; Catz, I.; Warren, K.G.; Dossetor, J.B.; Carnegie, P.R. Identification of myelin basic protein (MBP) in circulating immune complexes (CIC) from some multiple sclerosis (MS) patients. Prog. Clin. Biol. Res. 1984, 146, 353–358. [Google Scholar] [PubMed]
- Meinl, E.; Hohlfeld, R. Immunopathogenesis of multiple sclerosis: MBP and beyond. Clin. Exp. Immunol. 2002, 128, 395–397. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Simpson, S., Jr.; Charlesworth, J.C.; van der Mei, I.; Lucas, R.M.; Ponsonby, A.L.; Taylor, B.V.; AUSLONG Investigators Group. Variation within MBP gene predicts disease course in multiple sclerosis. Brain Behav. 2017, 7, e00670. [Google Scholar] [CrossRef] [PubMed]
- Geng, J.; Liu, G.; Ma, S.; Li, S.; Zhou, H. Effect and Mechanism of Treating Experimental Autoimmune Encephalomyelitis in Mice with Butylphthalide Combined with Bone Marrow Mesenchymal Stem Cells. J. Sichuan Univ. (Med. Sci.) 2021, 52, 759–766. [Google Scholar] [CrossRef]
- Laaker, C.; Hsu, M.; Fabry, Z.; Miller, S.D.; Karpus, W.J. Experimental Autoimmune Encephalomyelitis in the Mouse. Curr. Protoc. 2021, 1, e300. [Google Scholar] [CrossRef]
- Almad, A.A.; Taga, A.; Joseph, J.; Gross, S.K.; Welsh, C.; Patankar, A.; Richard, J.P.; Rust, K.; Pokharel, A.; Plott, C.; et al. Cx43 hemichannels contribute to astrocyte-mediated toxicity in sporadic and familial ALS. Proc. Natl. Acad. Sci. USA 2022, 119, e2107391119. [Google Scholar] [CrossRef]
- Place, D.E.; Kanneganti, T.-D. Recent advances in inflammasome biology. Curr. Opin. Immunol. 2018, 50, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.X.; Mat Nor, M.N.; Danesh-Meyer, H.V.; Vessey, K.A.; Fletcher, E.L.; O’Carroll, S.J.; Acosta, M.L.; Green, C.R. Connexin43 Mimetic Peptide Improves Retinal Function and Reduces Inflammation in a Light-Damaged Albino Rat Model. Investig. Ophthalmol. Vis. Sci. 2016, 57, 3961–3973. [Google Scholar] [CrossRef] [PubMed]
- Mat Nor, M.N.; Rupenthal, I.D.; Green, C.R.; Acosta, M.L. Connexin Hemichannel Block Using Orally Delivered Tonabersat Improves Outcomes in Animal Models of Retinal Disease. Neurotherapeutics 2020, 17, 371–387. [Google Scholar] [CrossRef] [PubMed]
- Gris, D.; Ye, Z.; Iocca, H.A.; Wen, H.; Craven, R.R.; Gris, P.; Huang, M.; Schneider, M.; Miller, S.D.; Ting, J.P. NLRP3 plays a critical role in the development of experimental autoimmune encephalomyelitis by mediating Th1 and Th17 responses. J. Immunol. 2010, 185, 974–981. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, B.A.; Mamik, M.K.; Saito, L.B.; Boghozian, R.; Monaco, M.C.; Major, E.O.; Lu, J.-Q.; Branton, W.G.; Power, C. Caspase-1 inhibition prevents glial inflammasome activation and pyroptosis in models of multiple sclerosis. Proc. Natl. Acad. Sci. USA 2018, 115, E6065–E6074. [Google Scholar] [CrossRef] [PubMed]
- Zirngibl, M.; Assinck, P.; Sizov, A.; Caprariello, A.V.; Plemel, J.R. Oligodendrocyte death and myelin loss in the cuprizone model: An updated overview of the intrinsic and extrinsic causes of cuprizone demyelination. Mol. Neurodegener. 2022, 17, 34. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; He, H.; Lin, B.; Chen, Y.; Deng, X.; Jiang, W.; Zhou, R. RRx-001 ameliorates inflammatory diseases by acting as a potent covalent NLRP3 inhibitor. Cell Mol. Immunol. 2021, 18, 1425–1436. [Google Scholar] [CrossRef]
- Shaw, P.J.; Lukens, J.R.; Burns, S.; Chi, H.; McGargill, M.A.; Kanneganti, T.D. Cutting edge: Critical role for PYCARD/ASC in the development of experimental autoimmune encephalomyelitis. J. Immunol. 2010, 184, 4610–4614. [Google Scholar] [CrossRef]
- Mittelbronn, M.; Dietz, K.; Schluesener, H.J.; Meyermann, R. Local distribution of microglia in the normal adult human central nervous system differs by up to one order of magnitude. Acta Neuropathol. 2001, 101, 249–255. [Google Scholar] [CrossRef]
- Wendimu, M.Y.; Hooks, S.B. Microglia Phenotypes in Aging and Neurodegenerative Diseases. Cells 2022, 11, 2091. [Google Scholar] [CrossRef]
- Wang, W.Y.; Tan, M.S.; Yu, J.T.; Tan, L. Role of pro-inflammatory cytokines released from microglia in Alzheimer’s disease. Ann. Transl. Med. 2015, 3, 136. [Google Scholar] [CrossRef] [PubMed]
- Goldmann, T.; Wieghofer, P.; Müller, P.F.; Wolf, Y.; Varol, D.; Yona, S.; Brendecke, S.M.; Kierdorf, K.; Staszewski, O.; Datta, M. A new type of microglia gene targeting shows TAK1 to be pivotal in CNS autoimmune inflammation. Nat. Neurosci. 2013, 16, 1618–1626. [Google Scholar] [CrossRef] [PubMed]
- Voet, S.; Mc Guire, C.; Hagemeyer, N.; Martens, A.; Schroeder, A.; Wieghofer, P.; Daems, C.; Staszewski, O.; Walle, L.V.; Jordao, M.J.C. A20 critically controls microglia activation and inhibits inflammasome-dependent neuroinflammation. Nat. Commun. 2018, 9, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Hart, C.G.; Karimi-Abdolrezaee, S. Recent insights on astrocyte mechanisms in CNS homeostasis, pathology, and repair. J. Neurosci. Res. 2021, 99, 2427–2462. [Google Scholar] [CrossRef] [PubMed]
- Linnerbauer, M.; Wheeler, M.A.; Quintana, F.J. Astrocyte Crosstalk in CNS Inflammation. Neuron 2020, 108, 608–622. [Google Scholar] [CrossRef] [PubMed]
- Claycomb, K.I.; Johnson, K.M.; Winokur, P.N.; Sacino, A.V.; Crocker, S.J. Astrocyte regulation of CNS inflammation and remyelination. Brain Sci. 2013, 3, 1109–1127. [Google Scholar] [CrossRef] [PubMed]
- Brambilla, L.; Martorana, F.; Rossi, D. Astrocyte signaling and neurodegeneration: New insights into CNS disorders. Prion 2013, 7, 28–36. [Google Scholar] [CrossRef]
- Blanco-Suarez, E.; Caldwell, A.L.; Allen, N.J. Role of astrocyte-synapse interactions in CNS disorders. J. Physiol. 2017, 595, 1903–1916. [Google Scholar] [CrossRef]
- Kato, H.; Okuno, T.; Isohashi, K.; Koda, T.; Shimizu, M.; Mochizuki, H.; Nakatsuji, Y.; Hatazawa, J. Astrocyte metabolism in multiple sclerosis investigated by 1-C-11 acetate PET. J. Cereb. Blood Flow Metab. 2021, 41, 369–379. [Google Scholar] [CrossRef]
- Holley, J.E.; Gveric, D.; Newcombe, J.; Cuzner, M.L.; Gutowski, N.J. Astrocyte characterization in the multiple sclerosis glial scar. Neuropathol. Appl. Neurobiol. 2003, 29, 434–444. [Google Scholar] [CrossRef]
- Barna, B.P.; Chou, S.M.; Ransohoff, R.M.; Jacobs, B. Astrocyte interactions with immune modifiers: A possible role for astrocytes in the immunopathogenesis of multiple sclerosis (MS). Prog. Clin. Biol. Res. 1984, 146, 153–157. [Google Scholar] [PubMed]
- Matute-Blanch, C.; Brito, V.; Midaglia, L.; Villar, L.M.; Garcia-Diaz Barriga, G.; Guzman de la Fuente, A.; Borras, E.; Fernandez-Garcia, S.; Calvo-Barreiro, L.; Miguez, A.; et al. Inflammation in multiple sclerosis induces a specific reactive astrocyte state driving non-cell-autonomous neuronal damage. Clin. Transl. Med. 2022, 12, e837. [Google Scholar] [CrossRef] [PubMed]
- Masvekar, R.; Wu, T.; Kosa, P.; Barbour, C.; Fossati, V.; Bielekova, B. Cerebrospinal fluid biomarkers link toxic astrogliosis and microglial activation to multiple sclerosis severity. Mult. Scler. Relat. Disord. 2019, 28, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Maeda, Y.; Ma, J.; Delgado, M.; Sohn, J.; Miers, L.; Ko, E.M.; Bannerman, P.; Xu, J.; Wang, Y. Macroglial plasticity and the origins of reactive astroglia in experimental autoimmune encephalomyelitis. J. Neurosci. 2011, 31, 11914–11928. [Google Scholar] [CrossRef]
- Alvarez, J.I.; Katayama, T.; Prat, A. Glial influence on the blood brain barrier. Glia 2013, 61, 1939–1958. [Google Scholar] [CrossRef]
- Silberstein, S.D.; Schoenen, J.; Gobel, H.; Diener, H.C.; Elkind, A.H.; Klapper, J.A.; Howard, R.A. Tonabersat, a gap-junction modulator: Efficacy and safety in two randomized, placebo-controlled, dose-ranging studies of acute migraine. Cephalalgia 2009, 29 (Suppl. 2), 17–27. [Google Scholar] [CrossRef]
- Pettinelli, C.B.; McFarlin, D.E. Adoptive transfer of experimental allergic encephalomyelitis in SJL/J mice after in vitro activation of lymph node cells by myelin basic protein: Requirement for Lyt 1+ 2-T lymphocytes. J. Immunol. 1981, 127, 1420–1423. [Google Scholar] [CrossRef]
- Calvo-Flores Guzman, B.; Elizabeth Chaffey, T.; Hansika Palpagama, T.; Waters, S.; Boix, J.; Tate, W.P.; Peppercorn, K.; Dragunow, M.; Waldvogel, H.J.; Faull, R.L.M.; et al. The Interplay Between Beta-Amyloid 1–42 (Aβ1–42)-Induced Hippocampal Inflammatory Response, p-tau, Vascular Pathology, and Their Synergistic Contributions to Neuronal Death and Behavioral Deficits. Front. Mol. Neurosci. 2020, 13, 522073. [Google Scholar] [CrossRef]
- Glenn, J.A.; Ward, S.A.; Stone, C.R.; Booth, P.L.; Thomas, W.E. Characterisation of ramified microglial cells: Detailed morphology, morphological plasticity and proliferative capability. J. Anat. 1992, 180, 109–118. [Google Scholar]
- Wilhelmsson, U.; Bushong, E.A.; Price, D.L.; Smarr, B.L.; Phung, V.; Terada, M.; Ellisman, M.H.; Pekny, M. Redefining the concept of reactive astrocytes as cells that remain within their unique domains upon reaction to injury. Proc. Natl. Acad. Sci. USA 2006, 103, 17513–17518. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwakowsky, A.; Chawdhary, B.; de Souza, A.; Meyer, E.; Kaye, A.H.; Green, C.R.; Stylli, S.S.; Danesh-Meyer, H. Tonabersat Significantly Reduces Disease Progression in an Experimental Mouse Model of Multiple Sclerosis. Int. J. Mol. Sci. 2023, 24, 17454. https://doi.org/10.3390/ijms242417454
Kwakowsky A, Chawdhary B, de Souza A, Meyer E, Kaye AH, Green CR, Stylli SS, Danesh-Meyer H. Tonabersat Significantly Reduces Disease Progression in an Experimental Mouse Model of Multiple Sclerosis. International Journal of Molecular Sciences. 2023; 24(24):17454. https://doi.org/10.3390/ijms242417454
Chicago/Turabian StyleKwakowsky, Andrea, Bhavya Chawdhary, Antonio de Souza, Emily Meyer, Andrew H. Kaye, Colin R. Green, Stanley S. Stylli, and Helen Danesh-Meyer. 2023. "Tonabersat Significantly Reduces Disease Progression in an Experimental Mouse Model of Multiple Sclerosis" International Journal of Molecular Sciences 24, no. 24: 17454. https://doi.org/10.3390/ijms242417454
APA StyleKwakowsky, A., Chawdhary, B., de Souza, A., Meyer, E., Kaye, A. H., Green, C. R., Stylli, S. S., & Danesh-Meyer, H. (2023). Tonabersat Significantly Reduces Disease Progression in an Experimental Mouse Model of Multiple Sclerosis. International Journal of Molecular Sciences, 24(24), 17454. https://doi.org/10.3390/ijms242417454






