Maackiain Mimics Caloric Restriction through aak-2-Mediated Lipid Reduction in Caenorhabditis elegans
Abstract
:1. Introduction
2. Results
2.1. Maackiain Acts as a Chemoattractant and Increases Locomotor Activity in C. elegans
2.2. Maackiain Reduces Lipid Accumulation in Glucose-Stimulated C. elegans
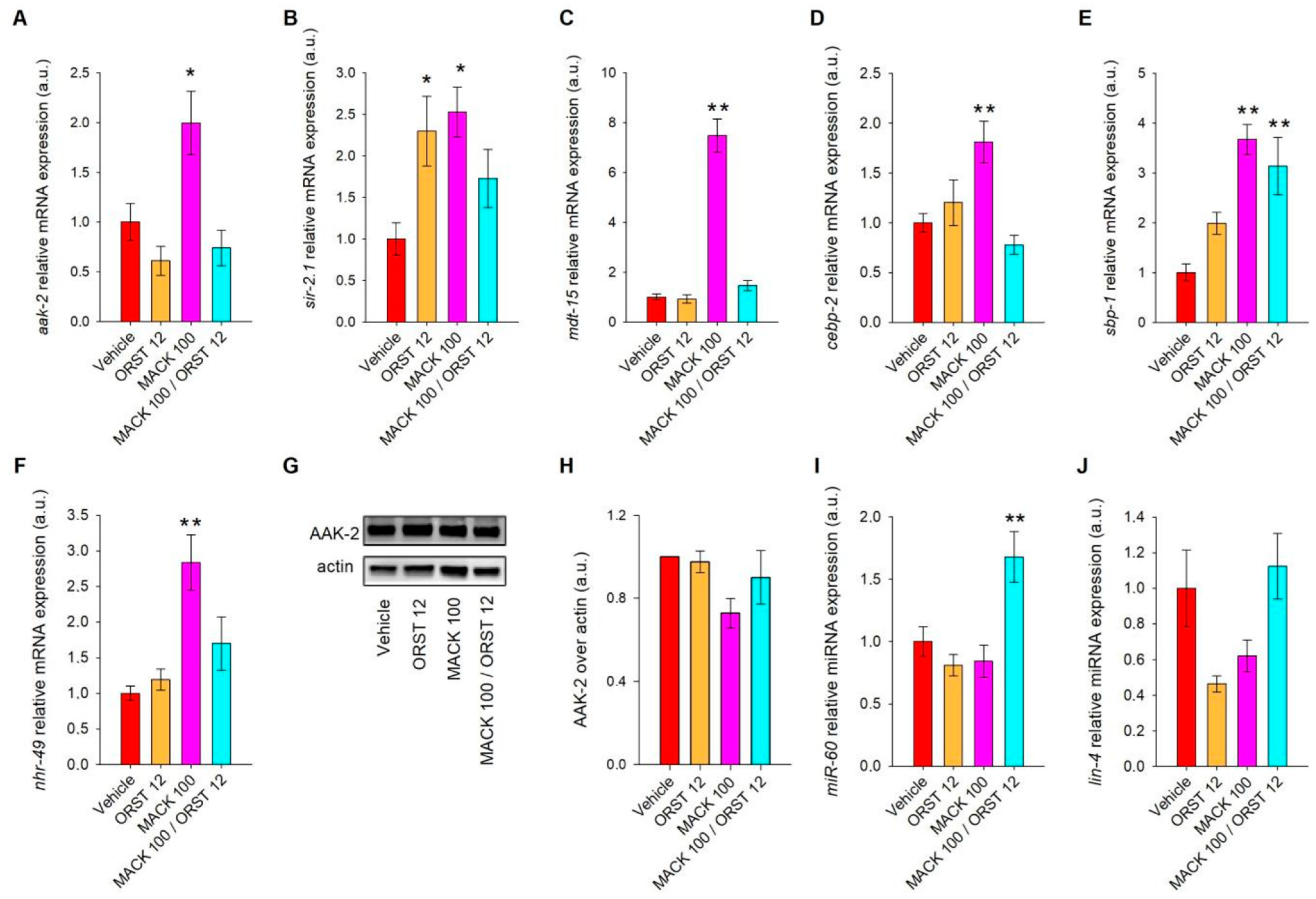
2.3. Maackiain Upregulates Genes Associated with the Nutrient Sensing aak-2/sir-2.1 Signaling Pathway
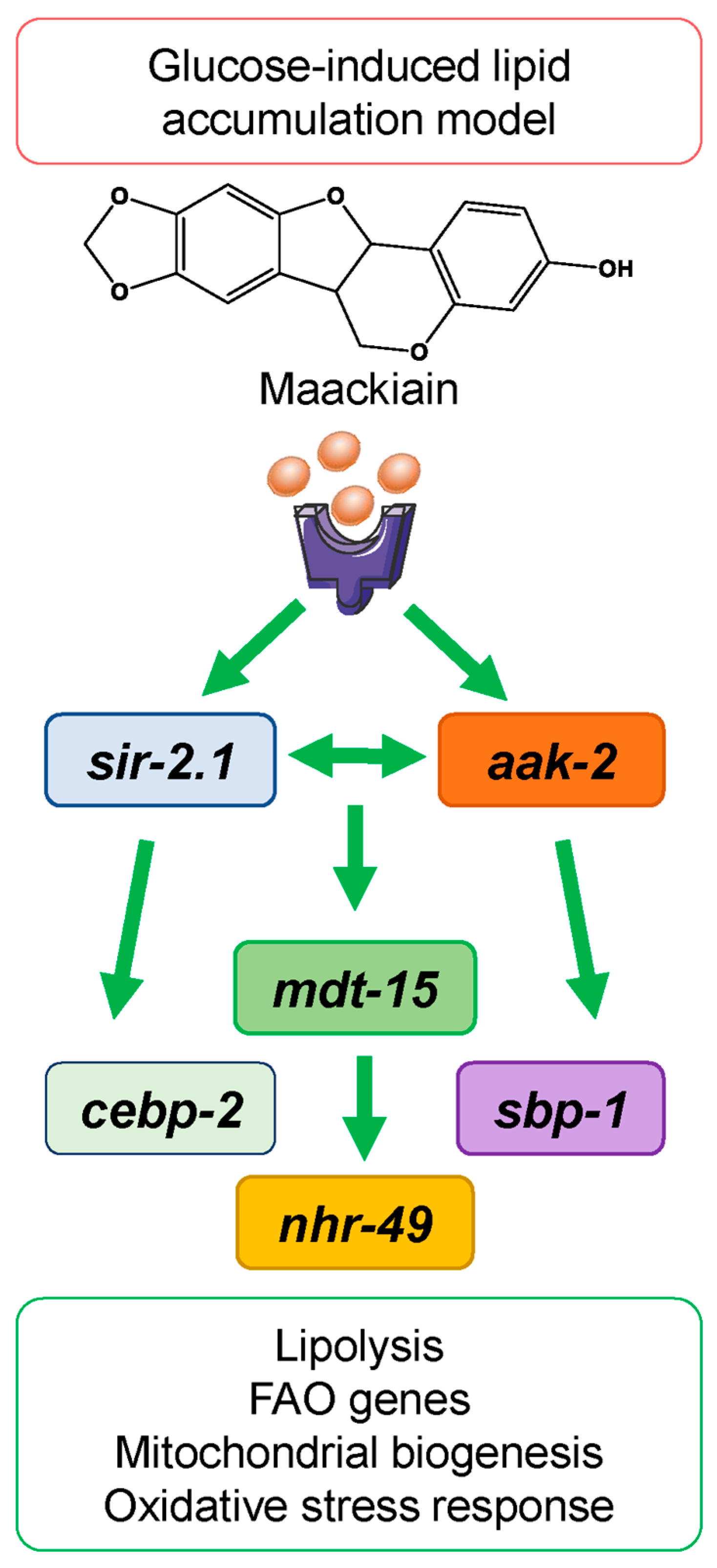
2.4. Proposed Mechanism of the Anti-Obesogenic Effect of Maackiain in C. elegans
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Caenorhabditis Elegans Maintenance and Treatment
4.3. Locomotion Assay
4.4. Chemotaxis Assay
4.5. Nile Red Triglyceride Staining and Confocal Imaging
4.6. Gene Expression Analysis through RT-qPCR of mRNA and miRNAs
4.7. Western Blot Analysis
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wen, X.; Zhang, B.; Wu, B.; Xiao, H.; Li, Z.; Li, R.; Xu, X.; Li, T. Signaling pathways in obesity: Mechanisms and therapeutic interventions. Signal Transduct. Target. Ther. 2022, 7, 298. [Google Scholar] [CrossRef] [PubMed]
- Muller, T.D.; Blüher, M.; Tschöp, M.H.; DiMarchi, R.D. Anti-obesity drug discovery: Advances and challenges. Nat. Rev. Drug Discov. 2022, 21, 201–223. [Google Scholar] [CrossRef] [PubMed]
- Baca, P.; Barajas-Olmos, F.; Mirzaeicheshmeh, E.; Zerrweck, L.; Guilbert, E.C.; Sánchez, M.; Flores-Huacuja, R.; Villafán, A.; Martínez-Hernández, H.; García-Ortiz, C.; et al. DNA methylation and gene expression analysis in adipose tissue to identify new loci associated with T2D development in obesity. Nutr. Diabetes 2022, 12, 50. [Google Scholar] [CrossRef] [PubMed]
- Bourebaba, Y.; Marycz, K.; Mularczyk, M.; Bourebaba, L. Postbiotics as potential new therapeutic agents for metabolic disorders management. Biomed. Pharmacother. 2022, 123, 113138. [Google Scholar] [CrossRef] [PubMed]
- Longo, V.D.; Di Tano, M.; Mattson, M.P.; Guidi, N. Intermittent and periodic fasting, longevity and disease. Nat. Aging 2021, 1, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Foley, K.P.; Chen, Y.; Barra, N.G.; Heal, M.; Kwok, K.; Tamrakar, A.K.; Chi, W.; Duggan, B.M.; Henriksbo, B.D.; Liu, Y.; et al. Inflammation promotes adipocyte lipolysis via IRE1 kinase. J. Biol. Chem. 2021, 296, 100440. [Google Scholar] [CrossRef]
- Bai, J.; Zhu, Y.; He, L.; Zhang, J.; Li, J.; Pan, R.; Zhang, J.; Zhao, Y.; Cui, L.; Lu, H.; et al. Saponins from bitter melon reduce lipid accumulation via induction of autophagy in C. elegans and HepG2 cell line. Curr. Res. Food Sci. 2022, 2, 1167–1175. [Google Scholar] [CrossRef]
- Qu, Z.; Zhang, L.; Huang, W.; Zheng, S. Vitamin K2 enhances fat degradation to improve the survival of C. elegans. Front. Nutr. 2022, 2, 858481. [Google Scholar] [CrossRef]
- Savova, M.S.; Mihaylova, L.V.; Tews, D.; Wabitsch, M.; Georgiev, M.I. Targeting PI3K/AKT signaling pathway in obesity. Biomed. Pharmacother. 2023, 159, 114244. [Google Scholar] [CrossRef]
- Shi, H.; Zhao, J.; Li, Y.; Li, J.; Li, Y.; Zhang, J.; Qiu, Z.; Wu, C.; Qin, M.; Liu, C.; et al. Ginsenosides Rg1 regulate lipid metabolism and temperature adaptation in Caenorhabditis elegans. J. Ginseng Res. 2023, 47, 524–533. [Google Scholar] [CrossRef]
- Yue, Y.; Hao, G.; Cho, J.; Park, Y. Curcumin reduced fat accumulation in Caenorhabditis elegans. Curr. Res. Food Sci. 2021, 4, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Madeo, F.; Carmona-Gutierrez, D.; Hofer, S.J.; Kroemer, G. Caloric restriction mimetics against age-associated disease: Targets, mechanisms, and therapeutic potential. Cell Metab. 2019, 29, 592–610. [Google Scholar] [CrossRef] [PubMed]
- Vasileva, L.V.; Marchev, A.S.; Georgiev, M.I. Causes and solutions to “globesity”: The new fa(s)t alarming global epidemic. Food Chem. Toxicol. 2018, 121, 173–193. [Google Scholar] [CrossRef] [PubMed]
- Mladenova, S.G.; Savova, M.S.; Marchev, A.S.; Ferrante, C.; Orlando, G.; Wabitsch, M.; Georgiev, M.I. Anti-adipogenic activity of maackiain and ononin is mediated via inhibition of PPARγ in human adipocytes. Biomed. Pharmacother. 2022, 149, 112908. [Google Scholar] [CrossRef]
- Huang, Y.; Hao, J.; Tian, D.; Wen, Y.; Zhao, P.; Chen, H.; Lv, Y.; Yang, X. Antidiabetic activity of a flavonoid-rich extract from Sophora davidii (Franch.) skeels in KK-Ay mice via activation of AMP-activated protein kinase. Front. Pharmacol. 2018, 9, 105358. [Google Scholar] [CrossRef]
- Anyanwu, G.O.; Iqbal, J.; Khan, S.U.; Zaib, S.; Rauf, K.; Onyeneke, C.E.; Ojo, O.O.; Ur-Rahman, N. Antidiabetic activities of chloroform fraction of Anthocleista vogelii Planch root bark in rats with diet- and alloxan-induced obesity-diabetes. J. Ethnopharmacol. 2019, 229, 293–302. [Google Scholar] [CrossRef]
- Bai, X.; Zhu, Y.; Jie, J.; Li, D.; Song, L.; Luo, J. Maackiain protects against sepsis via activating AMPK/Nrf2/HO-1 pathway. Int. Immunopharmacol. 2022, 108, 108710. [Google Scholar] [CrossRef] [PubMed]
- Savova, M.S.; Todorova, M.N.; Apostolov, A.G.; Yahubyan, G.T.; Georgiev, M.I. Betulinic acid counteracts the lipid accumulation in Caenorhabditis elegans by modulation of nhr-49 expression. Biomed. Pharmacother. 2022, 156, 113862. [Google Scholar] [CrossRef]
- Mudd, N.; Liceaga, A.M. Caenorhabditis elegans as an in vivo model for food bioactives: A review. Curr. Res. Food Sci. 2022, 5, 845–856. [Google Scholar] [CrossRef]
- Zhang, Y.; Lanjuin, A.; Chowdhury, S.R.; Mistry, M.; Silva-García, C.G.; Weir, H.J.; Lee, C.L.; Escoubas, C.C.; Tabakovic, E.; Mair, W.B. Neuronal TORC1 modulates longevity via AMPK and cell nonautonomous regulation of mitochondrial dynamics in C. elegans. eLife 2019, 8, e49158. [Google Scholar] [CrossRef]
- Weir, H.J.; Yao, P.; Huynh, F.K.; Escoubas, C.C.; Goncalves, R.L.; Burkewitz, K.; Laboy, R.; Hirschey, M.D.; Mair, W.D. Dietary restriction and AMPK increase lifespan via mitochondrial network and peroxisome remodeling. Cell Metab. 2017, 26, 884–896. [Google Scholar] [CrossRef]
- Goh, G.Y.S.; Winter, J.J.; Bhanshali, F.; Doering, K.R.S.; Lai, R.; Lee, K.; Veal, E.A.; Taubert, S. NHR-49/HNF4 integrates regulation of fatty acid metabolism with a protective transcriptional response to oxidative stress and fasting. Aging Cell 2018, 17, e12743. [Google Scholar] [CrossRef] [PubMed]
- Bouyanfif, A.; Jayarathne, S.; Koboziev, I.; Moustaid-Moussa, N. The nematode Caenorhabditis elegans as a model organism to study metabolic effects of ω-3 polyunsaturated fatty acids in obesity. Adv. Nutr. 2019, 10, 165–178. [Google Scholar] [CrossRef] [PubMed]
- Haerkens, F.; Kikken, C.; Kirkels, L.; van Amstel, M.; Wouters, W.; van Doornmalen, E.; Francke, C.; Hughes, S. A new use for old drugs: Identifying compounds with an anti-obesity effect using a high through-put semi-automated Caenorhabditis elegans screening platform. Heliyon 2022, 8, e10108. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Arriola, E.; El Hafidi, M.; Ortega-Cuéllar, D.; Carvajal, K. AMP-activated protein kinase regulates oxidative metabolism in Caenorhabditis elegans through the NHR-49 and MDT-15 transcriptional regulators. PLoS ONE 2016, 11, e0148089. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, F.; Karadeniz, Z.; Fischer-Rosinsnky, A.; Willmes, D.M.; Spranger, J.; Birkenfeld, A.L. Knockdown of Indy/CeNa2 extends Caenorhabditis elegans life span by inducing AMPK/aak-2. Aging 2015, 7, 553–567. [Google Scholar] [CrossRef] [PubMed]
- Nunez, S.; Moliner, C.; Valero, M.S.; Mustafa, A.M.; Maggi, F.; Gómez-Rincón, C.; López, V. Antidiabetic and anti-obesity properties of a polyphenol-rich flower extract from Tagetes erecta L. and its effects on Caenorhabditis elegans fat storages. J. Physiol. Biochem. 2023, 79, 427–440. [Google Scholar] [CrossRef] [PubMed]
- Tsai, R.T.; Tsai, C.W.; Liu, S.P.; Gao, J.X.; Kuo, Y.H.; Chao, P.M.; Hung, S.H.; Shyu, W.C.; Lin, S.Z.; Fu, R.H. Maackiain ameliorates 6-hydroxydopamine and SNCA pathologies by modulating the PINK1/Parkin pathway in models of Parkinson’s disease in Caenorhabditis elegans and the SH-SY5Y cell line. Int. J. Mol. Sci. 2020, 21, 4455. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Lin, Y.; Chen, Y.; Xu, J.; Li, Y.; Cao, Y.; Su, Z.; Chen, Y. Effects of Momordica saponin extract on alleviating fat accumulation in Caenorhabditis elegans. Food Funct. 2019, 10, 3237–3251. [Google Scholar] [CrossRef]
- Bai, J.; Farias-Pereira, R.; Zhang, Y.; Jang, M.; Park, Y.; Kim, K.H. C. elegans ACAT regulates lipolysis and its related lifespan in fasting through modulation of the genes in lipolysis and insulin/IGF-1 signaling. BioFactors 2020, 46, 754–765. [Google Scholar] [CrossRef]
- Queiros, L.; Marques, C.; Pereira, J.L.; Gonçalves, F.J.M.; Aschner, M.; Pereira, P. Overview of chemotaxis behavior assays in Caenorhabditis elegans. Curr. Protoc. 2021, 1, e120. [Google Scholar] [CrossRef]
- Margie, O.; Palmer, C.; Chin-Sang, I. C. elegans chemotaxis assay. J. Vis. Exp. 2013, 27, e50069. [Google Scholar]
- Farias-Pereira, R.; Savarese, J.; Yue, Y.; Lee, S.H.; Park, Y. Fat-lowering effects of isorhamnetin are via NHR-49-dependent pathway in Caenorhabditis elegans. Curr. Res. Food Sci. 2019, 2, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Stuhr, N.; Nhan, J.; Hammerquist, A.; Van Camp, B.; Reoyo, D.; Curran, S. Rapid lipid quantification in Caenorhabditis elegans by oil red O and Nile red staining. Bio-Protoc. 2022, 12, e4340. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.H.; Han, J.S.; Jung, Y.; Lee, S.M.; Park, S.H.; Park, M.; Shin, M.G.; Kim, N.; Kang, M.S.; Kim, S.; et al. A new AMPK isoform mediates glucose-restriction induced longevity non-cell autonomously by promoting membrane fluidity. Nat. Commun. 2023, 14, 288. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ding, W.; Li, C.Y.; Liu, Y. HLH-11 modulates lipid metabolism in response to nutrient availability. Nat. Commun. 2020, 11, 5959. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Ridzon, D.A.; Broomer, A.J.; Zhou, Z.; Lee, D.H.; Nguyen, J.T.; Barbisin, M.; Xu, N.L.; Mahuvakar, V.R.; Andersen, M.R.; et al. Real-time quantification of microRNAs by stem-loop RT-PCR. Nucleic Acids Res. 2005, 33, e179. [Google Scholar] [CrossRef]
- Shamsuzzama, S.; Kumar, L.; Nazir, A. Modulation of alpha-synuclein expression and associated effects by microRNA let-7 in transgenic C. elegans. Front. Mol. Neurosci. 2017, 10, 1662–5099. [Google Scholar] [CrossRef]
- Zhang, B.; Tian, L.; Xie, J.; Chen, G.; Wang, F. Targeting miRNAs by natural products: A new way for cancer therapy. Biomed. Pharmacother. 2020, 130, 110546. [Google Scholar] [CrossRef]
- Kato, M.; Kashem, M.A.; Cheng, C. An intestinal microRNA modulates the homeostatic adaptation to chronic oxidative stress in C. elegans. Aging 2016, 8, 1979–2005. [Google Scholar] [CrossRef]
- Brosnan, C.A.; Palmer, A.J.; Zuryn, S. Cell-type-specific profiling of loaded miRNAs from Caenorhabditis elegans reveals spatial and temporal flexibility in Argonaute loading. Nat. Commun. 2021, 12, 2194. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Orhan, I.E.; Banach, M.; Rollinger, J.M.; Barreca, D.; Weckwerth, W.; Bauer, R.; Bayer, E.A.; et al. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef] [PubMed]
- Chao, A.M.; Quigley, K.M.; Wadden, T.A. Dietary interventions for obesity: Clinical and mechanistic findings. J. Clin. Investig. 2021, 131, e140065. [Google Scholar] [CrossRef] [PubMed]
- Misra, S.; Ke, C.; Srinivasan, S.; Goyal, A.; Nyriyenda, M.J.; Florez, J.C.; Khunti, K.; Magliano, D.J.; Luk, A. Current insights and emerging trends in early-onset type 2 diabetes. Lancet Diabetes Endocrinol. 2023, 11, 768–782. [Google Scholar] [CrossRef]
- Wei, M.; Brandhorst, S.; Shelehchi, M.; Mirzaei, H.; Cheng, C.W.; Budniak, J.; Groshen, S.; Mack, W.J.; Guen, E.; Di Biase, S.; et al. Fasting-mimicking diet and markers/risk factors for aging, diabetes, cancer, and cardiovascular disease. Sci. Transl. Med. 2017, 15, eaai8700. [Google Scholar] [CrossRef]
- Rachakatla, A.; Kalashikam, R.R. Calorie restriction-regulated molecular pathways and its impact on various age groups: An overview. DNA Cell Biol. 2022, 41, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Vasileva, L.V.; Savova, M.S.; Amirova, K.M.; Balcheva-Sivenova, Z.; Ferrante, C.; Orlando, G.; Wabitsch, M.; Georgiev, M.I. Caffeic and chlorogenic acids synergistically activate browning program in human adipocytes: Implications of AMPK- and PPAR-mediated pathways. Int. J. Mol. Sci. 2020, 21, 9740. [Google Scholar] [CrossRef]
- Dou, Y.; Huang, R.; Li, Q.; Liu, Y.; Li, Y.; Chen, H.; Ai, G.; Xe, J.; Zeng, H.; Chen, J.; et al. Oxyberberine, an absorbed metabolite of berberine, possess superior hypoglycemic effect via regulating the PI3K/Akt and Nrf2 signaling pathways. Biomed. Pharmacother. 2021, 137, 111312. [Google Scholar] [CrossRef]
- Liu, Y.; Qiu, Y.; Chen, Q.; Han, X.; Cai, M. Puerarin suppresses the hepatic gluconeogenesis via activation of PI3K/Akt signaling pathway in diabetic rats and HepG2 cells. Biomed. Pharmacother. 2021, 137, 111325. [Google Scholar] [CrossRef]
- Bakar, M.H.A.; Shariff, K.A.; Tan, J.S.; Lee, L.K. Celastrol attenuates inflammatory responses in adipose tissues and improves skeletal muscle mitochondrial functions in high fat diet-induced obese rats via upregulation of AMPK/SIRT1 signaling pathways. Eur. J. Pharmacol. 2020, 883, 173371. [Google Scholar] [CrossRef]
- Ma, X.; Xu, L.; Alberobello, A.T.; Gavrilova, O.; Bagattin, A.; Skarulis, M.; Liu, J.; Finkel, T.; Mueller, E. Celastrol protects against obesity and metabolic dysfunction through activation of HSF1-PGC1α transcription axis. Cell Metabol. 2015, 22, 695–708. [Google Scholar] [CrossRef]
- Yoo, X.; Kang, M.; Pyo, S.; Chae, H.S.; Ryu, K.N.; Kim, J.; Chin, Y.W. SKI3301, a purified herbal extract from Sophora tonkinensis, inhibited airway inflammation and bronchospasm in allergic asthma animal models in vivo. J. Ethnopharmacol. 2017, 206, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Peng, F.; Wang, L.; Xiong, L.; Tang, H.; Du, J.; Peng, C. Maackiain modulates miR-374a/GADD45A axis to inhibit triple-negative breast cancer initiation and progression. Front. Pharmacol. 2022, 13, 806869. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; García, R. SIR-2.1 integrates metabolic homeostasis with the reproductive neuromuscular excitability in early aging male Caenorhabditis elegans. eLife 2014, 3, e01730. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Xia, M.; Yan, H.; Han, Y.; Zhang, F.; Hu, Z.; Cui, A.; Ma, F.; Liu, Z.; Gong, Q.; et al. Berberine attenuates hepatic steatosis and enhances energy expenditure in mice by inducing autophagy and fibroblast growth factor 21. Br. J. Pharmacol. 2018, 175, 374–387. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, K.; Tano, I.; Kaneko, N.; Matsumoto, T.; Kobayashi, T. Plant polyphenols morin and quercetin rescue nitric oxide production in diabetic mouse aorta through distinct pathways. Biomed. Pharmacother. 2020, 129, 110463. [Google Scholar] [CrossRef]
- Islam, M.T.; Tuday, E.; Allen, S.; Kim, J.; Trott, D.W.; Holland, W.L.; Donato, A.J.; Lesniewski, L.A. Senolytic drugs, dasatinib and quercetin, attenuate adipose tissue inflammation, and ameliorate metabolic function in old age. Aging Cell 2023, 22, e13767. [Google Scholar] [CrossRef]
- Novais, E.J.; Tran, V.A.; Johnston, S.N.; Darris, K.R.; Roupas, A.J.; Sessions, G.A.; Shapiro, I.M.; Diekman, B.O.; Risbud, M.V. Long-term treatment with senolytic drugs dasatinib and quercetin ameliorates age-dependent intervertebral disc degeneration in mice. Nat. Commun. 2021, 12, 5213. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mladenova, S.G.; Todorova, M.N.; Savova, M.S.; Georgiev, M.I.; Mihaylova, L.V. Maackiain Mimics Caloric Restriction through aak-2-Mediated Lipid Reduction in Caenorhabditis elegans. Int. J. Mol. Sci. 2023, 24, 17442. https://doi.org/10.3390/ijms242417442
Mladenova SG, Todorova MN, Savova MS, Georgiev MI, Mihaylova LV. Maackiain Mimics Caloric Restriction through aak-2-Mediated Lipid Reduction in Caenorhabditis elegans. International Journal of Molecular Sciences. 2023; 24(24):17442. https://doi.org/10.3390/ijms242417442
Chicago/Turabian StyleMladenova, Saveta G., Monika N. Todorova, Martina S. Savova, Milen I. Georgiev, and Liliya V. Mihaylova. 2023. "Maackiain Mimics Caloric Restriction through aak-2-Mediated Lipid Reduction in Caenorhabditis elegans" International Journal of Molecular Sciences 24, no. 24: 17442. https://doi.org/10.3390/ijms242417442
APA StyleMladenova, S. G., Todorova, M. N., Savova, M. S., Georgiev, M. I., & Mihaylova, L. V. (2023). Maackiain Mimics Caloric Restriction through aak-2-Mediated Lipid Reduction in Caenorhabditis elegans. International Journal of Molecular Sciences, 24(24), 17442. https://doi.org/10.3390/ijms242417442







