Unsymmetrical Pd(II) Pincer Complexes with Benzothiazole and Thiocarbamate Flanking Units: Expedient Solvent-Free Synthesis and Anticancer Potential
Abstract
:1. Introduction
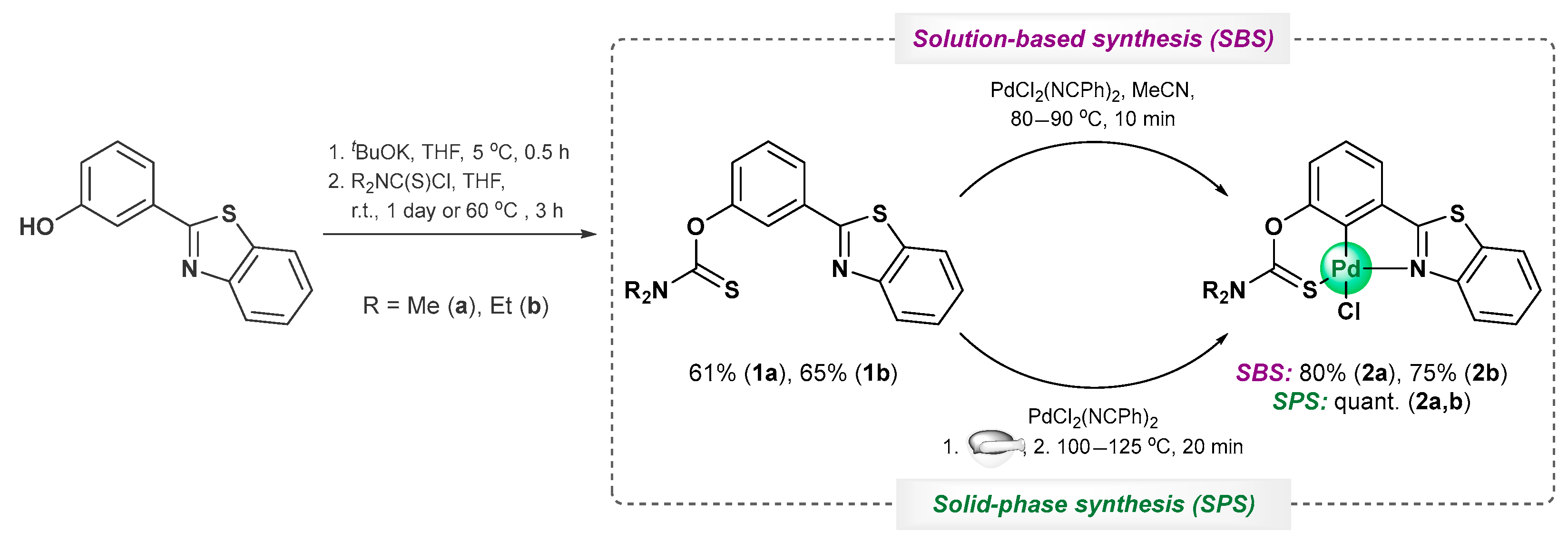
2. Results and Discussion
3. Materials and Methods
3.1. General Remarks
3.2. Syntheses
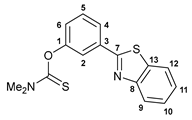
3.2.1. O-[3-(Benzo[d]thiazol-2-yl)phenyl] Dimethylthiocarbamate, 1a
3.2.2. O-[3-(Benzo[d]thiazol-2-yl)phenyl] Diethylthiocarbamate, 1b

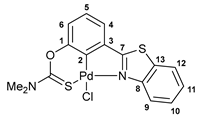
3.2.3. Solution-Based Synthesis and Characterization of [κ3-S,C,N-(L)Pd(II)Cl] Complex 2a
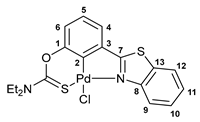
3.2.4. Solution-Based Synthesis and Characterization of [κ3-S,C,N-(L)Pd(II)Cl] Complex 2b
3.2.5. Solid-Phase Synthesis of [κ3-S,C,N-(L)Pd(II)Cl] Complexes 2a,b
3.3. X-ray Crystallography
3.4. Powder X-ray Diffraction Analysis
3.5. Bioactivity Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abdolmaleki, S.; Aliabadi, A.; Khaksar, S. Riding the metal wave: A review of the latest developments in metal-based anticancer agents. Coord. Chem. Rev. 2024, 501, 215579. [Google Scholar] [CrossRef]
- Lucaciu, R.L.; Hangan, A.C.; Sevastre, B.; Oprean, L.S. Metallo-drugs in cancer therapy: Past, present and future. Molecules 2022, 27, 6485. [Google Scholar] [CrossRef] [PubMed]
- Sen, S.; Won, M.; Levine, M.S.; Noh, Y.; Sedgwick, A.C.; Kim, J.S.; Sessler, J.L.; Arambula, J.F. Metal-based anticancer agents as immunogenic cell death inducers: The past, present, and future. Chem. Soc. Rev. 2022, 51, 1212–1233. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, M.G.; Piccolo, M.; Misso, G.; Santamaria, R.; Irace, C. Bioactivity and development of small non-platinum metal-based chemotherapeutics. Pharmaceutics 2022, 14, 954. [Google Scholar] [CrossRef] [PubMed]
- Shumi, G.; Desalegn, T.; Ramachandran, V.P.; Eswaramoorthy, R. Metal complexes in target-specific anticancer therapy: Recent trends and challenges. J. Chem. 2022, 9261683. [Google Scholar] [CrossRef]
- Zhang, C.; Xu, C.; Gao, X.; Yao, Q. Platinum-based drugs for cancer therapy and anti-tumor strategies. Theranostics 2022, 12, 2115–2132. [Google Scholar] [CrossRef] [PubMed]
- Paprocka, R.; Wiese-Szadkowska, M.; Janciauskiene, S.; Kosmalski, T.; Kulik, M.; Helmin-Basa, A. Latest developments in metal complexes as anticancer agents. Coord. Chem. Rev. 2022, 452, 214307. [Google Scholar] [CrossRef]
- Czarnomysy, R.; Radomska, D.; Szewczyk, O.K.; Roszczenko, P.; Bielawski, K. Platinum and palladium complexes as promising sources for antitumor treatments. Int. J. Mol. Sci. 2021, 22, 8271. [Google Scholar] [CrossRef]
- Rottenberg, S.; Disler, C.; Perego, P. The rediscovery of platinum-based cancer therapy. Nat. Rev. Cancer 2021, 21, 37–50. [Google Scholar] [CrossRef]
- Murray, B.S.; Dyson, P.J. Recent progress in the development of organometallics for the treatment of cancer. Curr. Opin. Chem. Biol. 2020, 56, 28–34. [Google Scholar] [CrossRef]
- Simpson, P.V.; Desai, N.M.; Casari, I.; Massi, M.; Falasca, M. Metal-based antitumor compounds: Beyond cisplatin. Future Med. Chem. 2019, 11, 119–135. [Google Scholar] [CrossRef] [PubMed]
- Englinger, M.; Pirker, C.; Heffeter, P.; Terenzi, A.; Kowol, C.R.; Keppler, B.K.; Berger, W. Metal drugs and the anticancer immune response. Chem. Rev. 2019, 119, 1519–1624. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, T.C.; Suntharalingam, K.; Lippard, S.J. The next generation of platinum drugs: Targeted Pt(II) agents, nanoparticle delivery, and Pt(IV) prodrugs. Chem. Rev. 2016, 116, 3436–3486. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Wu, Z.; Ge, Q.; Zheng, X.; Yang, Z. Antitumor activity of tridentate pincer and related metal complexes. Org. Biomol. Chem. 2021, 19, 5254–5273. [Google Scholar] [CrossRef] [PubMed]
- Bugarčić, Ž.D.; Bogojeski, J.; van Eldik, R. Kinetics, mechanism and equilibrium studies on the substitution reactions of Pd(II) in reference to Pt(II) complexes with bio-molecules. Coord. Chem. Rev. 2015, 292, 91–106. [Google Scholar] [CrossRef]
- Zhou, X.-Q.; Wang, P.; Ramu, V.; Zhang, L.; Jiang, S.; Li, X.; Abyar, S.; Papadopoulou, P.; Shao, Y.; Bretin, L.; et al. In vivo metallophilic self-assembly of a light-activated anticancer drug. Nat. Chem. 2023, 15, 980–987. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.-Q.; Xiao, M.; Ramu, V.; Hilgendorf, J.; Li, X.; Papadopoulou, P.; Siegler, M.A.; Kros, A.; Sun, W.; Bonnet, S. The self-assembly of a cyclometalated palladium photosensitizer into protein-stabilized nanorods triggers drug uptake in vitro and in vivo. J. Am. Chem. Soc. 2020, 142, 10383–10399. [Google Scholar] [CrossRef]
- Scattolin, T.; Pessotto, I.; Cavarzerani, E.; Canzonieri, V.; Orian, L.; Demitri, N.; Schmidt, C.; Casini, A.; Bortolamiol, E.; Visentin, F.; et al. Indenyl and allyl palladate complexes bearing N-heterocyclic carbene ligands: An easily accessible class of new anticancer drug candidates. Eur. J. Inorg. Chem. 2022, 2022, e202200103. [Google Scholar] [CrossRef]
- Scattolin, T.; Voloshkin, V.A.; Visentin, F.; Nolan, S.P. A critical review of palladium organometallic anticancer agents. Cell Rep. Phys. Sci. 2021, 2, 100446. [Google Scholar] [CrossRef]
- Vojtek, M.; Marques, M.P.M.; Ferreira, I.M.P.L.V.O.; Mota-Filipe, H.; Diniz, C. Anticancer activity of palladium-based complexes against triple-negative breast cancer. Drug Discov. Today 2019, 24, 1044–1058. [Google Scholar] [CrossRef]
- Bangde, P.; Prajapati, D.; Dandekar, P.; Fairlamb, I.J.S.; Kapdi, A.R. Chapter 9–Palladacycles as potential anticancer agents. In Palladacycles: Catalysis and Beyond; Kapdi, A.R., Maiti, D., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 343–370. [Google Scholar] [CrossRef]
- Mahapatra, R.; Hazra, K. A review on benzothiazole derivatives and their biological significances. Asian J. Res. Med. Pharm. Sci. 2023, 12, 13–19. [Google Scholar] [CrossRef]
- Kumar, S.; Dubey, B. A review on emerging benzothiazoles: Biological aspects. J. Drug Deliv. Ther. 2022, 12, 270–274. [Google Scholar] [CrossRef]
- Lihumis, H.S.; Alameri, A.A.; Zaooli, R.H. A review on recent development and biological applications of benzothiazole derivatives. Prog. Chem. Biochem. Res. 2022, 5, 147–164. [Google Scholar] [CrossRef]
- Haider, K.; Rehman, S.; Pathak, A.; Najmi, A.K.; Yar, M.S. Advances in 2-substituted benzothiazole scaffold-based chemotherapeutic agents. Arch. Pharm. 2021, 354, e2100246. [Google Scholar] [CrossRef]
- Ammazzalorso, A.; Carradori, S.; Amoroso, R.; Fernández, I.F. 2-Substituted benzothiazoles as antiproliferative agents: Novel insights on structure-activity relationships. Eur. J. Med. Chem. 2020, 207, 112762. [Google Scholar] [CrossRef]
- Irfan, A.; Batool, F.; Naqvi, S.A.Z.; Islam, A.; Osman, S.M.; Nocentini, A.; Alissa, S.A.; Supuran, C.T. Benzothiazole derivatives as anticancer agents. J. Enzym. Inhib. Med. Chem. 2020, 35, 265–279. [Google Scholar] [CrossRef]
- Keri, R.S.; Patil, M.R.; Patil, S.A.; Budagumpi, S. A comprehensive review in current developments of benzothiazole-based molecules in medicinal chemistry. Eur. J. Med. Chem. 2015, 89, 207–251. [Google Scholar] [CrossRef]
- Krause, M.; Friedel, J.; Buss, S.; Brünink, D.; Berger, A.; Strassert, C.A.; Doltsinis, N.L.; Klein, A. Isoelectronic Pt(II) complexes of cyclometalating C^N^N ligands with phenyl/(benzo)thiophenyl and pyridyl/(benzo)thiazolyl moieties. Dalton Trans. 2022, 51, 16181–16194. [Google Scholar] [CrossRef]
- Luconi, L.; Tuci, G.; Gafurov, Z.N.; Mercuri, G.; Kagilev, A.A.; Pettinari, C.; Morozov, V.I.; Yakhvarov, D.G.; Rossin, A.; Giambastiani, G. Unsymmetrical nickel (PCN) pincer complexes with a benzothiazole side-arm: Synthesis, characterization and electrochemical properties. Inorg. Chim. Acta 2021, 517, 120182. [Google Scholar] [CrossRef]
- Gafurov, Z.N.; Zueva, E.M.; Bekmukhamedov, G.E.; Kagilev, A.A.; Kantyukov, A.O.; Mikhailov, I.K.; Khayarov, K.R.; Petrova, M.M.; Dovzhenko, A.P.; Rossin, A.; et al. Benzothiazole- vs. pyrazole-based unsymmetrical (PCN) pincer complexes of nickel(II) as homogeneous catalysts in ethylene oligomerization. J. Organomet. Chem. 2021, 949, 121951. [Google Scholar] [CrossRef]
- Kuwabara, J.; Namekawa, T.; Sakabe, E.; Haga, M.-a.; Kanbara, T. Luminescent Ir(III) complexes bearing benzothiazole or benzoxazole-based pincer ligand. J. Organomet. Chem. 2017, 845, 189–195. [Google Scholar] [CrossRef]
- Lawrence, M.A.W.; Jackson, Y.A.; Mulder, W.H.; Martin Björemark, P.; Håkansson, M. Synthesis and structure of a novel substituted benzothiazolyl-N-phenyl-2-pyridinecarbothioamide; kinetics of formation and electrochemistry of two of its palladium pincer complexes. Aust. J. Chem. 2015, 68, 731–741. [Google Scholar] [CrossRef]
- Kuwabara, J.; Namekawa, T.; Haga, M.-a.; Kanbara, T. Luminescent Ir(III) complexes containing benzothiazole-based tridentate ligands: Synthesis, characterization, and application to organic light-emitting diodes. Dalton Trans. 2012, 41, 44–46. [Google Scholar] [CrossRef] [PubMed]
- Kozlov, V.A.; Aleksanyan, D.V.; Nelyubina, Y.V.; Lyssenko, K.A.; Petrovskii, P.V.; Vasil’ev, A.A.; Odinets, I.L. Hybrid thiophosphoryl–benzothiazole palladium SCN-pincer complexes: Synthesis and effect of structure modifications on catalytic performance in the Suzuki cross-coupling. Organometallics 2011, 30, 2920–2932. [Google Scholar] [CrossRef]
- Wongsuwan, S.; Chatwichien, J.; Sirisaksoontorn, W.; Chainok, K.; Songsasen, A.; Chotima, R. Novel Pd(II) pincer complexes bearing salicylaldimine-based benzothiazole derivatives: Synthesis, structural characterization, DNA/BSA binding, and biological evaluation. New J. Chem. 2023, 47, 10624–10637. [Google Scholar] [CrossRef]
- Kyhoiesh, H.A.K.; Al-Adilee, K.J. Synthesis, spectral characterization, antimicrobial evaluation studies and cytotoxic activity of some transition metal complexes with tridentate (N,N,O) donor azo dye ligand. Results Chem. 2021, 3, 100245. [Google Scholar] [CrossRef]
- Aleksanyan, D.V.; Churusova, S.G.; Rybalkina, E.Y.; Nelyubina, Y.V.; Kozlov, V.A. Pd(II) and Pt(II) pincer complexes of a benzothiazole-appended methylthioacetamide ligand: Synthesis and in vitro cytotoxicity. INEOS OPEN 2021, 4, 237–242. [Google Scholar] [CrossRef]
- Kozlov, V.A.; Aleksanyan, D.V.; Korobov, M.V.; Avramenko, N.V.; Aysin, R.R.; Maloshitskaya, O.A.; Korlyukov, A.S.; Odinets, I.L. The first solid phase synthesis of pincer palladium complexes. Dalton Trans. 2011, 40, 8768–8772. [Google Scholar] [CrossRef]
- Aleksanyan, D.V.; Kozlov, V.A. Mechanochemical tools in the synthesis of organometallic compounds. Mendeleev Commun. 2023, 33, 287–301. [Google Scholar] [CrossRef]
- Aleksanyan, D.V.; Churusova, S.G.; Dubasova, E.V.; Ananyev, I.V.; Artyushin, O.I.; Peregudov, A.S.; Klemenkova, Z.S.; Denisov, G.L.; Kozlov, V.A. Experimental and computational insights into the direct cyclopalladation of different unsymmetrical, yet closely related pincer ligands with thione sulfur donors. Polyhedron 2023, 233, 116303. [Google Scholar] [CrossRef]
- Aleksanyan, D.V.; Churusova, S.G.; Aysin, R.R.; Klemenkova, Z.S.; Nelyubina, Y.V.; Kozlov, V.A. The first example of mechanochemical synthesis of organometallic pincer complexes. Inorg. Chem. Commun. 2017, 76, 33–35. [Google Scholar] [CrossRef]
- Shaw, T.E.; Shultz, L.R.; Garayeva, L.R.; Blair, R.G.; Noll, B.C.; Jurca, T. Mechanochemical routes for the synthesis of acetyl- and bis-(imino)pyridine ligands and organometallics. Dalton Trans. 2018, 47, 16876–16884. [Google Scholar] [CrossRef]
- Shaw, T.E.; Mathivathanan, L.; Jurca, T. One-pot, one-step precatalysts through mechanochemistry. Organometallics 2019, 38, 4066–4070. [Google Scholar] [CrossRef]
- Pudovik, A.N.; Khairullin, V.K.; Vasyanina, M.A.; Kamalov, R.M.; Kataeva, O.N.; Litvinov, I.A.; Naumov, V.A. Synthesis and structure of the dimethylamide of chlorothioformic acid and its reaction with potassium thiocyanate. Bull. Acad. Sci. USSR Div. Chem. Sci. 1989, 38, 819–822. [Google Scholar] [CrossRef]
- Bellamy, L.J. The Infrared Spectra of Complex Molecules; Wiley: New York, NY, USA, 1975. [Google Scholar]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A: Found. Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Bruker AXS GmbH. DIFFRAC.EVA; Bruker AXS GmbH: Karlsruhe, Germany, 2011. [Google Scholar]
- Coelho, A. TOPIC 5.0; Bruker AXS GmbH: Karlsruhe, Germany, 2012. [Google Scholar]
- Aleksanyan, D.V.; Konovalov, A.V.; Churusova, S.G.; Rybalkina, E.Y.; Peregudov, A.S.; Aksenova, S.A.; Gutsul, E.I.; Klemenkova, Z.S.; Kozlov, V.A. Modulation of the cytotoxic properties of Pd(II) complexes based on functionalized carboxamides featuring labile phosphoryl coordination sites. Pharmaceutics 2023, 15, 1088. [Google Scholar] [CrossRef]




| Cell Lines | IC50 ± SD 1, μM | |||
|---|---|---|---|---|
| 2a | 2b | VI (X = O) | Cisplatin | |
| Solid cancer cell lines | ||||
| HCT116 | 1.3 ± 0.2 | 12.7 ± 1.5 | 10.3 ± 0.8 | 18.0 ± 2.0 |
| MCF7 | 3.3 ± 0.4 | 13.5 ± 2.1 | 17.0 ± 0.5 | 25.0 ± 4.0 |
| PC3 | 1.1 ± 0.3 | 8.5 ± 2.5 | 6.6 ± 0.6 | 16.0 ± 3.0 |
| Hematopoietic cancer cell lines | ||||
| K562 | 0.9 ± 0.2 | 2.9 ± 0.4 | 1.9 ± 0.3 | 15.5 ± 0.5 |
| K562/iS9 | 0.9 ± 0.1 | 2.6 ± 0.8 | 2.5 ± 0.4 | 16.0 ± 2.0 |
| AMO1 | 0.3 ± 0.1 | 4.4 ± 0.8 | 3.4 ± 0.5 | 3.2 ± 0.6 |
| H9 | 1.3 ± 0.1 | 2.1 ± 0.9 | 1.7 ± 0.3 | 3.0 ± 1.0 |
| Non-cancerous cell lines | ||||
| HEK293 | 2.5 ± 0.2 | 9.5 ± 0.7 | 13.0 ± 0.5 | 12.5 ± 1.5 |
| HBL100 | 3.2 ± 0.5 | 10.2 ± 3.3 | 9.4 ± 0.9 | 14.6 ± 3.6 |
| HBL100/dox | 4.4 ± 0.8 | 16.3 ± 3.8 | 28.6 ± 3.8 | 23.6 ± 3.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozlov, V.A.; Aleksanyan, D.V.; Churusova, S.G.; Spiridonov, A.A.; Rybalkina, E.Y.; Gutsul, E.I.; Aksenova, S.A.; Korlyukov, A.A.; Peregudov, A.S.; Klemenkova, Z.S. Unsymmetrical Pd(II) Pincer Complexes with Benzothiazole and Thiocarbamate Flanking Units: Expedient Solvent-Free Synthesis and Anticancer Potential. Int. J. Mol. Sci. 2023, 24, 17331. https://doi.org/10.3390/ijms242417331
Kozlov VA, Aleksanyan DV, Churusova SG, Spiridonov AA, Rybalkina EY, Gutsul EI, Aksenova SA, Korlyukov AA, Peregudov AS, Klemenkova ZS. Unsymmetrical Pd(II) Pincer Complexes with Benzothiazole and Thiocarbamate Flanking Units: Expedient Solvent-Free Synthesis and Anticancer Potential. International Journal of Molecular Sciences. 2023; 24(24):17331. https://doi.org/10.3390/ijms242417331
Chicago/Turabian StyleKozlov, Vladimir A., Diana V. Aleksanyan, Svetlana G. Churusova, Aleksandr A. Spiridonov, Ekaterina Yu. Rybalkina, Evgenii I. Gutsul, Svetlana A. Aksenova, Alexander A. Korlyukov, Alexander S. Peregudov, and Zinaida S. Klemenkova. 2023. "Unsymmetrical Pd(II) Pincer Complexes with Benzothiazole and Thiocarbamate Flanking Units: Expedient Solvent-Free Synthesis and Anticancer Potential" International Journal of Molecular Sciences 24, no. 24: 17331. https://doi.org/10.3390/ijms242417331