The Implication of Sphingolipids in Viral Infections
Abstract
:1. Introduction
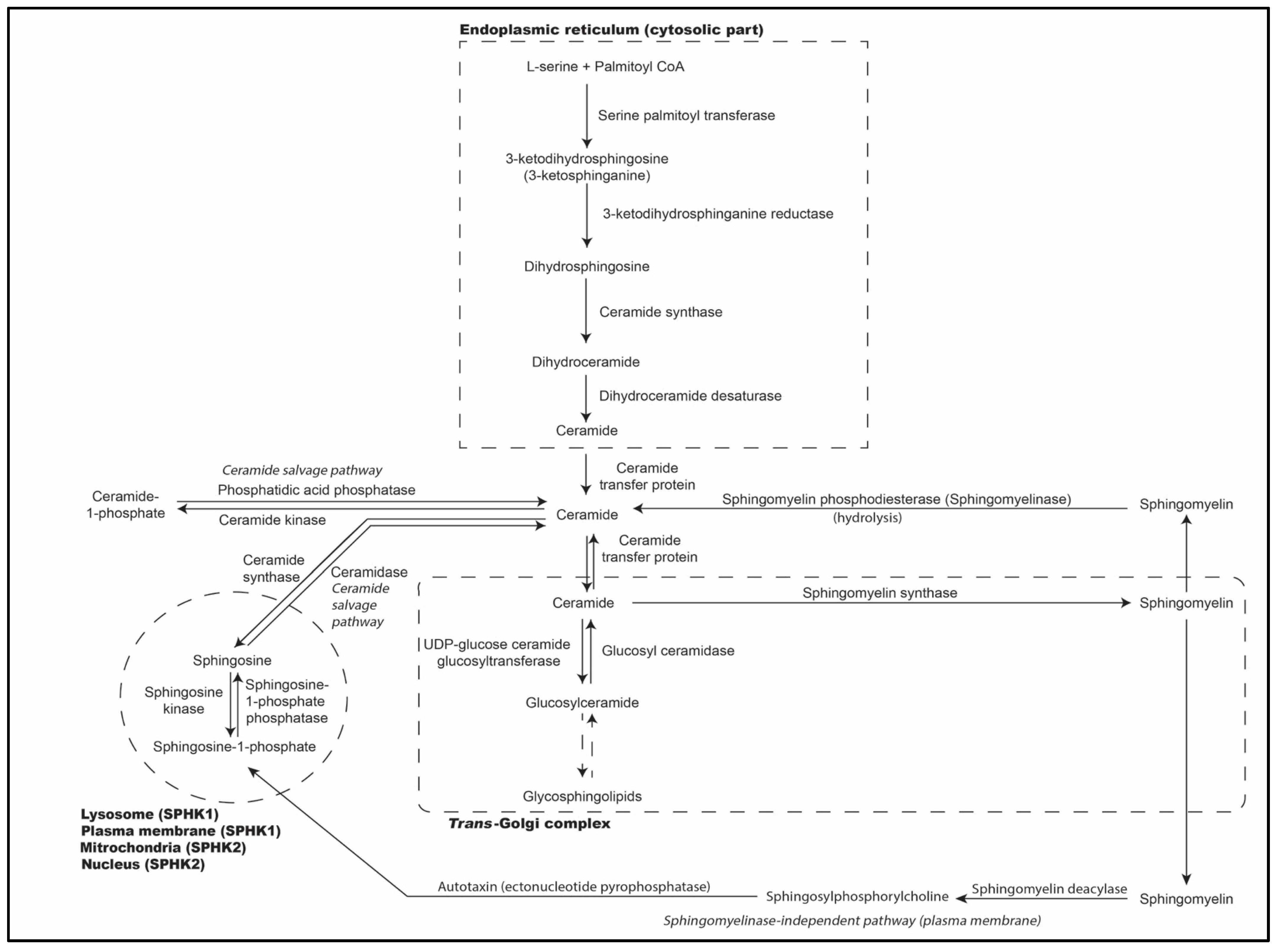
2. Biosynthesis of Sphingolipids
3. Role of Sphingolipid Metabolites in Immunometabolism
4. Role of Sphingolipids in Infectious Diseases
5. Knowledge Gaps in Sphingolipid Metabolism
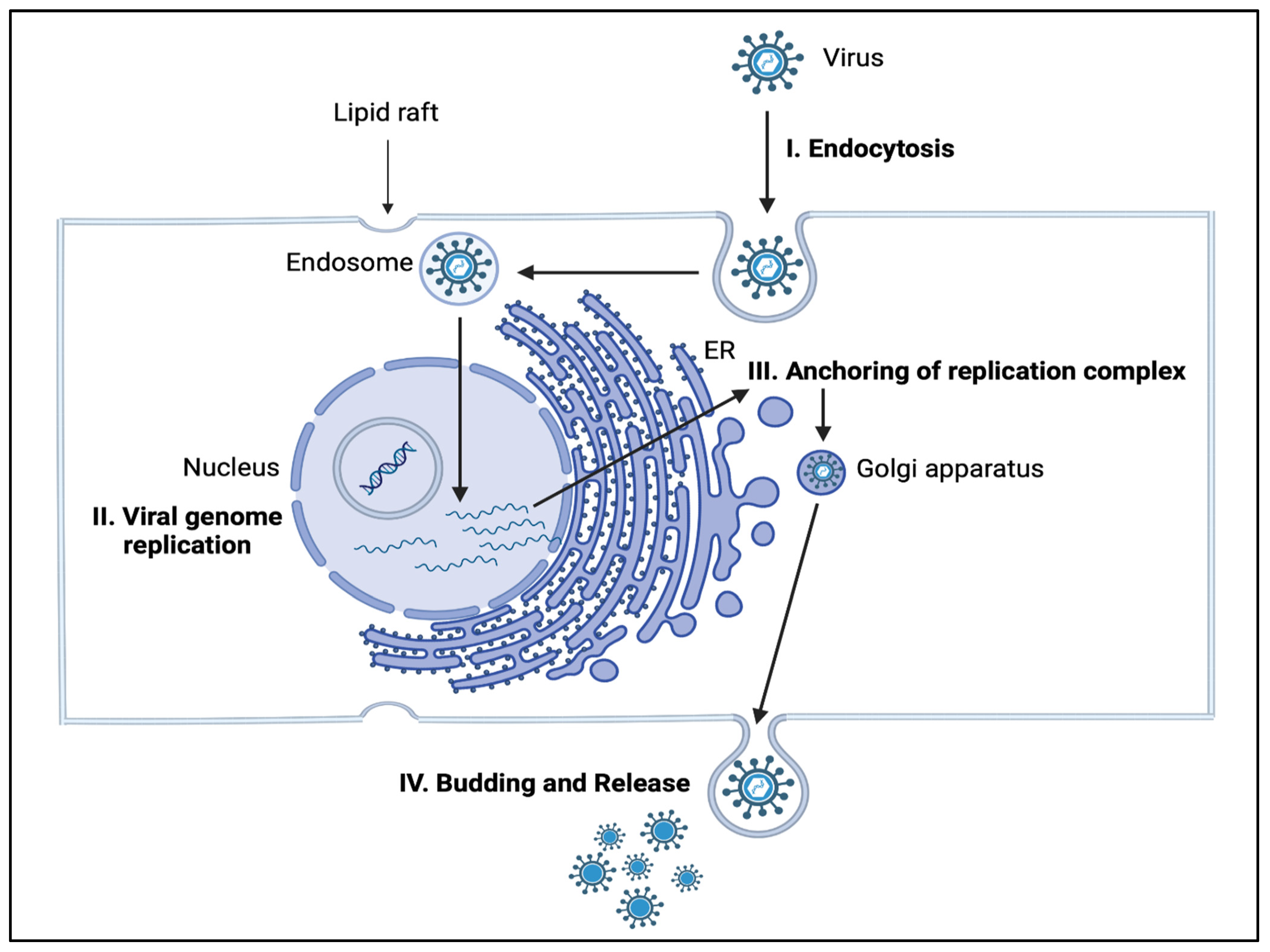
6. Sphingolipids as Potential Targets against Viruses
6.1. Influenza Virus
6.2. Dengue Virus
6.3. Zika Virus
6.4. Japanese Encephalitis Virus (JEV)
6.5. West Nile Virus
6.6. Hepatitis C Virus
6.7. Ebola Virus
6.8. Norovirus
6.9. Adenovirus
6.10. Human Immunodeficiency Virus (HIV)
6.11. Rhinoviruses
6.12. SARS-CoV-2
6.13. Rubella Virus
| Virus | Sphingolipid Targets/Component Involved | Effect |
|---|---|---|
| Ebola | Sphingomyelin | Promotion [72] |
| Ebola | Glucosylceramide | Promotion [55] |
| HIV | Sphingomyelin | Inhibition [77] |
| Influenza | Glucosylceramide | Promotion [55] |
| Influenza | Sphingomyelin | Inhibition [56] |
| JEV | Sphingomyelin | Promotion [62] |
| Measles | Glucosylceramide | Promotion [55] |
| Rhinovirus | Ceramide | Promotion [56,86] |
| Rhinovirus | Sphingomyelin | Promotion [56] |
| SARS-CoV-2 | Ceramide | Promotion [55,87] |
| SARS-CoV-2 | Glucosylceramide | Promotion [55,88] |
| SARS-CoV-2 | Sphingomyelin | Inhibition [56] |
| SARS-CoV-2 | Sphingosine | Inhibition [91] |
| Mechanism | Virus | Metabolites Involved |
|---|---|---|
| Alteration of lipid composition of cell surface for viral entry | Influenza virus | Sphingomyelin synthase 1 [46,47] |
| Japanese encephalitis | Sphingomyelin synthase 1, Sphingomyelinase [61,62] | |
| Norovirus | Sphingomyelin, Ceramide synthesis [73,74] | |
| Zika virus | NS4B protein causing sphingolipid alteration [59] | |
| Dengue virus | Upregulation of sphingomyelin and ceramide [57] | |
| Ebola virus | Sphingomyelin [72] | |
| Hepatitis C virus | Sphingomyelin [68] | |
| Human immunodeficiency virus (HIV) | Gp120, Sphingomyelinase, GM ganglioside, Galactocerebroside [76,77,80,81,82] | |
| Rhinovirus | Sphingomyelinase, Ceramide [56] | |
| SARS-CoV-2 | Glucosylceramide, Sphingomyelinase activation, Sphingosine binding to ACE receptor halting viral entry, Acid sphingomyelinase [87,88,91,93] | |
| Adenovirus | Ceramide, Sphingomyelin degradation altered [75] | |
| Rubella virus | Sphingomyelin synthase 1 [94,95] | |
| Alteration of replication machinery | Influenza virus | Sphingomyelinase, Sphingosine kinase-1 [50,56] |
| Zika virus | Glucosylceramide, Lactosylceramide [60] | |
| Japanese encephalitis virus | Sphingomyelinase activation [61,62] | |
| Hepatitis C virus | Sphingomyelin induced RNA polymerase [69,70,71] | |
| West Nile virus | Sphingomyelin [63] | |
| Rhinovirus | Sphingomyelinase [56] | |
| SARS-CoV-2 | Acid sphingomyelinase [56] | |
| Viral replication in endoplasmic reticulum | Dengue virus | Ganglioside GM3 [53] |
| Alteration in budding and release | Influenza virus | Neuraminidase trafficking aided by sphingomyelin synthesis [48] |
| HIV | Sphingomyelin, Gangliosides [78,83] | |
| West Nile virus | Altered sphingomyelinase activity [64] |
7. Targeting Sphingolipid Biomarkers for Vaccine or Therapeutic Development
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hannun, Y.A.; Obeid, L.M. Sphingolipids and their metabolism in physiology and disease. Nat. Rev. Mol. Cell Biol. 2018, 19, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Penno, A.; Reilly, M.M.; Houlden, H.; Laurá, M.; Rentsch, K.; Niederkofler, V.; Stoeckli, E.T.; Nicholson, G.; Eichler, F.; Brown, R.H., Jr.; et al. Hereditary Sensory Neuropathy Type 1 Is Caused by the Accumulation of Two Neurotoxic Sphingolipids. J. Biol. Chem. 2010, 285, 11178–11187. [Google Scholar] [CrossRef] [PubMed]
- Bezgovsek, J.; Gulbins, E.; Friedrich, S.-K.; Lang, K.S.; Duhan, V. Sphingolipids in early viral replication and innate immune activation. Biol. Chem. 2018, 399, 1115–1123. [Google Scholar] [CrossRef] [PubMed]
- Avota, E.; Bodem, J.; Chithelen, J.; Mandasari, P.; Beyersdorf, N.; Schneider-Schaulies, J. The Manifold Roles of Sphingolipids in Viral Infections. Front. Physiol. 2021, 12, 715527. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Lee, S.Y.; Bae, Y.-S. Functional roles of sphingolipids in immunity and their implication in disease. Exp. Mol. Med. 2023, 55, 1110–1130. [Google Scholar] [CrossRef] [PubMed]
- Harrison, P.J.; Dunn, T.M.; Campopiano, D.J. Sphingolipid biosynthesis in man and microbes. Nat. Prod. Rep. 2018, 35, 921–954. [Google Scholar] [CrossRef]
- Lowther, J.; Naismith, J.H.; Dunn, T.M.; Campopiano, D.J. Structural, mechanistic and regulatory studies of serine palmitoyltransferase. Biochem. Soc. Trans. 2012, 40, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Hanada, K. Serine palmitoyltransferase, a key enzyme of sphingolipid metabolism. Biochim. Biophys. Acta BBA—Mol. Cell Biol. Lipids 2003, 1632, 16–30. [Google Scholar] [CrossRef]
- Kihara, A.; Igarashi, Y. FVT-1 Is a Mammalian 3-Ketodihydrosphingosine Reductase with an Active Site That Faces the Cytosolic Side of the Endoplasmic Reticulum Membrane. J. Biol. Chem. 2004, 279, 49243–49250. [Google Scholar] [CrossRef]
- Mullen, T.D.; Hannun, Y.A.; Obeid, L.M. Ceramide synthases at the centre of sphingolipid metabolism and biology. Biochem. J. 2012, 441, 789–802. [Google Scholar] [CrossRef]
- Futerman, M.L.; Mammalian, A.H. Ceramide Synthases. IUBMB Life 2010, 62, 347–356. [Google Scholar]
- Ternes, P.; Franke, S.; Zähringer, U.; Sperling, P.; Heinz, E. Identification and Characterization of a Sphingolipid Δ4-Desaturase Family. J. Biol. Chem. 2002, 277, 25512–25518. [Google Scholar] [CrossRef] [PubMed]
- Yager, E.J.; Konan, K.V. Sphingolipids as Potential Therapeutic Targets against Enveloped Human RNA Viruses. Viruses 2019, 11, 912. [Google Scholar] [CrossRef]
- Venkataraman, K.; Futerman, A.H.; Venkataraman, K.; Futerman, A.H. Ceramide as a second messenger: Sticky solutions to sticky problems. Trends Cell Biol. 2000, 10, 408–412. [Google Scholar] [CrossRef]
- Futerman, A.H.; Hannun, Y.A. The complex life of simple sphingolipids. Embo Rep. 2004, 5, 777–782. [Google Scholar] [CrossRef] [PubMed]
- Rotthier, A.; Auer-Grumbach, M.; Janssens, K.; Baets, J.; Penno, A.; Almeida-Souza, L.; Van Hoof, K.; Jacobs, A.; De Vriendt, E.; Schlotter-Weigel, B.; et al. Mutations in the SPTLC2 Subunit of Serine Palmitoyltransferase Cause Hereditary Sensory and Autonomic Neuropathy Type I. Am. J. Hum. Genet. 2010, 87, 513–522. [Google Scholar] [CrossRef] [PubMed]
- Cowart, L.A.; Hannun, Y.A. Selective Substrate Supply in the Regulation of Yeast de Novo Sphingolipid Synthesis. J. Biol. Chem. 2007, 282, 12330–12340. [Google Scholar] [CrossRef]
- Siow, D.L.; Wattenberg, B.W. Mammalian ORMDL Proteins Mediate the Feedback Response in Ceramide Biosynthesis. J. Biol. Chem. 2012, 287, 40198–40204. [Google Scholar] [CrossRef]
- Xie, T.; Liu, P.; Wu, X.; Dong, F.; Zhang, Z.; Yue, J.; Mahawar, U.; Farooq, F.; Vohra, H.; Fang, Q.; et al. Ceramide sensing by human SPT-ORMDL complex for establishing sphingolipid homeostasis. Nat. Commun. 2023, 14, 3475. [Google Scholar] [CrossRef]
- Merrill, A.H.; Schmelz, E.-M.; Dillehay, D.L.; Spiegel, S.; Shayman, J.A.; Schroeder, J.J.; Riley, R.T.; Voss, K.A.; Wang, E. Sphingolipids—The Enigmatic Lipid Class: Biochemistry, Physiology, and Pathophysiology. Toxicol. Appl. Pharm. 1997, 142, 208–225. [Google Scholar] [CrossRef]
- Muir, A.; Ramachandran, S.; Roelants, F.M.; Timmons, G.; Thorner, J. TORC2-dependent protein kinase Ypk1 phosphorylates ceramide synthase to stimulate synthesis of complex sphingolipids. Elife 2014, 3, e03779. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Miao, Y.; Yamane, Y.; Zhang, C.; Shokat, K.M.; Takematsu, H.; Kozutsumi, Y.; Drubin, D.G. Orm protein phosphoregulation mediates transient sphingolipid biosynthesis response to heat stress via the Pkh-Ypk and Cdc55-PP2A pathways. Mol. Biol. Cell 2012, 23, 2388–2398. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, V.; Costa, V. Unraveling the role of the Target of Rapamycin signaling in sphingolipid metabolism. Prog. Lipid Res. 2016, 61, 109–133. [Google Scholar] [CrossRef]
- Sasset, L.; Chowdhury, K.H.; Manzo, O.L.; Rubinelli, L.; Konrad, C.; Maschek, J.A.; Manfredi, G.; Holland, W.L.; Di Lorenzo, A. Sphingosine-1-phosphate controls endothelial sphingolipid homeostasis via ORMDL. EMBO Rep. 2023, 24, e54689. [Google Scholar] [CrossRef] [PubMed]
- Sassa, T.; Hirayama, T.; Kihara, A. Enzyme Activities of the Ceramide Synthases CERS2–6 Are Regulated by Phosphorylation in the C-terminal Region. J. Biol. Chem. 2016, 291, 7477–7487. [Google Scholar] [CrossRef]
- Jensen, S.A.; Calvert, A.E.; Volpert, G.; Kouri, F.M.; Hurley, L.A.; Luciano, J.P.; Wu, Y.; Chalastanis, A.; Futerman, A.H.; Stegh, A.H. Bcl2L13 is a ceramide synthase inhibitor in glioblastoma. Proc. Natl. Acad. Sci. USA 2014, 111, 5682–5687. [Google Scholar] [CrossRef]
- Chakraborty, P.; Vaena, S.G.; Thyagarajan, K.; Chatterjee, S.; Al-Khami, A.; Selvam, S.P.; Nguyen, H.; Kang, I.; Wyatt, M.W.; Baliga, U.; et al. Pro-Survival Lipid Sphingosine-1-Phosphate Metabolically Programs T Cells to Limit Anti-tumor Activity. Cell Rep. 2019, 28, 1879–1893.e7. [Google Scholar] [CrossRef]
- Bryan, A.M.; Poeta, M.D. Sphingosine-1-phosphate receptors and innate immunity. Cell Microbiol. 2018, 20, e12836. [Google Scholar] [CrossRef]
- Liu, G.; Yang, K.; Burns, S.; Shrestha, S.; Chi, H. The S1P1-mTOR axis directs the reciprocal differentiation of TH1 and Treg cells. Nat. Immunol. 2010, 11, 1047–1056. [Google Scholar] [CrossRef]
- Shen, H.; Giordano, F.; Wu, Y.; Chan, J.; Zhu, C.; Milosevic, I.; Wu, X.; Yao, K.; Chen, B.; Baumgart, T.; et al. Coupling between endocytosis and sphingosine kinase 1 recruitment. Nat. Cell Biol. 2014, 16, 652–662. [Google Scholar] [CrossRef]
- Janneh, A.H.; Kassir, M.F.; Dwyer, C.J.; Chakraborty, P.; Pierce, J.S.; Flume, P.A.; Li, H.; Nadig, S.N.; Mehrotra, S.; Ogretmen, B. Alterations of lipid metabolism provide serologic biomarkers for the detection of asymptomatic versus symptomatic COVID-19 patients. Sci. Rep. 2021, 11, 14232. [Google Scholar] [CrossRef] [PubMed]
- Baeyens, A.; Bracero, S.; Chaluvadi, V.S.; Khodadadi-Jamayran, A.; Cammer, M.; Schwab, S.R. Monocyte-derived S1P in the lymph node regulates immune responses. Nature 2021, 592, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Oberholtzer, N.; Quinn, K.M.; Chakraborty, P.; Mehrotra, S. New Developments in T Cell Immunometabolism and Implications for Cancer Immunotherapy. Cells 2022, 11, 708. [Google Scholar] [CrossRef] [PubMed]
- Ghidoni, R.; Caretti, A.; Signorelli, P. Role of Sphingolipids in the Pathobiology of Lung Inflammation. Mediat. Inflamm. 2015, 2015, 487508. [Google Scholar] [CrossRef] [PubMed]
- Nowakowski, T.J.; Pollen, A.A.; Di Lullo, E.; Sandoval-Espinosa, C.; Bershteyn, M.; Kriegstein, A.R. Expression Analysis Highlights AXL as a Candidate Zika Virus Entry Receptor in Neural Stem Cells. Cell Stem Cell 2016, 18, 591–596. [Google Scholar] [CrossRef] [PubMed]
- Richard, A.S.; Shim, B.-S.; Kwon, Y.-C.; Zhang, R.; Otsuka, Y.; Schmitt, K.; Berri, F.; Diamond, M.S.; Choe, H. AXL-dependent infection of human fetal endothelial cells distinguishes Zika virus from other pathogenic flaviviruses. Proc. Natl. Acad. Sci. USA 2017, 114, 2024–2029. [Google Scholar] [CrossRef]
- Meertens, L.; Carnec, X.; Lecoin, M.P.; Ramdasi, R.; Guivel-Benhassine, F.; Lew, E.; Lemke, G.; Schwartz, O.; Amara, A. The TIM and TAM Families of Phosphatidylserine Receptors Mediate Dengue Virus Entry. Cell Host Microbe 2012, 12, 544–557. [Google Scholar] [CrossRef]
- Aligo, J.; Jia, S.; Manna, D.; Konan, K.V. Formation and function of hepatitis C virus replication complexes require residues in the carboxy-terminal domain of NS4B protein. Virology 2009, 393, 68–83. [Google Scholar] [CrossRef]
- Stone, M.; Jia, S.; Heo, W.D.; Meyer, T.; Konan, K.V. Participation of Rab5, an Early Endosome Protein, in Hepatitis C Virus RNA Replication Machinery. J. Virol. 2007, 81, 4551–4563. [Google Scholar] [CrossRef]
- Mackenzie, J.M.; Jones, M.K.; Westaway, E.G. Markers for trans -Golgi Membranes and the Intermediate Compartment Localize to Induced Membranes with Distinct Replication Functions in Flavivirus-Infected Cells. J. Virol. 1999, 73, 9555–9567. [Google Scholar] [CrossRef]
- Westaway, E.G.; Mackenzie, J.M.; Kenney, M.T.; Jones, M.K.; Khromykh, A.A. Ultrastructure of Kunjin virus-infected cells: Colocalization of NS1 and NS3 with double-stranded RNA, and of NS2B with NS3, in virus-induced membrane structures. J. Virol. 1997, 71, 6650–6661. [Google Scholar] [CrossRef]
- Miyanari, Y.; Atsuzawa, K.; Usuda, N.; Watashi, K.; Hishiki, T.; Zayas, M.; Bartenschlager, R.; Wakita, T.; Hijikata, M.; Shimotohno, K. The lipid droplet is an important organelle for hepatitis C virus production. Nat. Cell Biol. 2007, 9, 1089–1097. [Google Scholar] [CrossRef] [PubMed]
- Jackson, D.A.; Caton, A.J.; McCready, S.J.; Cook, P.R. Influenza virus RNA is synthesized at fixed sites in the nucleus. Nature 1982, 296, 366–368. [Google Scholar] [CrossRef] [PubMed]
- Bukrinsky, M. A Hard Way to the Nucleus. Mol. Med. 2004, 10, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Nayak, D.P.; Balogun, R.A.; Yamada, H.; Zhou, Z.H.; Barman, S. Influenza virus morphogenesis and budding. Virus Res. 2009, 143, 147–161. [Google Scholar] [CrossRef]
- Halter, D.; Neumann, S.; van Dijk, S.M.; Wolthoorn, J.; de Mazière, A.M.; Vieira, O.V.; Mattjus, P.; Klumperman, J.; van Meer, G.; Sprong, H. Pre- and post-Golgi translocation of glucosylceramide in glycosphingolipid synthesis. J. Cell Biol. 2007, 179, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Mizuike, A.; Sakai, S.; Katoh, K.; Yamaji, T.; Hanada, K. The C10orf76–PI4KB axis orchestrates CERT-mediated ceramide trafficking to the distal Golgi. J. Cell Biol. 2023, 222, e202111069. [Google Scholar] [CrossRef] [PubMed]
- Tafesse, F.G.; Sanyal, S.; Ashour, J.; Guimaraes, C.P.; Hermansson, M.; Somerharju, P.; Ploegh, H.L. Intact sphingomyelin biosynthetic pathway is essential for intracellular transport of influenza virus glycoproteins. Proc. Natl. Acad. Sci. USA 2013, 110, 6406–6411. [Google Scholar] [CrossRef] [PubMed]
- Takeda, M.; Leser, G.P.; Russell, C.J.; Lamb, R.A. Influenza virus hemagglutinin concentrates in lipid raft microdomains for efficient viral fusion. Proc. Natl. Acad. Sci. USA 2003, 100, 14610–14617. [Google Scholar] [CrossRef]
- Seo, Y.-J.; Pritzl, C.J.; Vijayan, M.; Bomb, K.; McClain, M.E.; Alexander, S.; Hahm, B. Sphingosine Kinase 1 Serves as a Pro-Viral Factor by Regulating Viral RNA Synthesis and Nuclear Export of Viral Ribonucleoprotein Complex upon Influenza Virus Infection. PLoS ONE 2013, 8, e75005. [Google Scholar] [CrossRef]
- Leser, G.P.; Lamb, R.A. Influenza virus assembly and budding in raft-derived microdomains: A quantitative analysis of the surface distribution of, H.A.; NA and M2 proteins. Virology 2005, 342, 215–227. [Google Scholar] [CrossRef] [PubMed]
- Barman, S.; Krylov, P.S.; Turner, J.C.; Franks, J.; Webster, R.G.; Husain, M.; Webby, R.J. Manipulation of neuraminidase packaging signals and hemagglutinin residues improves the growth of A/Anhui/1/2013 (H7N9) influenza vaccine virus yield in eggs. Vaccine 2017, 35, 1424–1430. [Google Scholar] [CrossRef]
- Wang, K.; Wang, J.; Sun, T.; Bian, G.; Pan, W.; Feng, T.; Wang, P.; Li, Y.; Dai, J. Glycosphingolipid GM3 is Indispensable for Dengue Virus Genome Replication. Int. J. Biol. Sci. 2016, 12, 872–883. [Google Scholar] [CrossRef]
- Baden, L.R.; Petersen, L.R.; Jamieson, D.J.; Powers, A.M.; Honein, M.A. Zika Virus. N. Engl. J. Med. 2016, 374, 1552–1563. [Google Scholar]
- Drews, K.; Calgi, M.P.; Harrison, W.C.; Drews, C.M.; Costa-Pinheiro, P.; Shaw, J.J.P.; Jobe, K.A.; Han, J.D.; Fox, T.E.; White, J.M.; et al. Glucosylceramide synthase maintains influenza virus entry and infection. PLoS ONE 2020, 15, e0228735. [Google Scholar] [CrossRef]
- Dissanayake, T.K.; Yan, B.; Ng, A.C.-K.; Zhao, H.; Chan, G.; Yip, C.C.-Y.; Sze, K.H.; To, K.K. Differential role of sphingomyelin in influenza virus, rhinovirus and SARS-CoV-2 infection of Calu-3 cells. J. Gen. Virol. 2021, 102, 001593. [Google Scholar] [CrossRef]
- Perera, R.; Riley, C.; Isaac, G.; Hopf-Jannasch, A.S.; Moore, R.J.; Weitz, K.W.; Pasa-Tolic, L.; Metz, T.O.; Adamec, J.; Kuhn, R.J. Dengue Virus Infection Perturbs Lipid Homeostasis in Infected Mosquito Cells. PLoS Pathog. 2012, 8, e1002584. [Google Scholar] [CrossRef]
- Turtle, L.; Solomon, T. Japanese encephalitis—The prospects for new treatments. Nat. Rev. Neurol. 2018, 14, 298–313. [Google Scholar] [CrossRef]
- Leier, H.C.; Weinstein, J.B.; Kyle, J.E.; Lee, J.-Y.; Bramer, L.M.; Stratton, K.G.; Kempthorne, D.; Navratil, A.R.; Tafesse, E.G.; Hornemann, T.; et al. A global lipid map defines a network essential for Zika virus replication. Nat. Commun. 2020, 11, 3652. [Google Scholar] [CrossRef]
- Konan, K.V.; Ogbamikael, S.A.; Yager, E.; Yamaji, T.; Cerone, J.; Monaco-Brown, M.; Barroso, M.; Hanada, K. Modulation of Zika virus replication via glycosphingolipids. Virology 2022, 572, 17–27. [Google Scholar] [CrossRef]
- Tani, H.; Shiokawa, M.; Kaname, Y.; Kambara, H.; Mori, Y.; Abe, T.; Moriishi, K.; Matsuura, Y. Involvement of Ceramide in the Propagation of Japanese Encephalitis Virus. J. Virol. 2010, 84, 2798–2807. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, M.; Tasaki, T.; Ninomiya, H.; Ueda, Y.; Kuremoto, K.; Mitsutake, S.; Igarashi, Y.; Okazaki, T.; Takegami, T. Sphingomyelin generated by sphingomyelin synthase 1 is involved in attachment and infection with Japanese encephalitis virus. Sci. Rep. 2016, 6, 37829. [Google Scholar] [CrossRef]
- Martín-Acebes, M.A.; Gabandé-Rodríguez, E.; García-Cabrero, A.M.; Sánchez, M.P.; Ledesma, M.D.; Sobrino, F.; Saiz, J.C. Host sphingomyelin increases West Nile virus infection in vivo. J. Lipid Res. 2016, 57, 422–432. [Google Scholar] [CrossRef] [PubMed]
- Martín-Acebes, M.A.; Merino-Ramos, T.; Blázquez, A.-B.; Casas, J.; Escribano-Romero, E.; Sobrino, F.; Saiz, J.C. The Composition of West Nile Virus Lipid Envelope Unveils a Role of Sphingolipid Metabolism in Flavivirus Biogenesis. J. Virol. 2014, 88, 12041–12054. [Google Scholar] [CrossRef] [PubMed]
- Manns, M.P.; Buti, M.; Gane, E.; Pawlotsky, J.-M.; Razavi, H.; Terrault, N.; Younossi, Z. Hepatitis C virus infection. Nat. Rev. Dis. Primers 2017, 3, 17006. [Google Scholar] [CrossRef] [PubMed]
- Aizaki, H.; Lee, K.-J.; Sung, V.M.-H.; Ishiko, H.; Lai, M.M.C. Characterization of the hepatitis C virus RNA replication complex associated with lipid rafts. Virology 2004, 324, 450–461. [Google Scholar] [CrossRef] [PubMed]
- Merz, A.; Long, G.; Hiet, M.-S.; Brügger, B.; Chlanda, P.; Andre, P.; Wieland, F.; Krijnse-Locker, J.; Bartenschlager, R. Biochemical and Morphological Properties of Hepatitis C Virus Particles and Determination of Their Lipidome. J. Biol. Chem. 2011, 286, 3018–3032. [Google Scholar] [CrossRef]
- Aizaki, H.; Morikawa, K.; Fukasawa, M.; Hara, H.; Inoue, Y.; Tani, H.; Saito, K.; Nishijima, M.; Hanada, K.; Matsuura, Y.; et al. Critical Role of Virion-Associated Cholesterol and Sphingolipid in Hepatitis C Virus Infection. J. Virol. 2008, 82, 5715–5724. [Google Scholar] [CrossRef]
- Konan, K.V.; Giddings, T.H., Jr.; Ikeda, M.; Li, K.; Lemon, S.M.; Kirkegaard, K. Nonstructural Protein Precursor NS4A/B from Hepatitis C Virus Alters Function and Ultrastructure of Host Secretory Apparatus. J. Virol. 2003, 77, 7843–7855. [Google Scholar] [CrossRef]
- Egger, D.; Wolk, B.; Gosert, R.; Bianchi, L.; Blum, H.E.; Moradpour, D.; Bienz, K. Expression of Hepatitis C Virus Proteins Induces Distinct Membrane Alterations Including a Candidate Viral Replication Complex. J. Virol. 2002, 76, 5974–5984. [Google Scholar] [CrossRef]
- Khan, I.; Katikaneni, D.S.; Han, Q.; Sanchez-Felipe, L.; Hanada, K.; Ambrose, R.L.; Mackenzie, J.M.; Konan, K.V. Modulation of Hepatitis C Virus Genome Replication by Glycosphingolipids and Four-Phosphate Adaptor Protein 2. J. Virol. 2014, 88, 12276–12295. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.E.; Adhikary, S.; Kolokoltsov, A.A.; Davey, R.A. Ebolavirus Requires Acid Sphingomyelinase Activity and Plasma Membrane Sphingomyelin for Infection. J. Virol. 2012, 86, 7473–7483. [Google Scholar] [CrossRef] [PubMed]
- Orchard, R.C.; Wilen, C.B.; Doench, J.G.; Baldridge, M.T.; McCune, B.T.; Lee, Y.-C.J.; Lee, S.; Pruett-Miller, S.M.; Nelson, C.A.; Fremont, D.H.; et al. Discovery of a proteinaceous cellular receptor for a norovirus. Science 2016, 353, 933–936. [Google Scholar] [CrossRef] [PubMed]
- Orchard, R.C.; Wilen, C.B.; Virgin, H.W. Sphingolipid biosynthesis induces a conformational change in the murine norovirus receptor and facilitates viral infection. Nat. Microbiol. 2018, 3, 1109–1114. [Google Scholar] [CrossRef] [PubMed]
- Luisoni, S.; Suomalainen, M.; Boucke, K.; Tanner, L.B.; Wenk, M.R.; Guan, X.L.; Grzybek, M.; Coskun, Ü.; Greber, U.F. Co-option of Membrane Wounding Enables Virus Penetration into Cells. Cell Host Microbe 2015, 18, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Mahfoud, R.; Garmy, N.; Maresca, M.; Yahi, N.; Puigserver, A.; Fantini, J. Identification of a Common Sphingolipid-binding Domain in Alzheimer, Prion, and HIV-1 Proteins. J. Biol. Chem. 2002, 277, 11292–11296. [Google Scholar] [CrossRef] [PubMed]
- Finnegan, C.M.; Rawat, S.S.; Cho, E.H.; Guiffre, D.L.; Lockett, S.; Merrill, A.H.; Blumenthal, R. Sphingomyelinase Restricts the Lateral Diffusion of CD4 and Inhibits Human Immunodeficiency Virus Fusion. J. Virol. 2007, 81, 5294–5304. [Google Scholar] [CrossRef]
- Brügger, B.; Glass, B.; Haberkant, P.; Leibrecht, I.; Wieland, F.T.; Kräusslich, H.-G. The HIV lipidome: A raft with an unusual composition. Proc. Natl. Acad. Sci. USA 2006, 103, 2641–2646. [Google Scholar] [CrossRef]
- Vieira, C.R.; Munoz-Olaya, J.M.; Sot, J.; Jiménez-Baranda, S.; Izquierdo-Useros, N.; Abad, J.L.; Apellániz, B.; Delgado, R.; Martinez-Picado, J.; Alonso, A.; et al. Dihydrosphingomyelin Impairs HIV-1 Infection by Rigidifying Liquid-Ordered Membrane Domains. Chem. Biol. 2010, 17, 766–775. [Google Scholar] [CrossRef]
- Rawat, S.S.; Gallo, S.A.; Eaton, J.; Martin, T.D.; Ablan, S.; KewalRamani, V.N.; Wang, J.M.; Blumenthal, R.; Puri, A. Elevated Expression of GM3 in Receptor-Bearing Targets Confers Resistance to Human Immunodeficiency Virus Type 1 Fusion. J. Virol. 2004, 78, 7360–7368. [Google Scholar] [CrossRef]
- Hammache, D.; Yahi, N.; Piéroni, G.; Ariasi, F.; Tamalet, C.; Fantini, J. Sequential Interaction of CD4 and HIV-1 gp120 with a Reconstituted Membrane Patch of Ganglioside GM3: Implications for the Role of Glycolipids as Potential HIV-1 Fusion Cofactors. Biochem. Biophys. Res. Commun. 1998, 246, 117–122. [Google Scholar] [CrossRef]
- Harouse, J.M.; Bhat, S.; Spitalnik, S.L.; Laughlin, M.; Stefano, K.; Silberberg, D.H.; Gonzalez-Scarano, F. Inhibition of Entry of HIV-1 in Neural Cell Lines by Antibodies Against Galactosyl Ceramide. Science 1991, 253, 320–323. [Google Scholar] [CrossRef] [PubMed]
- Lorizate, M.; Sachsenheimer, T.; Glass, B.; Habermann, A.; Gerl, M.J.; Kräusslich, H.; Brügger, B. Lipidomics of HIV-1 particles and producer plasma membranes. Cell Microbiol. 2013, 15, 292–304. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo-Useros, N.; Lorizate, M.; Contreras, F.-X.; Rodriguez-Plata, M.T.; Glass, B.; Erkizia, I.; Prado, J.G.; Casas, J.; Fabriàs, G.; Kräusslich, H.G.; et al. Sialyllactose in Viral Membrane Gangliosides Is a Novel Molecular Recognition Pattern for Mature Dendritic Cell Capture of HIV-1. PLoS Biol. 2012, 10, e1001315. [Google Scholar] [CrossRef] [PubMed]
- Hammonds, J.E.; Beeman, N.; Ding, L.; Takushi, S.; Francis, A.C.; Wang, J.-J.; Melikyan, G.B.; Spearman, P. Siglec-1 initiates formation of the virus-containing compartment and enhances macrophage-to-T cell transmission of HIV-1. PLoS Pathog. 2017, 13, e1006181. [Google Scholar] [CrossRef] [PubMed]
- Grassmé, H.; Riehle, A.; Wilker, B.; Gulbins, E. Rhinoviruses Infect Human Epithelial Cells via Ceramide-enriched Membrane Platforms. J. Biol. Chem. 2005, 280, 26256–26262. [Google Scholar] [CrossRef] [PubMed]
- Carpinteiro, A.; Edwards, M.J.; Hoffmann, M.; Kochs, G.; Gripp, B.; Weigang, S.; Adams, C.; Carpinteiro, E.; Gulbins, A.; Keitsch, S.; et al. Pharmacological Inhibition of Acid Sphingomyelinase Prevents Uptake of SARS-CoV-2 by Epithelial Cells. Cell Rep. Med. 2020, 1, 100142. [Google Scholar] [CrossRef]
- Vitner, E.B.; Achdout, H.; Avraham, R.; Politi, B.; Cherry, L.; Tamir, H.; Yahalom-Ronen, Y.; Paran, N.; Melamed, S.; Erez, N.; et al. Glucosylceramide synthase inhibitors prevent replication of SARS-CoV-2 and influenza virus. J. Biol. Chem. 2021, 296, 100470. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e8. [Google Scholar] [CrossRef]
- Mesquita, F.S.; Abrami, L.; Sergeeva, O.; Turelli, P.; Qing, E.; Kunz, B.; Raclot, C.; Paz Montoya, J.; Abriata, L.A.; Gallagher, T.; et al. S-acylation controls SARS-CoV-2 membrane lipid organization and enhances infectivity. Dev. Cell 2021, 56, 2790–2807.e8. [Google Scholar] [CrossRef]
- Edwards, M.J.; Becker, K.A.; Gripp, B.; Hoffmann, M.; Keitsch, S.; Wilker, B.; Soddemann, M.; Gulbins, A.; Carpinteiro, E.; Patel, S.H.; et al. Sphingosine prevents binding of SARS–CoV-2 spike to its cellular receptor ACE2. J. Biol. Chem. 2020, 295, 15174–15182. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Tan, Y.; Wang, L.; Su, X.; Shi, Y. Serum sphingosine-1-phosphate levels and Sphingosine-1-Phosphate gene polymorphisms in acute respiratory distress syndrome: A multicenter prospective study. J. Transl. Med. 2020, 18, 156. [Google Scholar] [CrossRef] [PubMed]
- Schloer, S.; Brunotte, L.; Goretzko, J.; Mecate-Zambrano, A.; Korthals, N.; Gerke, V.; Ludwig, S.; Rescher, U. Targeting the endolysosomal host-SARS-CoV-2 interface by clinically licensed functional inhibitors of acid sphingomyelinase (FIASMA) including the antidepressant fluoxetine. Emerg. Microbes Infect. 2020, 9, 2245–2255. [Google Scholar] [CrossRef] [PubMed]
- Otsuki, N.; Sakata, M.; Saito, K.; Okamoto, K.; Mori, Y.; Hanada, K.; Takeda, M. Both Sphingomyelin and Cholesterol in the Host Cell Membrane Are Essential for Rubella Virus Entry. J. Virol. 2018, 92, e01130-17. [Google Scholar] [CrossRef] [PubMed]
- Mori, Y.; Sakata, M.; Sakai, S.; Okamoto, T.; Nakatsu, Y.; Taguwa, S.; Otsuki, N.; Maeda, Y.; Hanada, K.; Matsuura, Y.; et al. Membrane Sphingomyelin in Host Cells Is Essential for Nucleocapsid Penetration into the Cytoplasm after Hemifusion during Rubella Virus Entry. mBio 2022, 13, e01698-22. [Google Scholar] [CrossRef] [PubMed]
- Diray-Arce, J.; Angelidou, A.; Jensen, K.J.; Conti, M.G.; Kelly, R.S.; Pettengill, M.A.; Liu, M.; van Haren, S.D.; McCulloch, S.D.; Michelloti, G.; et al. Bacille Calmette-Guérin vaccine reprograms human neonatal lipid metabolism in vivo and in vitro. Cell Rep. 2022, 39, 110772. [Google Scholar] [CrossRef]
- Koeken, V.A.C.M.; Qi, C.; Mourits, V.P.; de Bree, L.C.J.; Moorlag, S.J.C.F.M.; Sonawane, V.; Lemmers, H.; Dijkstra, H.; Joosten, L.A.B.; van Laarhoven, A.; et al. Plasma metabolome predicts trained immunity responses after antituberculosis BCG vaccination. PLoS Biol. 2022, 20, e3001765. [Google Scholar] [CrossRef]
- Maner-Smith, K.M.; Goll, J.B.; Khadka, M.; Jensen, T.L.; Colucci, J.K.; Gelber, C.E.; Albert, C.J.; Bosinger, S.E.; Franke, J.D.; Natrajan, M.; et al. Alterations in the Human Plasma Lipidome in Response to Tularemia Vaccination. Vaccines 2020, 8, 414. [Google Scholar] [CrossRef]
- Li, S.; Sullivan, N.L.; Rouphael, N.; Yu, T.; Banton, S.; Maddur, M.S.; McCausland, M.; Chiu, C.; Canniff, J.; Dubey, S.; et al. Metabolic Phenotypes of Response to Vaccination in Humans. Cell 2017, 169, 862–877.e17. [Google Scholar] [CrossRef]
- Zhang, Y.; Yue, Q.; Zhu, H.; Song, J.; Li, D.; Liu, W.; Jiang, S.; Jiang, N.; Qiu, C.; Ai, J.; et al. Serum Metabolic Correlates of the Antibody Response in Subjects Receiving the Inactivated COVID-19 Vaccine. Vaccines 2022, 10, 1890. [Google Scholar] [CrossRef]
- Schneider-Schaulies, S.; Schumacher, F.; Wigger, D.; Schöl, M.; Waghmare, T.; Schlegel, J.; Seibel, J.; Kleuser, B. Sphingolipids: Effectors and Achilles Heals in Viral Infections? Cells 2021, 10, 2175. [Google Scholar] [CrossRef] [PubMed]
- Wendt, C.H.; Castro-Pearson, S.; Proper, J.; Pett, S.; Griffin, T.J.; Kan, V.; Carbone, J.; Koulouris, N.; Reilly, C.; Neaton, J.D.; et al. Metabolite profiles associated with disease progression in influenza infection. PLoS ONE 2021, 16, e0247493. [Google Scholar] [CrossRef]
- Kurano, M.; Okamoto, K.; Jubishi, D.; Hashimoto, H.; Sakai, E.; Saigusa, D.; Kano, K.; Aoki, J.; Harada, S.; Okugawa, S.; et al. Dynamic modulations of sphingolipids and glycerophospholipids in COVID-19. Clin. Transl. Med. 2022, 12, e1069. [Google Scholar] [CrossRef] [PubMed]
- Schneider-Schaulies, J.; Schneider-Schaulies, S. Sphingolipids in viral infection. Biol. Chem. 2015, 396, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Beckmann, N.; Becker, K.A. Ceramide and Related Molecules in Viral Infections. Int. J. Mol. Sci. 2021, 22, 5676. [Google Scholar] [CrossRef]
- Wang, X.; Nijman, R.; Camuzeaux, S.; Sands, C.; Jackson, H.; Kaforou, M.; Emonts, M.; Herberg, J.A.; Maconochie, I.; Carrol, E.D.; et al. Plasma lipid profiles discriminate bacterial from viral infection in febrile children. Sci. Rep. 2019, 9, 17714. [Google Scholar] [CrossRef]
- Song, J.-W.; Lam, S.M.; Fan, X.; Cao, W.-J.; Wang, S.-Y.; Tian, H.; Chua, G.H.; Zhang, C.; Meng, F.P.; Xu, Z.; et al. Omics-Driven Systems Interrogation of Metabolic Dysregulation in COVID-19 Pathogenesis. Cell Metab. 2020, 32, 188–202.e5. [Google Scholar] [CrossRef]
- Studstill, C.J.; Pritzl, C.J.; Seo, Y.-J.; Kim, D.Y.; Xia, C.; Wolf, J.J.; Nistala, R.; Vijayan, M.; Cho, Y.B.; Kang, K.W.; et al. Sphingosine kinase 2 restricts T cell immunopathology but permits viral persistence. J. Clin. Investig. 2020, 130, 6523–6538. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thomas, S.; Samuel, S.V.; Hoch, A.; Syphurs, C.; Diray-Arce, J. The Implication of Sphingolipids in Viral Infections. Int. J. Mol. Sci. 2023, 24, 17303. https://doi.org/10.3390/ijms242417303
Thomas S, Samuel SV, Hoch A, Syphurs C, Diray-Arce J. The Implication of Sphingolipids in Viral Infections. International Journal of Molecular Sciences. 2023; 24(24):17303. https://doi.org/10.3390/ijms242417303
Chicago/Turabian StyleThomas, Sanya, Stephen Varghese Samuel, Annmarie Hoch, Caitlin Syphurs, and Joann Diray-Arce. 2023. "The Implication of Sphingolipids in Viral Infections" International Journal of Molecular Sciences 24, no. 24: 17303. https://doi.org/10.3390/ijms242417303
APA StyleThomas, S., Samuel, S. V., Hoch, A., Syphurs, C., & Diray-Arce, J. (2023). The Implication of Sphingolipids in Viral Infections. International Journal of Molecular Sciences, 24(24), 17303. https://doi.org/10.3390/ijms242417303






