The Influence of the Nature of the Polymer Incorporating the Same A3B Multifunctional Porphyrin on the Optical or Electrical Capacity to Recognize Procaine
Abstract
:1. Introduction
2. Results and Discussion
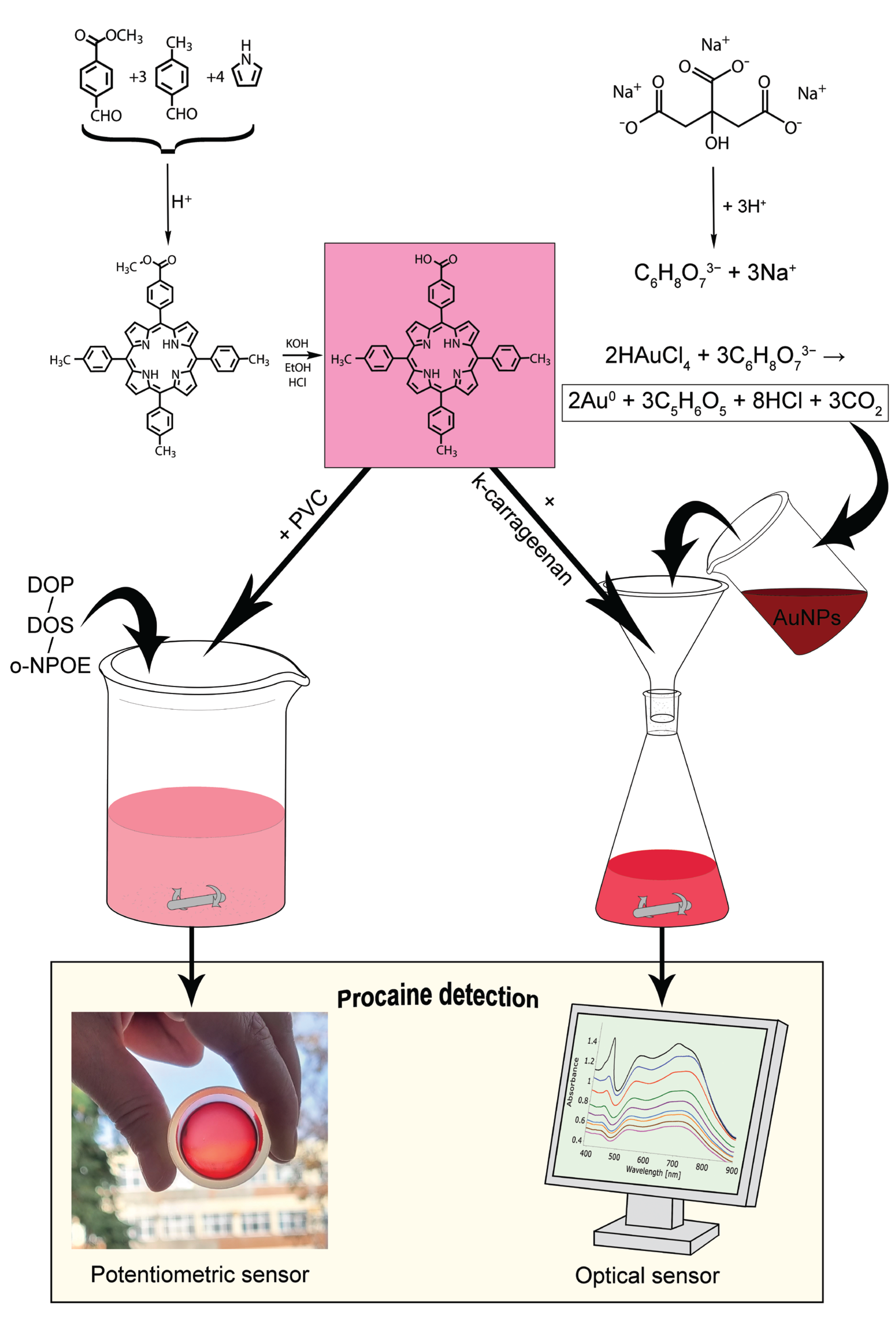
2.1. Optical Detection of Procaine with 5-COOH-3MPP-k-Caragenan-AuNPs Nanomaterial
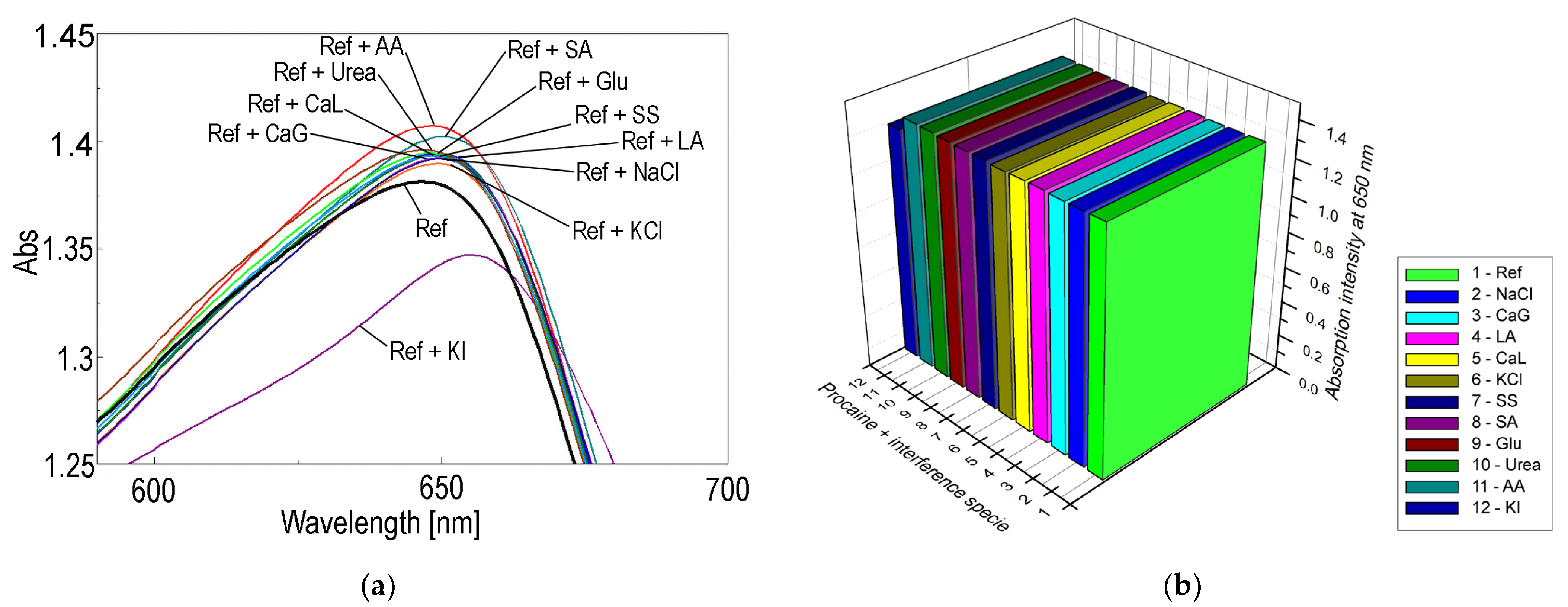
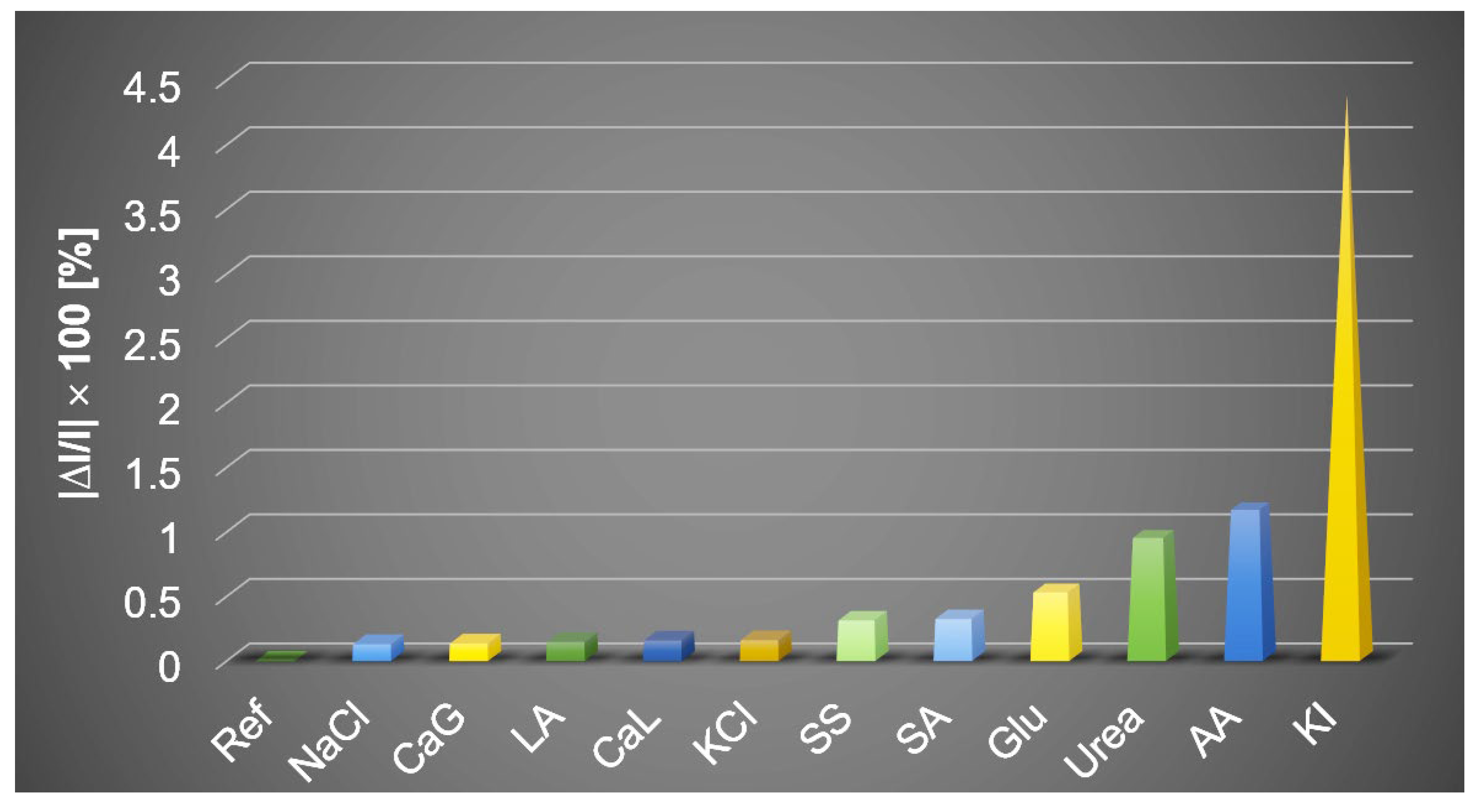
The Effects of Interferent Species in the Optical Detection of Procaine
2.2. FT-IR Analysis for Presuming the Mechanism of Detection
Presumed Mechanism of Optical Detection
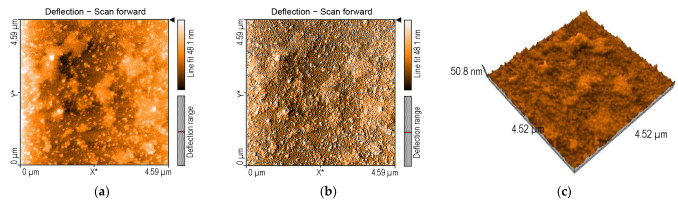
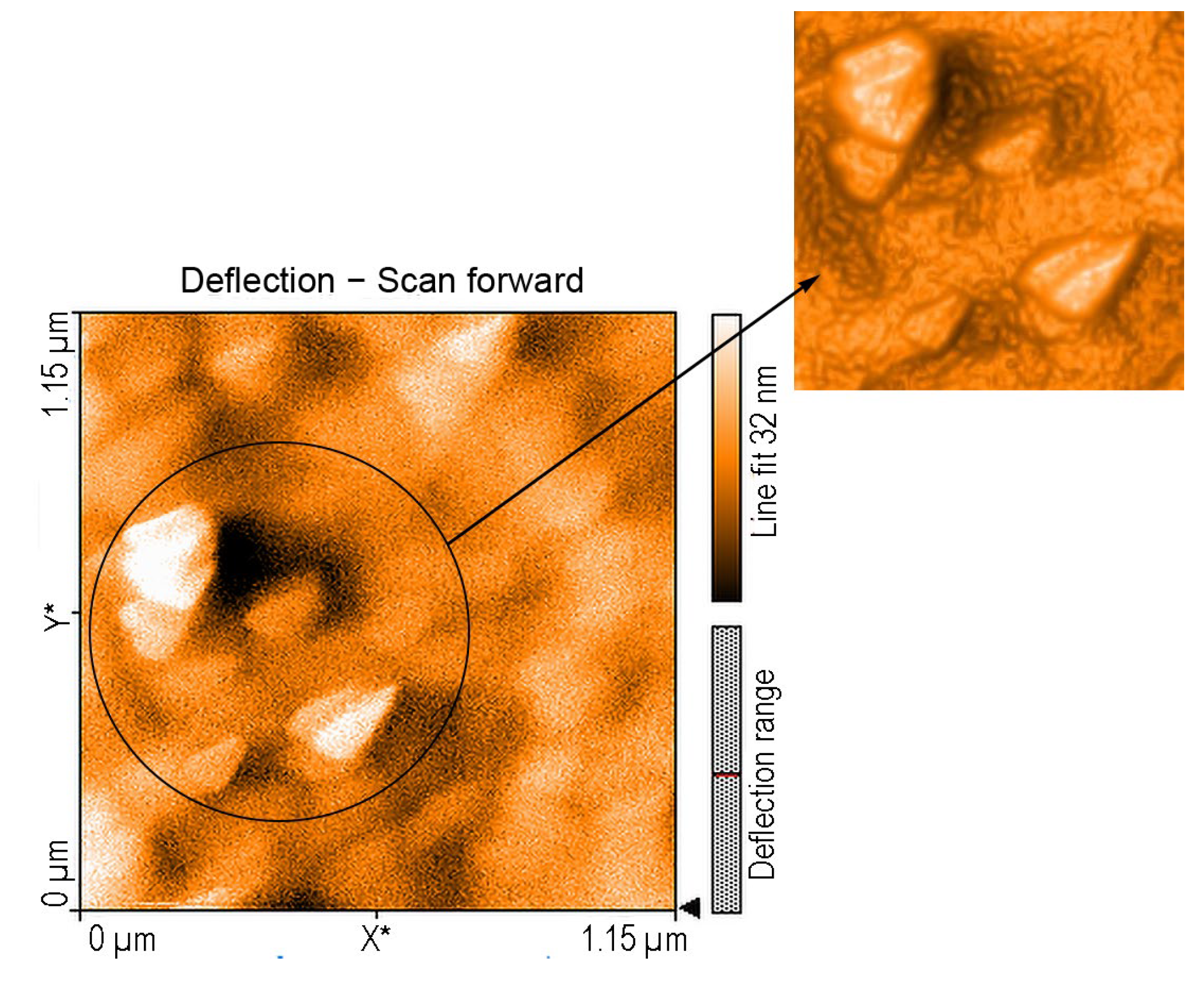
2.3. AFM Analysis to Evidence Morphological Changes after 5-COOH-3MPP-k-Carrageenan-AuNPs Material Interaction with Procaine
2.3.1. AFM of 5-COOH-3MPP-k-Carrageenan-AuNPs Nanomaterial
2.3.2. AFM of Composite Nanomaterial 5-COOH-3MPP-k-Carrageenan-AuNPs after Exposure to Procaine
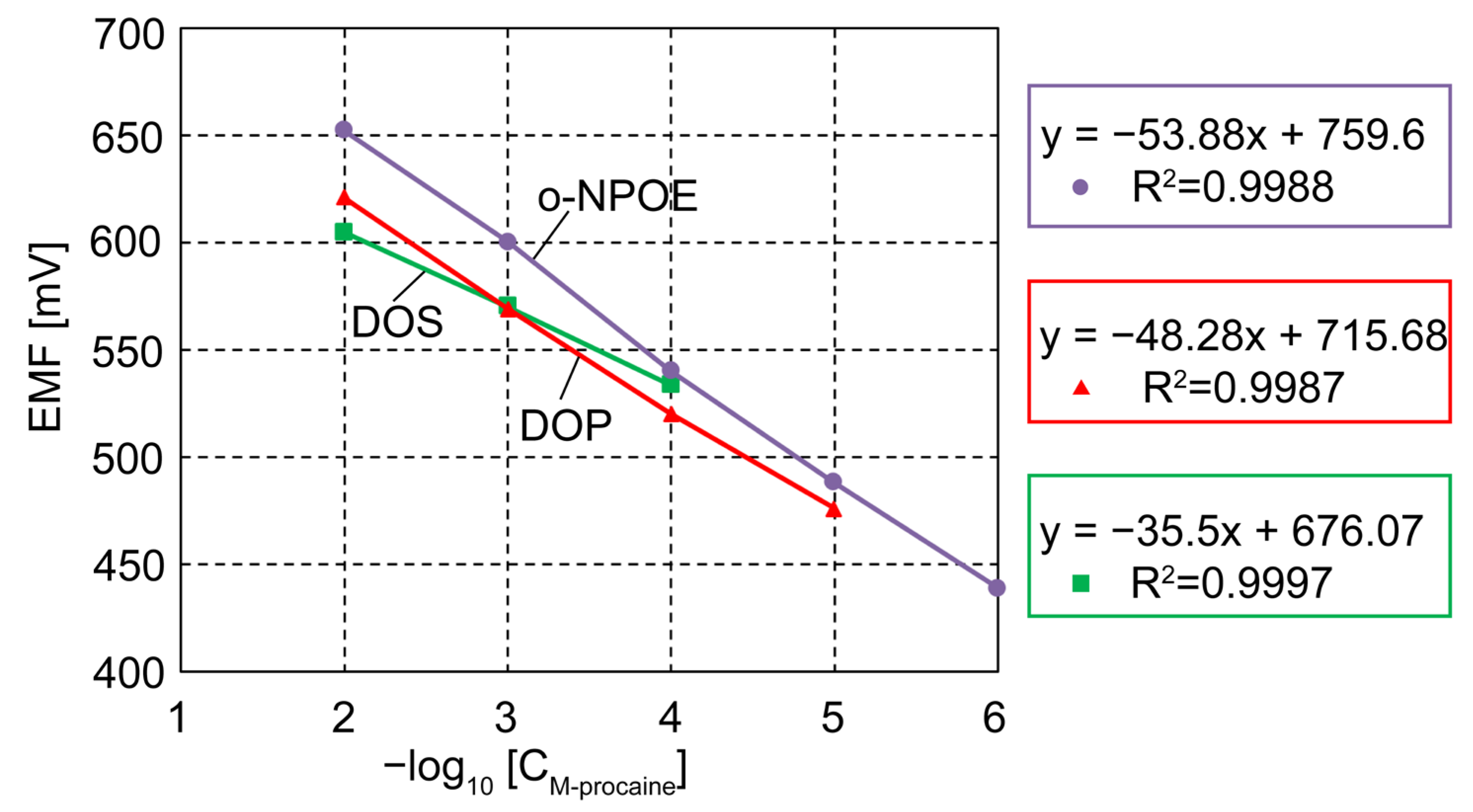
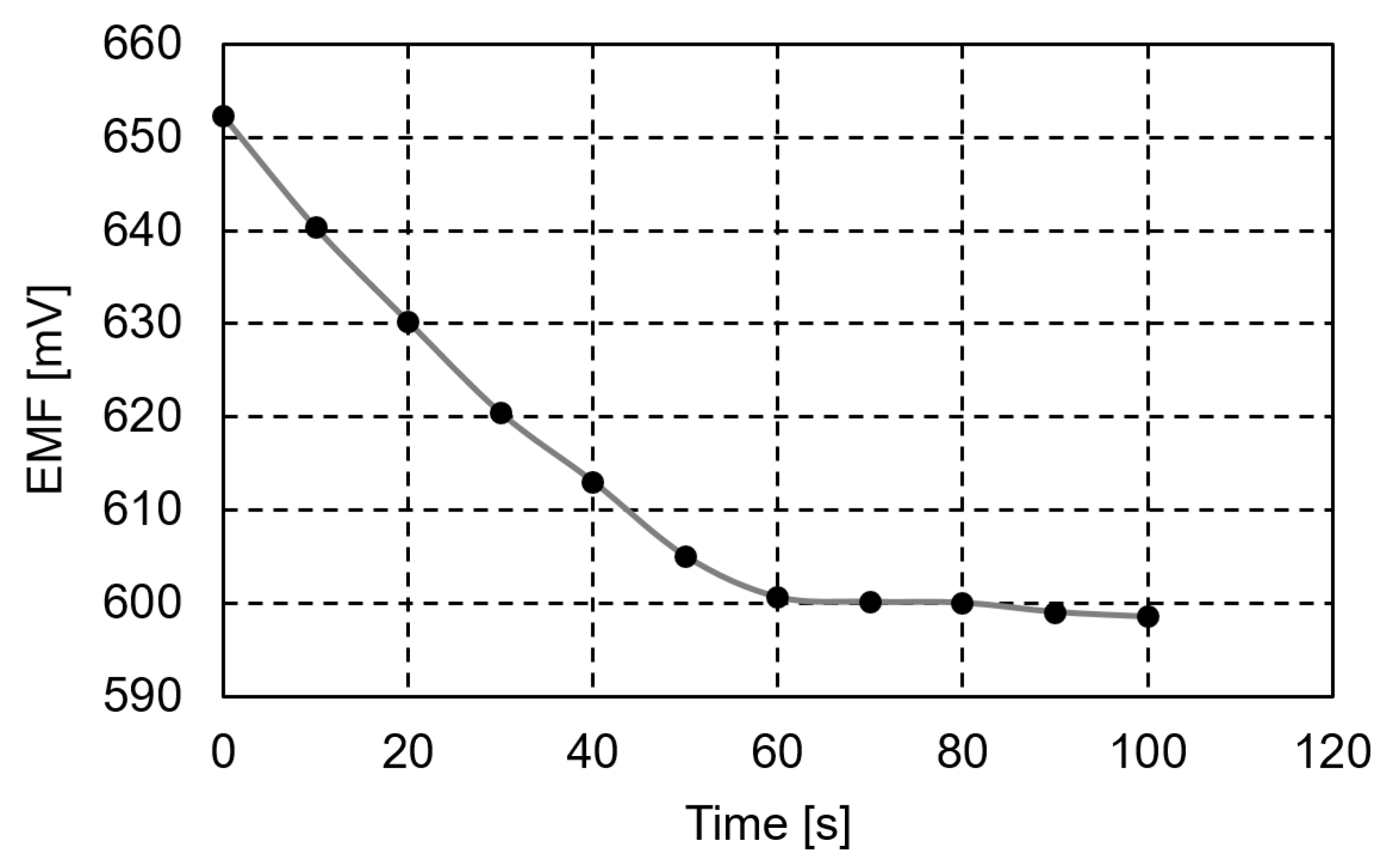
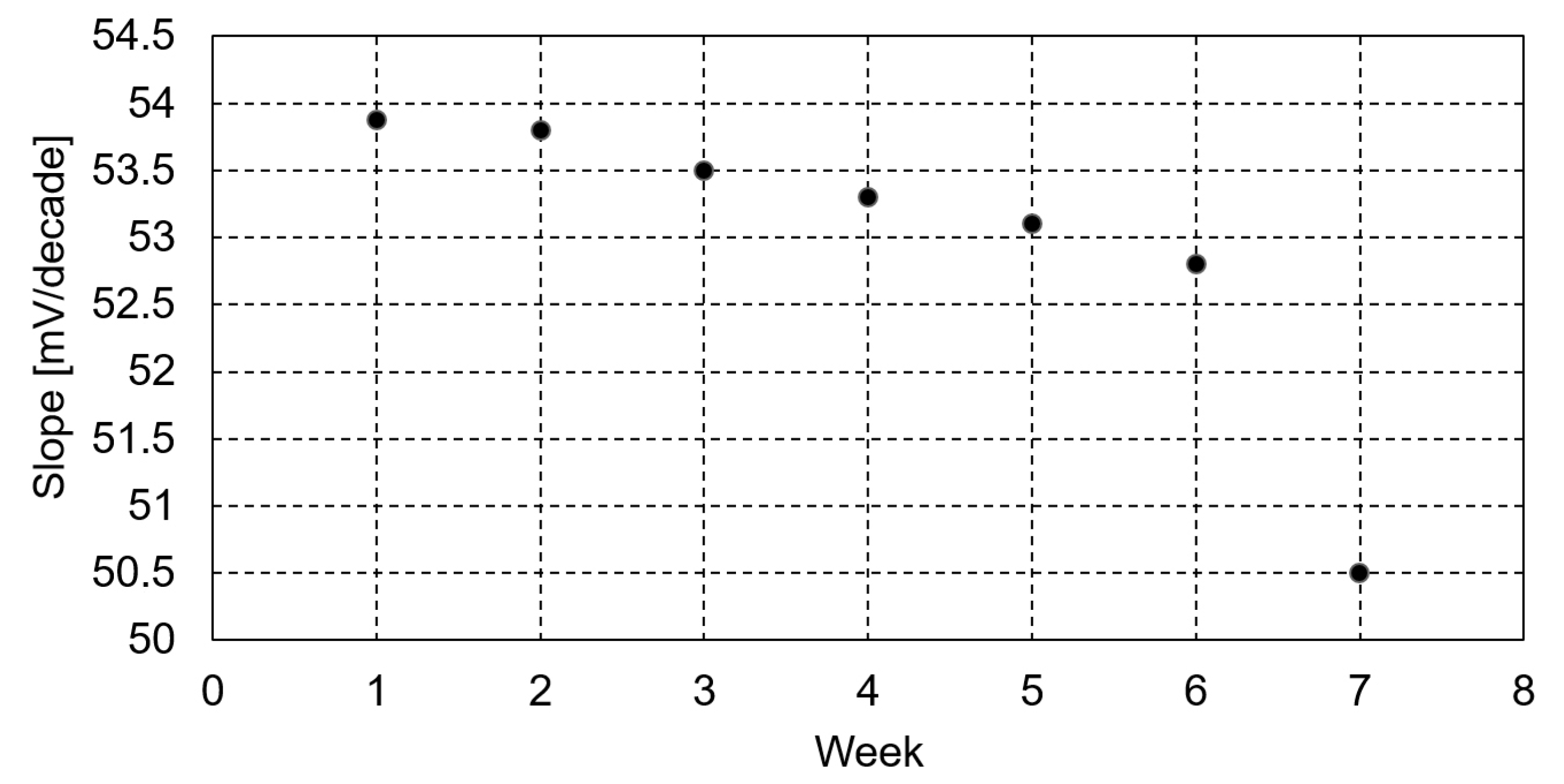
2.4. Potentiometric Detection of Procaine
Proposed Mechanism for the Potentiometric Detection of Procaine
2.5. Analytical Applications
3. Materials and Methods
3.1. Obtaining the 5-COOH-3MPP-k-Carrageenan-AuNPs Nanomaterial
3.2. Polymeric Membrane Obtaining and Measurements
3.3. Apparatus
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ayuse, T.; Kurata, S.; Ayuse, T. Successful Dental Treatments Using Procaine Hydrochloride in a Patient Afraid of Local Anesthesia but Consenting for Allergic Testing with Lidocaine: A Case Report. Local Reg. Anesth. 2020, 13, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Coutant, M.; Malnkvist, J.; Keiser, M.; Foldager, L.; Herskin, M.S. Piglets’ acute responses to procaine-based local anesthetic injection and surgical castration: Effects of two volumes of aesthetic. Front. Pain Res. 2022, 3, 943138. [Google Scholar] [CrossRef] [PubMed]
- Gradinaru, D.; Ungurianu, A.; Margina, D.; Moreno-Villanueva, M.; Bürkle, A. Procaine-The Controversial Geroprotector Candidate: New Insights Regarding Its Molecular and Cellular Effects. Oxidat. Med. Cell. Longev. 2021, 2021, 3617042. [Google Scholar] [CrossRef] [PubMed]
- Ungurianu, A.; Margina, D.; Borsa, C.; Ionescu, C.; von Scheven, G.; Oziol, L.; Faure, P.; Artur, Y.; Burkle, A.; Gradinaru, D.; et al. The radioprotective effect of procaine and procaine-derived product gerovital H3 in lymphocytes from young and aged individuals. Oxidat. Med. Cell. Longev. 2020, 2020, 3580934. [Google Scholar] [CrossRef] [PubMed]
- Malamed, S.F. Section V: Intravenous Sedation Chapter 25-Pharmacology. In Sedation, 6th ed.; Malamed, S.F., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 319–358. [Google Scholar] [CrossRef]
- Zhu, Y.; Xu, Y.; Liu, G. Electrochemical Detection of the Anesthetic Drug Procaine Hydrochloride Based on Molecularly Imprinted Polymer/Diamond-Graphite Composite Electrode. Int. J. Electrochem. Sci. 2021, 16, 211237. [Google Scholar] [CrossRef]
- Vladescu, L.; Moja, D.; Badea, I.A.; Ciomaga, C. Determination of procaine hydrochloride by potentiometric titration. Rev. Roum. Chim. 2022, 47, 911–915. [Google Scholar]
- Haghighian, F.; Ghoreishi, S.M.; Attaran, A.; Kashani, F.Z.; Khoobi, A. Electrochemical study for simultaneous detection of procaine hydrochloride and its metabolite in biological samples using a nanostructured strong sensor. Korean J. Chem. Eng. 2023, 40, 650–656. [Google Scholar] [CrossRef]
- Lascu, A.; Palade, A.; Birdeanu, M.; Fagadar-Cosma, E. Procaine detection using hybrids of cobalt-metalloporphyrin with gold and silver nanoparticles. J. Chem. Soc. Pak. 2019, 41, 43–51. [Google Scholar]
- Tantirungrotechai, Y.; Syananondh, A.; Youngvises, N. Flow Analysis with Cerium (IV) Colorimetric Reagent for Determination of Procaine and Elucidation of the Color Species by Computational Investigation. Sci. Technol. Asia 2023, 28, 1–16. Available online: https://ph02.tci-thaijo.org/index.php/SciTechAsia/article/view/248863 (accessed on 20 September 2023).
- Al-Saadi, A.A.; Haroon, M.; Popoola, S.A.; Saleh, T.A. Sensitive SERS detection and characterization of procaine in aqueous media by reduced gold nanoparticles. Sens. Actuators B Chem. 2020, 304, 127057. [Google Scholar] [CrossRef]
- Haroon, M.; Abdulazeez, I.; Saleh, T.A.; Al-Saadi, A.A. Electrochemically modulated SERS detection of procaine using FTO electrodes modified with silver-decorated carbon nanosphere. Electrochim. Acta 2021, 387, 138463. [Google Scholar] [CrossRef]
- Haroon, M.; Ashraf, M.; Ullah, N.; Tahir, M.N.; Al-Saadi, A.A. SERS and EC-SERS detection of local anesthetic procaine using Pd loaded highly reduced graphene oxide nanocomposite substrate. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 278, 121381. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.Y.; Shi, F.; Zhao, Y.G.; Wang, H.W. Determination of Local Anesthetic Drugs in Human Plasma Using Magnetic Solid-Phase Extraction Coupled with High-Performance Liquid Chromatography. Molecules 2022, 27, 5509. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Wang, C.; Liu, P.; Zhang, G.; Song, L.; Fu, Y. Achieving synergistically enhanced dual-mode electrochemiluminescent and electrochemical drug sensors via a multi-effect porphyrin-based metal-organic framework. Sens. Actuators B Chem. 2021, 330, 129388. [Google Scholar] [CrossRef]
- Zhao, B.; Li, Y.; Zhao, Y.; Ma, Y.; Li, F.; Han, H.; Wang, N.; Wang, X. A sensing platform based on zinc-porphyrin derinative in hexadecyl trimethyl ammonium bromide (CTAB) microemulsion for highly sensitive detection of theophylline. Spectrochim. Acta—A Mol. Biomol. Spectrosc. 2022, 281, 121592. [Google Scholar] [CrossRef] [PubMed]
- Lazoviskiy, D.A.; Mamardashvili, G.M.; Khodov, I.A.; Mamardashvili, N.Z. Water soluble porphyrin-fluorescein triads: Design, DFT calculation and pH-change-triggered fluorescence response. J. Photochem. Photobiol. A Chem. 2020, 402, 112832. [Google Scholar] [CrossRef]
- Malecka, K.; Kaur, B.; Cristaldi, D.A.; Chay, C.S.; Mames, I.; Radecka, H.; Radecki, J.; Stulz, E. Silver or gold? A comparison of nanoparticle modified electrochemical genosensors based on cobalt porphyrin-DNA. Bioelectrochemistry 2021, 138, 107723. [Google Scholar] [CrossRef] [PubMed]
- Abouzar, M.H.; Poghossian, A.; Cherstvy, A.G.; Pedraza, A.M.; Ingebrandt, S.; Schöning, M.J. Label-free electrical detection of DNA by means of field-effect nanoplate capacitors: Experiments and modeling. Phys. Status Solidi A 2012, 209, 925–934. [Google Scholar] [CrossRef]
- Comuzzi, C.; Marino, M.; Poletti, D.; Boaro, M.; Strazzolini, P. New antimicrobial PVC composites. Porphyrins self-aggregation in tuning surface morphologies and photodynamic inactivation towards sustainable water disinfection. J. Photochem. Photobiol. 2022, 430, 113967. [Google Scholar] [CrossRef]
- Ghafuri, H.; Hanifehnejad, P.; Felfelian, Z. Synthesis and characterization of porphyrin-modified chitosan biopolymer and its application in the degradation of methylene blue under visible light. J. Appl. Chem. 2023, 18, 173–186. [Google Scholar] [CrossRef]
- Sewid, F.A.; Annas, K.I.; Dubavik, A.; Veniaminov, A.V.; Maslov, V.G.; Orlov, A.O. Chitosan Nanocomposites with CdSe/ZnS Quantum Dots and Porphyrin. RSC Adv. 2022, 12, 899–906. [Google Scholar] [CrossRef]
- Monteiro, C.J.P.; Neves, M.G.P.M.S.; Nativi, C.; Almeida, A.; Faustino, M.A.F. Porphyrin Photosensitizers Grafted in Cellulose Supports: A Review. Int. J. Mol. Sci. 2023, 24, 3475. [Google Scholar] [CrossRef] [PubMed]
- Hino, S.; Funada, R.; Sugikawa, K.; Kawasaki, R.; Koumoto, K.; Suzuki, T.; Nagasaki, T.; Ikeda, A. Mechanism Toward Turn-on of Polysaccharide–Porphyrin Complexes for Fluorescence Probes and Photosensitizers in Photodynamic Therapy in Living Cells. ChemMedChem 2020, 16, 793–803. [Google Scholar] [CrossRef] [PubMed]
- Epuran, C.; Fratilescu, I.; Macsim, A.M.; Lascu, A.; Ianasi, C.; Birdeanu, M.; Fagadar-Cosma, E. Excellent Cooperation between Carboxyl-Substituted Porphyrins, k-Carrageenan and AuNPs for Extended Application in CO2 Capture and Manganese Ion Detection. Chemosensors 2022, 10, 133. [Google Scholar] [CrossRef]
- Birdeanu, M.; Fratilescu, I.; Epuran, C.; Murariu, A.C.; Socol, G.; Fagadar-Cosma, E. Efficient Decrease in Corrosion of Steel in 0.1 M HCl Medium Realized by a Coating with Thin Layers of MnTa2O6 and Porphyrins Using Suitable Laser- Type Approaches. Nanomaterials 2022, 12, 1118. [Google Scholar] [CrossRef] [PubMed]
- Mei, Q.; Shi, Y.; Hua, Q.; Tong, B. Phosphorescent chemosensor for Hg2+ based on iridium(III) complex coordinated with 4-phenylquinazoline and sodium carbazole dithiocarbamate. RSC Adv. 2015, 5, 74924–74931. [Google Scholar] [CrossRef]
- Borkhodoev, V.Y. About the limit of detection in X-ray fluorescence analysis. J. Anal. Chem. 2014, 69, 1041–1046. [Google Scholar] [CrossRef]
- Fulias, A.; Ledeti, I.; Vlase, G.; Popoiu, C.; Heghes, A.; Bilanin, M.; Vlase, T.; Gheorgheosu, D.; Craina, M.; Ferechide, D.; et al. Thermal behaviour of procaine and benzocaine Part II: Compatibility study with some pharmaceutical excipients used in solid dosage forms. Chem. Cent. J. 2013, 7, 140. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, W.A.K.; Khan, M.M.R.; Yee, T.C. Effects of reaction temperature on the synthesis and thermal properties of carrageenan ester. J. Phys. Sci. 2014, 25, 123–138. [Google Scholar]
- Lai, W.; Lau, M.K.; Chong, V.; Wong, W.T.; Leung, W.H.; Yu, N.T. The metal–carbon stretching frequencies in methyl complexes of Rh, Ir, Ga and In with porphyrins and a tetradentate pyridine–amide ligand. J. Organomet. Chem. 2001, 634, 61–68. [Google Scholar] [CrossRef]
- Hong, T.; Yin, J.Y.; Nie, S.P.; Xie, M.Y. Applications of infrared spectroscopy in polysaccharide structural analysis: Progress, challenge and perspective. Food Chem. X 2021, 12, 100168. [Google Scholar] [CrossRef]
- Lewis, P.D.; Lewis, K.E.; Ghosal, R.; Bayliss, S.; Lloyd, A.J.; Wills, J.; Godfrey, R.; Kloer, P.; Mur, L.A. Evaluation of FTIR spectroscopy as a diagnostic tool for lung cancer using sputum. BMC Cancer 2010, 10, 640. [Google Scholar] [CrossRef] [PubMed]
- Hermán, V.; Takacs, H.; Duclairoir, F.; Renault, O.; Tortai, J.H.; Viala, B. Core double–shell cobalt/graphene/polystyrene magnetic nanocomposites synthesized by in situ sonochemical polymerization. RSC Adv. 2015, 5, 51371–51381. [Google Scholar] [CrossRef]
- D’Souza, L.; Devi, P.; Divya Shridhar, M.P.; Naik, C.G. Use of Fourier Transform Infrared (FTIR) spectroscopy to study cadmium-induced changes in Padina tetrastromatica (Hauck). Anal. Chem. Insights 2008, 3, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Sharma, L.; Kimura, T. FT-IR Investigation into Miscible Interactions in New Materials for Optical Devices. Polym. Adv. Technol. 2003, 14, 392–399. [Google Scholar] [CrossRef]
- Gloaguen, E.; Mons, M.; Schwing, K.; Gerhards, M. Neutral Peptides in the Gas Phase: Conformation and Aggregation Issues. Chem. Rev. 2020, 120, 12490–12562. [Google Scholar] [CrossRef] [PubMed]
- Umezawa, Y.; Buhlmann, P.; Umezawa, K.; Tohda, K.; Amemiya, S. Potentiometric selectivity coefficients of ion-selective electrodes. Part I. Inorganic cations. Pure Appl. Chem. 2002, 74, 923–994. [Google Scholar] [CrossRef]
- Fagadar-Cosma, E.; Lascu, A.; Shova, S.; Zaltariov, M.-F.; Birdeanu, M.; Croitor, L.; Balan, A.; Anghel, D.; Stamatin, S. X-ray Structure Elucidation of a Pt-Metalloporphyrin and Its Application for Obtaining Sensitive AuNPs-Plasmonic Hybrids Capable of Detecting Triiodide Anions. Int. J. Mol. Sci. 2019, 20, 710. [Google Scholar] [CrossRef]
- Fagadar-Cosma, E.; Tarabukina, E.; Zakharova, N.; Birdeanu, M.; Taranu, B.; Palade, A.; Creanga, I.; Lascu, A.; Fagadar-Cosma, G. Hybrids formed between polyvinylpyrrolidone and an A3B porphyrin dye: Behaviour in aqueous solutions and chemical response to CO2 presence. Polym. Int. 2016, 65, 200–209. [Google Scholar] [CrossRef]
- Muthukumar, P.; Abraham, J.S. Gold nanoparticles decorated on cobalt porphyrin-modified glassy carbon electrode for the sensitive determination of nitrite ion. J. Colloid Interface Sci. 2014, 421, 78–84. [Google Scholar] [CrossRef]
- Fringu, I.; Lascu, A.; Macsim, A.M.; Fratilescu, I.; Epuran, C.; Birdeanu, M.; Fagadar-Cosma, E. Pt(II)-A2B2 metalloporphyrin-AuNPS hybrid material suitable for optical detection of 1-anthraquinonsulfonic acid. Chem. Pap. 2022, 76, 2513–2527. [Google Scholar] [CrossRef]
- Vlascici, D.; Fagadar-Cosma, G.; Plesu, N.; Lascu, A.; Petric, M.; Crisan, M.; Belean, A.; Fagadar-Cosma, E. Potentiometric sensors for iodide and bromide based on Pt(II)-porphyrin. Sensors 2018, 18, 2297. [Google Scholar] [CrossRef] [PubMed]
- Hibbard, A.J.; Burnley, H.; Michaela, J.; Rubin, H.N.; Miera, J.A.; Reynolds, M.M. Porphyrin-based metal-organic framework and polyvinylchloride composites for fluorescence sensing of divalent cadmium ions in water. Inorg. Chem. Commun. 2020, 115, 107861. [Google Scholar] [CrossRef]
- Itagaki, Y.; Deki, K.; Nakashima, S.I.; Sadaoka, Y. Toxic gas detection using porphyrin dispersed polymer composites. Sens. Actuators B Chem. 2005, 108, 393–397. [Google Scholar] [CrossRef]
- Özbek, O.; Isildak, Ö. Potentiometric determination of copper(II) ions based on a porphyrin derivative. J. Chin. Chem. Soc. 2022, 69, 1060–1069. [Google Scholar] [CrossRef]
- Amini, M.K.; Shahrokhian, S.; Tangestaninejad, S. Porphyrins as carriers in poly(vinyl chloride)-based membrane potentiometric sensors for histamine. Analyst 1999, 124, 1319–1322. [Google Scholar] [CrossRef]
- Avossa, J.; Paolesse, R.; Di Natale, C.; Zampetti, E.; Bertoni, G.; De Cesare, F.; Scarascia-Mugnozza, G.; Macagnano, A. Electrospinning of Polystyrene/Polyhydroxybutyrate Nanofibers Doped with Porphyrin and Graphene for Chemiresistor Gas Sensors. Nanomaterials 2019, 9, 280. [Google Scholar] [CrossRef]
- Özbek, O. A potentiometric sensor for the determination of potassium in different baby follow–on milk, water, juice and pharmaceutical samples. J. Food Compos. Anal. 2023, 115, 104937. [Google Scholar] [CrossRef]
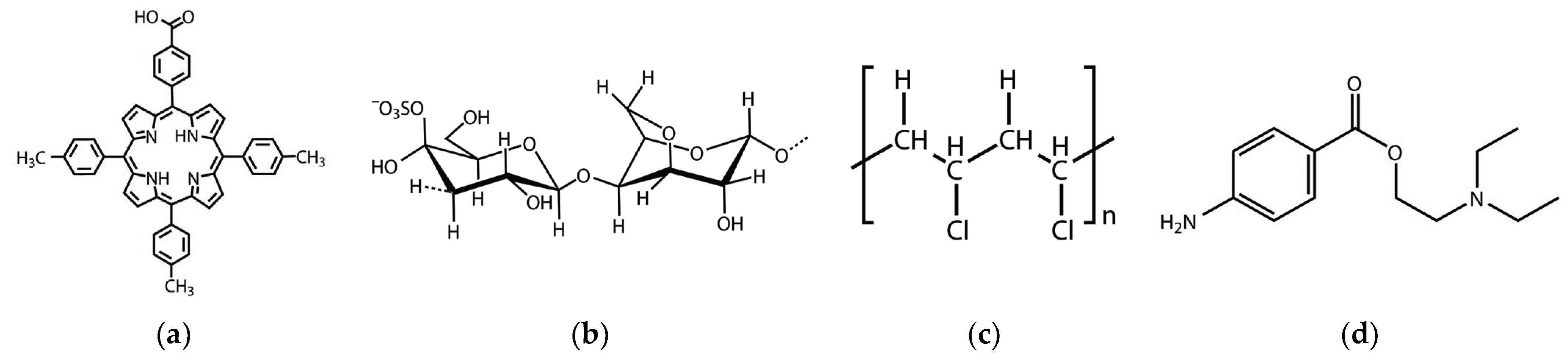


| Detection Method | Materials | Fully Linear Detected Concentration Range [M] | LOD [M] | Ref. |
|---|---|---|---|---|
| Potentiometric detection | diamond/graphite hybrid with a molecular imprint membrane | 4 × 10−8–2.5 × 10−5 | 1.5 × 10−8 | [6] |
| Potentiometric titration | 10−1 mol/L cerium(IV) sulfate solution in sulfuric acid | 10−2–10−4 | - | [7] |
| Potentiometric detection | 5-COOH-3MPP in PVC membrane | 10−6–10−2 | 7 × 10−7 | This work |
| Cyclic voltammetry | electrode of carbon paste modified with multi-walled carbon nanotubes | 2.4 × 10−6–10−4 | 62.0 × 10−9 | [8] |
| UV-vis spectroscopy | Co(II)-tetra(3-hydroxyphenyl)porphyrin/AgNPs | 5.39 × 10−5–28.04 × 10−5 | 1.1 × 10−5 | [9] |
| UV-vis spectroscopy | 5-COOH-3MPP + k-carrageenan + AuNPs nanomaterial | 5.7 × 10−6–2.7 × 10−7 | 1.3 × 10−7 | This work |
| Colorimetric detection | reaction with cerium (IV) sulfate tetrahydrate (sodium dodecyl sulfate as a sensitizer) | 4.2 × 10−6–6.3 × 10−4 | 3.1× 10−6 | [10] |
| Surface-enhanced Raman scattering (SERS) spectroscopic technique | gold nanoparticles | 10−3–10−8 | 10−10 | [11] |
| Surface-enhanced Raman scattering (SERS) spectroscopic technique | FTO electrodes modified with silver-decorated carbon nanospheres | 10−6–10−12 | 10−13 | [12] |
| SERS and EC-SERS | Pd-loaded highly reduced graphene oxide nanocomposite substrate | 10−2–10−7 | 10−8 | [13] |
| Magnetic solid-phase extraction coupled with high-performance liquid chromatography | - | 8.46 × 10−8–2.12 × 10−5 | 1.68 × 10−8 | [14] |
| Sensor | Interferent (X) | Glucose | NH4Cl | KCl | Lactose | Urea | Procaine |
|---|---|---|---|---|---|---|---|
| 1 | logKProcaine, X | −2.52 | −0.60 | −0.63 | −0.76 | −0.76 | 0 |
| 2 | −2.48 | −2.36 | −2.53 | −2.50 | −2.42 | 0 | |
| 3 | −0.58 | 0.62 | −0.61 | −0.65 | −0.58 | 0 |
| Samples | Potentiometric Detection (mg ± S a) | Amount (mg) |
|---|---|---|
| Procaine ampoules | 98 ± 1 | 100 |
| Synthetic samples | 197 ± 1.4 | 200 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lascu, A.; Vlascici, D.; Birdeanu, M.; Epuran, C.; Fratilescu, I.; Fagadar-Cosma, E. The Influence of the Nature of the Polymer Incorporating the Same A3B Multifunctional Porphyrin on the Optical or Electrical Capacity to Recognize Procaine. Int. J. Mol. Sci. 2023, 24, 17265. https://doi.org/10.3390/ijms242417265
Lascu A, Vlascici D, Birdeanu M, Epuran C, Fratilescu I, Fagadar-Cosma E. The Influence of the Nature of the Polymer Incorporating the Same A3B Multifunctional Porphyrin on the Optical or Electrical Capacity to Recognize Procaine. International Journal of Molecular Sciences. 2023; 24(24):17265. https://doi.org/10.3390/ijms242417265
Chicago/Turabian StyleLascu, Anca, Dana Vlascici, Mihaela Birdeanu, Camelia Epuran, Ion Fratilescu, and Eugenia Fagadar-Cosma. 2023. "The Influence of the Nature of the Polymer Incorporating the Same A3B Multifunctional Porphyrin on the Optical or Electrical Capacity to Recognize Procaine" International Journal of Molecular Sciences 24, no. 24: 17265. https://doi.org/10.3390/ijms242417265
APA StyleLascu, A., Vlascici, D., Birdeanu, M., Epuran, C., Fratilescu, I., & Fagadar-Cosma, E. (2023). The Influence of the Nature of the Polymer Incorporating the Same A3B Multifunctional Porphyrin on the Optical or Electrical Capacity to Recognize Procaine. International Journal of Molecular Sciences, 24(24), 17265. https://doi.org/10.3390/ijms242417265








