Culture Shock: An Investigation into the Tolerance of Pathogenic Biofilms to Antiseptics in Environments Resembling the Chronic Wound Milieu
Abstract
:1. Introduction
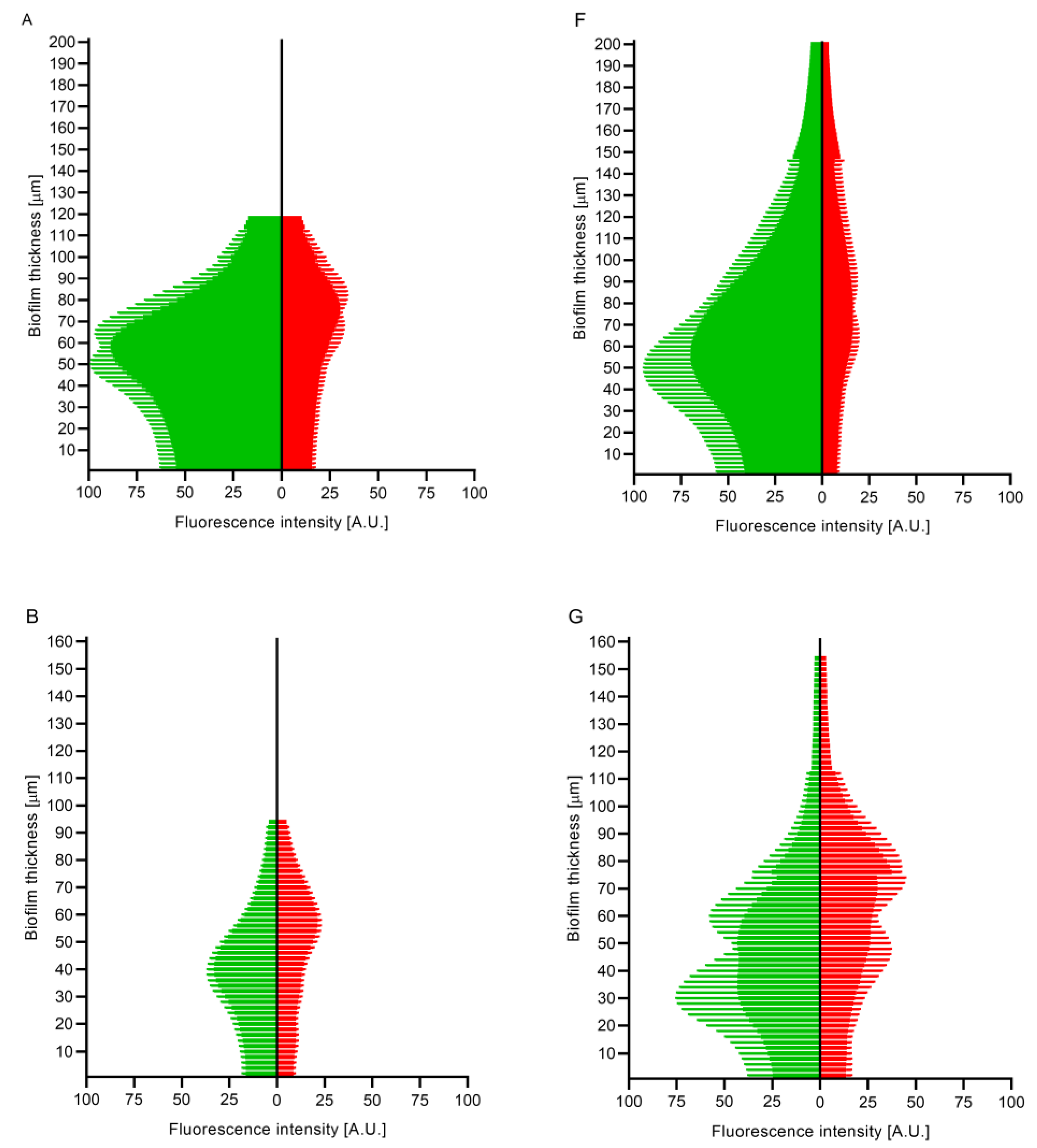
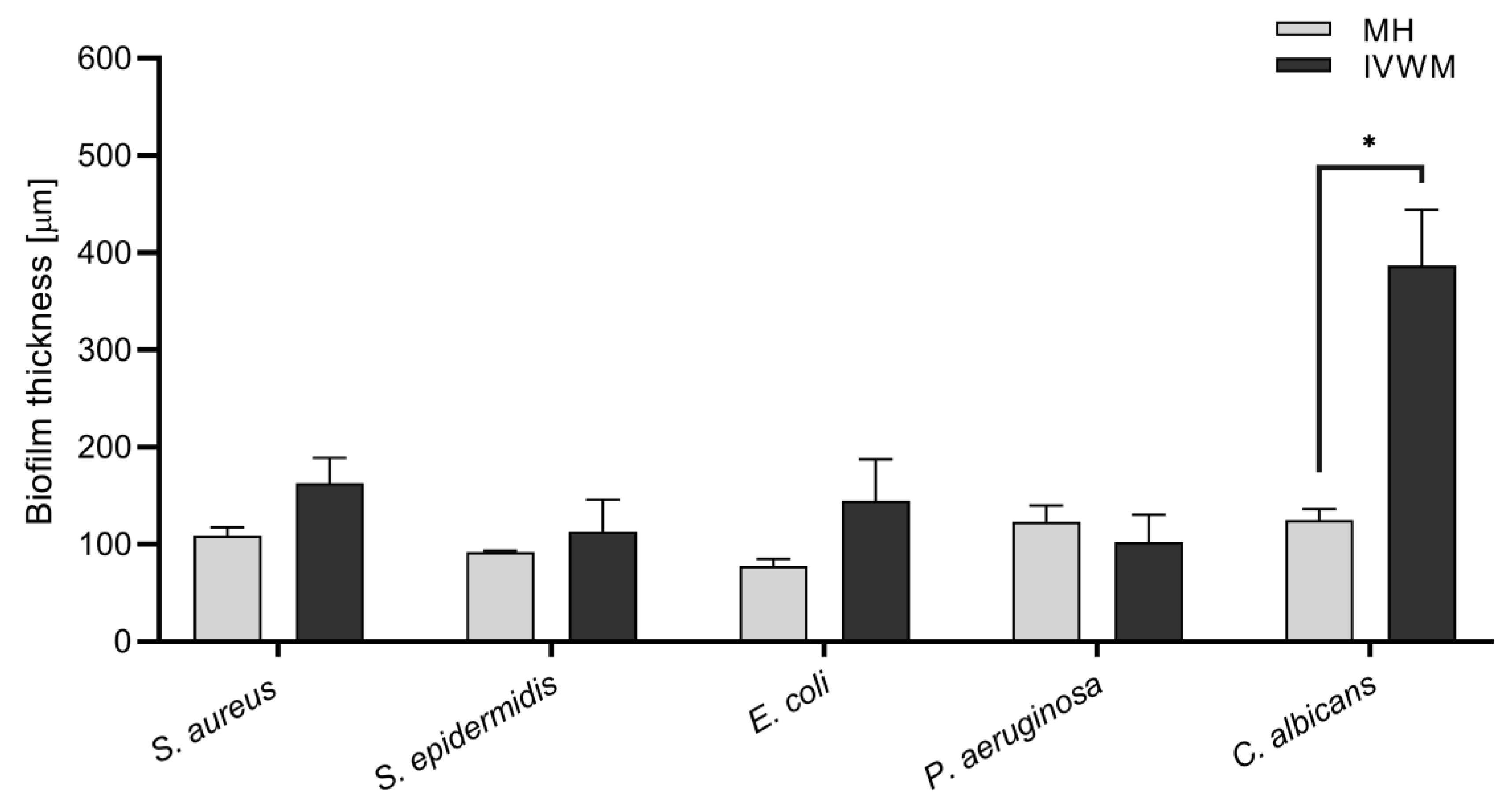
2. Results
3. Discussion
4. Materials and Methods
4.1. Microorganisms and Culture Conditions
- Staphylococcus aureus ATCC 6538;
- Staphylococcus epidermidis PCM 2118;
- Pseudomonas aeruginosa ATCC 27853;
- Escherichia coli ATCC 25922;
- Candida albicans ATCC 10231.
4.2. Ability of the Strains to Form Biofilms
4.2.1. Biomass Measurement
4.2.2. Metabolic Activity Measurement (Richards Method)
4.2.3. Confocal Microscopy
4.3. Antimicrobial and Antibiofilm Activities of Selected Antimicrobials
4.3.1. Antimicrobials
- Polyhexamethylene biguanide hydrochloride (PHMB)—Prontosan® wound irrigation solution (B. Braun, Melsungen, Germany), containing polyhexamethylene biguanide (0.1%), undecylenamidopropyl betaine (0.1%), and purified water.
- Super-oxidized solution with hypochlorites (SOHs)—Granudacyn® wound irrigation solution (Molnycke, Gothenburg, Sweden) containing water, sodium chloride, hypochlorous acid (0.005%), and sodium hypochlorite (0.005%).
- Povidone-iodine (PVP-I)—Braunol® skin solution liquid (B. Braun, Melsungen, Germany), containing 7.5% of iodized povidone with 10% available iodine, sodium dihydrogen phosphate dihydrate, sodium iodate, macrogol 9 lauryl ether, sodium hydroxide, and purified water.
4.3.2. Minimal Inhibitory Concentration (MIC)
4.3.3. Minimal Biofilm Eradication Concentration (MBEC)
4.3.4. Cellulose-Based Biofilm (CBB) Model
4.4. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Percival, S.L.; Emanuel, C.; Cutting, K.F.; Williams, D.W. Microbiology of the Skin and the Role of Biofilms in Infection. Int. Wound J. 2012, 9, 14–32. [Google Scholar] [CrossRef] [PubMed]
- Omar, A.; Wright, J.B.; Schultz, G.; Burrell, R.; Nadworny, P. Microbial Biofilms and Chronic Wounds. Microorganisms 2017, 5, 9. [Google Scholar] [CrossRef]
- Römling, U.; Kjelleberg, S.; Normark, S.; Nyman, L.; Uhlin, B.E.; Åkerlund, B. Microbial Biofilm Formation: A Need to Act. J. Intern. Med. 2014, 276, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Leaper, D.; Assadian, O.; Edmiston, C.E. Approach to Chronic Wound Infections. Br. J. Dermatol. 2015, 173, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Malone, M.; Bjarnsholt, T.; McBain, A.; James, G.; Stoodley, P.; Leaper, D.; Tachi, M.; Schultz, G.; Swanson, T.; Wolcott, R. The Prevalence of Biofilms in Chronic Wounds: A Systematic Review and Meta-Analysis of Published Data. J. Wound Care 2017, 26, 20–25. [Google Scholar] [CrossRef]
- Siddiqui, A.R.; Bernstein, J.M. Chronic Wound Infection: Facts and Controversies. Clin. Dermatol. 2010, 28, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Kramer, A.; Dissemond, J.; Kim, S.; Willy, C.; Mayer, D.; Papke, R.; Tuchmann, F.; Assadian, O. Consensus on Wound Antisepsis: Update 2018. Ski. Pharmacol. Physiol. 2018, 31, 28–58. [Google Scholar] [CrossRef]
- Hübner, N.O.; Kramer, A. Review on the Efficacy, Safety and Clinical Applications of Polihexanide, a Modern Wound Antiseptic. Ski. Pharmacol. Physiol. 2010, 23, 17–27. [Google Scholar] [CrossRef]
- Pachla, J.; Kopiasz, R.J.; Marek, G.; Tomaszewski, W.; Głogowska, A.; Drężek, K.; Kowalczyk, S.; Podgórski, R.; Butruk-Raszeja, B.; Ciach, T.; et al. Polytrimethylenimines: Highly Potent Antibacterial Agents with Activity and Toxicity Modulated by the Polymer Molecular Weight. Biomacromolecules 2023, 24, 2237–2249. [Google Scholar] [CrossRef]
- Hübner, N.O.; Matthes, R.; Koban, I.; Rändler, C.; Müller, G.; Bender, C.; Kindel, E.; Kocher, T.; Kramer, A. Efficacy of Chlorhexidine, Polihexanide and Tissue-Tolerable Plasma against Pseudomonas Aeruginosa Biofilms Grown on Polystyrene and Silicone Materials. Ski. Pharmacol. Physiol. 2010, 23 (Suppl. S1), 28–34. [Google Scholar] [CrossRef]
- Gray, M.J.; Wholey, W.Y.; Jakob, U. Bacterial Responses to Reactive Chlorine Species. Annu. Rev. Microbiol. 2013, 67, 141–160. [Google Scholar] [CrossRef] [PubMed]
- Krasowski, G.; Junka, A.; Paleczny, J.; Czajkowska, J.; Makomaska-Szaroszyk, E.; Chodaczek, G.; Majkowski, M.; Migdał, P.; Fijałkowski, K.; Kowalska-Krochmal, B.; et al. In Vitro Evaluation of Polihexanide, Octenidine and NaClO/HClO-Based Antiseptics against Biofilm Formed by Wound Pathogens. Membranes 2021, 11, 62. [Google Scholar] [CrossRef] [PubMed]
- Severing, A.L.; Rembe, J.D.; Koester, V.; Stuermer, E.K. Safety and Efficacy Profiles of Different Commercial Sodium Hypochlorite/Hypochlorous Acid Solutions (NaClO/HClO): Antimicrobial Efficacy, Cytotoxic Impact and Physicochemical Parameters In Vitro. J. Antimicrob. Chemother. 2019, 74, 365–372. [Google Scholar] [CrossRef]
- Paleczny, J.; Junka, A.F.; Krzyżek, P.; Czajkowska, J.; Kramer, A.; Benkhai, H.; Żyfka-Zagrodzińska, E.; Bartoszewicz, M. Comparison of Antibiofilm Activity of Low-Concentrated Hypochlorites vs Polyhexanide-Containing Antiseptic. Front. Cell Infect. Microbiol. 2023, 13, 1119188. [Google Scholar] [CrossRef] [PubMed]
- Shin, A.-R.; Nam, S.-H. The Effects of Various Mouthwashes on the Oral Environment Change for Oral Health Care. Available online: https://www.biomedres.info (accessed on 15 November 2023).
- Thaarup, I.C.; Bjarnsholt, T. Current In Vitro Biofilm-Infected Chronic Wound Models for Developing New Treatment Possibilities. Adv. Wound Care 2021, 10, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Vyas, H.K.N.; Xia, B.; Mai-Prochnow, A. Clinically Relevant In Vitro Biofilm Models: A Need to Mimic and Recapitulate the Host Environment. Biofilm 2022, 4, 100069. [Google Scholar] [CrossRef] [PubMed]
- Kadam, S.; Madhusoodhanan, V.; Dhekane, R.; Bhide, D.; Ugale, R.; Tikhole, U.; Kaushik, K.S. Milieu Matters: An In Vitro Wound Milieu to Recapitulate Key Features of, and Probe New Insights into, Mixed-Species Bacterial Biofilms. Biofilm 2021, 3, 100047. [Google Scholar] [CrossRef]
- Di Domenico, E.G.; Oliva, A.; Guembe, M. The Current Knowledge on the Pathogenesis of Tissue and Medical Device-Related Biofilm Infections. Microorganisms 2022, 10, 1259. [Google Scholar] [CrossRef]
- Thaarup, I.C.; Iversen, A.K.S.; Lichtenberg, M.; Bjarnsholt, T.; Jakobsen, T.H. Biofilm Survival Strategies in Chronic Wounds. Microorganisms 2022, 10, 775. [Google Scholar] [CrossRef]
- Beyene, R.T.; Derryberry, S.L.; Barbul, A. The Effect of Comorbidities on Wound Healing. Surg. Clin. N. Am. 2020, 100, 695–705. [Google Scholar] [CrossRef]
- Oropallo, A.R.; Andersen, C.; Abdo, R.; Hurlow, J.; Kelso, M.; Melin, M.; Serena, T.E. Guidelines for Point-of-Care Fluorescence Imaging for Detection of Wound Bacterial Burden Based on Delphi Consensus. Diagnostics 2021, 11, 1219. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Lorenzen, J.; Xu, Y.; Jonikaite, M.; Thaarup, I.C.; Bjarnsholt, T.; Kirketerp-Møller, K.; Thomsen, T.R. A Novel Chronic Wound Biofilm Model Sustaining Coexistence of Pseudomonas Aeruginosa and Staphylococcus Aureus Suitable for Testing of Antibiofilm Effect of Antimicrobial Solutions and Wound Dressings. Wound Repair Regen. 2021, 29, 820–829. [Google Scholar] [CrossRef] [PubMed]
- Spear, M. Wound Exudate-the Good, the Bad, and the Ugly. Plast. Surg. Nurs. 2012, 32, 77–79. [Google Scholar] [CrossRef] [PubMed]
- Cullen, B.; Gefen, A. The Biological and Physiological Impact of the Performance of Wound Dressings. Int. Wound J. 2023, 20, 1292–1303. [Google Scholar] [CrossRef] [PubMed]
- Prame Kumar, K.; Nicholls, A.J.; Wong, C.H.Y. Partners in Crime: Neutrophils and Monocytes/Macrophages in Inflammation and Disease. Cell Tissue Res. 2018, 371, 551–565. [Google Scholar] [CrossRef] [PubMed]
- Guryanova, S.V.; Ovchinnikova, T.V. Immunomodulatory and Allergenic Properties of Antimicrobial Peptides. Int. J. Mol. Sci. 2022, 23, 2499. [Google Scholar] [CrossRef] [PubMed]
- Dauner, M.; Skerra, A. Scavenging Bacterial Siderophores with Engineered Lipocalin Proteins as an Alternative Antimicrobial Strategy. ChemBioChem 2020, 21, 601–606. [Google Scholar] [CrossRef]
- Maheswary, T.; Nurul, A.A.; Fauzi, M.B. The Insights of Microbes’ Roles in Wound Healing: A Comprehensive Review. Pharmaceutics 2021, 13, 981. [Google Scholar] [CrossRef]
- Rosa, L.; Cutone, A.; Lepanto, M.S.; Paesano, R.; Valenti, P. Lactoferrin: A Natural Glycoprotein Involved in Iron and Inflammatory Homeostasis. Int. J. Mol. Sci. 2017, 18, 1985. [Google Scholar] [CrossRef]
- Sharma, S.; Mohler, J.; Mahajan, S.D.; Schwartz, S.A.; Bruggemann, L.; Aalinkeel, R. Microbial Biofilm: A Review on Formation, Infection, Antibiotic Resistance, Control Measures, and Innovative Treatment. Microorganisms 2023, 11, 1614. [Google Scholar] [CrossRef]
- Krasowski, G.; Migdał, P.; Woroszyło, M.; Fijałkowski, K.; Chodaczek, G.; Czajkowska, J.; Dudek, B.; Nowicka, J.; Oleksy-Wawrzyniak, M.; Kwiek, B.; et al. The Assessment of Activity of Antiseptic Agents against Biofilm of Staphylococcus Aureus Measured with the Use of Processed Microscopic Images. Int. J. Mol. Sci. 2022, 23, 3524. [Google Scholar] [CrossRef] [PubMed]
- Guzmán-Soto, I.; Mctiernan, C.; Gonzalez-Gomez, M.; Ross, A.; Gupta, K.; Suuronen, E.J.; Mah, T.-F.; Griffith, M.; Alarcon, E.I. Mimicking Biofilm Formation and Development: Recent Progress in In Vitro and In Vivo Biofilm Models. iScience 2021, 24, 102443. [Google Scholar] [CrossRef] [PubMed]
- Paleczny, J.; Junka, A.; Brożyna, M.; Dydak, K.; Oleksy-Wawrzyniak, M.; Ciecholewska-Juśko, D.; Dziedzic, E.; Bartoszewicz, M. The High Impact of Staphylococcus Aureus Biofilm Culture Medium on In Vitro Outcomes of Antimicrobial Activity of Wound Antiseptics and Antibiotic. Pathogens 2021, 10, 1385. [Google Scholar] [CrossRef]
- Stuermer, E.K.; Besser, M.; Brill, F.; Geffken, M.; Plattfaut, I.; Severing, A.L.; Wiencke, V.; Rembe, J.D.; Naumova, E.A.; Kampe, A.; et al. Comparative Analysis of Biofilm Models to Determine the Efficacy of Antimicrobials. Int. J. Hyg. Environ. Health 2021, 234, 113744. [Google Scholar] [CrossRef] [PubMed]
- Sauer, K.; Stoodley, P.; Goeres, D.M.; Hall-Stoodley, L.; Burmølle, M.; Stewart, P.S.; Bjarnsholt, T. The Biofilm Life Cycle: Expanding the Conceptual Model of Biofilm Formation. Nat. Rev. Microbiol. 2022, 20, 608–620. [Google Scholar] [CrossRef] [PubMed]
- Brożyna, M.; Kozłowska, W.; Malec, K.; Paleczny, J.; Fabianowska-Majewska, K.; Junka, A. Chronic Wound Milieu Challenges Essential Oils’ Antibiofilm Activity. bioRxiv 2023. [Google Scholar] [CrossRef]
- Rembe, J.D.; Huelsboemer, L.; Plattfaut, I.; Besser, M.; Stuermer, E.K. Antimicrobial Hypochlorous Wound Irrigation Solutions Demonstrate Lower Anti-Biofilm Efficacy Against Bacterial Biofilm in a Complex in-Vitro Human Plasma Biofilm Model (HpBIOM) Than Common Wound Antimicrobials. Front. Microbiol. 2020, 11, 564513. [Google Scholar] [CrossRef] [PubMed]
- Krzyżewska-Dudek, E.; Kotimaa, J.; Kapczyńska, K.; Rybka, J.; Meri, S. Lipopolysaccharides and Outer Membrane Proteins as Main Structures Involved in Complement Evasion Strategies of Non-Typhoidal Salmonella Strains. Mol. Immunol. 2022, 150, 67–77. [Google Scholar] [CrossRef]
- Blanchard, C.; Barnett, P.; Perlmutter, J.; Dunman, P.M. Identification of Acinetobacter Baumannii Serum-Associated Antibiotic Efflux Pump Inhibitors. Antimicrob. Agents Chemother. 2014, 58, 6360–6370. [Google Scholar] [CrossRef]
- Tasse, J.; Cara, A.; Saglio, M.; Villet, R.; Laurent, F. A Steam-Based Method to Investigate Biofilm. Sci. Rep. 2018, 8, 13040. [Google Scholar] [CrossRef]
- Žiemytė, M.; Carda-Diéguez, M.; Rodríguez-Díaz, J.C.; Ventero, M.P.; Mira, A.; Ferrer, M.D. Real-Time Monitoring of Pseudomonas Aeruginosa Biofilm Growth Dynamics and Persister Cells’ Eradication. Emerg. Microbes Infect. 2021, 10, 2062–2075. [Google Scholar] [CrossRef] [PubMed]
- Stiefel, P.; Rosenberg, U.; Schneider, J.; Mauerhofer, S.; Maniura-Weber, K.; Ren, Q. Is Biofilm Removal Properly Assessed? Comparison of Different Quantification Methods in a 96-Well Plate System. Appl. Microbiol. Biotechnol. 2016, 100, 4135–4145. [Google Scholar] [CrossRef] [PubMed]
- Dydak, K.; Junka, A.; Dydak, A.; Brożyna, M.; Paleczny, J.; Fijalkowski, K.; Kubielas, G.; Aniołek, O.; Bartoszewicz, M. In Vitro Efficacy of Bacterial Cellulose Dressings Chemisorbed with Antiseptics against Biofilm Formed by Pathogens Isolated from Chronic Wounds. Int. J. Mol. Sci. 2021, 22, 3996. [Google Scholar] [CrossRef]
- Attinger, C.; Wolcott, R. Clinically Addressing Biofilm in Chronic Wounds. Adv. Wound Care 2012, 1, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Crivello, G.; Fracchia, L.; Ciardelli, G.; Boffito, M.; Mattu, C. In Vitro Models of Bacterial Biofilms: Innovative Tools to Improve Understanding and Treatment of Infections. Nanomaterials 2023, 13, 904. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, K.; Preem, L.; Sagor, K.; Putrinš, M.; Tenson, T.; Kogermann, K. Development of In Vitro and Ex Vivo Biofilm Models for the Assessment of Antibacterial Fibrous Electrospun Wound Dressings. Mol. Pharm. 2023, 20, 1230–1246. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Dudek-Wicher, R.; Paleczny, J.; Kowalska-Krochmal, B.; Szymczyk-Ziółkowska, P.; Pachura, N.; Szumny, A.; Brożyna, M. Activity of Liquid and Volatile Fractions of Essential Oils against Biofilm Formed by Selected Reference Strains on Polystyrene and Hydroxyapatite Surfaces. Pathogens 2021, 10, 515. [Google Scholar] [CrossRef]




| Minimal Inhibitory Concentration (mg/L) | ||||||
|---|---|---|---|---|---|---|
| PHMB | SOHs | PVP-I | ||||
| MH | IVWM | MH | IVWM | MH | IVWM | |
| S. aureus | 2 | 2 | R | R | 1172 | 937.5 |
| S. epidermidis | 0.5 | 0.5 | 5 | R | 586 | 4688 |
| E. coli | 2 | 9 | R | R | 2344 | 9375 |
| P. aeruginosa | 8 | 63 | R | R | 9375 | 9375 |
| C. albicans | 0.5 | 0.5 | R | R | 2344 | 9375 |
| Minimal Biofilm Eradication Concentration (mg/L) | ||||||
|---|---|---|---|---|---|---|
| PHMB | SOHs | PVP-I | ||||
| MH | IVWM | MH | IVWM | MH | IVWM | |
| S. aureus | 125 | 250 | 0.100 | 100 | 938 | 18,750 |
| S. epidermidis | ND | ND | ND | ND | ND | ND |
| E. coli | 63 | 125 | 100 | 100 | 9380 | 18,750 |
| P. aeruginosa | 500 | 250 | R | R | 18,750 | 18,750 |
| C. albicans | 15 | 500 | 100 | R | 9380 | 18,750 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paleczny, J.; Brożyna, M.; Dudek, B.; Woytoń, A.; Chodaczek, G.; Szajnik, M.; Junka, A. Culture Shock: An Investigation into the Tolerance of Pathogenic Biofilms to Antiseptics in Environments Resembling the Chronic Wound Milieu. Int. J. Mol. Sci. 2023, 24, 17242. https://doi.org/10.3390/ijms242417242
Paleczny J, Brożyna M, Dudek B, Woytoń A, Chodaczek G, Szajnik M, Junka A. Culture Shock: An Investigation into the Tolerance of Pathogenic Biofilms to Antiseptics in Environments Resembling the Chronic Wound Milieu. International Journal of Molecular Sciences. 2023; 24(24):17242. https://doi.org/10.3390/ijms242417242
Chicago/Turabian StylePaleczny, Justyna, Malwina Brożyna, Bartłomiej Dudek, Aleksandra Woytoń, Grzegorz Chodaczek, Marta Szajnik, and Adam Junka. 2023. "Culture Shock: An Investigation into the Tolerance of Pathogenic Biofilms to Antiseptics in Environments Resembling the Chronic Wound Milieu" International Journal of Molecular Sciences 24, no. 24: 17242. https://doi.org/10.3390/ijms242417242
APA StylePaleczny, J., Brożyna, M., Dudek, B., Woytoń, A., Chodaczek, G., Szajnik, M., & Junka, A. (2023). Culture Shock: An Investigation into the Tolerance of Pathogenic Biofilms to Antiseptics in Environments Resembling the Chronic Wound Milieu. International Journal of Molecular Sciences, 24(24), 17242. https://doi.org/10.3390/ijms242417242






